Abstract
Background:
Recent evidence of high systemic absorption of sunscreen ingredients has raised concerns regarding the safety of sunscreen products. Oxybenzone (BP-3) and octinoxate (OMC), two common sunscreen ingredients, were recently banned in Key West and Hawaii due to their toxic effects on marine ecosystems. Their impact on human health requires a careful assessment.
Objective:
To summarize the current evidence on the association between the systemic level of BP-3 or OMC and its health impact.
Methods:
A primary literature search was conducted using PubMed database in February 2019.
Results:
There are 29 studies that address the impact of these ingredients on human health. Studies show that elevated systemic level of BP-3 has no adverse effect on male and female fertility, female reproductive hormone level, adiposity, fetal growth, child’s neurodevelopment and sexual maturation. However, the association of BP-3 level on thyroid hormone, testosterone level, kidney function and pubertal timing has been reported and prompts further investigations to validate a true association. The systemic absorption of OMC has no reported effect on thyroid and reproductive hormone levels.
Conclusions:
Current evidence is not sufficient to support the causal relationship between the elevated systemic level of BP-3 or OMC and adverse health outcomes. There are either contradictory findings among different studies or insufficient number of studies to corroborate the observed association. To accurately evaluate the long-term risk of exposure to BP-3 and OMC from sunscreen, a well-designed longitudinal randomized controlled trial needs to be conducted.
Keywords: benzophenone-3, oxybenzone, sunscreen, octyl methoxycinnamate, octinoxate, toxicity, endocrine, reproductive, fertility, endocrine disrupting compounds, EDC, systemic absorption
1. Introduction
The concern for safety and effectiveness of sunscreen ingredients has been heightened after recent evidence of their measurable systemic absorption following topical application 1,2. Indeed, the US Food and Drug Administration (FDA) emphasized the importance of the safety assessment on all sunscreen ingredients with systemic absorption greater than 0.5 ng/mL 3. In the recent randomized controlled trial, 4 out of 4 tested sunscreen ingredients were found in the subjects’ blood at concentrations exceeding the FDA threshold for waiving toxicology assessment, thereby raising concerns among sunscreen users 1. In fact, concerns regarding the safety of sunscreen ingredients were growing even before the study was published. In 2018 and 2019, the state of Hawaii and Key West, Florida banned the sale of sunscreen containing oxybenzone (BP-3) or octinoxate (OMC) due to their detrimental threat to the marine ecosystems 4-6. Their toxic effects on other species has alarmed the general public about their potential to impact human health when applied to human skin 7.
BP-3 and OMC are two common sunscreen ingredients that are also known to have endocrine-disrupting potential 8. While they are mainly used as UV filter in sunscreens, they are also prevalent in air, drinking water, cosmetics, fragrances and plastic packagings, providing additional routes of exposure to humans 9. Numerous studies have shown that they can penetrate through the skin and enter the systemic circulation 1,10-15. They have been detected in urine, blood, semen, and even amniotic fluid and breast milk, thereby raising concerns about their negative consequences on fetal development as well as other organ systems 9,16. However, whether the presence of these ingredients in systemic circulation poses risks to human health is unclear. To answer whether systemic absorption of sunscreen poses risks to human health, further investigation is needed.
In this review, we evaluated current evidence on the association between the systemic levels of BP-3 and OMC in humans, and altered health outcomes and fetal development to help provide an insight into the potential clinical significance of the sunscreens’ systemic absorption.
2. Methods
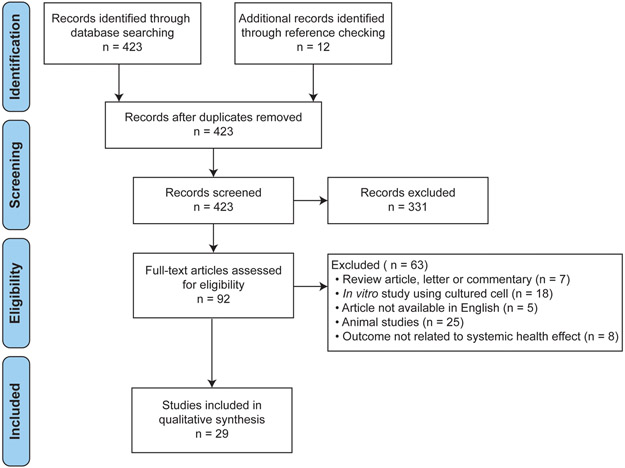
A literature search was conducted for all relevant publications in the Pubmed using the following search term: (benzophenone-3 OR oxybenzone OR octinoxate OR octyl methoxycinnamate) AND (endocrine OR reproductive OR kidney OR liver OR cancer OR brain OR hormone OR skin OR hair OR gastrointestinal OR defect OR embryo OR pregnant OR nervous system OR autoimmune disease) AND (animal OR mice OR rat OR rabbit OR human). All articles were searched from January 1, 1979 to February 22, 2019. A total of 423 studies were retrieved, and two independent reviewers screened all titles and abstracts in accordance to the Preferred Reporting System for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Figure 1). The following exclusion criteria were applied: 1) non-evidence-based studies including review articles, letters and commentaries; 2) in vitro studies using cultured cell or tissue; 3) animal studies using non-mammalian species such as insects and amphibians; 4) studies written in languages other than English; 5) studies that do not focus on physiological outcome. The following inclusion criteria were applied: 1) studies conducted on either humans or mammalian species (e.g. mice, rats); 2) studies that included univariate analysis between BP-3 (or OMC) level and physiological outcome.
Figure 1. PRISMA Diagram.
Process of inclusion of studies
3. Results
3.1. Selection of Studies
29 studies met our criteria. 16 studies evaluated the association between the systemic levels of BP-3 (n=16) or OMC (n=2, also included in BP-3) and various outcomes in organ systems, whereas 13 studies evaluated the association between the prenatal systemic levels of BP-3 (n=13) and outcomes in child development (Table 1). We quantified the number of supporting and refuting evidences into Tables 2 and 3.
Table 1.
Human studies on the association between the systemic levels of oxybenzone (BP-3)/octinoxate (OMC) and health outcome
| Author, Year | Study type (LOE) |
# of Subjectsa | Study Characteristics | Measure of Outcome |
Conclusion | |
|---|---|---|---|---|---|---|
| Thyroid Hormone Level | Przybyla et al., 2018 20 | Cross-sectional study (4) | 850 M, 710 F | Measurement of urinary BP-3 concentration and serum thyroid hormone levels | T3, T4, TSH levels | No significant association between BP-3 and thyroid hormone levels |
| Aker et al., 2018 19 | Case-control study (3) | 439 pregnant F | Measurement of urinary BP-3 concentration and serum thyroid hormone levels | T3, T4, T3/T4 ratio, TSH, FT4 levels | No significant association between BP-3 and thyroid hormone levels, although the author reported an inverse trend with T3 and T4 | |
| Kim et al., 2017 17 | Retrospective cohort study (3) | 1829 M/F | Measurement of urinary BP-3 concentration and serum thyroid hormone levels | T3, T4, FT3, FT4, TSH levels | High BP-3 concentration was associated with decrease FT4 and T4 | |
| Aker et al., 2016 18 | Prospective cohort study (2) | 106 pregnant F | Measurement of urinary BP-3 concentration and serum hormone levels at two time points (16-20 wks and 24-28 wks) during pregnancy | FT3, FT4, TSH levels | An IQR increase in BP-3 was significantly associated with 3% decrease in free T3; no significance with TSH or T4 | |
| Janjua et al., 2007 21 | Single-blinded clinical trial (4) | 32 M/F | Measurement of serum thyroid hormone levels before and after whole-body topical application of sunscreen + 10% of BP3, OMC and 4-MBC for QD for one week | TBG, TSH, T3, T4, FT3, FT4 levels | No biologically significant change in thyroid hormone levels, although statistical difference was observed likely due to normal hormonal variation | |
| Fertility | Buck Louis et al., 2015 25 | Case-control study (3) | 413 M | Measurement of urinary BP-3 concentration and semen analysis | Semen parameters | No significant association between BP-3 concentration and semen quality |
| Buck Louis et al., 2014 22 | Prospective cohort study (2) | 501 couples | Measurement of urinary BP-3 concentration and fecundity | The number of menstrual cycles required to achieve pregnancy | No significant association between BP-3 concentration and fecundity | |
| Chen et al., 2013 23 | Case-control study (3) | 877 idiopathic infertile male, 713 controls | Measurement of urinary BP-3 concentration and semen analysis | Semen parameters (volume, sperm concentration and number) | No significant association between BP-3 concentrations and idiopathic male infertility and semen parameters | |
| Chen et al., 2013 24 | Case-control study (3) | 70 couples with spontaneous abortions, 180 control couples | Measurement of urinary BP-3 concentration and the rate of spontaneous abortions | Rate of spontaneous abortions | No significant association between BP-3 and spontaneous abortions | |
| Reproductive hormone level | Pollack et al., 2018 26 | Prospective cohort study (2) | 143 premenopausal F | Measurement of urinary BP-3 concentration and reproductive hormones over two menstrual cycles | Reproductive hormone levels (estradiol, progesterone, FSH, LH) | Single-chemical exposure model: No significant association between BP-3 and reproductive hormones Multi-chemical exposure model: Negative association between BP-3 and FSH and LH |
| Scinicariello et al., 2016 27 | Cross-sectional study (4) | 588 M/F | Measurement of urinary BP-3 concentration and serum testosterone levels | Serum testosterone | Male adolescents in the 3rd and 4th quartiles of BP-3 had significantly lower total testosterone (−38.74%; 95% CI: −58.52, −10.42) and (−36.87%; 95% CI: −59.34, −1.98) | |
| Aker et al., 2016 18 | Prospective cohort Study (2) | 106 pregnant F | Measurement of urinary BP-3 concentration and serum hormone levels at two time points (16-20 wks and 24-28 wks) during pregnancy | estradiol, progesterone, SHBG levels | IQR inc in BP-3 was signif. associated with 10.3% dec. in SHBG at second time point but not first time point; no significance w/ estradiol or progesterone | |
| Janjua et al., 2004 14 | Single-blinded clinical trial (1) | 32 M/F | Plasma reproductive hormone levels were measured before and after whole-body topical application of sunscreen for a week | Reproductive hormone levels (testosterone, estradiol, progesterone, FSH, LH) | Decrease in plasma testosterone after the application, but likely due to normal hormonal variation. No association with other female reproductive hormones | |
| Pubertal development | Binder et al., 2018 28 | Prospective cohort study (2) | 200 F | Measurement of urinary BP-3 concentration at two time points: prior to the onset of breast development (B1) and during adolescence (B4); age of menarche was recorded | Age of menarche | A higher BP-3 concentration at B1 was associated with earlier menarche (HR: 1.17; 95% CI: 1.06, 1.29) |
| Wolff et al., 2015 29 | Prospective cohort study (2) | 1239 girls at age 6-8 years old | Measurement of urinary BP-3 concentration at enrollment. Subjects were followed annually for 7 years to determine the age at first breast or pubic hair development. | Pubertal onset | BP-3 concentration was associated with later onset of breast development 5-6 months later, (AOR: 0.80; 95% CI: 0.65, 0.98) | |
| Adiposity | Xue et al., 2015 31 | Case-control study (3) | 49 obese children 27 non-obese control | Measurement of urinary BP-3 concentration and clinical evaluation of obesity | Rate of obesity | No significant association between BP-3 concentration and the likelihood of obesity |
| Renal function | Kang et al., 2019 30 | Cross-sectional study (4) | 441 F | Measurement of urinary BP-3 concentratoin and albumin-to-creatine ratio (ACR) at a single time point | Urinary albumin-to-creatine ratio (AR) | Positive association between the concentration of BP-1, a metabolite of BP-3, and ACR |
| Child's birth weight and size | Messerlian et al., 2018 35 | Prospective cohort study (2) | 346 mother-child cohorts | Measurement of urinary BP-3 concentration from both men and women prior to conception and second measurement from women during pregnancy; birth weight and head circumferences of infants were assessed | birth weight and size | No significant association between maternal BP-3 and the birth weight |
| Ferguson et al., 2018 36 | Prospective cohort study (2) | 476 mother-child cohorts | Measurement of paternal and maternal prenatal urinary BP-3 concentration and newborn's size and weight at delivery | birth weight and size | No significant association between maternal BP-3 and the birth weight | |
| Tang et al., 2013 50 | Prospective cohort study (2) | 567 mother-child cohorts | Measurement of maternal prenatal urinary BP-3 concentration prior to delivery and newborn's weight, length and gestational age | birth weight, size and length of gestation | No significant association between BP-3 concentrations and the birth weight and size. Prenatal BP-3 concentration was associated with decreased length of gestation (−0.45 week; 95% CI: −0.87, −0.04) p-value=0.03 | |
| Philippat et al., 2012 38 | Prospective cohort study (2) | 191 mother-child cohorts | Measurement of paternal and maternal prenatal urinary BP-3 concentration and newborn's size and weight at delivery | birth weight and size | No significant association between maternal BP3 and the birth weight (26g; CI: −2, 54), but author mentioned that a positive trend exists. | |
| Wolff et al., 2008 45 | Prospective cohort study (2) | 404 mother-child cohorts | Measurement of paternal and maternal prenatal urinary BP-3 concentration and newborn's size and weight at delivery | birth weight and size | BP3 was associated with increasing birth weight in boys (44 g; 95% CI: 5.4, 84) per 1 ln-transformed biomarker, but decreasing birthweight in girls (but no statistical data shown for girls) | |
| Child's sexual maturation | Harley et al., 2019 40 | Prospective cohort study (2) | 179 mother-daughter cohorts 159 mother-son cohorts | Measurement of maternal prenatal urinary BP-3 concentration. Children were followed up for pubertal assessments | Pubertal onset in children | No significant association of prenatal urinary BP3 concentrations with pubertal onset |
| Child's birth abnormality | Huo et al., 2016 46 | Case-control study (3) | 101 Hirschsprug's disease (HSCR) patient's mother-child 322 control | Measurement of urinary BP-3 concentration from HSCR patient’s mother and control | The odds of giving birth to child with HSCR | Higher BP-3 exposure groups were more likely to give birth of HSCR children (adjusted OR = 2.39; 95% CI=1.10, 5.21). However, the urine sample was not collected during pregnancy. |
| Chevrier et al., 2012 41 | Case-control study (3) | 5200 mother-child cohorts | Urine samples were collected from mothers who gave birth to boys with hypospadias and undescended testis, and three controls per case were selected among male singleton live births | The odds of giving birth to child with hypospadias and undescended testes | No significant association between maternal BP-3 and male genital abnormalities | |
| Child's body fat mass | Buckley et al., 2016 42 | Prospective cohort study (2) | 173 mother-child cohorts | Prenatal BP-3 was measured from pregnant women's urine samples; body fat mass of child was measured from 1998 to 2002. | Percent body fat | No significant association, but prenatal BP-3 concentrations were inversely associated with percent fat mass in girls (beta=−1.51, 95% CI=−3.06, 0.01) but not in boys (beta=−0.20, 95% CI=−1.69, 1.26) |
| Child's intelligence quotient (IQ) | Nakiwala et al., 2018 43 | Prospective cohort study (2) | 452 mother-son cohorts | Urine samples were collected from pregnant women at 22-29 gestational weeks. Verbal and performance IQ of children were assessed at 5-6 years old | verbal and performance IQ | No significant association between prenatal BP-3 exposure and the boys' verbal or performance IQ (uncorrected p-value ≥ 0.09) |
| Child's behavior | Philippat et al., 2017 44 | Prospective cohort study (2) | 529 mother-son cohorts | Prenatal BP-3 was measured from mother's urine samples; child's behavior was assessed by the Strength and Difficulties Questionnaire (SDQ) completed by mothers | Child's behavior | No significant association between BP-3 and child's behavior |
| Child's sex determination | Bae et al., 2016 39 | Prospective cohort study (2) | 220 parent-child cohorts | Preconception urinary BP-3 was measured from both father and mother, and the sex of child was reported | Sex ratio of child | No significant association between BP-3 and secondary sex ratio |
| Child's immune system | Buckley et al., 2018 47 | Prospective cohort study (2) | 164 mother-child cohorts | Urine samples were collected from pregnant women in the third trimester; allergy and immune system of children was assessed by questionnaire | Risk of developing atopic dermatitis, asthma, and wheeze | Wheeze was inversely associated with low BP-3 exposure in all children. No significant association exists for other allergic conditions. |
All subjects are adults unless otherwise stated.
Abbreviations: LOE, Level of Evidence; M, male; F, female; y/o, year-old; T3, Total triiodothyronine; T4, Total thyroxine; TSH, Thyroid Stimulating Hormone; FT3, Free triiodothyronine; FT4, Free thyroxine; QD, once a day; 4-MBC, 4-Methylbenzylidene camphor; QD, once a day; FSH, Follicle Stimulating Hormone; LH, Leutinizing Hormone; IQR, Interquartile Range; SHBG, sex hormone-binding globulin; AOR, adjusted odds ratio
Table 2.
Number of human studies about the association between urinary concentration of oxybenzone (BP-3) or octinoxate (OMC) and the physiological outcomes. n=16 (duplicate excluded)
| Target effect | Statistically significant association |
No statistically significant association |
||
|---|---|---|---|---|
| BP-3 | OMC | BP-3 | OMC | |
| Thyroid hormone level | 2 (↓↓) | 0 | 3 | 1a |
| Pubertal onset | 2 (↓↑) | 0 | 0 | 0 |
| Male reproductive hormone level | 1 (↓) | 0 | 1 | 1a |
| Female reproductive hormone level | 0 | 0 | 2 | 1a |
| Kidney function | 1 (↓) | 0 | 0 | 0 |
| Fertility | 0 | 0 | 4 | 0 |
| Childhood adiposity | 0 | 0 | 1 | 0 |
In parenthesis, ↓ indicates a negative correlation with elevated BP-3 level. ↑ indicates a positive correlation with elevated BP-3 level.
The results are from the same paper that is also included in BP-3 column.
Table 3.
Number of human studies about the association between maternal prenatal urinary concentration of oxybenzone (BP-3) or octinoxate (OMC) and the target effects in child. n=13 (duplicate excluded)
| Target effect | Statistically significant association |
No statistically significant association |
||
|---|---|---|---|---|
| BP-3 | OMC | BP-3 | OMC | |
| Offspring's birth weight | 1 (↑ in boys) | 0 | 4 | 0 |
| Offspring's birth defect | 1 (↑) | 0 | 1 | 0 |
| Offspring's allergic outcome | 1 (↓) | 0 | 0 | 0 |
| Offspring's pubertal onset | 0 | 0 | 1 | 0 |
| Offspring's body fat mass | 0 | 0 | 1 | 0 |
| Offspring's intelligence quotient (IQ) | 0 | 0 | 1 | 0 |
| Offspring's behavior | 0 | 0 | 1 | 0 |
| Offspring's sex determination | 0 | 0 | 1 | 0 |
In parenthesis, ↓ indicates a negative correlation with elevated BP-3 level. ↑ indicates a positive correlation with elevated BP-3 level.
3.2. Oxybenzone
3.2.1. Effects on Organ Systems
We have found 29 studies that assess the impact of high systemic level of BP-3 on endocrine, reproductive, metabolic, renal systems and neonatal development during pregnancy (Table 1). The effect on thyroid hormone levels was most extensively studied (n=5) most likely due to its endocrine disrupting potential shown in animal studies. Despite the amount of evidence, the association between elevated systemic level of BP-3 and thyroid hormone levels in humans is still inconclusive, because the observed association from each study was not consistent throughout all other studies. Two studies showed an association of higher urinary BP-3 concentrations with decreased thyroid hormones, yet one study showed an inverse association with T4, and another showed the association with T3 only 17,18. Interestingly, the two most recent studies with a cohort of 1560 and 439 demonstrated no statistically significant association (p<0.05) between urinary BP-3 concentration and thyroid hormone levels, disputing the results from two previous studies 19,20. Of the five studies referenced above, only one study assessed the thyroid hormone level after sunscreen application. In this single-blinded clinical trial, 32 volunteers applied cream formulated with 10% of three active ingredients in sunscreen (OMC, BP-3, and 4-methylbenzylcathinone) for one week, and the plasma thyroid levels before and after treatment were measured 21. No biologically significant effects on thyroid hormone levels were observed after one week, indicating that the maximum allowable concentration of BP-3 in sunscreen was not capable of disturbing the homeostasis of thyroid hormones, at least during the short duration of the study.
The effect of BP-3 concentration on fertility was the second most common potential outcome studied (n=4 studies) 22-25. All four studies demonstrated that there was no association between BP-3 exposure and fertility although different parameters were used for measuring fertility, which included semen analysis, the number of menstrual cycles required to achieve pregnancy, and a hazard ratio for spontaneous abortion.
The estrogenic and anti-androgenic activities of BP-3 have frequently been reported in in vitro and animal studies, but its impact on human reproductive endocrine systems has not been addressed in great detail. We found four studies that investigated the effect of elevated systemic level of BP-3 on reproductive hormone levels 14,18,26,27. In males, the urinary BP-3 concentration was found to be associated with significantly lower serum total testosterone levels in a cross-sectional study with 588 adolescents 27. The same association was also observed in a single-blinded study, which measured the plasma testosterone levels of 15 males after topical application of BP-3 containing cream 14. The author, however, indicated that the difference was more likely due to normal biological variations in hormone levels, because the statistical difference was also evident in blood samples drawn prior to sunscreen application. The clinical significance of BP-3 effect on testosterone level may require further studies as two other studies found no association between BP-3 level and male semen quality or infertility 23,25. In females, on the other hand, there was no statistically significant association between urinary BP-3 concentration and reproductive hormone levels, such as estradiol, progesterone, FSH and LH 14,18,26.
Besides analysing the reproductive hormone levels, the endocrine disrupting potential of BP-3 was evaluated by measuring the age of pubertal onset by two prospective cohort studies. Each study found contradicting association, one showing a positive and the other showing a negative association between the BP-3 urine levels and the age of pubertal onset 28,29. Therefore, the effect on pubertal development is inconclusive.
There is growing evidence suggesting that exposure to environmental chemicals, such as phthalates, bisphenol A and multiple endocrine disrupting chemicals (EDCs), could have adverse consequences on renal function and might contribute to cumulative renal injury. One case-control study evaluated whether the exposure to BP-3 can be a risk factor for chronic kidney diseases 30. When they measured the albumin-to-creatinine ratio (ACR), a kidney function marker, and the urinary concentrations of BP-1, a BP-3 metabolite, of 441 female participants, they found a significant association between BP-1 and ACR, suggesting BP-3 as a potential contributing factor to kidney injury.
Xue et al. compared the urinary concentration of BP-3 in 49 obese Indian children and 27 non-obese controls to examine whether exposure to BP-3 is associated with childhood obesity 31. The results showed no significant association between BP-3 and childhood obesity.
3.2.2. Effects on Fetal and Neonatal Development
The developing embryo and fetus are particularly vulnerable to endocrine disrupting chemicals (EDCs) such as BP-3, because they can potentially interfere with the hormones, neurotransmitters and growth factors that are critical for normal development 32,33. Since BP-3 is known to cross blood-placenta barrier and enter amniotic fluid and breast milk, the safety concern regarding the use of sunscreen in pregnant women has increased 34. Thirteen studies assessed the association between prenatal BP-3 exposure and offspring’s development. In all studies, the level of prenatal BP-3 exposure was determined by the mother’s urinary concentration of BP-3 during pregnancy, and the target outcome was evaluated from the offspring through post-birth follow-up. Overall, the prenatal concentration of BP-3 did not have biologically significant effects on the development of offspring (Table 3). Studies found no statistically significant association between prenatal BP-3 exposure and the offspring’s sex ratio, birth weight, pubertal development, body fat mass, intelligence quotient and behaviour 35-44. However, a few studies reported the potential risk of prenatal BP-3 exposure on offspring development 45-47.
The study by Wolff et al. found that prenatal BP-3 exposure was associated with increased birth weight in boys, but four other studies reported no association between prenatal BP-3 and offspring’s birth weight 45. In a case control study, Huo et al. compared the urinary concentration of BP-3 from 101 mothers with children diagnosed with Hirschsprug’s disease (HSCR) and 322 controls, and reported that maternal BP-3 urinary level was associated with higher odds of having a child with HSCR 46. However, the urine samples from mothers were collected after child was born, and therefore the observed concentration does not necessarily reflect the prenatal BP-3 level. Buckley et al. studied the association between prenatal BP-3 exposure and the risk of developing allergy or immune disorders in offspring 47. The results actually shows that the higher prenatal exposure of BP-3 was associated with a lower risk of wheeze in children.
3.3. Octinoxate
3.3.1. Effects on Organ System
Octinoxate (octyl methoxycinnamate; OMC), like BP-3, is also considered as EDC 8,48,49. However, the effect of OMC exposure on human health is insufficiently investigated compared to that of BP-3. A paucity of studies may be attributed to its relatively low dermal penetration and systemic absorption compared to that of BP-3 11. When female participants applied the same amount of cream containing either 10% OMC or BP-3 containing cream, the maximum plasma concentration was 7 ng/mL for OMC, whereas 187 ng/mL for BP-3 11.
Only two studies were found on the effect of OMC on human physiology, which were published by the same author. In both studies, 32 participants applied a cream with 10% of BP-3, OMC and 4-MBC for a week, and its effect on the levels of reproductive hormones and thyroid hormones was reported in 2004 and 2007, respectively 14,21. Neither reproductive hormone nor thyroid hormone levels were remarkably affected, indicating that short-term topical application of OMC did not disrupt the regulation of thyroid hormone.
3.3.2. Effects on Fetal and Neonatal Development
We could not find any human study that evaluated the effect of high systemic levels of OMC on fetal and neonatal development.
4. Discussion
The primary goal of our review was to understand whether elevated systemic levels of BP-3 and OMC can cause negative health consequences in humans. Among many sunscreen ingredients, we focused on BP-3 and OMC as their toxicity to aquatic species and their endocrine-disrupting potential make them high-priority candidates for safety assessment. The endocrine disruptive effect and developmental toxicity of BP-3 and OMC in cell line and animals are well documented, but their impact on humans has not been addressed in great detail. Aside from sunscreens, BP-3 and OMC are frequently used in cosmetics, shampoo, lip balms and fragrances, so the safety assessment of these compounds are of particular importance. As previously mentioned, the number of studies that investigated the health impact from systemic absorption of sunscreen is significantly scarce. On the other hand, we found 29 studies that investigated the impact of high systemic level of BP-3 (n=29) or OMC (n=2, also included in BP-3) on a wide range of health outcomes in endocrine, reproductive, metabolic, renal, dermatologic, and developmental systems. Our systematic review demonstrated that current evidence does not strongly support a causal relationship between the systemic level of BP-3 or OMC and adverse health outcome in humans.
The major limitation of our study, however, is that we could not determine the actual contribution of sunscreen on the systemic levels of BP-3 and OMC, and therefore cannot verify the long-term risk of using sunscreen containing BP-3 or OMC. Most studies included an analysis of urinary concentration as an exposure biomarker, which may result from exposure to environmental means other than sunscreen absorption. None of the studies, except 2, evaluated the health outcome using sunscreen as a main route of exposure. It remains to identify the systemic levels of BP-3 and OMC among chronic sunscreen users and use those values to re-evaluate its association with health effects.
In fact, the previous study by Janjua et al. provides an insight into the contribution of transdermal absorption on the systemic level of BP-3 and OMC by comparing the plasma and urine concentration before and after sunscreen application in a group of 32 volunteers 11. Prior to sunscreen application, the plasma and urine concentration for BP-3 and OMC were below the level of detection (3.9 ng/mL) in most subjects. However, after the first whole-body application of sunscreens containing 10% of BP-3 and OMC, the median plasma concentrations rose to 238 ng/mL for BP-3 and 16 ng/mL for OMC within the first two hours. The dramatic increase of plasma concentrations after sunscreen application suggests that a large amount of systemic circulation of BP-3 and OMC could be attributed to sunscreen absorption.
The most recent study by Matta et al. indeed replicates the results reported by Janjua et al. When participants applied sunscreen products containing 6% BP-3, the geometric mean maximum plasma concentration was elevated to 209.6 ng/mL from undetectable level of concentration within two hours, resembling the pharmacokinetics observed from the previous study. Their study also highlighted a long terminal half-life of BP-3 and drug accumulation by measuring the plasma concentration at constant intervals over 7 days after first sunscreen application.
A substantial accumulation of BP-3 in the body from repeated sunscreen application was also mentioned in a previous study by Gonzalez et al. When subjects applied sunscreen containing 4% BP-3 for five days, they continued to excrete significant amounts of BP-3 up to five days after the last application 10.
The evidence of substantial systemic absorption and accumulation of BP-3 prompts a thorough investigation of the long-term risks of BP-3 containing sunscreen use. Although current evidence does not show a significant correlation between the systemic level of BP-3 and adverse health effects, further studies need to be done to determine the systemic effects resulting from long-term sunscreen use and whether steady state levels exceed the threshold for toxic biological effects.
OMC, unlike BP-3, exhibits low dermal penetration and systemic absorption compared to that of BP-3, which may explain a lack of investigation into its potential health impact 11. Nevertheless, the median toxic dose (TD50), the dose at which toxicity occurs in 50% of cases, is different for each compound, so the low systemic absorption of OMC does not prove its relative safety. More importantly, the topical application of OMC results in systemic absorption greater than 0.5 ng/mL, a threshold established by the FDA for waiving toxicology assessment, and therefore further drug safety assessment on OMC is crucial.
5. Conclusion
In this systematic review, we did not find a strong support for a causal relationship between the systemic level of BP-3 or OMC and adverse health outcomes. The elevated systemic level of BP-3 did not have adverse effect on fertility, childhood adiposity, and fetal and neonatal development, but its impact on thyroid and reproductive hormone levels, pubertal development, kidney function and the immune system will require further investigations. The health consequences of an elevated OMC level has been less extensively studied presumably due to its poor dermal absorption and low serum concentrations relative to other sunscreen compounds. The current evidence shows that topical application of OMC does not have biologically significant effect on thyroid and reproductive hormone levels. To evaluate the long-term risk of exposure to BP-3 or OMC from sunscreens, a well-designed longitudinal randomized controlled trial is of high priority.
6. Questions (answers highlighted)
- Which two sunscreen ingredients did Hawaii and Key West ban?
- Avobenzone and octisalate
- Oxybenzone and octinoxate
- Zinc oxide and titanium dioxide
- Octocrylene and trolamine salicylate
- Why did Hawaii and Key West ban two sunscreen ingredients?
- Studies showed that they can cause damage on coral reef and marine species.
- Studies showed that they can lead to higher risk of melanoma.
- Studies showed that they can monopolize the sale of sunscreens.
- Studies showed that they can cause digestive problems when ingested.
- Oxybenzone and octinoxate can penetrate through the skin and enter the systematic circulation.
- True
- False
- FDA recommends toxicology assessment on sunscreen ingredients with systemic absorption greater than which of the following?
- 0.1 ng/mL
- 0.5 ng/mL
- 10 ng/mL
- 50 ng/mL
- Humans can be exposed to oxybenzone and octinoxate from other sources besides sunscreen.
- True
- False
- Oxybenzone has been detected in which of the following in human body?
- Blood
- Urine
- Breast milk
- All of the above
- The evidence exists for an association between systemic level of oxybenzone and all of the following except ___.
- Thyroid hormone level
- Testosterone level
- Kidney function
- Heart failure risk
- Oxybenzone and octinoxate are considered as endocrine disrupting chemicals (EDCs).
- True
- False
- Current evidence shows that there is a significant correlation between the systemic level of oxybenzone and fertility.
- True
- False
- There is sufficient amount of evidence that sunscreen absorption causes detrimental effect on human health.
- True
- False
Footnotes
The authors have no conflicts of interest to disclose. The authors did not receive financial support to complete this research
References
- 1.Matta MK, Zusterzeel R, Pilli NR, et al. Effect of Sunscreen Application Under Maximal Use Conditions on Plasma Concentration of Sunscreen Active Ingredients: A Randomized Clinical TrialEffect of Sunscreen Application on Plasma Concentration of Active IngredientsEffect of Sunscreen Application on Plasma Concentration of Active Ingredients. JAMA. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Califf RM, Shinkai K. Filling in the Evidence About SunscreenFilling in the Evidence About SunscreenEditorial. JAMA. 2019. [DOI] [PubMed] [Google Scholar]
- 3.Sunscreen drug products for over-the-counter human use: proposed rule In: (FDA) UFaDA, ed: Fed Regist; 2019. [Google Scholar]
- 4.Schneider SL, Lim HW. Review of environmental effects of oxybenzone and other sunscreen active ingredients. J Am Acad Dermatol. 2019;80(1):266–271. [DOI] [PubMed] [Google Scholar]
- 5.Downs CA, Kramarsky-Winter E, Segal R, et al. Toxicopathological Effects of the Sunscreen UV Filter, Oxybenzone (Benzophenone-3), on Coral Planulae and Cultured Primary Cells and Its Environmental Contamination in Hawaii and the U.S. Virgin Islands. Arch Environ Contam Toxicol. 2016;70(2):265–288. [DOI] [PubMed] [Google Scholar]
- 6.Zhou R, Lu G, Yan Z, Jiang R, Shen J, Bao X. Parental transfer of ethylhexyl methoxy cinnamate and induced biochemical responses in zebra fi sh. Aquat Toxicol. 2019;206:24–32. [DOI] [PubMed] [Google Scholar]
- 7.Wang SQ, Burnett ME, Lim HW. Safety of Oxybenzone: Putting Numbers Into Perspective. Archives of dermatology. 2011;147(7):865–866. [DOI] [PubMed] [Google Scholar]
- 8.Schlumpf M, Cotton B, Conscience M, Haller V, Steinmann B, Lichtensteiger W. In vitro and in vivo estrogenicity of UV screens. Environ Health Perspect. 2001;109(3):239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calafat AM, Wong LY, Ye X, Reidy JA, Needham LL. Concentrations of the sunscreen agent benzophenone-3 in residents of the United States: National Health and Nutrition Examination Survey 2003--2004. Environ Health Perspect. 2008;116(7):893–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez H, Farbrot A, Larko O, Wennberg AM. Percutaneous absorption of the sunscreen benzophenone-3 after repeated whole-body applications, with and without ultraviolet irradiation. The British journal of dermatology. 2006;154(2):337–340. [DOI] [PubMed] [Google Scholar]
- 11.Janjua NR, Kongshoj B, Andersson AM, Wulf HC. Sunscreens in human plasma and urine after repeated whole-body topical application. J Eur Acad Dermatol Venereol. 2008;22(4):456–461. [DOI] [PubMed] [Google Scholar]
- 12.Hayden CGJ, Roberts MS, Benson HAE. Systemic absorption of sunscreen after topical application. The Lancet. 1997;350(9081):863–864. [DOI] [PubMed] [Google Scholar]
- 13.Jiang Roberts, Collins Benson. Absorption of sunscreens across human skin: an evaluation of commercial products for children and adults. British Journal of Clinical Pharmacology. 1999;48(4):635–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janjua NR, Mogensen B, Andersson AM, et al. Systemic absorption of the sunscreens benzophenone-3, octyl-methoxycinnamate, and 3-(4-methylbenzylidene) camphor after whole-body topical application and reproductive hormone levels in humans. The Journal of investigative dermatology. 2004;123(1):57–61. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Ganley CJ. Safety Threshold Considerations for Sunscreen Systemic Exposure: A Simulation Study. Clinical Pharmacology & Therapeutics. 2019;105(1):161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DiNardo JC, Downs CA. Dermatological and environmental toxicological impact of the sunscreen ingredient oxybenzone/benzophenone-3. J Cosmet Dermatol. 2018;17(1):15–19. [DOI] [PubMed] [Google Scholar]
- 17.Kim S, Kim S, Won S, Choi K. Considering common sources of exposure in association studies - Urinary benzophenone-3 and DEHP metabolites are associated with altered thyroid hormone balance in the NHANES 2007-2008. Environ Int. 2017;107:25–32. [DOI] [PubMed] [Google Scholar]
- 18.Aker AM, Watkins DJ, Johns LE, et al. Phenols and parabens in relation to reproductive and thyroid hormones in pregnant women. Environ Res. 2016;151:30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aker AM, Johns L, McElrath TF, Cantonwine DE, Mukherjee B, Meeker JD. Associations between maternal phenol and paraben urinary biomarkers and maternal hormones during pregnancy: A repeated measures study. Environ Int. 2018;113:341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Przybyla J, Geldhof GJ, Smit E, Kile ML. A cross sectional study of urinary phthalates, phenols and perchlorate on thyroid hormones in US adults using structural equation models (NHANES 2007-2008). Environ Res. 2018;163:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janjua NR, Kongshoj B, Petersen JH, Wulf HC. Sunscreens and thyroid function in humans after short-term whole-body topical application: a single-blinded study. The British journal of dermatology. 2007;156(5):1080–1082. [DOI] [PubMed] [Google Scholar]
- 22.Buck Louis GM, Maisog J, Sapra KJ, Kannan K, Sundaram R. Urinary Concentrations of Benzophenone-Type Ultraviolet Radiation Filters and Couples' Fecundity. American Journal of Epidemiology. 2014;180(12):1168–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen M, Tang R, Fu G, et al. Association of exposure to phenols and idiopathic male infertility. Journal of Hazardous Materials. 2013;250-251:115–121. [DOI] [PubMed] [Google Scholar]
- 24.Chen X, Chen M, Xu B, et al. Parental phenols exposure and spontaneous abortion in Chinese population residing in the middle and lower reaches of the Yangtze River. Chemosphere. 2013;93(2):217–222. [DOI] [PubMed] [Google Scholar]
- 25.Buck Louis GM, Chen Z, Kim S, Sapra KJ, Bae J, Kannan K. Urinary concentrations of benzophenone-type ultraviolet light filters and semen quality. Fertil Steril. 2015;104(4):989–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pollack AZ, Mumford SL, Krall JR, et al. Exposure to bisphenol A, chlorophenols, benzophenones, and parabens in relation to reproductive hormones in healthy women: A chemical mixture approach. Environ Int. 2018;120:137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scinicariello F, Buser MC. Serum Testosterone Concentrations and Urinary Bisphenol A, Benzophenone-3, Triclosan, and Paraben Levels in Male and Female Children and Adolescents: NHANES 2011-2012. Environ Health Perspect. 2016;124(12):1898–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Binder AM, Corvalan C, Calafat AM, et al. Childhood and adolescent phenol and phthalate exposure and the age of menarche in Latina girls. Environ Health. 2018;17(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolff MS, Teitelbaum SL, McGovern K, et al. Environmental phenols and pubertal development in girls. Environ Int. 2015;84:174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang H, Kim S, Lee G, et al. Urinary metabolites of dibutyl phthalate and benzophenone-3 are potential chemical risk factors of chronic kidney function markers among healthy women. Environ Int. 2019;124:354–360. [DOI] [PubMed] [Google Scholar]
- 31.Xue J, Wu Q, Sakthivel S, Pavithran PV, Vasukutty JR, Kannan K. Urinary levels of endocrine-disrupting chemicals, including bisphenols, bisphenol A diglycidyl ethers, benzophenones, parabens, and triclosan in obese and non-obese Indian children. Environ Res. 2015;137:120–128. [DOI] [PubMed] [Google Scholar]
- 32.Zoeller RT, Brown TR, Doan LL, et al. Endocrine-Disrupting Chemicals and Public Health Protection: A Statement of Principles from The Endocrine Society. Endocrinology. 2012;153(9):4097–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mallozzi M, Bordi G, Garo C, Caserta D. The effect of maternal exposure to endocrine disrupting chemicals on fetal and neonatal development: A review on the major concerns. Birth defects research Part C, Embryo today : reviews. 2016;108(3):224–242. [DOI] [PubMed] [Google Scholar]
- 34.Krause M, Frederiksen H, Sundberg K, et al. Presence of benzophenones commonly used as UV filters and absorbers in paired maternal and fetal samples. Environ Int. 2018;110:51–60. [DOI] [PubMed] [Google Scholar]
- 35.Messerlian C, Mustieles V, Minguez-Alarcon L, et al. Preconception and prenatal urinary concentrations of phenols and birth size of singleton infants born to mothers and fathers from the Environment and Reproductive Health (EARTH) study. Environ Int. 2018;114:60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferguson KK, Meeker JD, Cantonwine DE, et al. Environmental phenol associations with ultrasound and delivery measures of fetal growth. Environ Int. 2018;112:243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang R, Chen MJ, Ding GD, et al. Associations of prenatal exposure to phenols with birth outcomes. Environ Pollut. 2013;178:115–120. [DOI] [PubMed] [Google Scholar]
- 38.Philippat C, Mortamais M, Chevrier C, et al. Exposure to phthalates and phenols during pregnancy and offspring size at birth. Environ Health Perspect. 2012;120(3):464–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bae J, Kim S, Kannan K, Buck Louis GM. Couples' urinary concentrations of benzophenone-type ultraviolet filters and the secondary sex ratio. Science of The Total Environment. 2016;543:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harley KG, Berger KP, Kogut K, et al. Association of phthalates, parabens and phenols found in personal care products with pubertal timing in girls and boys. Human reproduction (Oxford, England). 2019;34(1):109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chevrier C, Petit C, Philippat C, et al. Maternal urinary phthalates and phenols and male genital anomalies. Epidemiology (Cambridge, Mass). 2012;23(2):353–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buckley JP, Herring AH, Wolff MS, Calafat AM, Engel SM. Prenatal exposure to environmental phenols and childhood fat mass in the Mount Sinai Children's Environmental Health Study. Environ Int. 2016;91:350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakiwala D, Peyre H, Heude B, et al. In-utero exposure to phenols and phthalates and the intelligence quotient of boys at 5 years. Environ Health. 2018;17(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Philippat C, Nakiwala D, Calafat AM, et al. Prenatal Exposure to Nonpersistent Endocrine Disruptors and Behavior in Boys at 3 and 5 Years. Environ Health Perspect. 2017;125(9):097014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolff MS, Engel SM, Berkowitz GS, et al. Prenatal phenol and phthalate exposures and birth outcomes. Environ Health Perspect. 2008;116(8):1092–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huo W, Cai P, Chen M, et al. The relationship between prenatal exposure to BP-3 and Hirschsprung's disease. Chemosphere. 2016;144:1091–1097. [DOI] [PubMed] [Google Scholar]
- 47.Buckley JP, Quiros-Alcala L, Teitelbaum SL, Calafat AM, Wolff MS, Engel SM. Associations of prenatal environmental phenol and phthalate biomarkers with respiratory and allergic diseases among children aged 6 and 7years. Environ Int. 2018;115:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klammer H, Schlecht C, Wuttke W, et al. Effects of a 5-day treatment with the UV-filter octyl-methoxycinnamate (OMC) on the function of the hypothalamo-pituitary-thyroid function in rats. Toxicology. 2007;238(2-3):192–199. [DOI] [PubMed] [Google Scholar]
- 49.Klammer H, Schlecht C, Wuttke W, Jarry H. Multi-organic risk assessment of estrogenic properties of octyl-methoxycinnamate in vivo A 5-day sub-acute pharmacodynamic study with ovariectomized rats. Toxicology. 2005;215(1-2):90–96. [DOI] [PubMed] [Google Scholar]
- 50.Tang R, Chen M-j, Ding G-d, et al. Associations of prenatal exposure to phenols with birth outcomes. Environmental Pollution. 2013;178:115–120. [DOI] [PubMed] [Google Scholar]