Abstract
Objectives
Cytokine release syndrome (CRS) is a potentially severe complication of COVID-19 most commonly resulting in respiratory failure. This ten-patient study was designed to determine the efficacy of therapeutic plasma exchange (TPE) in improving oxygenation and in reducing the cytokine load in a critically ill subset of patients.
Methods
Five single volume plasma exchanges over eight days within a 14-day study period. In mechanically ventilated patients, oxygenation was measured via the PaO2/FiO2 (P/F) ratio and the oxygenation index (OI) daily for 14 days. Supplemental oxygen requirements were tracked daily for non-ventilated patients.
Results
Non-ventilated patients were liberated from supplemental oxygen after TPE. The response was rapid with an 87% average reduction in oxygenation requirements following and average time to return to room air of 5.25 days. All mechanically ventilated patients demonstrated improvement in oxygenation with a 78% average improvement in the P/F ratio and a 43% improvement in OI. C-reactive protein (CRP) and serum levels of IL-6, IL-8, IL-10, TNFα, IFNγ and GM-CSF, were measured daily with immediate post TPE levels drawn on days 1, 2, 4, 6 and 8. All patients demonstrated significant reductions in CRP, IL-6, IL-10 and TNFα.
Conclusions
In the majority of patients with Penn class 3 and 4 CRS complicating COVID-19, TPE demonstrated a prompt improvement in oxygenation and reduction in cytokine load without compromising patient safety. As this pilot study was envisioned to be hypothesis generating, expanded trials using TPE alone and in conjunction with novel pharmacologic agents are warranted.
Registration
ClinicalTrials.gov NCT04374149.
Keywords: Therapeutic plasma exchange, COVID-19, Cytokine release syndrome, Respiratory failure, Oxygenation, Therapeutic benefit
1. Introduction
The virally mediated pandemic of 2020 is linked to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causing coronavirus disease 2019 (COVID-19). The predominant modes of transmission are respiratory droplets, aerosolization and contaminated surface contact [[1], [2], [3]]. COVID-19 is characterized by a spectrum of severity and disease manifestations ranging from asymptomatic to multiorgan failure. While definitive treatment is lacking, there is an increasing awareness of the associated systemic cascade of inflammatory molecules which warrants the exploration of possible therapeutic intervention.
Therapeutic plasma exchange (TPE) has long played a successful role in the depletion of injurious immunoglobulins and immune complexes across a diverse spectrum of diseases [4]. Its efficacy in addressing syndromes of cytokine excess has been more widely debated with inconclusive results in ameliorating sepsis [5,6], while both disputed and supported as efficacious in vasculitis and other inflammatory states [7]. Lyu and colleagues [8] postulated that centrifugal TPE may be more effective than membrane filtration to remove smaller protein molecules. While cytokines possess a molecular weight amenable to reduction by TPE, their extravascular distribution and potentially high rate of production make them less than ideal solutes for depletion by apheresis.
Conti and colleagues [9] have outlined the binding of COVID-19 to the Toll-like receptor (TLR) with subsequent multistep generation of mature IL-1β to trigger fever and pneumonitis. Many investigators draw similarity to the evolving understanding of cytokine storm or the cytokine release syndrome. Huang [10] reported excessive elaboration of IL-2, IL-10, TNFα and macrophage inflammatory protein 1-alpha among others, while Mehta et al. [11] noted the resemblance of severe COVID-19 expression to secondary hemophagocytic lymphohistiocytosis (sHLH) with its potentially fatal hypercytokinemic state. Additionally, TPE has been shown to have clinical benefit in various hypercytokinemic states including CRS following CAR T-cell treatment [12] and in pH1N1 influenza A respiratory failure and hemodynamic shock [13].
Since completion of our pilot study, there have been several publications pertinent to the use of TPE in COVID-19 patients including a single case reporting benefit [14], advocation for clinical trials from theoretical benefit [15], a non-randomized trial reporting higher extubation rates and lower mortality [16], and a report of five patients with improved oxygenation and a decrease in inflammatory markers [17]. Additionally, there has been discussion of potential adverse events of TPE in COVID-19 including compromised immunity, worsening coagulopathy, and depletion of blood bank resources [18].
The cytokine release syndrome is characterized by fever, pneumonitis, and elaboration of a proinflammatory cascade of molecules complicating approximately 20% of COVID-19 cases [19]. Mortality for patients requiring high flow oxygen but short of mechanical ventilation is variably reported. Mortality of critically ill patients requiring mechanical ventilation, while initially reported to exceed 50%, has been more recently reported as ‘only’ 35.7% [20].
We utilized the Penn classification for grading the severity of CRS [21]. Penn class 3 is of clinical severity requiring hospitalization to manage organ dysfunction secondary to CRS. Penn class 4 is life-threatening CRS which requires high dose vasopressors or hypoxemia requiring mechanical ventilation. Here we share the results of a single arm, non-placebo-controlled pilot trial of TPE in ten patients with Penn class 3 or 4 CRS complicating COVID-19. Endpoints included clinical benefit, impact on oxygenation, time course of recovery from respiratory failure, daily CRP, and cytokine mapping for the 14-day study period and the impact of TPE on those blood levels.
2. Materials and methods
2.1. Study population
Eligible patients were COVID-19 positive by polymerase chain reaction (PCR), met criteria for Penn class 3 or 4 CRS, were aged 12 to 80 and required supplemental oxygen or mechanical ventilation. Patients were excluded if pregnant, breastfeeding, categorized as Class 3 or 4 New York Heart Association heart failure, stage 4 obstructive lung disease or interstitial lung disease with chronic hypoxic respiratory failure requiring supplemental oxygen at baseline. Patients were also excluded for current use of disease-modifying anti-rheumatic drugs (with the exception of hydroxychloroquine or IL-6 inhibitors), chronic corticosteroids if in excess of prednisone 10 mg/day or equivalent, suspected or confirmed clinically significant bacterial infection, history of tuberculosis, HIV, or irritable bowel disease, creatinine clearance of less than 15 mL/min, absolute neutrophil count less than 1000/μL, platelet count less than 50,000/μL and AST or ALT greater than five times the institutional upper limit of normal.
2.2. Trial design
The trial, approved by the health system's Institutional Review Board (IRB), included a single cohort of ten patients on a single arm study without placebo randomization. All patients providing informed consent underwent TPE with one plasma volume exchange daily for two consecutive days then every other day times three for a total of five exchanges using the Spectra Optia Apheresis System (Terumo BCT Inc., Lakewood, Colorado, USA) employing centrifugal blood component separation. Patients received isovolemic replacement with 5% albumin or, in the setting of underlying coagulopathy, fresh frozen plasma (FFP).
2.3. Study endpoints
The primary endpoint of the trial was to document the efficacy of TPE in decreasing the CRP and cytokine load (IL-6, IL-8, IL-10, TNFα, IFNγ, GM-CSF) by measuring these levels daily and immediately post TPE over a 14-day period. Secondary endpoints included the number of patients achieving clinical benefit, time to improvement in oxygenation and time to independence from mechanical ventilation or supplemental oxygen.
2.4. Oxygenation metrics
Supplemental oxygen requirements were tracked daily for non-ventilated patients. In mechanically ventilated patients, oxygenation was measured via the PaO2/FiO2 (P/F) ratio and the oxygenation index (OI) daily for 14 days. The OI (FiO2 x mean airway pressure/PaO2) is used to assess the intensity of ventilatory support required to maintain oxygenation.
2.5. Laboratory assessments
CRP and fibrinogen levels were analyzed by the hospital laboratory. Cytokine assays were performed in the cancer institute research laboratory. Serum levels of IL-6, IL-8, IL-10, TNFα, GM-CSF, and IFNγ were measured using a Magnetic Luminex Performance Assay (R&D Systems) according to the manufacturer's protocol [22]. Data were analyzed using Bio-Plex Manager Software and GraphPad Prism. COVID-19 serology was assayed at baseline, day 7 and day 14 utilizing Bio-Rad's Platelia SARS-CoV-2 total antibody assay.
2.6. Clinical benefit
Clinical benefit was defined as those patients who no longer required supplemental oxygen or who were successfully extubated within the 14-day study window.
2.7. Safety
Study related adverse events were graded using NCI Common terminology criteria for adverse events (CTCAE Version 5.0). The safety analysis included all patients who underwent at least one TPE.
2.8. Statistical analysis plans
This pilot study was conceived to be largely hypothesis generating as the small sample size was felt unlikely to allow for statistically significant inferences. However, the observation of sharp reductions in several molecular markers prompted analysis of the data. Median comparisons were performed using the Wilcoxon Signed Rank test.
3. Results
3.1. Patients
Ten patients were enrolled between April and June of 2020 onto this single cohort pilot study to assess the impact of TPE on oxygenation status, clinical response, and laboratory parameters of inflammatory mediators. The patient demographics, Penn class of CRS and pertinent comorbid conditions are shown in Table 1 . No patients received previous or concurrent convalescent plasma, remdesivir, corticosteroids, or IL-6 inhibitors. Two patients received a short course (less than four days) of hydroxychloroquine prior to enrollment. All patients received 5% human albumin as replacement fluid except for a single patient who received FFP for only two of five plasma exchanges. Nine of the ten patients completed all five planned TPE. One patient completed four of the five planned TPE but stopped early due to intercurrent staphylococcal pneumonia. There was no 14-day mortality.
Table 1.
Demographic and clinical characteristics of patients.
| N | 10 |
|---|---|
| Age, Mean ± SD | 51.8 ± 12.6 |
| Body Mass Index (kg/m2), Mean ± SD | 34.4 ± 10.2 |
| Gender, N (%) | |
| Female | 7 (70) |
| Male | 3 (30) |
| Race/Ethnicity, N (%) | |
| Hispanic | 6 (60) |
| White | 2 (20) |
| Asian | 1 (10) |
| Black | 1 (10) |
| Days from COVID + test to 1st TPE, Median (IQR) | 4.5 (3, 6) |
| ABO Blood Group, N (%) | |
| A- | 1 (10) |
| A+ | 2 (20) |
| B+ | 2 (20) |
| O+ | 5 (50) |
| Respiratory Status, N (%) | |
| Penn Class 3 | |
| Nasal Cannula or High Flow Nasal Cannula | 4 (40) |
| Penn Class 4 | |
| Invasive Mechanical Ventilation | 6 (60) |
| Comorbidities, N (%) | |
| Diabetes | 3 (30) |
| Hypertension | 5 (50) |
| Obesity | 6 (60) |
3.2. Efficacy
All four non-ventilated patients were liberated from supplemental oxygen after TPE. The response was rapid with an 87% average reduction of oxygenation requirements following the second TPE (day 3) and average time to return to room air of 5.25 days. Similarly, all ventilated patients demonstrated improvement in oxygenation by day 3 with an average improvement of 78% in the P/F ratio and an average improvement of 43% in the OI (Table 2 ). Two of the six ventilated patients were extubated within 14 days.
Table 2.
Oxygenation status of patients.
| Nasal Cannula or High Flow Nasal Cannula (n = 4) | ||||||
|---|---|---|---|---|---|---|
| Patient | Baseline O2 L/min on day of TPE | O2 L/min after 2nd TPE (day 3) | % Reduction at day 3 | Time to Room Air (in days) | ||
| 101 | 10 | 4 | 60 | 11 | ||
| 102 | 10 | 1 | 90 | 4 | ||
| 104 | 6 | 0 | 100 | 3 | ||
| 110 | 3 | 0 | 100 | 3 | ||
| Average | 7.25 | 1.25 | 87.5 | 5.25 | ||
| Mechanical Ventilation (n = 6) | ||||||
| Patient | Baseline P/F ratio on day of TPE | P/F ratio after 2nd TPE (day 3) | % Change | Baseline OI | OI after 2nd TPE (day 3) | % Change |
| 103 | 255 | 245 | −3.92 | 5.1 | 4.9 | −3.92 |
| 105 | 135 | 152 | 12.59 | 14.07 | 9.54 | −32.2 |
| 106 | 56 | 143 | 155.36 | 29.7 | 12.63 | −57.47 |
| 107 | 57 | 150 | 163.16 | 29.82 | 11 | −63.11 |
| 108 | 95 | 194 | 104.21 | 14.74 | 8.76 | −40.57 |
| 109a | 174 | 240 | 37.93 | 8.03 | 2.92 | −63.64 |
| Average | 78.22 | −43.49 | ||||
Patient 109-Patient was extubated on study day 3, therefore values were taken from the day prior (after TPE).
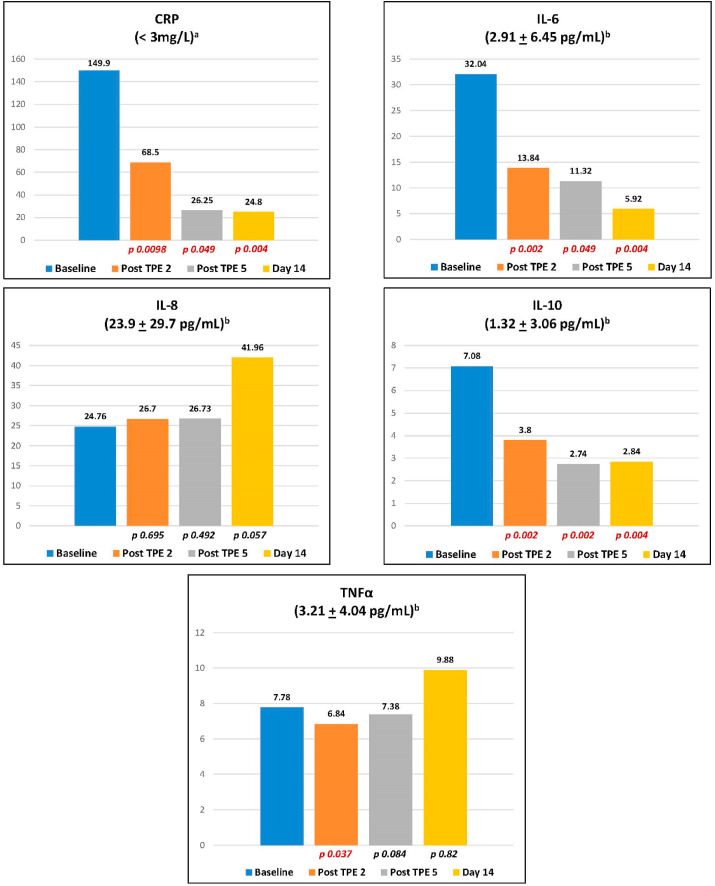
TPE produced sharp and immediate reductions in CRP, IL-6, and IL-10 levels from baseline to completion of the second and fifth TPE and at study day 14. No incremental reductions in CRP, IL-6 and IL-10 were generated beyond the second TPE. TPE reduced levels of TNFα by completion of the second exchange but did not impact levels after the fifth TPE or at day 14 compared to baseline. TPE did not significantly alter IL-8 levels from baseline through day 14 (Fig. 1 ). GM-CSF levels were barely detectable or completely undetectable for all ten patients at baseline and through day 14. IFNγ levels were remarkable for only one of ten patients with an elevation at baseline and rendered undetectable after the second TPE. Given the small size of the study, no subset analysis was planned.
Fig. 1.
Two-sample Median Comparisons for CRP and Cytokine Levels.
The median of CRP and cytokine levels was compared in all ten patients following the second and fifth (final) plasma exchange and at day 14.
a Non-cardiac (inflammatory) CRP reference range.
b Cytokine levels represent average concentrations + standard deviation [Kim et al. J TranslMed 9,113 (2011) https://doi.org/10.1186/1479-5876-9-113].
Six of the ten patients, including all four Penn class 3 and two of the six Penn class 4 patients, experienced a clinical benefit. There were no study related adverse events. TPE appears well tolerated with a notable absence of hypotension, ectopy, and autonomic lability. In several patients, we noted a transient need to titrate the fentanyl infusion likely due to the high plasma protein binding of the drug. A careful review of other medications was necessary to avoid inadvertent depletion. The development of bacterial pneumonia prompted TPE discontinuation following the fourth of five planned exchanges in one patient. There was no evidence of bacteremia related to the apheresis catheter. The schedule of TPE was not altered in any patient due to hypofibrinogenemia.
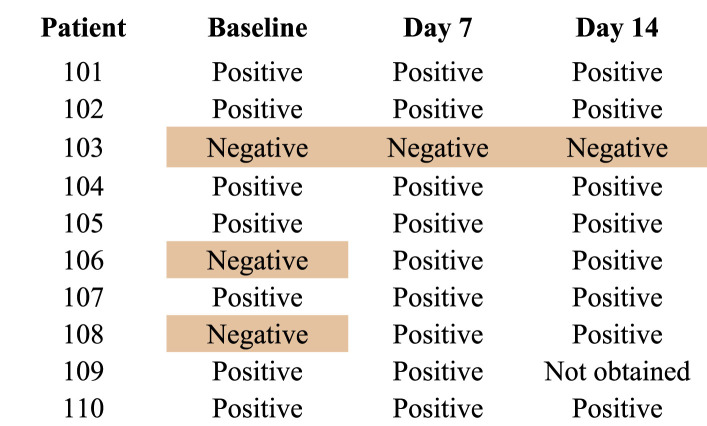
Positive serology for COVID-19 was present in seven of ten patients at baseline, two of 10 negative at baseline but becoming positive by day 7 and only one patient with a history of immunosuppressive therapy for non-Hodgkin's lymphoma demonstrated no evidence of humoral immunity throughout the study window. Once positive, all patients remained persistently positive (Table 3 ).
Table 3.
COVID-19 Serology.
4. Discussion
This study included critically ill patients with severe disease burden. Six of the ten (60%) patients started TPE while on invasive mechanical ventilation with an average PEEP of 11.7 cm H2O and an average FiO2 of 0.68. Of the four patients who required oxygenation via nasal cannula, the average starting oxygenation was 7.3 L/min. In comparison, only 25.6% of patients in the ACTT-1 trial required mechanical ventilation while 11.9% of the patients did not require supplemental oxygen [25]. Unlike studies reported to date, these ten patients were remarkable for their pristine freedom from confounding variables such as antecedent or concomitant convalescent plasma, remdesivir, glucocorticoids and anti-IL-6 agents. This gave us a unique opportunity to define the impact of TPE more clearly.
In keeping with the findings of Morath et al. [17], all patients, irrespective of ventilatory status, demonstrated improvement in oxygenation parameters by day 3 (after TPE #2). This contrasts with the data from a similar group of patients treated with Tocilizumab in the setting of COVID-19 related CRS who worsened clinically over the course of treatment [26].
The assessment of oxygenation status and thus improvement in the setting of ARDS is complex. We herein report data measured by the commonly used P/F ratio and by the OI which has been shown to reflect patient status more accurately by accounting for the high levels of PEEP [[27], [28], [29], [30]]. Regardless, as reflected in Table 2, five of six ventilated patients exhibited a trend of improving oxygenation by day 3 with rapid extubation possible in two. The findings were similarly striking for non-ventilated patients as all were free of supplemental oxygen at a mean of 5.3 days.
No patients died at the 14-day mark which is noteworthy considering the severity of our cohort. In the ACTT-1 trial, the 14-day mortality with remdesivir was 7.1% compared with 11.9% with placebo [25]. In the tocilizumab trial, the mortality rate of all patients was 14% at 14 days [26]. Mortality in patients with COVID-19 who require mechanical ventilation is high with reports from 35.7% in a U.S. based population [20] to 97% in the original Wuhan outbreak [31]. While our sample size is small, our results demonstrate a signal of remarkable improvement in a critically ill patient cohort and should be further evaluated to determine if the results are reproducible.
The lack of safety issues was reassuring but remains in need of further validation. No bleeding complications were noted. The development of positive serology for COVID-19 and its persistence were evident in all but one severely immunocompromised patient. We chose 5% albumin as the replacement fluid as coagulation factor deficiency was not evident by laboratory screening or clinical assessment. Its successful use addresses the admonition by Stahl et al. [18] regarding potential exhaustion of blood bank reserves.
The molecular mapping in this trial was intensive with CRP and all six cytokines assessed 20 times per patient within a 14-day period. All ten patients demonstrated a reduction in CRP, IL-6, IL-10 and TNFα by completion of the second TPE in temporal synchrony with all ten patients demonstrating an improvement in oxygenation by day three. Regarding IL-6, some investigators have emphasized its “king pin” importance [23] while others are dismissive [24]. We await clear incrimination of IL-6 specifically or cytokines in general as opposed to being markers functioning as epiphenomenal surrogates. Our results are at least consistent with other reports that suggest IL-6 is a significant molecular mediator of CRS [23]. Interpretation of these values along with low levels of IFNγ and GM-CSF remains conjectural as serum levels may be a poor reflection of extravascular, tissue centric levels. Of note, our definition of clinical benefit was a “hard stop” without nuance. Patients were either free of supplemental oxygen, extubated or were not.
IL-10 is commonly regarded as an anti-inflammatory cytokine of monocytic and other lineage production, likely playing a broader role in immunoregulation as well as within the inflammatory cascade. IL-10's molecular weight of 18 kDa is less than optimal for removal but did undergo significant reduction by conclusion of the second TPE, fifth TPE and was persistently reduced at day 14. The depletion of a cytokine that may play a role in attenuating the CRS is of theoretical concern.
There were no hypercoagulable events despite the high thrombotic risk in severe COVID-19 patients. The role of the endothelium in COVID-19 systemic inflammatory excess as both victim and villain remains minimally explored. Goshua et al. [32] assessed markers of endothelial cell and platelet activation across a spectrum of COVID-19 disease severity and reported increased levels of von Willebrand factor (VWF) antigen, soluble P antigen and soluble thrombomodulin. These markers are excellent candidates to be removed via TPE and remain a plausible explanation of the efficacy of TPE.
5. Conclusions
We chose to publish this pilot study in the spirit of rapid communication. In the absence of definitive drug treatment or vaccination, the value of TPE should be further explored for validation and delineation of the optimal intensity and periodicity of the procedure. Should subsequent studies confirm the clinical benefit, TPE would rise to a truly meaningful therapeutic intervention employing a procedure that remains untapped and underutilized amidst a raging pandemic but ironically available throughout the world and without need for expensive upgrades to deploy. The timing of this prospective trial, early in the pandemic, allowed for freedom from confounding treatments with antiviral and anti-inflammatory agents that could have altered laboratory findings. This study is the first to report on the development and preservation of immune response to COVID-19 in patients undergoing TPE. The clinical benefit as portrayed by the cytokine panel was equally reflected through the measurement of CRP, which is universally available and more cost effective. Finally, the CRS complicating COVID-19 provides an unforeseen opportunity to define the molecular orchestration likely shared in part by sHLH and possibly ARDS and is certainly timely given the anticipated surge in incidence as CAR-T therapy and additional generations of T-cell engagement therapies unfold.
Source of funding
The funders of the study had no role in the study design, data collection, data analysis, or writing of the manuscript. Terumo BCT, Inc. provided the disposable apheresis exchange kits. Prisma Health Office of Philanthropy and Partnership assisted in securing funding for the laboratory analysis. All authors had full access to all study data and had final responsibility for the decision to submit the manuscript for publication. W. Jeffery Edenfield serves as a consultant for Chimerix, Inc., unrelated to the submitted work. The remaining authors have disclosed that they do not have any conflicts of interest.
CRediT authorship contribution statement
W. Larry Gluck: Conceptualization, Methodology, Formal analysis, Data curation, Writing - original draft, Funding acquisition. Sean P. Callahan: Investigation, Supervision, Writing - review & editing. Robert A. Brevetta: Investigation, Supervision, Writing - review & editing. Antine E. Stenbit: Investigation, Supervision, Writing - review & editing. Wesley M. Smith: Investigation, Visualization, Data curation, Writing - original draft, Formal analysis. Julie C. Martin: Project administration, Visualization, Data curation, Writing - original draft, Formal analysis. Anna V. Blenda: Data curation, Validation, Writing - review & editing. Sergio Arce: Data curation, Validation, Writing - review & editing. W. Jeffery Edenfield: Visualization, Funding acquisition, Writing - review & editing.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: W. Jeffery Edenfield serves as a consultant for Chimerix, Inc., unrelated to the submitted work. The remaining authors have disclosed that they do not have any conflicts of interest.
Acknowledgments
We thank the following individuals from for their contributions: Research Manager Jan Kueber for leading the on-site implementation; Jennifer Caldwell and Noreen Denham for patient case management; Erin Hudgins and Katie McKelvey for their expertise and care of the participants undergoing therapeutic plasma exchange; the staff of the Prisma Health Cancer Institute Biorepository and Phase I unit for diligent specimen deidentification, processing, storage and data management; and Alex Ewing for guidance regarding the data analysis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmed.2020.106188.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zhou P., Yang X., Wang X., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Novel Coronavirus Situation Report-2. World Health Organization; Geneva: January 2020. https://www.who.int/docs/default-source/coronavirus/situation-reports/20200122-sitrep-2-2019-ncov.pdf [Google Scholar]
- 3.Transmission of SARS-CoV-2: Implications for Infection Prevention Precautions. World Health Organization; Geneva: July 2020. https://www.who.int/publications/i/item/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations [Google Scholar]
- 4.Padmanabhan A., Connelly-Smith L., Aqui N., et al. Guidelines on the use of therapeutic apheresis in clinical practice- evidence-based approach from the writing committee of the American Society for Apheresis: the eighth special issue. J. Chromatogr., A. 2019;34(3):167–354. doi: 10.1002/jca.21705. [DOI] [PubMed] [Google Scholar]
- 5.Busund R., Koukline V., Utrobin U., et al. Plasmapheresis in severe sepsis and septic shock: a prospective, randomised, controlled trial. Intensive Care Med. 2002;28:1434–1439. doi: 10.1007/s00134-002-1410-7. [DOI] [PubMed] [Google Scholar]
- 6.Rimmer E., Houston B.L., Kumar A., et al. The efficacy and safety of plasma exchange in patients with sepsis and septic shock; a systematic review and meta-analysis. Crit. Care. 2014;18(6):699. doi: 10.1186/s13054-014-0699-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tesar V., Jelinkova E., Masek Z., et al. The effect of plasmapheresis on cytokine storm levels and adhesion molecules in ANCA-positive renal vasculitis. Cas. Lek. Cesk. 1997;136(20):627–632. [PubMed] [Google Scholar]
- 8.Lyu R.K., Chen W.H., Hsieh S.T. Plasma exchange versus double filtration plasmapheresis in the treatment of Gillain-Barre syndrome. Ther. Apher. 2002;6(2):163–166. doi: 10.1046/j.1526-0968.2002.00382.x. [DOI] [PubMed] [Google Scholar]
- 9.Conti P., Ronconi G., Caraffa A., et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19: anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents. 2020;34(2) doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 10.Huang C., Wang Y., Ki X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta P., McAuley D.F., Brown M., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;295(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao X., Xiaoyuan H., Li Q., et al. Plasma exchange can be an alternative therapeutic modality for severe cytokine release syndrome after chimeric antigen receptor-T cell infusion: a case report. Clin. Canc. Res. January 1 2019;(25):29–34. doi: 10.1158/1078-0432.CCR-18-1379. 2019, 1. [DOI] [PubMed] [Google Scholar]
- 13.Patel P., Nandwani V., Vanchiere J., et al. Use of therapeutic plasma exchange as a rescue therapy in 2009 pH1N1 influenza A-An associated respiratory failure and hemodynamic shock. Pediatr. Crit. Care Med. 2011;12:e87–e89. doi: 10.1097/PCC.0b013e3181e2a569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi H., Zhou C., He P., et al. Successful treatment with plasma exchange followed by intravenous immunoglobulin in a critically ill patient with COVID-19. Int. J. Antimicrob. Agents. 2020;56(2):105974. doi: 10.1016/j.ijantimicag.2020.105974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tabibi S., Tabibi T., Conic R.R.Z., et al. Therapeutic plasma exchange: a potential management strategy for critically ill COVID-19 patients. J. Intensive Care Med. 2020;35(9):827–835. doi: 10.1177/0885066620940259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khamis F., Al-Zakwani I., Al Hashmi S., et al. Therapeutic plasma exchange in adults with severe COVID-19 infection [published online ahead of print, 2020 jun 22] Int. J. Infect. Dis. 2020;S1201–9712(20) doi: 10.1016/j.ijid.2020.06.064. 30499-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morath C., Weigand M.A., Zeier M., et al. Plasma exchange in critically ill COVID-19 patients. Crit. Care. 2020;24(1):481. doi: 10.1186/s13054-020-03171-3. Published 2020 Aug 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stahl K., Bode C., David S. First do no harm-beware the risk of therapeutic plasma exchange in severe COVID-19. Crit. Care. 2020;24(1):363. doi: 10.1186/s13054-020-03070-7. Published 2020 Jun 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cantanzaro M., Fagiani F., Racchi M., et al. Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal Transduct. Target Ther. 2020;5:84. doi: 10.1038/s41392-020-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Auld S.C., Caridi-Scheible M., Blum J.M., et al. 2019. ICU and Ventilator Mortality Among Critically Ill Adults with Coronavirus Disease. Crit Care Med 2020, [published online ahead of print, 2020 May 26] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Porter D., Frey N., Wood P., et al. Grading of cytokine release syndrome associated with the CAR T cell therapy tisagenlecleucel. J. Hematol. Oncol. 2018;11:35. doi: 10.1186/s13045-018-0571-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magnetic Luminex Performance Assay. https://www.rndsystems.com/products/luminex-high-performance-assays Minneapolis, MN: R&D Systems, Inc.
- 23.Zhang C., Wu Z., Li J.-W., et al. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int. J. Antimicrob. Agents. 2020 May;55(5):105954. doi: 10.1016/j.ijantimicag.2020.105954. [published online ahead of print, 2020 Mar 29] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sinha P., Matthay M.A., Calfee C.S. Is a “cytokine storm” relevant to COVID-19? JAMA Int. Med. 2020;180(9):1152–1154. doi: 10.1001/jamainternmed.2020.3313. [DOI] [PubMed] [Google Scholar]
- 25.Beigel J.H., Tomashek K.M., Dodd L.E., et al. Remdesivir for the treatment of covid-19 - preliminary report. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2007764. NEJMoa2007764, [published online ahead of print, 2020 May 22] [DOI] [PubMed] [Google Scholar]
- 26.Price C.C., Altice F.L., Shyr Y., et al. Tocilizumab treatment for Cytokine Release Syndrome in hospitalized COVID-19 patients: survival and clinical outcomes. Chest. 2020 doi: 10.1016/j.chest.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seeley E., McAuley D.F., Eisner M., et al. Predictors of mortality in acute lung injury during the era of lung protective ventilation. Thorax. 2008;63:994–998. doi: 10.1136/thx.2007.093658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balzer F., Menk M., Ziegler J., et al. Predictors of survival in critically ill patients with acute respiratory distress syndrome (ARDS): an observational study. BMC Anesthesiol. 2016;16:108. doi: 10.1186/s12871-016-0272-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monchi M., Bellenfant F., Cariou A., et al. Early predictive factors of survival in the acute respiratory distress syndrome. A multivariate analysis. Am. J. Respir. Crit. Care Med. 1998;158:1076–1081. doi: 10.1164/ajrccm.158.4.9802009. [DOI] [PubMed] [Google Scholar]
- 30.Villar J., Pérez-Méndez L., López J., et al. An early PEEP/FIO2 trial identifies different degrees of lung injury in patients with acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2007;176(8):795–804. doi: 10.1164/rccm.200610-1534OC. [DOI] [PubMed] [Google Scholar]
- 31.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [published correction appears in Lancet. 2020 Mar 28;395(10229):1038] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goshua G., Pine A.B., Meizlish M.L., et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7(8):e575–e582. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.