Definition of disseminated intravascular coagulation
Disseminated intravascular coagulation (DIC) represents a serious condition in which systemic and persistent coagulation activation occurs in the presence of an underlying pathology, resulting in the formation of microthrombi in microvessels. Activation of fibrinolysis is observed alongside coagulation, but the degree of fibrinolytic activation varies considerably depending on the underlying diseases. As DIC progresses, hemostatic factors such as platelets and coagulation factors decrease, leading to the pathophysiology of consumption coagulopathy [1].
The two major symptoms of DIC are bleeding and organ failure, but the prognosis is dire once clinical symptoms appear. Ideally, therefore, appropriate treatment for the condition should be started before such symptoms arise. For that reason, blood tests are crucial (See this Progress in Hematology < PIH > : Madoiwa’s paper). Many diseases capable of causing DIC are known, with acute leukemia, solid tumor, and sepsis considered the three major underlying pathologies.
A definition of DIC has been proposed by the International Society on Thrombosis and Haemostasis (ISTH) [2]. This definition takes into account DIC associated with sepsis, but not those DICs associated with acute leukemia, aortic aneurysm, obstetric complications, etc. (where bleeding symptoms are more likely to be seen than organ failure).
In this PIH, experts in each field wrote on the theme of hematopoietic malignancies, sepsis, aortic aneurysm, COVID-19, and clinical laboratory medicine under the unifying theme of DIC.
Pathophysiology and classification of DIC
The pathogenic mechanisms underlying DIC differ depending on the specific disease, but tissue factor (TF) plays an important role in many cases. Although the pathogenic mechanisms of DIC in aortic aneurysm have not been fully elucidated, drugs that suppress active coagulation factors such as heparin are known to clinically improve DIC (warfarin, which lowers coagulation factors as a substrate, is ineffective and thus contraindicated). (See this PIH: Yamada’s paper).
Suppressed-fibrinolytic-type DIC
Inflammatory cytokines play a major role in the development of DIC caused by severe infectious diseases such as sepsis. In sepsis, lipopolysaccharide (LPS) and cytokines (tumor necrosis factor < TNF > , interleukin < IL > -1, etc.) produce large amounts of TF from monocytes/macrophages and vascular endothelial cells, resulting in significant activation of coagulation. Furthermore, LPS and cytokines suppress the expression of thrombomodulin, an important anticoagulant protein present on vascular endothelial cells, thus accelerating the activation of coagulation.
Multiple microthrombi resulting from coagulation activation would normally be dissolved by the activation of fibrinolysis. However, plasminogen activator inhibitor (PAI), a fibrinolytic inhibitor, is overexpressed from vascular endothelial cells through the actions of LPS and cytokines, resulting in the strong suppression of fibrinolysis. As a result, multiple microthrombi remain difficult to dissolve, and multiple-organ failure progresses due to microcirculatory disorders (see this PIH: Iba’s paper). Such DIC is called “suppressed-fibrinolytic-type DIC” [3].
As for findings from blood coagulation tests, the coagulation activation marker thrombin-antithrombin complex (TAT) is markedly elevated, but plasmin-α2 plasmin inhibitor complex (PIC), a fibrinolytic activation marker, is only slightly elevated. Fibrin/fibrinogen degradation products (FDP) and D-dimer reflect the dissolution of microthrombi, and are also only slightly increased.
Enhanced-fibrinolytic-type DIC
In hematological malignancies such as acute leukemia, activation of the extrinsic coagulation pathway by TF from leukemia cells is considered to be the main cause of DIC (see this PIH: Ikezoe’s paper). In fact, when chemotherapy destroys leukemic cells, DIC may be transiently exacerbated due to the rapid release of TF from leukemic cells into the bloodstream [4]. The involvement of vascular endothelial cells and inflammation is relatively small; instead, the condition is a more direct pathological condition of coagulation activation. Fibrinolytic activation remains sufficient due to the low expression of PAI. Since multiple microthrombi are sufficiently dissolved by fibrinolysis, no microcirculatory disorder is present and few organ symptoms arise.
However, dissolution of not only a large number of microthrombi (so-called “bad thrombi”), but also hemostatic thrombi (so-called “good thrombi”) causes severe bleeding symptoms.
Bleeding symptoms are particularly severe in acute promyelocytic leukemia (APL). Acute leukemia other than APL, aortic aneurysm, giant hemangioma, obstetric complications (amniotic fluid embolism, placental abruption of the placenta), etc. show similar pathological conditions. This type of DIC is called “enhanced-fibrinolytic-type DIC”.
As for blood coagulation test findings, plasma levels of both TAT and PIC increase markedly, and FDP and D-dimer levels also rise (particularly FDP levels). Since degradation of not only fibrin but also fibrinogen progresses, a divergence phenomenon is observed between levels of FDP and D-dimer. When fibrinogen and α2 plasmin inhibitor decrease markedly with increasing PIC levels, major bleeding is more likely to occur.
Balanced-fibrinolytic-type DIC
Among malignant tumors, solid tumors display a slower course of progression than acute leukemia. Coagulation activation and fibrinolysis activation are thus enhanced in a well-balanced manner. Bleeding symptoms and organ failure are relatively unlikely unless the disease has progressed significantly. This type of DIC is called “balanced-fibrinolytic-type DIC”.
However, among solid cancers, some cancers such as advanced prostate cancer with metastasis show enhanced-fibrinolytic-type DIC.
COVID-19
In 2020, coagulation abnormalities and DIC associated with COVID-19 started drawing attention. Mild cases of COVID-19 rarely show DIC, but severe cases leading to death show a high rate of DIC.
With the progression of cytokine storm, COVID-19 patients become highly hypercoagulable and clinically exhibit arterial and venous thrombosis, with pulmonary thrombosis being the most common. In addition to such macrothrombosis, the existence of microthrombosis at the microscopic level has also been noted at autopsy.
However, bleeding symptoms are often a problem at the end of COVID-19 and during extracorporeal membrane oxygenation (ECMO) [5]. One of the causes is that COVID-19 is complicated by suppressed-fibrinolytic-type DIC in the early-to-middle stage, but may be transformed into the pathological condition of enhanced-fibrinolytic-type DIC in the advanced stage (See this PIH: Asakura’s paper).
Diagnostic criteria for DIC
Until relatively recently, the most frequently used diagnostic criteria in Japan were those of the Japanese Ministry of Health and Welfare (JMHW criteria) [6]. With these diagnostic criteria, the presence or absence of DIC was diagnosed after scoring the underlying disease, clinical symptoms, platelet count, FDP level, fibrinogen level, and prothrombin time (PT) ratio (for diseases with myelosuppression, bleeding symptoms and platelet counts were excluded from scoring). These diagnostic criteria covered clinical and laboratory findings in typical DIC, but were identified as unsuitable for early diagnosis of DIC.
The acute-stage DIC diagnostic criteria of the Japanese Association for Acute Medicine [7] have been expected in the emergency field as diagnostic criteria to enable earlier diagnosis. These criteria are effective for diagnosing DIC associated with infectious diseases, but cannot be applied to hematological malignancies. The diagnostic criteria of the ISTH [2] not only are modeled after the JMHW criteria, but are also unsuitable for early diagnosis.
The DIC Diagnostic Criteria of the Japanese Society on Thrombosis and Hemostasis (2017 edition) [8, 9] are diagnostic criteria that have been revised to address the problems of the JMHW criteria. These criteria are at least superior to the JMHW criteria because of the process of creating new diagnostic criteria.
Treatment for DIC
Preventing the development of DIC requires suppression of coagulation activation, as the main pathophysiology underlying DIC, as well as treatment of the underlying disorder. Even with treatment of the underlying disorder, cure within a few days is the exception rather that the rule. Avoiding exacerbation of the condition due to DIC is thus necessary during treatment of the underlying disorder. In addition, the pathophysiology of DIC is diverse, and use of different treatment methods according to the specific pathophysiology in the individual is necessary, rather than application of some uniform treatment.
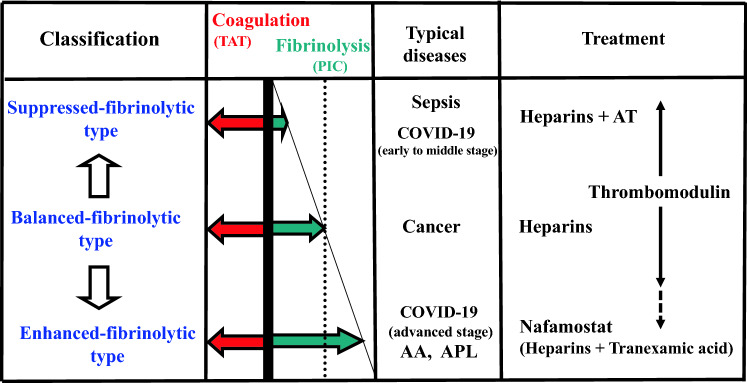
A suitable figure for DIC classification has already been published in another journal [10], so this time I would like to publish a figure regarding DIC treatment considering the disease classification (Fig. 1).
Fig. 1.
Selection of treatment according to DIC type. TAT, thrombin-antithrombin complex; PIC, plasmin-α2 plasmin inhibitor complex; AA, aortic aneurysm; APL, acute promyelocytic leukemia; AT, antithrombin concentrate. Heparins include low molecular weight heparin, unfractionated heparin, and danaparoid. 1) Suppressed-fibrinolytic-type: Treat with heparins. In Japan, AT concentrate can also be used for AT activity ≤ 70%. In sepsis, only AT concentrates can be used without heparins. 2) Balanced-fibrinolytic-type: When DIC is complicated by solid cancer, the solid cancer is often at an advanced stage. Heparin treatment may extend over a long period. 3) Enhanced-fibrinolytic-type: Nafamostat (NM) works well for this type, but may be inadequate for this type of DIC associated with solid tumors, in which case heparin plus tranexamic acid (TA) is effective. However, since TA for DIC has the side effect of thrombosis, confirming that this combination therapy lowers TAT and PIC levels is important. 4) Thrombomodulin (TM) preparations: Effective against DIC caused by many underlying diseases. For enhanced-fibrinolytic-type DIC, the above treatments may be preferable. However, TM preparations are effective when using ATRA for APL. 5) AT concentrates: AT concentrates are frequently used in sepsis, because AT activity is likely to decrease. In DIC caused by acute leukemia and solid tumors, AT activity is unlikely to decrease without a decrease in hepatic reserve
Treatment of the underlying disorder
Treatment of the underlying disorder is of paramount importance in all cases of DIC. For example, chemotherapy for acute leukemia and advanced cancer, and antibiotic treatment for sepsis are treatments for the underlying disorder.
Anticoagulant therapy
Anticoagulant therapy is performed to prevent coagulation activation, as the main pathophysiology behind DIC.
-
Heparins and antithrombin concentrates
Heparins (e.g., low molecular weight heparin, unfractionated heparin, danaparoid) display a common characteristic, in that they exert anticoagulant activity in an antithrombin (AT)-dependent manner. However, differences in anti-Xa/thrombin activity ratio and half-life in blood.
Since heparins cannot be expected to exert sufficient effect when AT activity decreases, AT concentrates are used in combination with heparins. Insurance coverage for AT concentrates in Japan is for DIC cases with AT activity ≤ 70%.
-
Thrombomodulin preparation
Thrombomodulin exerts not only anticoagulant effects, but also anti-inflammatory effects, and is thus expected to prove particularly useful for DIC associated with inflammatory disease [11, 12]. In fact, thrombomodulin is frequently used for DIC in Japan [13].
-
Synthetic protease inhibitor
Synthetic protease inhibitors exert antithrombin activity in an AT-independent manner. Representative drugs are nafamostat and gabexate [14]. These drugs show few side effects of bleeding, and are thus indicated when heparins are difficult to use due to concerns about side effects of bleeding.
Nafamostat also shows strong antifibrinolytic activity and is particularly effective for enhanced-fibrinolytic-type DIC. Nafamostat is known to block SARS-CoV-2 from invading host cells and appears to hold particular promise as a drug for DIC associated with COVID-19. Heparins have frequently been used as anticoagulant therapy for COVID-19. In addition, the combined use of nafamostat and heparin may be significant for enhancing positive effects on patients [15].
However, potential side effects of hyperkalemia should be noted for nafamostat.
Replacement therapy
If bleeding is observed due to a significant decrease in platelets and coagulation factors (consumption coagulopathy), replacement therapy is warranted. Platelet concentrate is used for platelet replacement, while fresh frozen plasma is used for coagulation factor replacement.
Antifibrinolytic therapy
Fibrinolytic activation in DIC is another aspect of the body’s defense response to dissolve microthrombi, and antifibrinolytic therapies such as tranexamic acid are contraindicated in principle. In particular, antifibrinolytic therapies are absolutely contraindicated for the suppressed-fibrinolytic-type DIC associated with sepsis.
Although tranexamic acid is being clinically studied for COVID-19, careful selection of cases and combined use of anticoagulant therapy are considered essential [16].
Administration of tranexamic acid in combination with heparins is often effective against the severe, potentially fatal bleeding tendencies seen with enhanced-fibrinolytic-type DIC (see this PIH: Yamada’s paper). Tranexamic acid for DIC has dramatic effects on bleeding when used properly with anticoagulant therapy. However, misuse of tranexamic acid can lead to fatal systemic thrombosis, making tranexamic acid a double-edged sword for DIC.
Tranexamic acid is absolutely contraindicated when using all-trans retinoic acid (ATRA) for APL, because ATRA transforms the character of APL from enhanced to suppressed-fibrinolytic-type DIC (see this PIH: Ikezoe’s paper). Several reports have described thrombosis-related deaths when tranexamic acid was used with ATRA for APL.
Conclusion
This PIH took up the theme of DIC. The pathophysiology of DIC varies depending on the underlying disease, and treatment policies thus need to be changed in accordance with the pathophysiology.
This discussion covered COVID-19-related coagulopathies and DIC, as well as hematological malignancies, sepsis, and aortic aneurysm, as typical diseases underlying DIC. The pathophysiology of COVID-19 is currently unclear, but will be elucidated in the future.
In addition, the theme of clinical laboratory medicine, which plays an extremely important role from the perspective of understanding the pathophysiology of DIC, is also included in this PIH.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Levi M, Ten Cate H. Disseminated intravascular coagulation. N Engl J Med. 1999;341(8):586–592. doi: 10.1056/NEJM199908193410807. [DOI] [PubMed] [Google Scholar]
- 2.Taylor FB Jr, Toh CH, Hoots WK, Wada H, Levi M; Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH). Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001; 86(5): 1327–30. [PubMed]
- 3.Asakura H. Classifying types of disseminated intravascular coagulation: clinical and animal models. J Intensive Care. 2014;2(1):20. doi: 10.1186/2052-0492-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harada-Shirado K, Wang X, Mori H, Fukatsu M, Takahashi H, Ikezoe T, et al. Circulating intranuclear proteins may play a role in development of disseminated intravascular coagulation in individuals with acute leukemia. Int J Hematol. 2020;111(3):378–387. doi: 10.1007/s12185-019-02798-5. [DOI] [PubMed] [Google Scholar]
- 5.Asakura H, Ogawa H. Overcoming bleeding events related to extracorporeal membrane oxygenation in COVID-19. Lancet Respir Med, in press. [DOI] [PMC free article] [PubMed]
- 6.Kobayashi N, Maekawa T, Takada M, Tanaka H, Gonmori H. Criteria for diagnosis of DIC based on the analysis of clinical and laboratory findings in 345 DIC patients collected by the research committee on DIC in Japan. Bibl Haematol. 1983;49:265–275. doi: 10.1159/000408467. [DOI] [PubMed] [Google Scholar]
- 7.Gando S, Iba T, Eguchi Y, Ohtomo Y, Okamoto K, Koseki K, et al. A multicenter, prospective validation of disseminated intravascular coagulation diagnostic criteria for critically ill patients: comparing current criteria. Crit Care Med. 2006;34(3):625–631. doi: 10.1097/01.CCM.0000202209.42491.38. [DOI] [PubMed] [Google Scholar]
- 8.Asakura H, Takahashi H, Uchiyama T, Eguchi Y, Okamoto K, Wada H, et al. Proposal for new diagnostic criteria for DIC from the Japanese Society on Thrombosis and Hemostasis. Thromb J. 2016;14:42. doi: 10.1186/s12959-016-0117-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wada H, Takahashi H, Uchiyama T, Eguchi Y, Okamoto K, Asakura H, et al. The approval of revised diagnostic criteria for DIC from the Japanese Society on Thrombosis and Hemostasis. Thromb J. 2017;15:17. doi: 10.1186/s12959-017-0142-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asakura H, Ogawa H. Perspective on fibrinolytic therapy in COVID-19: the potential of inhalation therapy against suppressed-fibrinolytic-type DIC. J Intensive Care. 2020;8:71. doi: 10.1186/s40560-020-00491-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saito H, Maruyama I, Shimazaki S, Yamamoto Y, Aikawa N, Ohno R, et al. Efficacy and safety of recombinant human soluble thrombomodulin (ART-123) in disseminated intravascular coagulation: results of a phase III, randomized, double-blind clinical trial. J Thromb Haemost. 2007;5(1):31–41. doi: 10.1111/j.1538-7836.2006.02267.x. [DOI] [PubMed] [Google Scholar]
- 12.Ito T, Thachil J, Asakura H, Levy JH, Iba T. Thrombomodulin in disseminated intravascular coagulation and other critical conditions-a multi-faceted anticoagulant protein with therapeutic potential. Crit Care. 2019;23(1):280. doi: 10.1186/s13054-019-2552-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osone S, Fukushima K, Yano M, Kakazu M, Sano H, Kato Y, et al. Supportive care for hemostatic complications associated with pediatric leukemia: a national survey in Japan. Int J Hematol. 2019;110(6):743–750. doi: 10.1007/s12185-019-02740-9. [DOI] [PubMed] [Google Scholar]
- 14.Minakata D, Fujiwara SI, Ikeda T, Kawaguchi SI, Toda Y, Ito S, et al. Comparison of gabexate mesilate and nafamostat mesilate for disseminated intravascular coagulation associated with hematological malignancies. Int J Hematol. 2019;109(2):141–146. doi: 10.1007/s12185-018-02567-w. [DOI] [PubMed] [Google Scholar]
- 15.Asakura H, Ogawa H. Potential of heparin and nafamostat combination therapy for COVID-19. J Thromb Haemost. 2020;18(6):1521–1522. doi: 10.1111/jth.14858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogawa H, Asakura H. Consideration of tranexamic acid administration to COVID-19 patients. Physiol Rev. 2020;100(4):1595–1596. doi: 10.1152/physrev.00023.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]