Abstract
BACKGROUND
In the Catheter Ablation for Atrial Fibrillation with Heart Failure (CASTLE-AF) trial, catheter ablation reduced the risk of death and heart failure (HF) hospitalization in patients with atrial fibrillation and HF by 40%.
OBJECTIVES
The study aimed to assess the generalizability of CASTLE-AF to routine clinical practice.
METHODS
Using a large US administrative database, we identified 289,831 patients with atrial fibrillation and HF treated with ablation (n = 7465) or medical therapy alone (n = 282,366) from January 1, 2008, through August 31, 2018. Patients were divided into 3 groups on the basis of trial eligibility: (1) eligible for CASTLE-AF, (2) failing to meet the inclusion criteria, and (3) meeting at least 1 of the exclusion criteria. Propensity score overlap weighting was used to balance ablated and drug-treated patients on 90 baseline characteristics. Cox proportional hazards regression was used to compare ablation with medical therapy for the primary outcome of a composite end point of all-cause mortality and HF hospitalization.
RESULTS
Only 7.8% of patients would have been eligible for the trial; 91.0% failed to meet the trial inclusion criteria;and 15.5% met the exclusion criteria. Ablation was associated with a lower risk of the primary outcome in the overall cohort (hazard ratio [HR] 0.81; 95% confidence interval [CI] 0.76–0.87; P < .001), in the trial-eligible cohort (HR 0.82; 95% CI 0.70–0.96; P = .01), and in patients who failed to meet inclusion criteria (HR 0.79; 95% CI 0.73–0.86; P < .001) but not in patients who met the exclusion criteria (HR 0.97; 95% CI 0.81–1.17). The relative risk reduction was consistent regardless of whether patients had HF with reduced left ventricular ejection fraction.
CONCLUSION
The benefit associated with ablation appears to be more modest in practice than that reported in the CASTLE-AF trial.
Keywords: Atrial fibrillation, Catheter ablation, Heart failure, Trial generalizability
Introduction
Atrial fibrillation (AF) and heart failure (HF) commonly coexist—one-third of patients with AF have HF, and more than half of patients with HF have AF.1 It has long been hypothesized that restoring sinus rhythm may reduce cardiovascular events, but rhythm control with antiarrhythmic drugs (AADs) has not been found to be superior to rate control.2 The inability of AADs to improve outcomes may be due to limited efficacy as well as challenges with safety, including adverse events and proarrhythmia, especially in patients with HF.
Catheter ablation has emerged as a more effective approach to maintain sinus rhythm than AADs. Ablation has also been shown to improve left ventricular ejection fraction (LVEF) and N-terminal pro-brain natriuretic peptide levels, suggesting potential cardiovascular benefits in patients with HF.3 Recently, in the Catheter Ablation for Atrial Fibrillation with Heart Failure (CASTLE-AF) trial, ablation reduced all-cause mortality and HF hospitalization by ~40%.4 In light of this trial, the 2019 focused update on the American guidelines added a class IIb recommendation for ablation in patients with HF with reduced LVEF (HFrEF).5 However, little is known about whether the trial results are generalizable to routine clinical practice, especially considering that CASTLE-AF included only 363 patients who had LVEF ≤ 35% and implantable cardioverter-defibrillator (ICD).
Therefore, this study aimed to assess the generalizability of the CASTLE-AF trial in a large cohort of US patients with AF and HF. We examined the proportion of patients who would have met trial eligibility and the associations between ablation and clinical outcomes, stratified by trial eligibility.
Methods
The Mayo Clinic Institutional Review Board exempted this study from review, because the study used preexisting, de-identified data.
Study population
This study was a retrospective cohort analysis using Optum-Labs Data Warehouse, which contains >130 million patients with private insurance or Medicare Advantage of all ages and races from all 50 states throughout the United States.6,7 The study population consisted of adult patients (age ≥18 years) with AF who were treated with ablation or drug therapy alone (either with AADs or rate-control drugs or both) from January 1, 2008, through August 31, 2018. The ablation cohort included patients who underwent an ablation procedure with a primary diagnosis of AF on the procedure claim. When a patient received multiple AF ablation procedures, the first one was selected as their index date. Patients who received their first AF ablation before January 1, 2008, were excluded. Patients were also required to have an HF diagnosis on or before the index date. The drug cohort included patients who did not undergo ablation but filled a study drug during the study period and had diagnoses of both HF and AF on or before the prescription fill date. Many patients may have used multiple drugs during the study period, and the initiation date of one of the drugs was randomly selected as the index date. In other words, for the drug cohort, the index date was the first fill date of a specific drug, but patients could have used a different drug before the index date. This method assured that both the ablated and drug-treated cohorts included patients with prior drug use, which was considered in the propensity score model. Patients were required to have at least 12 months of continuous enrollment in health insurance plans before the index date in order to capture an adequate medical history. Patients with invalid demographic data were excluded. The patient selection flow diagram is given in Supplemental Figure 1.
Exposure
A list of rhythm- and rate-control drugs is given in Supplemental Table 1. Ablation was identified using procedure codes (Supplemental Table 2). The same methods have been used in prior studies.8–12
Outcomes
Patients were followed until the end of the study period (August 31, 2018), the end of enrollment in health insurance plans, or death, whichever occurred first. The primary outcome was a composite end point of all-cause mortality or HF hospitalization. Mortality was identified on the basis of the Social Security Administration’s Death Master File and discharge status. HF hospitalization was defined as a hospitalization with HF as the primary diagnosis. The secondary outcomes included all-cause mortality and HF hospitalization considered separately, cardiovascular hospitalization, and hospitalization for any cause, and cerebrovascular accident (defined as ischemic or hemorrhagic stroke as a primary diagnosis during an emergency department visit or an inpatient stay). The diagnosis codes used to define cohorts and outcomes are given in Supplemental Table 2.
Statistical analysis
Patients were divided into 3 groups on the basis of trial eligibility as defined using the operational definitions given in Supplemental Table 3: (1) patients who would be eligible for CASTLE-AF; (2) patients who failed to meet the inclusion criteria, that is, HFrEF and ICD; and (3) patients who met at least one of the exclusion criteria. HFrEF was defined using the systolic HF diagnosis codes. In a previous internal validation using a subgroup of patients with linked electronic health records in OptumLabs, the codes performed relatively well in identifying patients with HFrEF, with a specificity of 91% and a sensitivity of 81%. Some patients both failed to meet the inclusion criteria and met the exclusion criteria. In the stratified analyses for clinical outcomes, such patients were classified as those who met the exclusion criteria.
Propensity score overlap weighting was used to account for the differences in baseline characteristics between patients who underwent catheter ablation and those who were treated with medical therapy alone.13 Standardized mean difference was used to assess the balance of covariates after weighting, and a difference of <0.1 was considered acceptable.14 Cox proportional hazards regression was used to compare outcomes in patients treated with ablation and medical therapy in the propensity score–weighted cohort with a robust sandwich estimator for variance estimation. The Fine and Gray method was used to consider death as a competing risk when assessing nonfatal outcomes.15 The proportional hazards assumption was tested using Schoenfeld residuals.16 When the assumption was violated, cumulative risks and hazard ratios (HRs) at different points were presented in addition to the overall HR.
Subgroup analyses
Prespecified subgroup analyses included stratification by age, sex, race, HFrEF, cardiomyopathy, prior cardioversion, implanted device, diabetes, hypertension with left ventricular hypertrophy, prior amiodarone use, concurrent use of digitalis, concurrent use of β-blockers, and the number of prior AADs. In patients who were eligible for the trial, all patients had HFrEF and ICD; therefore, the subgroup analyses stratified by HFrEF and ICD were removed and 2 additional subgroup analyses were added: cardiac resynchronization therapy and indication for defibrillator (primary or secondary).
Sensitivity analyses
First, a stratified analysis was performed on the basis of whether the drug-treated patients were treated with AADs or with rate-control drugs only. Propensity score weights were recalculated to compare ablation with AADs and to compare ablation with rate-control drugs. Second, a similar stratified analysis was performed on the basis of the adherence to drug therapy (defined as proportion of days covered ≥80%). Third, post hoc exploratory analyses were performed on the basis of HF hospitalization, as the reduction was not significant in the present study. Poisson regression was used to allow multiple hospitalizations and performed another sensitivity analysis blanking the first 30 days of follow-up. Fourth, falsification end points were used to test for residual confounding. Three end points that are unlikely to be a result of undergoing ablation—emergency department visit or hospitalization related to chronic obstructive pulmonary disease, pneumonia, and fracture—were selected. A significant relationship between ablation and a falsification end point would suggest a possibility of residual confounding.
The prespecified analysis plan, including more details of the methods, is available in the Supplement. A P value of <.05 was considered statistically significant for all tests. All tests were 2-sided. No adjustment for multiple testing was performed. All the analyses except those related to the primary outcome were considered to be exploratory. All analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC) and Stata 14.1 (Stata Corp, College Station, TX).
Results
Patient characteristics
This study identified 289,831 patients with AF and HF treated with ablation (n = 7465) or medical therapy alone (n = 282,366). Approximately 21.8% of the drug-treated patients were treated with AADs, 68.4% of whom were amiodarone (Supplemental Table 4). In the overall group before propensity score weighting, the mean age was 73.2 ± 10.3 years and 47.6% were female (Table 1). Approximately 91.0% of patients did not meet the trial inclusion criteria, that is, HFrEF and ICD; 15.5% of patients met at least one of the exclusion criteria (eg, renal failure, contraindication to anticoagulation, and a recent myocardial infarction or stroke); and 7.8% would have been eligible for the trial (Supplemental Table 3). After propensity score weighting, patients treated with ablation and those treated with medical therapy alone were identical on 90 dimensions (Table 1, Supplemental Tables 5–7, and Supplemental Figure 2).
Table 1.
Selected baseline characteristics before and after PS weighting in the overall cohort
| Before PS weighting |
After PS weighting |
|||||
|---|---|---|---|---|---|---|
| Characteristic | Drug treated (n = 282,366) | Ablation (n = 7465) | Total (N = 289,831) | Drug treated (n = 282,366) | Ablation (n = 7465) | Standard mean difference |
| Age (y) | 73.4 ± 10.2 | 65.9 ± 10.2 | 73.2 ± 10.3 | 67.1 ± 11.1 | 67.1 ± 10.0 | 0.00 |
| 18–64 | 18.7 | 42.3 | 19.3 | 37.3 | 37.3 | 0.00 |
| 65–74 | 27.1 | 36.7 | 27.3 | 37.5 | 37.5 | 0.00 |
| ≥75 | 54.2 | 21.0 | 53.4 | 25.2 | 25.2 | 0.00 |
| Female sex | 48.0 | 34.5 | 47.6 | 36.7 | 36.7 | 0.00 |
| Race | ||||||
| Asian | 1.7 | 1.4 | 1.7 | 1.4 | 1.4 | 0.00 |
| Black | 12.9 | 8.8 | 12.8 | 9.6 | 9.6 | 0.00 |
| Hispanic/Latino | 6.4 | 5.0 | 6.4 | 5.2 | 5.2 | 0.00 |
| White | 74.2 | 81.0 | 74.4 | 79.9 | 79.9 | 0.00 |
| Other/unknown | 4.8 | 3.7 | 4.7 | 4.0 | 4.0 | 0.00 |
| Medical history | ||||||
| HFrEF | 37.9 | 43.0 | 38.0 | 43.0 | 43.0 | 0.00 |
| Cardiomyopathy | ||||||
| None | 47.5 | 38.1 | 47.3 | 38.8 | 38.8 | 0.00 |
| Hypertrophic | 1.9 | 3.6 | 1.9 | 3.4 | 3.4 | 0.00 |
| Ischemic | 9.0 | 12.1 | 9.1 | 11.9 | 11.9 | 0.00 |
| Dilated | 41.6 | 46.2 | 41.8 | 45.9 | 45.9 | 0.00 |
| Implanted device | ||||||
| None | 73.7 | 74.6 | 73.7 | 72.2 | 72.2 | 0.00 |
| CRT – defibrillator | 1.6 | 2.1 | 1.6 | 2.3 | 2.3 | 0.00 |
| ICD | 11.7 | 12.6 | 11.7 | 13.8 | 13.8 | 0.00 |
| CRT – pacemaker | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.00 |
| Dual-chamber pacemaker | 8.1 | 7.4 | 8.1 | 8.1 | 8.1 | 0.00 |
| Single-chamber pacemaker | 4.7 | 3.1 | 4.7 | 3.3 | 3.3 | 0.00 |
| Cardioversion | 11.6 | 60.9 | 12.9 | 53.6 | 53.6 | 0.00 |
| Hypertension | 95.9 | 94.7 | 95.9 | 95.0 | 95.0 | 0.00 |
| Diabetes mellitus | 48.7 | 39.0 | 48.5 | 40.9 | 40.9 | 0.00 |
| Thromboembolism | 27.2 | 19.3 | 27.0 | 20.4 | 20.4 | 0.00 |
| CAD | 74.8 | 73.8 | 74.8 | 74.2 | 74.2 | 0.00 |
| Myocardial infarction | 31.3 | 26.6 | 31.2 | 27.8 | 27.8 | 0.00 |
| Prior valve procedure | 5.6 | 4.0 | 5.6 | 4.2 | 4.2 | 0.00 |
| Intracranial bleeding | 3.6 | 2.2 | 3.6 | 2.3 | 2.3 | 0.00 |
| Stage 3–5 CKD | 27.1 | 17.3 | 26.8 | 19.0 | 19.0 | 0.00 |
| Obstructive sleep apnea | 23.2 | 45.0 | 23.8 | 41.9 | 41.9 | 0.00 |
| Previous drug treatment | ||||||
| No. of previous AADs | ||||||
| 0 | 76.9 | 21.6 | 75.5 | 28.0 | 28.0 | 0.00 |
| 1 | 20.5 | 48.6 | 21.2 | 50.1 | 50.1 | 0.00 |
| 2 | 2.2 | 22.6 | 2.8 | 17.4 | 17.4 | 0.00 |
| ≥3 | 0.3 | 7.1 | 0.5 | 4.5 | 4.5 | 0.00 |
| Prior use of amiodarone | 15.5 | 45.5 | 16.2 | 42.0 | 42.0 | 0.00 |
| No. of previous rate-control drugs | ||||||
| 0 | 14.8 | 5.9 | 14.6 | 6.9 | 6.9 | 0.00 |
| 1 | 45.1 | 32.1 | 44.8 | 34.0 | 34.0 | 0.00 |
| 2 | 27.9 | 34.6 | 28.1 | 34.2 | 34.2 | 0.00 |
| ≥3 | 12.2 | 27.4 | 12.5 | 24.9 | 24.9 | 0.00 |
| Concurrent use of digitalis | 21.5 | 16.4 | 21.3 | 17.7 | 17.7 | 0.00 |
| Concurrent medication | ||||||
| Oral anticoagulant | ||||||
| None | 60.7 | 23.8 | 59.7 | 28.4 | 28.4 | 0.00 |
| Warfarin | 27.2 | 34.7 | 27.4 | 35.0 | 35.0 | 0.00 |
| NOAC | 12.1 | 41.4 | 12.9 | 36.6 | 36.6 | 0.00 |
| ACE inhibitors | 31.0 | 33.0 | 31.0 | 33.1 | 33.1 | 0.00 |
| ARB | 16.9 | 22.1 | 17.0 | 21.4 | 21.4 | 0.00 |
| Other β-blockers | 83.3 | 66.1 | 82.8 | 68.7 | 68.7 | 0.00 |
| Statin | 45.1 | 46.6 | 45.2 | 47.4 | 47.4 | 0.00 |
| CHA2DS2-VASc score | 5.6 ± 1.7 | 4.6 ± 1.7 | 5.6 ± 1.7 | 4.8 ± 1.7 | 4.8 ± 1.7 | 0.00 |
| Baseline period duration (y) Trial eligibility | 3.2 ± 3.3 | 4.4 ± 3.4 | 3.2 ± 3.3 | 4.0 ± 4.2 | 4.0 ± 3.0 | 0.00 |
| Ineligible for CASTLE-AF | 92.3 | 90.6 | 92.2 | 10.4 | 10.4 | 0.00 |
| Lack systolic HF | 37.9 | 43.0 | 38.0 | 57.0 | 57.0 | 0.00 |
| Lack of defibrillator | 86.7 | 85.3 | 86.7 | 72.2 | 72.2 | 0.00 |
| Met one of the exclusion criteria | 15.7 | 6.0 | 15.5 | 6.7 | 6.7 | 0.00 |
| Contraindication for anticoagulation | 5.4 | 3.3 | 5.3 | 3.5 | 3.5 | 0.00 |
Values are presented as mean ± SD or as percentage.
AAD = antiarrhythmic drug;ACE = angiotensin-converting enzyme;ARB = angiotensin II receptor blocker;CAD = coronary artery disease;CASTLE-AF = Catheter Ablation for Atrial Fibrillation with Heart Failure;CKD = chronic kidney disease;CRT = cardiac resynchronization therapy;HF = heart failure;HFrEF = heart failure with reduced ejection fraction;ICD = implantable cardioverter-defibrillator;NOAC = non-vitamin K antagonist oral anticoagulant; PS = propensity score.
Outcomes
The mean follow-up period was 1.8 ± 1.8 years. In the overall cohort, ablation was associated with a lower risk of the primary outcome of all-cause mortality and HF hospitalization (HR 0.81; 95% confidence interval [CI] 0.76–0.87; P < .001), a lower risk of all-cause mortality (HR 0.67; 95% CI 0.62–0.74; P < .001) but not HF hospitalization (HR 1.02; 95% CI 0.94–1.10; P = .67) or cerebrovascular accident (HR 0.98; 95% CI 0.82–1.17; P = .81) (Table 2 and Supplemental Table 8). The cumulative risks and HRs over time are illustrated in Figure 1 and Supplemental Table 9.
Table 2.
Outcomes in propensity score-weighted patients stratified by trial eligibility
| Variable | No. of events | Person-years | Event rate | No. of events | Person-years | Event rate | Absolute reduction in event rate (95% CI) | Hazard ratio (95% CI) | P |
|---|---|---|---|---|---|---|---|---|---|
| Overall | Drug treated (n = 282,366) | Ablated (n = 7465) | |||||||
| Composite of all-cause mortality and HF hospitalization | 1099 | 88.49 | 12.42 | 927 | 93.24 | 9.94 | −2.48 (−3.18 to −1.79) | 0.81(0.76 to 0.87) | <.001 |
| All-cause mortality | 696 | 95.46 | 7.29 | 495 | 101.18 | 4.89 | −4.33 (−5.16 to −3.51) | 0.67 (0.62 to 0.74) | <.001 |
| HF hospitalization | 576 | 88.49 | 6.50 | 587 | 93.24 | 6.30 | −0.21 (−0.74 to −0.33) | 1.02 (0.94 to 1.10) | .666 |
| Trial eligible | Drug treated (n = 21,824) | Ablated (n = 704) | |||||||
| Composite of all-cause mortality and HF hospitalization | 187 | 7.68 | 24.32 | 155 | 7.90 | 19.64 | −4.68 (−8.19 to −1.17) | 0.82 (0.70 to 0.96) | .014 |
| All-cause mortality | 101 | 9.04 | 11.18 | 76 | 9.13 | 8.32 | −4.60 (−7.75 to −1.44) | 0.75 (0.60 to 0.94) | .011 |
| HF hospitalization | 131 | 7.68 | 17.00 | 112 | 7.90 | 14.22 | −2.79 (−5.70 to 0.13) | 0.87 (0.72 to 1.04) | .127 |
| Failed to meet inclusion | Drug treated (n = 5 216,095) | Ablated (n = 6314) | |||||||
| Composite of all-cause mortality and HF hospitalization | 801 | 75.60 | 10.60 | 661 | 79.90 | 8.27 | −2.33 (−3.01 to −1.65) | 0.79 (0.73 to 0.86) | <.001 |
| All-cause mortality | 520 | 80.65 | 6.45 | 353 | 85.72 | 4.12 | −4.27 (−5.12 to −3.43) | 0.64 (0.58 to 0.71) | <.001 |
| HF hospitalization | 389 | 75.60 | 5.15 | 401 | 79.90 | 5.02 | −0.13 (−0.64 to 0.38) | 1.02 (0.93 to 1.13) | .630 |
| Met exclusion criteria | Drug treated (n = 5 44,447) | Ablated (n = 447) | |||||||
| Composite of all-cause mortality and HF hospitalization | 112 | 5.21 | 21.41 | 111 | 5.44 | 20.40 | −1.02 (−5.20 to 3.17) | 0.97 (0.81 to 1.17) | .746 |
| All-cause mortality | 75 | 5.77 | 13.06 | 65 | 6.33 | 10.33 | −4.48 (−8.78 to −0.19) | 0.80 (0.63 to 1.02) | .072 |
| HF hospitalization | 56 | 5.21 | 10.68 | 74 | 5.44 | 13.53 | 2.85 (−0.42 to 6.13) | 1.35 (1.07 to 1.70) | .012 |
Event rate was calculated as the number of events per 100 person-years. Propensity score weight was applied when calculating number of events, person-years, event rates, absolute reduction, and hazard ratios. CI = confidence interval;HF = heart failure.
Figure 1.
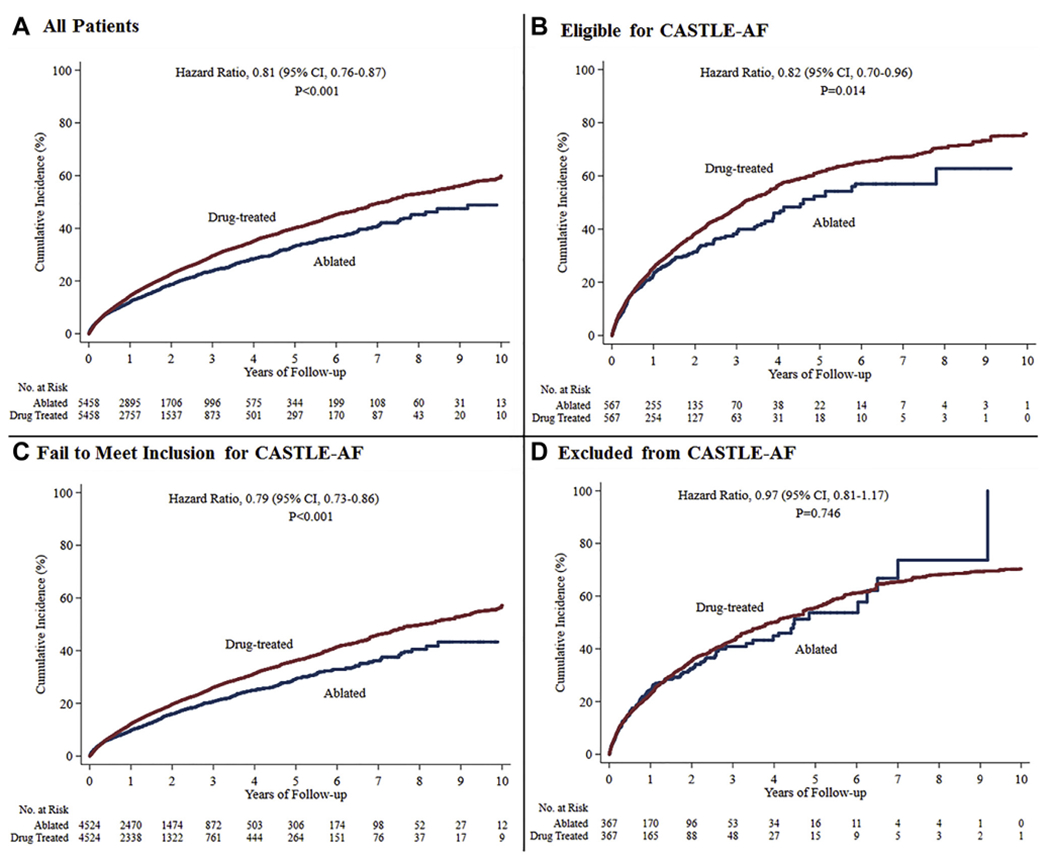
Primary end point (composite of all-cause mortality and heart failure hospitalization) in ablated or drug-treated patients with atrial fibrillation and heart failure, stratified by Catheter Ablation for Atrial Fibrillation with Heart Failure (CASTLE-AF) trial eligibility criteria. The cumulative incidence in the overall cohort (A), in patients who would be potentially eligible for CASTLE-AF (B), in patients who failed to meet the inclusion criteria (C), and in patients who met at least one of the trial exclusion criteria (D). Drug-treated patients are the reference group in Cox proportional hazards regression analyses. All the curves and numbers were generated using propensity score weighting. CI = confidence interval.
In analyses stratified by trial eligibility, ablation was associated with a lower risk of the primary end point in patients eligible for CASTLE-AF (HR 0.82; 95% CI 0.70–0.96; P = .01) and those who would have failed to meet the inclusion criteria for CASTLE-AF (HR 0.79; 95% CI 0.73–0.86; P < .001) but not in those who would have been excluded from the CASTLE-AF trial (HR 0.97; 95% CI 0.81–1.17; P = .75).
Subgroup analyses
In the overall cohort, the relative risk reduction associated with ablation was greater in patients younger than 65 years, men, patients without any implanted devices, and patients without diabetes (Figure 2). The effect was consistent regardless of whether patients had HFrEF. In trial-eligible patients, the effect was consistent across subgroups (Supplemental Figure 3). In patients who failed to meet trial inclusion criteria, the relative risk reduction associated with ablation was greater in patients without any implanted devices and patients without diabetes (Supplemental Figure 4). In patients with trial exclusions, the relative risk reduction associated with ablation was greater in patients younger than 65 years, men, and patients without concurrent use of β-blockers (Supplemental Figure 5).
Figure 2.
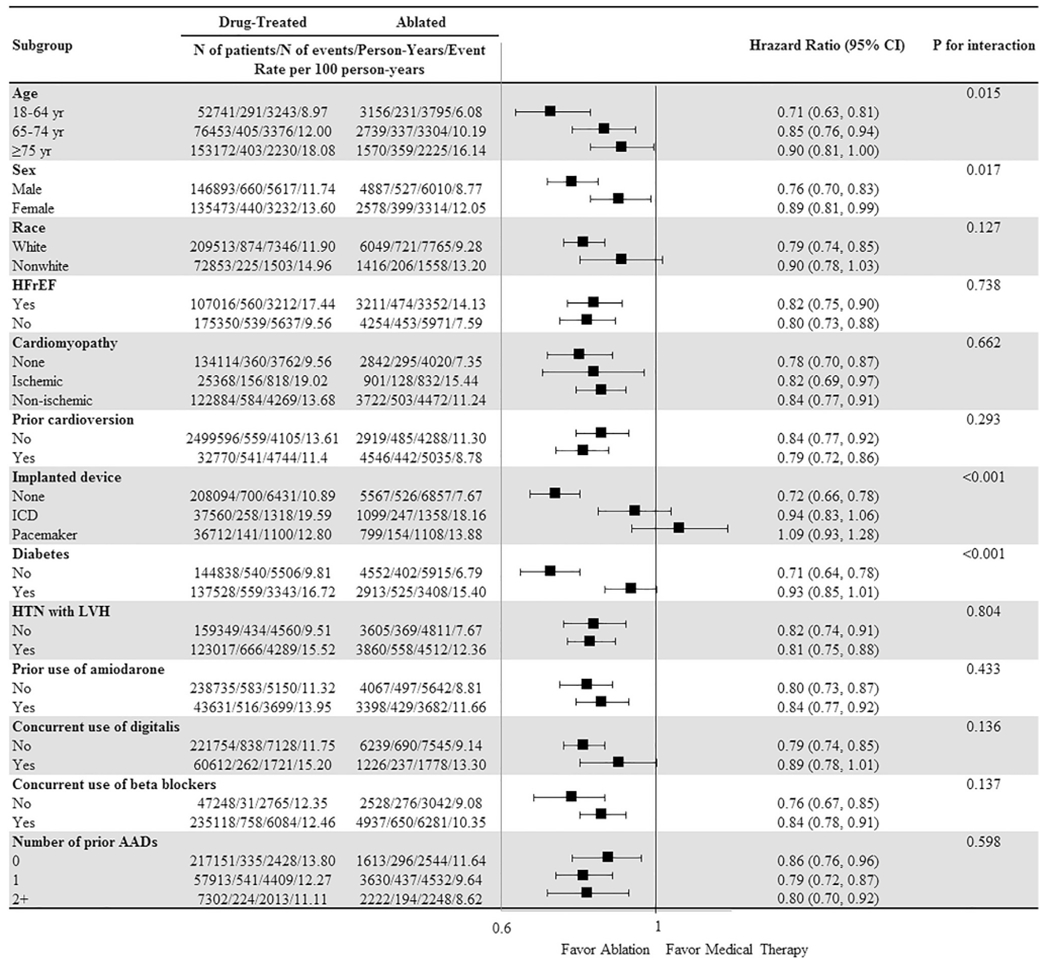
Subgroup analyses in the overall cohort for the primary end point (composite of all-cause mortality and heart failure hospitalization). AAD = anti-arrhythmic drug; CI = confidence interval; HFrEF = heart failure with reduced left ventricular ejection fraction; HTN = hypertension; ICD = implantabl cardioverter-defibrillator; LVH = left ventricular hypertrophy.
Sensitivity analyses
The relative risk reduction associated with ablation was similar when comparing ablation with AADs (HR 0.82; 95% CI 0.77–0.88; P < .001) or with rate-control drugs (HR 0.81; 95% CI 0.76–0.87; P < .001) (Supplemental Table 10). In the analysis stratified by the adherence to treatment in the medical therapy cohort, ablation was associated with a lower risk of the primary end point in both adherent and nonadherent patients, but the magnitude was greater when comparing ablation between patients who did not adhere to medical therapy (HR 0.69; 95% CI 0.65–0.74; P < .001) and patients who adhered to medical therapy (HR 0.90; 95% CI 0.84–0.96; P = .001) (Supplemental Table 11). When considering multiple HF hospitalizations during follow-up, ablation was associated with a lower rate of HF hospitalizations (incident rate ratio 0.78; 95% CI 0.67–0.92; P = .002) (Supplemental Table 12) in trial-eligible patients. The results for HF hospitalizations blanking the first 30 days were similar (Supplemental Table 13). There were no significant relationships between ablation and any of the falsification end points (Supplemental Table 14).
Discussion
In this large cohort of patients with AF and HF encountered in routine practice, only 1 in 13 would have been eligible for the CASTLE-AF trial. Ablation was associated with a lower risk of HF hospitalization and all-cause mortality. However, the magnitude appears to be more modest than that reporter in CASTLE-AF.
The small proportion of patients in practice eligible for the trial is consistent with the fact that CASTLE-AF screened nearly 10 times as many patients as it enrolled.17 Although clinical trials are the criteria standard for evaluating treatment effects, the external validity is often low because of the strict eligibility criteria. However, the present study provides reassurance tha ablation is associated with a lower risk of the CASTLE-AF primary outcome in the majority of patients with AF and HF including those who do not have HFrEF or ICD.
In the CASTLE-AF trial, ablation reduced the primary end point by 38%—more than twice the benefit observed in the present study (18% relative risk reduction in the trial-eligible cohort). Some have questioned the findings of CASTLE-AF because of the small number of patients, low event rates, unbalanced baseline characteristics, and high rates of loss to follow-up. There have been examples in the literature that large treatment effects reported by small trials were subsequently invalidated by larger trials.18,19 Unfortunately, CASTLE-AF stands alone with few other randomized trials to increase the certainty in its findings. In such cases, large observational studies such as this one may provide useful complementary evidence or even more realistic estimations of treatment effects. In fact, a recent study by this group using the same administrative database successfully replicated the results of both the intention-to-treat and per-protocol analyses of the Catheter Ablation vs Antiarrhythmic Drug Therapy for Atrial Fibrillation (CABANA) trial.8 However, as with all observational studies, there is a potential for residual confounding.
Approximately 1 in 6 patients in practice would have met at least one of the CASTLE-AF trial exclusion criteria such as contraindication to anticoagulation, recent cardiac event or surgery, and renal failure. Many of these patients are considered poor candidates for ablation. In such excluded patients, ablation was not associated with a lower risk of the composite end point of all-cause mortality and HF hospitalization and was even associated with an increased risk of HF hospitalization.
Limitations
This study does have several limitations. First, despite careful adjustment, all observational studies are subject to unmeasured confounding. However, many of the measured characteristics are highly correlated with unmeasured ones. For example, age, valvular heart disease, hypertension, cardioversion, and previous AADs are associated with unmeasured characteristics, such as left atrial diameter, AF pattern, and AF burden. Therefore, groups that were identical on 90 baseline characteristics were unlikely to substantially differ in other measurements. The falsification end points also provide some reassurance that there was no evidence for substantial residual confounding. Second, administrative data are subject to misclassification. The billing codes used in this study have been commonly used in previous studies and demonstrated good performance in validation studies.20–26 Third, HFrEF codes were used for the inclusion criteria LVEF ≤ 35%. However, this would not likely substantially affect the results, because in the subgroup analyses, the treatment effect did not differ between patients with and without HFrEF. Fourth, unlike in a trial, not all patients in routine practice are regularly monitored, and thus, the recurrence of AF after ablation or drug therapy is not known. Even when monitoring is performed, it is challenging to accurately ascertain arrhythmia outcomes such as AF recurrence or AF burden within administrative data sets such as OptumLabs. The success of ablation in achieving rhythm control may influence long-term hard clinical outcomes, but this cannot be assessed in the present study. However, previous trials have demonstrated that ablation is superior to drug therapy in maintaining sinus rhythm.27 Similarly, we do not have information on AF pattern (paroxysmal, persistent, or long-standing persistent AF), which is known to have a substantial impact on ablation outcomes. Last, the mean follow-up period was ~ 2 years, shorter than observed in the CASTLE-AF trial (~ 3 years), partly because patients with HF and AF managed in routine practice have a high mortality risk. Although the sample is large, we note that there is considerable decrease in the rate of follow-up beyond 2 years.
Conclusion
For most patients with AF and HF, catheter ablation was associated with a lower risk of all-cause mortality and HF hospitalization than did medical therapy alone. However, the risk reduction was more modest than that observed in the CASTLE-AF trial. Future large randomized controlled trials are needed to confirm the benefit of ablation in a broad population of patients with AF and HF.
Supplementary Material
Acknowledgments
This study was supported by the National Institutes of Health (grant no. R21 HL140205, to Dr Noseworthy and Dr Yao) and by the Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery (to Dr Noseworthy, Ms Van Houten, Dr Shah, and Dr Yao). The study sponsor had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. Dr Piccini reports relationships with Abbott Medical, Boston Scientific, Johnson & Johnson, Medtronic, and Sanofi. Dr Packer reports grants from the National Institutes of Health, St. Jude Medical, Biosense Webster, Medtronic, Boston Scientific, Aperture Diagnostics, Spectrum Dynamics, MediaSphere Medical, CardioInsight, Siemens, Thermedical, and Wiley & Sons, Oxford (outside the submitted work). Dr Gersh serves on the data and safety monitoring board for Janssen Research & Development, Mount Sinai St. Luke’s, Boston Scientific, Teva, St. Jude Medical, Thrombosis Research Institute, Duke Clinical Research Institute, Kowa Research Institute, and Cardiovascular Research Foundation and provides general consulting for Janssen Scientific Affairs, Xenon Pharmaceuticals, and Sirtex Medical. The rest of the authors report no conflicts of interest.
Footnotes
Appendix
Supplementary data
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hrthm.2020.02.030.
References
- 1.Santhanakrishnan R, Wang N, Larson MG, et al. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation 2016;133:484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roy D, Talajic M, Nattel S, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med 2008;358:2667–2677. [DOI] [PubMed] [Google Scholar]
- 3.Anselmino M, Matta M, D’Ascenzo F, et al. Catheter ablation of atrial fibrillation in patients with left ventricular systolic dysfunction: a systematic review and meta-analysis. Circ Arrhythm Electrophysiol 2014;7:1011–1018. [DOI] [PubMed] [Google Scholar]
- 4.Marrouche NF, Brachmann J, Andresen D, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 2018;378:417–427. [DOI] [PubMed] [Google Scholar]
- 5.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2019;74:104–132. [DOI] [PubMed] [Google Scholar]
- 6.Wallace PJ, Shah ND, Dennen T, Bleicher PA, Crown WH. Optum Labs: building a novel node in the learning health care system. Health Aff (Millwood) 2014; 33:1187–1194. [DOI] [PubMed] [Google Scholar]
- 7.Optum. Optum Research Data Assets. https://www.optum.com/content/dam/optum/resources/productSheets/5302_Data_Assets_Chart_Sheet_ISPOR.pdf. Accessed March 30, 2020.
- 8.Noseworthy PA, Gersh BJ, Kent DM, et al. Atrial fibrillation ablation in practice: assessing CABANA generalizability. Eur Heart J 2019;40:1257–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noseworthy PA, Kapa S, Deshmukh AJ, et al. Risk of stroke after catheter ablation versus cardioversion for atrial fibrillation: a propensity-matched study of 24,244 patients. Heart Rhythm 2015;12:1154–1161. [DOI] [PubMed] [Google Scholar]
- 10.Noseworthy PA, Kapa S, Haas LR, et al. Trends and predictors of readmission after catheter ablation for atrial fibrillation, 2009–2013. Am Heart J 2015; 170:483–489. [DOI] [PubMed] [Google Scholar]
- 11.Noseworthy PA, Van Houten HK, Sangaralingham LR, et al. Effect of antiarrhythmic drug initiation on readmission after catheter ablation for atrial fibrillation. JACC Clin Electrophysiol 2015;1:238–244. [DOI] [PubMed] [Google Scholar]
- 12.Noseworthy PA, Yao X, Deshmukh AJ, et al. Patterns of anticoagulation use and cardioembolic risk after catheter ablation for atrial fibrillation. J Am Heart Assoc 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li F, Morgan KL, Zaslavsky AM. Balancing covariates via propensity score weighting. J Am Stat Assoc 2018;113:390–400. [Google Scholar]
- 14.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 16.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994;81:515–526. [Google Scholar]
- 17.Packer M, Kowey PR. Building castles in the sky: catheter ablation in patients with atrial fibrillation and chronic heart failure. Circulation 2018;138:751–753. [DOI] [PubMed] [Google Scholar]
- 18.Armstrong PW, Granger CB, Adams PX, et al. Pexelizumab for acute ST-elevation myocardial infarction in patients undergoing primary percutaneous coronary intervention: a randomized controlled trial. JAMA 2007;297:43–51. [DOI] [PubMed] [Google Scholar]
- 19.Granger CB, Mahaffey KW, Weaver WD, et al. Pexelizumab, an anti-C5 complement antibody, as adjunctive therapy to primary percutaneous coronary intervention in acute myocardial infarction: the COMplement inhibition in Myocardial infarction treated with Angioplasty (COMMA) trial. Circulation 2003;108:1184–1190. [DOI] [PubMed] [Google Scholar]
- 20.Kumamaru H, Judd SE, Curtis JR, et al. Validity of claims-based stroke algorithms in contemporary Medicare data REasons for Geographic And Racial Differences in Stroke (REGARDS) study linked with medicare claims. Circ Cardiovasc Qual Outcomes 2014;7:611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kokotailo RA, Hill MD. Coding of stroke and stroke risk factors using International Classification of Diseases, Revisions 9 and 10. Stroke 2005;36:1776–1781. [DOI] [PubMed] [Google Scholar]
- 22.Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R, Dublin S. Identifying atrial fibrillation from electronic medical data: a systematic review. Pharmacoepi-demiol Drug Saf 2012;21:141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan J, Arruda-Olson AM, Leibson CL, et al. Billing code algorithms to identify cases of peripheral artery disease from administrative data. J Am Med Inform Assoc 2013;20:e349–e354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao X, Gersh BJ, Holmes DR Jr, et al. Association of surgical left atrial appendage occlusion with subsequent stroke and mortality among patients undergoing cardiac surgery. JAMA 2018;319:2116–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yao X, Shah ND, Sangaralingham LR, Gersh BJ, Noseworthy PA. Non-vitamin K antagonist oral anticoagulant dosing in patients with atrial fibrillation and renal dysfunction. J Am Coll Cardiol 2017;69:2779–2790. [DOI] [PubMed] [Google Scholar]
- 26.Yao X, Tangri N, Gersh BJ, et al. Renal outcomes in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol 2017;70:2621–2632. [DOI] [PubMed] [Google Scholar]
- 27.Di Biase L, Mohanty P, Mohanty S, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation 2016;133:1637–1644. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


