Abstract
Introduction: Hospitals are in a unique position to promote, protect, and support breastfeeding. However, the association between in-hospital events and breastfeeding success within population-based samples has not been well studied.
Materials and Methods: A stratified (by education and birth weight) systematic sample of 5,770 mothers taking part in the Utah Pregnancy Risk Assessment Monitoring System, 2012–2015, were included. Mothers, 2–4 months postpartum, completed the 82-item questionnaire, including if they had ever breastfed their new baby, and if so, current breastfeeding status. Relationships between in-hospital experiences and breastfeeding termination and duration were evaluated via Poisson and Cox proportional hazard regression models, respectively, adjusting for other in-hospital experiences, maternal age, race/ethnicity, maternal education, marital status, smoking, physical activity, delivery method, pregnancy complications, and length of hospital stay.
Results: Of all, 94.4% of mothers self-reported breastfeeding initiation, of whom 18.8% had breastfed <2 months, having breastfed on average 3.2 weeks (standard error: 0.07). In fully adjusted models, mothers who reported receiving a pacifier, receiving formula, or had staff help them learn how to breastfeed had a higher prevalence of terminating breastfeeding before 2 months (adjusted prevalence ratio [aPR] = 1.13, 95% confidence interval [CI]: 0.97–1.32; aPR = 1.20, 95% CI: 1.07–1.36; and aPR = 1.25, 95% CI: 1.08–1.34). Conversely, mothers who reported starting and feeding only breast milk in the hospital and receiving a phone number to call for help with breastfeeding had a lower prevalence of breastfeeding termination before 2 months (aPR = 0.72, 95% CI: 0.61–0.86; aPR = 0.57, 95% CI: 0.51–0.64; and aPR = 0.91, 95% CI: 0.80–1.03). Adjusted Cox models showed similar direction of associations.
Conclusions: Encouraging mothers to exclusively breastfeed in the hospital, and reducing gift packs containing pacifiers and formula, may be key areas United States hospitals can focus on to increase breastfeeding success. Prospective assessment in other geographical regions is needed to corroborate these findings.
Keywords: breastfeeding, lactation, infant formula, pacifiers, postpartum period, hospital
Introduction
The World Health Organization (WHO), the American Academy of Pediatrics (AAP), the American College of Obstetricians and Gynecologists, and the United States Preventive Services Task Force recommend exclusive breastfeeding for the first 6 months of life, with the AAP recommending continued breastfeeding up to 1 year and WHO up to 2 years.1–4 In addition, a systematic review of the literature strongly encourages continued breastfeeding due to the known empirical benefits such as improved immune and gastrointestinal function, enhanced dietary nutrition, and overall psychological well-being for both the infant and mother.1,5,6
In contrast, the negative effects of not being breastfed as an infant include increased incidence of infectious morbidity and elevated risks of childhood obesity, type 2 diabetes, and sudden infant death syndrome.7 For mothers, breastfeeding is associated with lower risk of maternal bleeding after delivery, more rapid postpartum weight loss, lower levels of stress, lower incidence of breast and ovarian cancer, type 2 diabetes, and cardiovascular disease.7–9 A cost analysis conducted in 2010 predicted that if 90% of American families exclusively breastfed for 6 months, the United States would save $13 billion per year and prevent 911 pediatric deaths.10
Many measures take place within hospitals to enhance the care of the newborn infant. Some measures may promote breastfeeding, such as helping mothers initiate breastfeeding within 1 hour of birth or encouraging breastfeeding on demand, while others may deter such as providing gift packs with formula or a pacifier. Measures that enhance breastfeeding have been largely informed by the 10 Steps to Successful Breastfeeding, developed by a team of experts from the Baby-Friendly Hospital Initiative (BFHI).
While the United States has seen a 22% rise in births taking place in BFHI designated facilities since 2007, three-quarters of births still occur outside of BFHI facilities. Previous research, among populations drawn from mail/telephone/internet sampling frames, has clearly documented how the 10 steps increase breastfeeding duration, including initiation of breastfeeding within the first hour of birth (Step 4), exclusive breastfeeding (Step 6), and not providing pacifiers (Step 9).11–14 More recent research has begun to tease apart the modifying effects of age, race, psychosocial and lifestyle factors, and offspring health status on the relationship between hospital practices and breastfeeding duration.6,14–19
Further research, particularly among population-based samples that include at-risk women, regarding how hospital practices influence breastfeeding success is needed. Such research is particularly needed within states that fall behind BFHI designation, including Utah that makes up only one of the 539 (0.002%) BFHI designated facilities in the United States despite having the highest birthrate in the country (Utah: 16.9 births/1,000 women; United States: 12.4 births/1,000 women).20 In addition, while nationally representative samples are important for informing national and global policies, analyses of specific regions within the United States are important for targeted interventions.
To further enhance the knowledge of breastfeeding success within at-risk populations, an analysis of the Utah Pregnancy Risk Assessment and Monitoring System data (UT-PRAMS, 2012–2015) was conducted to determine the association between in-hospital newborn care enhancement measures (IHNCEM) and early postpartum breastfeeding continuation. Our study population, oversampled by low birth weight infants and low maternal education, may inform optimal places for intervention among new mothers who are susceptible to neither initiating nor continuing breastfeeding.
Methods
Study population
Study participants were mothers who participated in the UT-PRAMS between 2012 and 2015. PRAMS is a multistate, population-based surveillance system funded by the Centers for Disease Control and Prevention (CDC) in collaboration with state health departments. PRAMS surveys include both core questions that are administered in all participating states, and state-specific questions. PRAMS collects data on maternal experiences and behaviors that occur before, during, and after pregnancy as well as in early infancy, and is administered at 2–6 months after delivery. Topics addressed in the PRAMS questionnaire include barriers to and content of prenatal care, obstetric history, maternal use of alcohol and cigarettes, physical abuse, contraception, economic status, maternal stress, and early infant development and health status. Details of PRAMS methodology and protocols have been published elsewhere.21
In Utah, a sample of ∼200 new mothers per month is randomly selected from birth certificate data to participate in PRAMS. Each state taking part in PRAMS has a unique stratified sampling scheme, with Utah stratifying on maternal education and infant birth weight, to ensure that adequate data are available in smaller but higher risk populations. In Utah, this sample represents ∼5% of all live births.
Mothers who are selected receive via mail a pre-letter introducing PRAMS to the mother and, 3–7 days later, the initial mail questionnaire packet. Nonrespondents are mailed up to three follow-up ticklers or questionnaire packets (sent ∼1–2 weeks after each nonresponse) and telephone contact (1–2 weeks after non-response from last questionnaire, up to 15 attempts). Following this CDC-developed protocol, a 60% response rate was expected. Response rates for the UT sample were 72% in 2012, 66% in 2013, 69% in 2014, and 67% in 2015.
The survey is available in both English and Spanish, with mothers marked as Hispanic on birth certificate receiving both Spanish and English versions. Mothers of twins and triplets have one infant randomly selected by the state's department of health to be the index infant. Mothers whose pregnancy ended in stillbirth are excluded.
Breastfeeding initiation/duration measures
As part of the Utah Phase 7 (2012–2015) questionnaire, women were asked “Did you ever breastfeed or pump breast milk to feed your new baby, even for a short period of time?” Women who answered yes were then asked “Are you currently breastfeeding or feeding pumped milk to your new baby?” and if no, “How many weeks or months did you breastfeed or pump milk to feed your baby?”
In-hospital newborn care enhancement measures
Women who confirmed to have ever breastfed or pumped breast milk for their new baby, even for a short period of time, were then asked about events that may have happened at the hospital where their new baby was born, requiring a yes/no response for each: (1) “Hospital staff gave me information about breastfeeding”; (2) “My baby stayed in the same room with me at the hospital”; (3) “Hospital staff helped me learn how to breastfeed”; (4) “I breastfed in the first hour after my baby was born”; (4) “I breastfed my baby in the hospital”; (5) “My baby was fed only breast milk at the hospital”; (6) “Hospital staff told me to breastfeed whenever my baby wanted”; (7) “The hospital gave me a breast pump to use”; (8) “The hospital gave me a gift pack with formula”; (9) “The hospital gave me a telephone number to call for help with breastfeeding”; and (10) “Hospital staff gave my baby a pacifier.”
Covariates
Guided by the existing literature,15,16,22–24 we took into account potential covariates likely to impact the link between IHNCEM and early postpartum breastfeeding continuation. Factors captured via the linked birth certificates included maternal age, education, marital status, race/ethnicity, plurality (singleton versus multiple birth), pregnancy complications (preterm birth [gestational age <37 weeks], hypertensive disorders of pregnancy, gestational diabetes, and birth defect), and delivery method. Factors captured via the PRAMS survey responses included smoking, physical activity, postpartum depressive symptoms, and length of hospital stay for most recent birth.
We defined smoking (yes/no) as any woman reporting smoking any cigarettes in the past 2 years. Physical activity was categorized into four groups (<1 day/week, 1–2 days/week, 3–4 days/week, ≥5 days/week) based on women's reported participation in any physical activities or exercise during the last 3 months of pregnancy. Finally, we defined women to have postpartum depressive symptoms if they answered “always” or “often” to either of the following two questions: (1) “Since your new baby was born, how often have you felt down, depressed, or hopeless?” and 2) “Since your new baby was born, how often have you had little interest or little pleasure in doing things?”
Statistical analysis
Of the total of 204,293 live births that occurred in Utah between 2012 and 2015, 5,770 women completed the PRAMS survey. Of these, we excluded 113 mothers (2.0%) due to their child being born outside of the hospital and an additional 156 (2.7%) who did not answer whether they had ever breastfed their infant leaving a total sample of 5,501 mothers.
Descriptive characteristics of the Utah mothers who completed the survey by breastfeeding termination <2 months versus ≥2 months were calculated and compared by chi square or t-tests as appropriate, taking into account the stratified systematic sampling in the analyses.
Relationships between IHNCEMs and breastfeeding termination <2 months versus ≥2 months were evaluated via unadjusted and adjusted Poisson regression to generate prevalence ratios (PRs) and 95% confidence intervals (CIs) taking into account stratified systematic sampling.25 In addition, to model time to breastfeeding cessation, adjusted Cox regression analyses were used to generate hazard ratios (HR) and 95% CI also taking into account stratified systematic sampling. Cox proportional hazards regression models correspond to the week-specific probability of breastfeeding termination after initiation at hospital birth. Women who were still breastfeeding at the time of the questionnaire were right censored.
Factors thought to possibly impact IHNCEM and breastfeeding termination and time to breastfeeding termination were considered as potential confounders. In our fully adjusted models, we account for other IHNCEM measures, maternal age (continuous), race/ethnicity (white/non-Hispanic, white/Hispanic, nonwhite/non-Hispanic, nonwhite/Hispanic), maternal education (0–8 years, 9–11 years, 12 years, 13–15 years, ≥16 years), marital status (yes/no), smoking (yes/no), exercise in last trimester (<1 days/week, 1–2 days/week, >3–4 days/week, ≥5 days/week), vaginal delivery (yes/no), multiples (yes/no), preterm birth (yes/no), hypertensive disorder of pregnancy (yes/no), gestational diabetes (yes/no), birth defect (yes/no), and length of hospital stay (<1 day, 1–2 days, 3–5 days, 6–14 days, >14 days, still in the hospital). Analyses were completed using SAS version 9.4 (SAS Institute, Inc.) and Stata version 14.2 (StataCorp, LLC).
Results
Mothers completed the 2012–2015 UT-PRAMS survey on average at 16 weeks, interquartile range: 13–19 weeks, range: 8–42 weeks. The majority of mothers self-reported breastfeeding initiation (94.4%), and of those, 18.8% had breastfed <2 months, having breastfed on average for 3.2 weeks (standard error: 0.07), compared to 81.2% of mothers who breastfed ≥2 months or were still breastfeeding at the time of the survey. Women who breastfed <2 months versus ≥2 months were more likely to be younger, nonwhite, Hispanic, of lower education, nonmarried, smoker, and whose labor was complicated by a cesarean section delivery, multiple birth, preterm birth, and a longer hospital stay (Table 1).
Table 1.
Population Characteristics: Utah Pregnancy Risk Assessment Monitoring System 2012–2015 by Breastfeeding Duration Status
| Characteristics | Overall | Breastfed <2 months 19.8% | Breastfed ≥2 months 80.2% | p-Value |
|---|---|---|---|---|
| Age, mean (SE) | 28.1 (0.8) | 26.8 (0.2) | 28.5 (0.1) | <0.001 |
| Race/ethnicity (%) | <0.001 | |||
| White, non-Hispanic | 78.9 | 71.1 | 80.7 | |
| White, Hispanic | 6.3 | 9.3 | 5.6 | |
| Other, non-Hispanic | 5.8 | 6.4 | 5.7 | |
| Other, Hispanic | 9.0 | 13.2 | 8.1 | |
| Maternal education level (%) | <0.001 | |||
| 0–8 Years | 1.7 | 1.7 | 1.7 | |
| 9–11 Years | 7.7 | 15.4 | 6.0 | |
| 12 Years | 17.8 | 26.6 | 15.8 | |
| 13–15 Years | 36.0 | 37.2 | 35.8 | |
| ≥16 Years | 36.7 | 19.1 | 40.8 | |
| Married (%) | 84.1 | 68.6 | 87.7 | <0.001 |
| Smoker (%) | 3.4 | 7.9 | 2.3 | <0.001 |
| Exercise last trimester (%) | 0.40 | |||
| <1 Days/week | 29.3 | 31.2 | 28.9 | |
| 1–2 Days/week | 33.1 | 32.4 | 33.3 | |
| 3–4 Days/week | 26.1 | 24.0 | 26.6 | |
| ≥5 Days/week | 11.5 | 12.4 | 11.3 | |
| Delivery method (%) | ||||
| First C-section | 11.1 | 14.8 | 10.2 | <0.001 |
| Forceps delivery | 1.3 | 1.9 | 1.2 | 0.10 |
| Repeated C-section | 10.7 | 13.1 | 10.1 | 0.02 |
| Vacuum delivery | 2.9 | 3.5 | 2.8 | 0.26 |
| Vaginal delivery (VD) | 75.2 | 70.6 | 76.3 | 0.002 |
| VD after C-section | 3.1 | 1.6 | 3.4 | 0.01 |
| Plurality (%) | 0.02 | |||
| Single child birth | 98.4 | 97.5 | 98.6 | |
| Twin/triplet | 1.6 | 2.4 | 1.4 | |
| Preterm birth (%) | 7.7 | 10.0 | 7.2 | 0.002 |
| Hypertensive pregnancy (%) | 6.1 | 7.1 | 5.9 | 0.20 |
| Gestational diabetes (%) | 4.6 | 5.3 | 4.4 | 0.23 |
| Birth defect (%) | 0.7 | 0.4 | 0.8 | 0.07 |
| Hospital length of stay (%) | <0.001 | |||
| <1 Day | 4.9 | 4.2 | 5.0 | |
| 1–2 Days | 65.4 | 60.0 | 66.7 | |
| 3–5 Days | 22.8 | 27.0 | 21.9 | |
| 6–14 Days | 4.1 | 5.8 | 3.7 | |
| >14 Days | 2.7 | 2.8 | 2.6 | |
| Still in hospital | 0.1 | 0.2 | 0.1 |
Descriptive characteristics of the Utah mothers by breastfeeding duration <2 months versus ≥2 months were calculated by Chi Square or t-tests as appropriate, taking into account the stratified random sampling in the analysis. Analysis includes mothers who ever breastfed their infant and completed the question on whether they were still breastfeeding at time of survey, and if not, breastfeeding duration (n = 5,102). Missing n = 0 for maternal age, n = 124 for race/ethnicity, n = 261 for maternal education, n = 11 for marital status, n = 463 for exercise, n = 11 for smoking status, n = 1 for delivery method, n = 0 for maternal hypertension, n = 0 for gestational diabetes, n = 0 for preterm birth, n = 7 for birth defect, and n = 35 for length of hospital stay.
SE, standard error.
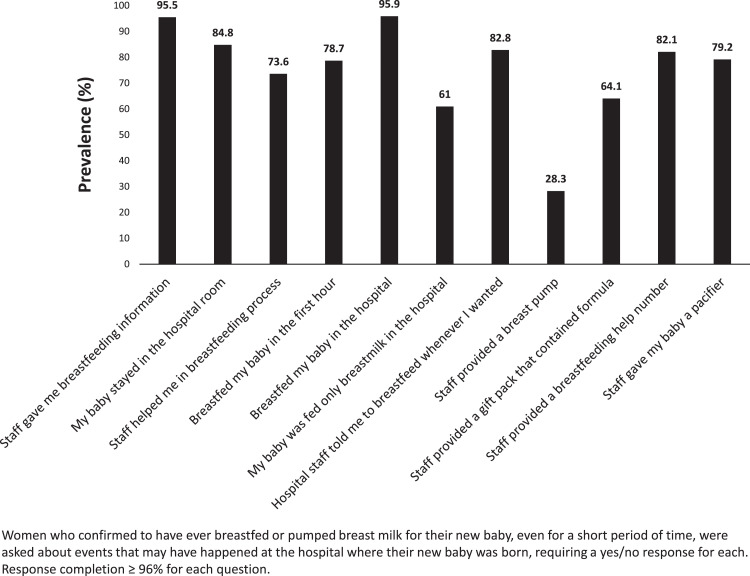
The frequency of receiving each of the IHNCEMs ranged from 28.3% (staff provided a breast pump) to 95.9% (breastfed my baby in the hospital) (Fig. 1). Adjusting for the other IHNCEMs, we found that mothers who reported receiving a pacifier, a gift pack with formula, or had staff help them learn how to breastfeed had a higher prevalence of terminating breastfeeding at <2 months (adjusted prevalence ratio [aPR] = 1.18, 95% CI: 1.02–1.36; aPR = 1.21, 95% CI: 1.08–1.36; and aPR = 1.37, 95% CI: 1.20–1.57, respectively) (Table 2). Conversely, mothers who reported starting breastfeeding in the hospital, feeding only breast milk in the hospital, and receiving a telephone number to call for help with breastfeeding had a lower prevalence of terminating breastfeeding at <2 months (aPR = 0.61, 95% CI: 0.52–0.71; aPR = 0.54, 95% CI: 0.49–0.61; aPR: 0.85, 95% CI: 0.76–0.96 respectively) (Table 2).
FIG. 1.
Prevalence of in-hospital newborn care enhancement measures (UT-PRAMS 2012–2015). UT-PRAMS, Utah Pregnancy Risk Assessment Monitoring System.
Table 2.
Breastfeeding Duration (<2 Months Versus ≥2 Months) Prevalence Ratios by In-Hospital Newborn Care Enhancement Measures: Utah Pregnancy Risk Assessment Monitoring System 2012–2015, N = 657
| Contributing factors | Unadjusted | Model 1 | Model 2 |
|---|---|---|---|
| PR (95% CI) | PR (95% CI) | PR (95% CI) | |
| “Hospital staff gave me information about breastfeeding” | 0.93 (0.75–1.15) | 0.83 (0.66–1.05) | 0.97 (0.76–1.24) |
| “My baby stayed in the same room with me at the hospital” | 0.82 (0.73–0.93) | 1.14 (0.99–1.30) | 1.05 (0.88–1.26) |
| “Hospital staff helped me learn how to breastfeed” | 1.35 (1.19–1.53) | 1.37 (1.20–1.57) | 1.25 (1.08–1.44) |
| “I breastfed in the first hour after my baby was born” | 0.68 (0.61–0.75) | 0.91 (0.81–1.04) | 0.94 (0.82–1.08) |
| “I breastfed my baby in the hospital” | 0.46 (0.41–0.52) | 0.61 (0.52–0.71) | 0.72 (0.61–0.86) |
| “My baby was fed only breast milk in the hospital” | 0.48 (0.43–0.53) | 0.54 (0.49–0.61) | 0.57 (0.51–0.64) |
| “Hospital staff told me to breastfeed whenever my baby wanted” | 0.81 (0.72–0.91) | 0.97 (0.86–1.10) | 0.99 (0.88–1.13) |
| “Hospital staff gave me a breast pump to use” | 1.40 (1.26–1.54) | 1.09 (0.98–1.23) | 1.04 (0.91–1.18) |
| “Hospital gave me a gift pack with formula” | 1.41 (1.26–1.58) | 1.21 (1.08–1.36) | 1.20 (1.07–1.36) |
| “Hospital gave me telephone number to call for help with breastfeeding” | 0.85 (0.75–0.96) | 0.85 (0.76–0.96) | 0.91 (0.80–1.03) |
| “Hospital staff gave my baby a pacifier” | 1.41 (1.22–1.63) | 1.18 (1.02–1.36) | 1.13 (0.97–1.32) |
Model 1: Adjusted for other IHNCEM measures.
Model 2: Adjusted for other IHNCEM measures, maternal age (continuous), race/ethnicity (white/non-Hispanic, white/Hispanic, nonwhite/non-Hispanic, nonwhite/Hispanic), maternal education (0–8 years, 9–11 years, 12 years, 13–15 years, ≥16 years), marital status (yes/no), smoking (yes/no), exercise in last trimester (<1 day/week, 1–2 days/week, 3–4 days/week, ≥5 days/week), vaginal delivery (yes/no), multiples (yes/no), preterm birth, hypertensive disorder of pregnancy, gestational diabetes, birth defect, and length of hospital stay (<1 day, 1–2 days, 3–5 days, 6–14 days, >14 days, still in the hospital).
IHNCEM, in-hospital newborn care enhancement measures; CI, confidence interval; PR, prevalence ratio.
Direction and magnitude of estimates were similar in our fully adjusted models compared with IHNCEM-adjusted models with pacifier, gift packs, and help associated with a higher prevalence of terminating breastfeeding at <2 months (aPR = 1.13, 95% CI: 0.97–1.32; aPR = 1.20, 95% CI: 1.07–1.36; and aPR = 1.25, 95% CI: 1.08–1.34, respectively); and starting breastfeeding in the hospital, feeding only breast milk in the hospital, and receiving a phone number to call for help associated with a lower prevalence of terminating breastfeeding at <2 months (aPR = 0.72, 95% CI: 0.61–0.86, aPR = 0.57, 95% CI: 0.51–0.64; aPR = 0.91, 95% CI: 0.80–1.03, respectively).
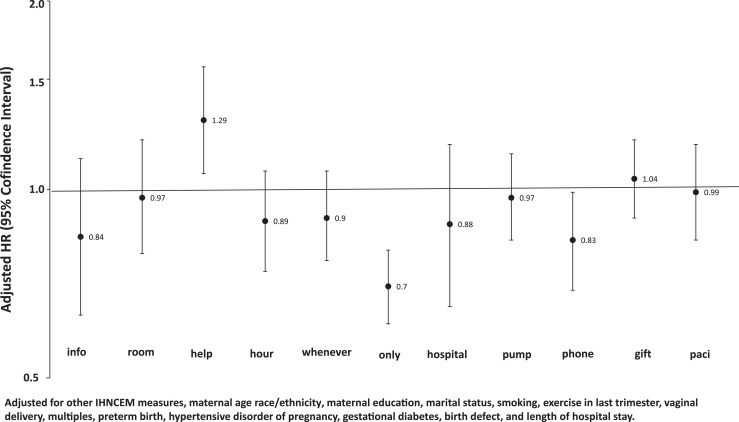
Adjusted Cox regression models (modeling week-specific probability of breastfeeding termination after initiation) showed similar direction of effects (Fig. 2). However, feeding only breast milk in the hospital (aHR: 0.70; 95% CI: 0.61–0.80) and receiving a telephone number to call for help with breastfeeding (aHR: 0.83; 95% CI: 0.69–0.99) showed the strongest enhancing association, while having staff help mothers learn how to breastfeed (aHR: 1.29; 95% CI: 1.06–1.57) showed the strongest detrimental association.
FIG. 2.
Adjusted HR for breastfeeding discontinuation by in-hospital newborn care enhancement measures (UT-PRAMS 2012–2015). HR, hazard ratios; Paci, pacifier.
Discussion
In this population-based study, representative of Utah mothers who gave birth during 2012–2015, we found that women who started breastfeeding in the hospital, exclusively breastfed in the hospital, and had a phone number to call for help with breastfeeding had a 28%, 43%, and 9% reduced probability, respectively, of terminating breastfeeding <2 months versus ≥2 months postdelivery after adjusting for important confounding factors. Conversely, women who received a pacifier, gift pack with formula, or had breastfeeding help by hospital staff had a 13%, 20%, and 25% increased probability, respectively, of terminating breastfeeding by 2 months postdelivery.
Given the physiological and psychological benefits of breastfeeding, most professional maternal and infant health organizations recommend exclusive breastfeeding for at least the first 6 months of a newborn's life. Similar to findings from the United States National Immunization Surveys (2016–2017) that reported 89.7% of Utah babies were ever breastfed and 62.5% were still being breastfed at 6 months,26 we found that 94.4% of UT-PRAMS participants reported initiating breastfeeding and 69.0% of mothers reported still breastfeeding at the time of survey completion (2–4 months postpartum). Utah ranks in the top third in the nation in regard to both breastfeeding initiation and duration to at least 6 months26 and is comfortably above the Healthy People 2020 goal of 81.9% ever breastfed and 60.6% breastfed to 6 months. However, Utah still has room to improve. Utah has dropped 5% in babies ever breastfed and 7% in babies still being breastfed at 6 months from the 2014–2015 to the 2016–2017 national surveys.26 In addition, Utah ranks worse than the national average in all of CDCs Breastfeeding Support Indicators, including birth facilities providing maternity care supportive of breastfeeding (via Maternity Practices in Infant Nutrition and Care score), number of designated Baby Friendly Hospitals, percent of infants receiving formula before 2 days of age, and number of lactation consultants per 1,000 live births.26 Despite having the highest birth rate in the nation (16.5 births/1,000 women aged 15–44),20 Utah is one of four states to have only one BFHI facility.27 Indeed, in opposition to Baby-Friendly United States of America recommendations to give newborn infants “no food or drink other than human milk, unless medically indicated” and “no artificial teats or pacifiers,” 78.8% of women participating in the UT-PRAMS survey reported receiving a pacifier by hospital staff and 67.0% reported receiving a gift pack that contained formula by hospital staff.
While prior observational and randomized control trials have shown that pacifier use is associated with early breast weaning,28,29 one trial among 281 mother–infant pairs showed that advice to avoid pacifier use did not significantly reduce weaning at 3 months.30 Authors of this study concluded that pacifier use may be an indicator of breastfeeding difficulties rather than a cause of early weaning.31 This reasoning may explain the increased risk of weaning <2 versus ≥2 months postpartum among women in our study who reported that hospital staff helped them learn how to breastfeed. Reverse causation is a likely explanation for the significant link found between hospital support of breastfeeding and early weaning—women who are having difficulties breastfeeding are more likely to terminate early and are simultaneously more likely to be receiving breastfeeding support.
The evidence is more convincing that hospital-associated provisions of non-breast milk supplements without medical necessity contributes to early weaning.30,32 Because there are so few absolute contraindications to breastfeeding, breast milk supplementation should only occur in the presence of a clear medical indication.1,33,34 While UT-PRAMS did not collect information regarding whether infants were fed formula within the hospital, providing formula to women was marginally associated with an increased risk of breastfeeding termination by 2 months postpartum, suggesting that one possible change that would encourage exclusive breastfeeding for a longer period is to have more hospitals follow the Baby-Friendly guideline to abstain from such practice unless supplementation is medically warranted. While the use of pasteurized donor milk is another option for women whose supply of maternal milk is insufficient, currently its low availability and high cost make it an unviable option for the majority of women.35
Regarding protective factors for breastfeeding, we found that starting breastfeeding in the hospital and exclusively breastfeeding in the hospital were the two key measures linked with postpartum breastfeeding continuance. Key practices that hospitals and birthing facilities implement, such as the 10-step BFHI, have been shown to increase breastfeeding initiation and exclusivity.14,21 However, more research is needed to clarify how these practices lead to successful breastfeeding, and the adverse impacts of differential implementation of these practices by age or other sociodemographic factors.
Strengths of our study included using a population-based survey, which was weighted to represent all mothers who gave birth from 2012 to 2015 while living in Utah. The sample size of the study was reflective of the Utah population with weights to ensure inclusivity of at-risk women.
Limitations of our study include potential selection bias. While PRAMs follows a rigorous protocol for obtaining surveys from sampled mothers, Utah's average response rate for 2012–2015 was (69%), limiting generalizability for all UT mothers. In addition, the survey uses self-report measures, which allows for potential reporting bias. Given that breastfeeding is widely promoted by maternal and infant health organizations, our use of a self-reported breastfeeding status and duration of breastfeeding may be prone to social desirability bias. However, while we cannot rule out misclassification of outcome, prior research showing strong agreement between birth certificate and PRAMs self-reported breastfeeding initiation status (κ = 0.72) gives us confidence that this bias would be minimal and not likely to be associated with IHNCEM predictors. Finally, we were not able to assess whether women were given gift packs with formula and/or pacifiers because they asked for them (which may indicate they were having problems with breastfeeding or did not intend to continue breastfeeding) or whether they were given them based on standard practice. Not knowing makes it difficult to rule out reverse causation. Future PRAMs questionnaires may wish to consider asking questions to better assess breastfeeding difficulties and breastfeeding intention.
Conclusion
We found that the hospital providing postpartum mothers with free pacifiers and gift packs containing formula were the two events most strongly associated with breastfeeding discontinuation before 2 months. Initiating and exclusively breastfeeding in the hospital were the two events most strongly associated with continued breastfeeding. While receiving help, breastfeeding is most likely to occur among mothers having difficulties (thus reverse causation cannot be ruled out), the inverse relationship we found between receiving help and breastfeeding success signals that proper training among the appropriate health care staff is essential.36
Acknowledgments
Data were provided by the Utah Pregnancy Risk Assessment Monitoring System (PRAMS), a project of the Utah Department of Health (UDOH), the Office of Vital Records and Health Statistics of the UDOH, and the Center for Disease Control and Prevention (CDC) of the United States Health and Human Services Department. This report does not represent the official views of the CDC or the Utah Department of Health.
Disclosure Statement
No competing financial interests exist.
References
- 1. Eidelman AI. Breastfeeding and the use of human milk: An analysis of the American Academy of Pediatrics 2012 Breastfeeding Policy Statement. Breastfeed Med 2012;7:323–324 [DOI] [PubMed] [Google Scholar]
- 2. Committee on Health Care for Underserved Women. ACOG committee opinion no. 361: Breastfeeding: Maternal and infant aspects. Obstet Gynecol 2007;109:479–480 [DOI] [PubMed] [Google Scholar]
- 3. Calonge N, Petitti DB, DeWitt TG, et al. Primary care interventions to promote breastfeeding: US Preventive Services Task Force recommendation statement. Ann Intern Med 2008;149:560–564 [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization and UNICEF. Global Strategy for Infant and Young Child Feeding. Geneva, Switzerland: World Health Organization, 2003. [Google Scholar]
- 5. Hauk L. AAFP releases position paper on breastfeeding. Am Fam Physician 2015;91:56–57 [Google Scholar]
- 6. Victora CG, Bahl R, Barros AJ, et al. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016;387:475–490 [DOI] [PubMed] [Google Scholar]
- 7. Dieterich CM, Felice JP, O'Sullivan E, et al. Breastfeeding and health outcomes for the mother-infant dyad. Pediatr Clin North Am 2013;60:31–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sibolboro Mezzacappa E, Endicott J. Parity mediates the association between infant feeding method and maternal depressive symptoms in the postpartum. Arch Womens Ment Health 2007;10:259–266 [DOI] [PubMed] [Google Scholar]
- 9. Godfrey JR, Lawrence RA. Toward optimal health: The maternal benefits of breastfeeding. J Womens Health (Larchmt) 2010;19:1597–1602 [DOI] [PubMed] [Google Scholar]
- 10. Bartick M, Reinhold A. The burden of suboptimal breastfeeding in the United States: A pediatric cost analysis. Pediatrics 2010;125:e1048–e1056 [DOI] [PubMed] [Google Scholar]
- 11. Nickel NC, Labbok MH, Hudgens MG, et al. The extent that noncompliance with the ten steps to successful breastfeeding influences breastfeeding duration. J Hum Lact 2013;29:59–70 [DOI] [PubMed] [Google Scholar]
- 12. Declercq E, Labbok MH, Sakala C, et al. Hospital practices and women's likelihood of fulfilling their intention to exclusively breastfeed. Am J Public Health 2009;99:929–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. DiGirolamo AM, Grummer-Strawn LM, Fein SB. Effect of maternity-care practices on breastfeeding. Pediatrics 2008;122:S43–S49 [DOI] [PubMed] [Google Scholar]
- 14. Munn AC, Newman SD, Mueller M, et al. The impact in the United States of the baby-friendly hospital initiative on early infant health and breastfeeding outcomes. Breastfeed Med 2016;11:222–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sipsma HL, Jones K, Nickel NC. Hospital practices to promote breastfeeding: The effect of maternal age. Birth 2017;44:272–280 [DOI] [PubMed] [Google Scholar]
- 16. Kair LR, Colaizy TT. Association between in-hospital pacifier use and breastfeeding continuation and exclusivity: Neonatal intensive care unit admission as a possible effect modifier. Breastfeed Med 2017;12:12–19 [DOI] [PubMed] [Google Scholar]
- 17. Pounds L, Shostrom V. Analyzing factors that impact breastfeeding duration in the postpartum period: A secondary analysis of PRAMS data. Breastfeed Med 2018;13:335–340 [DOI] [PubMed] [Google Scholar]
- 18. Chung M, Raman G, Chew P, et al. Breastfeeding and maternal and infant health outcomes in developed countries. Evid Technol Asses (Full Rep) 2007;153:1–186 [PMC free article] [PubMed] [Google Scholar]
- 19. Centers for Disease Control and Prevention. Breastfeeding among US children born 2000–2010, CDC National Immunization Survey. Available at https://cdc.gov/breastfeeding/data/nis_data/index.htm (acccessed July 9, 2018)
- 20. Martin JA, Hamilton BE, Osterman MJK, et al. Births: Final Data for 2015. National Vital Statistics Report (vol. 66, no. 1). Hyattsville, MD: National Center for Health Statistics, 2017. [PubMed] [Google Scholar]
- 21. Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion. Pregnancy risk assessment measurement scale (PRAMS). Available at https://cdc.gov/prams/index.htm (accessed July9, 2018)
- 22. Boies EG, Vaucher YE. ABM clinical protocol #10: Breastfeeding the late preterm (34–36 6/7 weeks of gestation) and early term infants (37–38 6/7 weeks of gestation), second revision 2016. Breastfeed Med 2016;11:494–500 [DOI] [PubMed] [Google Scholar]
- 23. Mathews ME, Leerkes EM, Lovelady CA, et al. Psychosocial predictors of primiparous breastfeeding initiation and duration. J Hum Lact 2014;30:480–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wallwiener S, Muller M, Doster A, et al. Predictors of impaired breastfeeding initiation and maintenance in a diverse sample: What is important? Arch Gynecol Obstet 2016;294:455–466 [DOI] [PubMed] [Google Scholar]
- 25. Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol 2005;162:199–200 [DOI] [PubMed] [Google Scholar]
- 26. Centers for Disease Control and Prevention. Breastfeeding report card: United States. 2018. Atlanta, GA. Available at https://cdc.gov/breastfeeding/data/reportcard.htm (accessed November11, 2018)
- 27. Baby-Friendly USA. USA designated baby friendly facilities by state. Available at https://babyfriendlyusa.org/for-parents/find-a-baby-friendly-facility (accessed November11, 2018)
- 28. Vogel A, Hutchison B, Mitchell E. The impact of pacifier use on breastfeeding: A prospective cohort study. J Paediatr Child Health 2001;37:58–63 [DOI] [PubMed] [Google Scholar]
- 29. Howard CR, Howard FM, Lanphear B, et al. Randomized clinical trial of pacifier use and bottle-feeding or cupfeeding and their effect on breastfeeding. Pediatrics 2003;111:511–518 [DOI] [PubMed] [Google Scholar]
- 30. Kramer MS, Barr RG, Dagenais S, et al. Pacifier use, early weaning, and cry/fuss behavior: A randomized controlled trial. JAMA. 2001;286:322–326 [DOI] [PubMed] [Google Scholar]
- 31. Sexton S, Natale R. Risks and benefits of pacifiers. Am Fam Physician 2009;79:681–685 [PubMed] [Google Scholar]
- 32. Chantry CJ, Dewey KG, Peerson JM, et al. In-hospital formula use increases early breastfeeding cessation among first-time mothers intending to exclusively breastfeed. J Pediatr 2014;164:1339–1345.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nelson JM, Perrine CG, Scanlon KS, et al. Provision of non-breast milk supplements to healthy breastfed newborns in U.S. hospitals, 2009 to 2013. Matern Child Health J 2016;20:2228–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tender JA, Janakiram J, Arce E, et al. Reasons for in-hospital formula supplementation of breastfed infants from low-income families. J Hum Lact 2009;25:11–17 [DOI] [PubMed] [Google Scholar]
- 35. Committee on Nutrition, Section on Breastfeeding, Committee on Fetus and Newborn. Donor human milk for the high-risk infant: Preparation, safety, and usage options in the United States. Pediatrics 2017;139 [Epub ahead of print]; DOI: 10.1542/peds.2016-3440 [DOI] [PubMed] [Google Scholar]
- 36. Meyers D, Turner-Maffei C. Improved breastfeeding success through the baby-friendly hospital initiative. Am Fam Physician 2008;78:180, 182 [PubMed] [Google Scholar]