Polycomb group (PcG) genes are an important group of epigenetic regulators that act to repress transcription. In Drosophila, the PcG group protein complex PRC2 is recruited to discrete DNA elements called Polycomb response elements. PRC2 .....
Keywords: PRE, PcG, topological domain, ChIP-seq
Abstract
Polycomb group (PcG) proteins are an important group of transcriptional repressors that act by modifying chromatin. PcG target genes are covered by the repressive chromatin mark H3K27me3. Polycomb repressive complex 2 (PRC2) is a multiprotein complex that is responsible for generating H3K27me3. In Drosophila, PRC2 is recruited by Polycomb Response Elements (PREs) and then trimethylates flanking nucleosomes, spreading the H3K27me3 mark over large regions of the genome, the “Polycomb domains.” What defines the boundary of a Polycomb domain? There is experimental evidence that insulators, PolII, and active transcription can all form the boundaries of Polycomb domains. Here we divide the boundaries of larval Polycomb domains into six different categories. In one category, genes are transcribed toward the Polycomb domain, where active transcription is thought to stop the spreading of H3K27me3. In agreement with this, we show that introducing a transcriptional terminator into such a transcription unit causes an extension of the Polycomb domain. Additional data suggest that active transcription of a boundary gene may restrict the range of enhancer activity of a Polycomb-regulated gene.
POLYCOMB group (PcG) genes encode a group of transcriptional repressors that act to maintain the “OFF” transcription state for developmentally important genes in metazoans. Genetic and biochemical studies have identified ∼15 PcG genes. Many of these genes encode protein components of three protein complexes: Pho Repressive Complex (PhoRC), Polycomb Repressive Complex 1 (PRC1) and Polycomb Repressive Complex 2 (PRC2). PRC2 trimethylates histone H3 on lysine 27 (H3K27me3)—a repressive chromatin mark (Cao et al. 2002). PRC1 acts to compact chromatin (Francis et al. 2004). PRC1 and PRC2 are recruited to PcG target genes through DNA elements called Polycomb-group Response Elements (PREs) (Kassis et al. 2017). PREs are made up of binding sites for a number of different DNA-binding proteins, including Pho. Pho binds in a complex with Sfmbt (Pho-RC), in part through an interaction with Scm, and plays a role in recruiting PRC1 and PRC2 to PREs (Kang et al. 2015; Frey et al. 2016).
Many Drosophila PcG target genes have complex regulatory regions that extend over tens to hundreds of kilobases, with multiple enhancers stimulating expression for temporal- and spatial-specific expression during development. Typically, in the silenced state, H3K27me3 covers the entire gene, including the transcription unit and all the regulatory DNA. The accumulation of H3K27me3 over a PcG target gene starts at the nucleosomes flanking the PREs, and proceeds to flanking nucleosomes in a positive feed-back loop (Li et al. 2014). PRC2 binds to H3K27me3, and this stimulates the activity of the catalytic subunit of PRC2, E(z) (Margueron et al. 2009). Thus, H3K27me3 can spread for many kilobases from a PRE.
What stops the spreading of this mark? In mammals, the insulator protein CTCF plays a key role (Narendra et al. 2015; Hnisz et al. 2016). In Drosophila, three studies using transgenic ectopic Polycomb domains have been informative. Most definitively, Fujioka et al. (2013) showed that insulators can block the spreading of H3K27me3 from PREs using a transgene containing even-skipped (eve) regulatory DNA and reporter genes (Fujioka et al. 2013). In agreement with this, deletion of a presumed insulator at the left border of the endogenous Psc-Su(z)2 Polycomb domain allowed H3K27me3 to spread to the flanking gene (Park et al. 2012). Two other transgenic studies suggest that insulators are not always required to stop the spreading of H3K27me3. In one study, RNA polymerase was present at the boundary of the domain, while in the other, H3K36me3, a mark of active transcription, was present (Schuettengruber and Cavalli 2013; De et al. 2019).
Extensive genetic studies from flies, worms, and mammalian cells have documented an antagonistic relationship between H3K27me3 and H3K36me2/3. Early studies in flies demonstrated that the Trithorax-group protein Ash1, a H3K36 dimethyltransferase, antagonized H3K27me3 levels at the transcriptionally active Ubx promoter (Papp and Muller 2006). Genome-wide studies examining the effect of ash1 mutation and a transcriptional inhibitor on H3K27me3 and H3K36me2 in Drosophila polytene chromosomes showed little-to-no colocalization of these two chromatin marks (Srinivasan et al. 2008; Dorighi and Tamkun 2013). Genomic studies in Caenorhabditis elegans germ cells also showed that H3K36me3 and H3K27me3 occupy different regions of the genome (Gaydos et al. 2012). In mouse embryonic stem cells, knocking down the H3K36me2 methyltransferase Nsd1 led to an expansion of H3K27me3 domains (Streubel et al. 2018). Biochemical studies provide a mechanistic explanation for these observations; the enzymatic activity of PRC2 is inhibited by active chromatin marks including H3K36me2/3 (Schmitges et al. 2011; Yuan et al. 2011).
What is the configuration of genes and insulators flanking Polycomb domains genome-wide in Drosophila? What stops the spreading of the H3K27me3 chromatin mark? These are the questions we address in this study. Active transcription units flank the majority of Polycomb domains, often in the absence of an insulator. Here we show that insertion of a transcriptional terminator into a gene transcribed toward a Polycomb domain causes a spreading of H3K27me3. We also suggest that actively transcribed genes can act as a boundary for enhancer activity of Polycomb target genes. Overall, our data show that active transcription is an important Polycomb domain boundary in Drosophila.
Materials and Methods
Fly strains
Transgenic lines were made using φC31 recombinase-mediated cassette exchange (RMCE) (Bateman et al. 2006). The stocks used were BDSC #52163- inv-MiMIC inserted at 2R:7366718, BDSC #58531- en-MiMIC inserted at 2R:7441305 and BDSC #33074- tou-MiMIC inserted at 2R:7471605. BDSC #59378-bchs-MiMIC inserted at 2L:5910275. All coordinates are dm5.
qRT-PCR and RT-PCR
Total RNA was collected from 10 third-instar larval brains and imaginal discs using Trizol (Invitrogen) according to manufacturer’s protocol. DNA was digested with amplification grade DNase I (Sigma-Aldrich). One-step qRT-PCR was performed with the SensiFAST SYBR No-ROX One-Step kit (Bioline) on a Roche LightCycler 480 according to manufacturer instructions. For RT-PCR, we used a Qiagen one-step RT-PCR kit. Primer sequences: qRT-PCR- GGTGGCGACATTTGGTTAGT and CATGTAAACGCCTGCCAGT; RT-PCR- CATTTGGTTAGTGGCCGTTC and GGTCCGGGTTCCAGTTAAATA.
RNA-FISH
yellow mRNA antisense probe was synthesized by PCR amplifying the genomic sequence (X:253,703-254283) using the primer sequences: GGGGTACCCGGAGCTAATTCCGTATCCA and GGGGGATCCTCTTCCGTCCTGGTTTCATC. This PCR product was digested with KpnI and BamHI (NEB) and cloned into the pBluescript II SK(+) plasmid. The plasmid was linearized with KpnI and in vitro transcribed with T3 polymerase. The antisense probes to tou transcripts were made by amplifying genomic DNA with tou primers, one with a T7 promoter sequence added, and transcribing the PCR product with T7 polymerase in vitro. tou-all (2R:7467055-7467358) primer sequences:
F: GAAATTAATACGACTCACTATAGGCTGTGCTTCTCGACCCTCTC and
R: CGTGCGTCAGATCTTCGATA.
tou-big (2R:7473673-7474181): F: GAAATTAATACGACTCACTATAGGCGCACGTTTCTAGACTTCC
and R: TGCACGATGCTTTAATGAGC.
The RNA in situ protocol was described by Tian et al. (2019).
Immunostaining experiments
Antibody staining of imaginal discs and embryos has been described previously (Cheng et al. 2014). Rabbit anti-GFP (1:2000, A-11122; Invitrogen) and mouse anti-β-gal (1:500; Life Technologies) were used for the immunostaining experiments.
ChIP and ChIP-seq
Protocol for carrying out chromatin immunoprecipitation (ChIP) has been described (De et al. 2019). Total PolII (Covance, 8WG16, MMS-126), CP190, and CTCF antibodies were used at 1:100 dilution, anti-H3K27me3 (17–622; Millipore, Bedford, MA) and anti-H3K36me3 (ABE435; Millipore) antibodies were used at 1:200 dilution. CP190 and CTCF antibodies were a kind gift from Elissa Lei, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). ChIP-sequencing (ChIP-seq) libraries were made with NEBNext Ultra II DNA Library Prep kit. Analysis of the next-generation sequencing data has been described (De et al. 2019). To visualize the distribution of histone modification marks within the transgenes, we created a custom reference gene track by extracting the 400 kb sequence surrounding the tou-MiMIC insertion site, 2R:7471605 (Gene Disruption Project database) (Venken et al. 2011). The sequence of the MiMIC insert (GenBank: GU370067.1) was reverse complemented and inserted at 2R:7471605. This sequence was used as the reference genome for subsequent Bowtie 2 alignment with ChIP-seq data from WT and tou-MiMIC.
Data availability
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article. Sequencing data generated in this study (CTCF, CP190, Pol II from WT and H3K27me3 and H3K36me3 from the transgenic stocks) is deposited in the NCBI BioProject database- PRJNA564118. We also used our previously published ChIP-seq data: WT H3K27me3- GSE77342 and WT H3K36me3- PRJNA494709 (De et al. 2019). All other data and materials are available on request. Supplemental material available at figshare: https://doi.org/10.25386/genetics.12890870.
Results
Actively transcribed genes flank most Polycomb domains
A total of 203 H3K27me3 domains were previously identified bioinformatically in ChIP-seq data from third-instar larval samples [made from brains and imaginal discs, (Brown et al. 2018)]. We visually compared the H3K27me3 domains with ChIP-seq datasets for the insulator proteins CP190 and CTCF (generated for this study), PolII (this study), and H3K36me3 (De et al. 2019), also from brains and discs of third-instar larvae. Of the many insulator proteins in Drosophila, we chose CP190 because it was shown to be present at 95% of domain boundaries in embryos (Stadler et al. 2017) and CTCF because it is important for mammalian Polycomb boundaries. Although these samples contain a mixed cell population, and many genes within H3K27me3 domains are expressed in a subset of cells, the H3K27me3 domains are robust, and the boundaries are easy to discern by visual inspection. Of the 203 bioinformatically determined canonical H3K27me3 domains, 21 were false positives, and 8 large domains were incorrectly broken into multiple domains. In addition, visual examination showed that one of the bioinformatically called domains was actually two merged domains (see Supplemental Material, Table S1 for details). Each Polycomb domain has two boundaries (left and right), so the total number of boundaries examined was 340. We note that CTCF was not present at any boundaries that did not also have CP190. Therefore, in our study, when we refer to insulator, we are specifically referring to CP190 peaks.
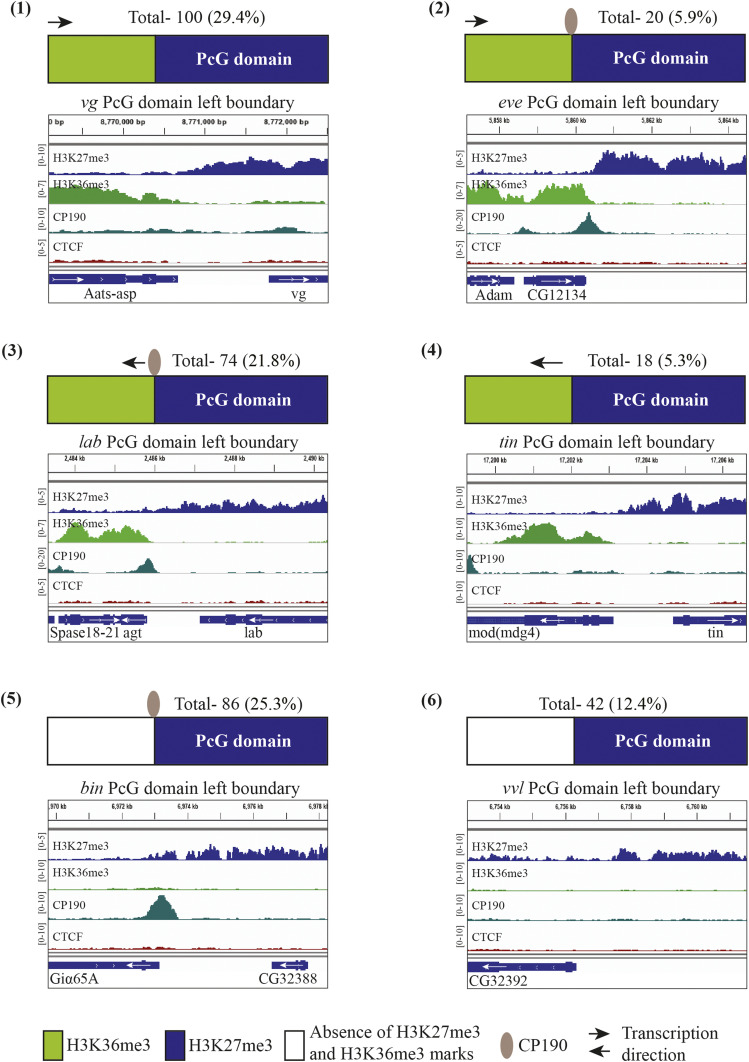
When we classified each boundary by the presence or absence of CP190 (insulator) and H3K36me3, six different types of Polycomb boundaries were identified by visual examination and quantitated (Figure 1). In type 1, a flanking gene is transcribed toward the Polycomb domain with no insulator at the border. In this case, H3K36me3 occurs next to, or within, 1 kb of the H3K27me3 boundary. Often, but not always, there are insulator proteins at the promoter or within these flanking genes but never present at the border where the H3K27me3 domain ends (for example, see the tou gene in Figure 2A). In type 2, a gene is transcribed toward the Polycomb domain, with an insulator protein at the border, with H3K36me3 extending to the insulator protein binding site. In type 3, an insulator and a promoter are next to the Polycomb domain, transcribing away from the domain, with H3K36me3 extending to the insulator. A type 4 gene is transcribed away from the Polycomb domain but no CP190 is present. Type 5 has insulator proteins only, with no H3K36me3 adjacent to it. For type 5 genes, the insulator protein is often near a promoter, but there is no H3K36me3 present next to the insulator binding site. In type 6, neither H3K36me3 nor CP190 is found near the boundary of the Polycomb domain. It is possible that another insulator protein delineates a type 6 boundary. For the majority of Polycomb domains in Drosophila (62%), H3K36me3 occurs at the boundary. Thus, the inhibition of PRC2 by H3K36me3 and/or transcription is likely very important for domain boundaries, at least in Drosophila. From these data we predict that blocking transcription from a type 1 gene will cause the H3K27me3 domain to spread into the flanking transcription unit.
Figure 1.
PcG domain boundaries in Drosophila larvae. Schematic of six types of domain boundaries are shown (1–6). H3K27me3 is shown as blue boxes (PcG domain), green boxes show H3K36me3, arrow denotes direction of transcription, and insulator protein (CP190) is depicted with a gray ellipse. The total number of boundaries of each type is shown above each diagram. Below each diagram is an Integrative Genomics Viewer (IGV) track of H3K27me3, H3K36me3, CP190, and CTCF ChIP-seq data and gene locations for one example of that domain boundary type. Scales on the side of ChIP-seq data show reads per million.
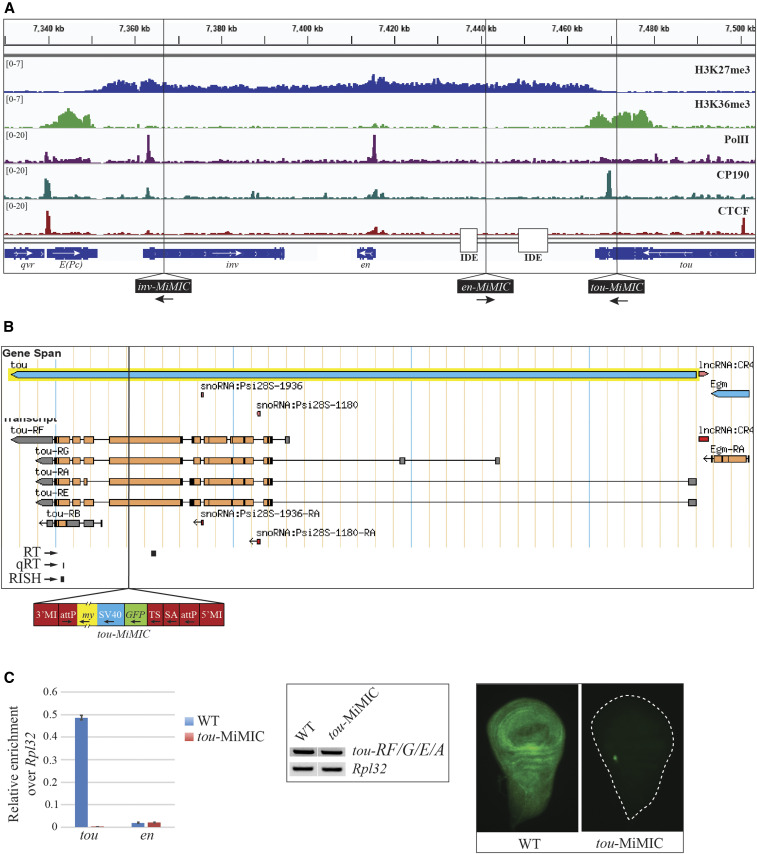
Figure 2.
tou-MiMIC stops tou transcription. (A) ChIP-seq distribution of H3K27me3, H3K36me3, PolII, CP190, and CTCF over the inv-en domain in WT. ChIP-seq data shown here and throughout this study are derived from brains and discs from third-instar larvae. H3K27me3 covers the entire inv-en domain, from the 3′ end of E(Pc) to the 3′ end of tou. In contrast, H3K36me3, a mark of actively transcribed genes, is strong over E(Pc) and tou but undetectable over the inv and en transcription units. Vertical black lines show the insertion site of the three MiMIC transgenes used in this study (bottom). The locations of the imaginal disc enhancers (IDE) are also shown. (B) tou gene and annotated transcript isoforms from FlyBase with the tou-MiMIC insertion site indicated. Positions of the tou qRT-PCR amplicon (qRT), the tou RT-PCR amplicon (RT), and the tou-all in situ probe (RISH) used in this figure are shown below the tou isoforms. The MiMIC element is diagrammed below (my: mini-yellow, SV: SV40 terminator, TS: translation stop, SA: splice acceptor), (C) Left panel: qRT-PCR analysis of tou and en transcripts in WT and tou-MiMIC. Middle panel: RT-PCR analysis of tou-RF/G/E/A, designed to amplify all tou transcripts except tou-RB. Rpl32 amplicons were used as a loading control. Right panel: RISH experiments with tou-all probe targeting both tou-RB and the 3′-end of all other tou isoforms in WT and tou-MiMIC larval wing discs.
Insertion of a transcriptional stop within a type 1 transcribed gene causes an extension of the H3K27me3 mark
inv and en are coregulated homeodomain transcription factors that form a 113 kb Polycomb domain. The inv-en Polycomb domain is flanked by the genes E(Pc) and tou (Figure 2A). For these two type 1 genes we hypothesized that stopping transcription would lead to an extension of the H3K27me3 mark. The E(Pc) gene has a relatively simple structure, with three closely spaced promoters in the vicinity of overlapping CTCF and CP190 binding peaks. We hypothesized that deleting the E(Pc) promoter region would eliminate E(Pc) transcription and cause an extension of H3K27me3 into the E(Pc) transcription unit. We tried to delete the E(Pc) promoter region using CRISPR-Cas9 and four different guide RNA pairs. These experiments failed due to high larval lethality. We do not know whether this failure was due to technical or biological reasons.
We next turned our attention to the tou gene. The tou gene encodes a protein with similarity to subunits of chromatin remodeling complexes (Vanolst et al. 2005). tou mutants are reported to be mostly lethal, with some escapers having bristle defects (Vanolst et al. 2005). The tou gene has four transcription start sites spread over a 37 kb region and is expressed nearly ubiquitously in larval brains and discs (Figure 2B). Deleting this promoter region using CRISPR-Cas9 did not seem like a viable option. Fortuitously, the Drosophila Gene Disruption project had isolated a MiMIC element inserted into the tou transcription unit (Venken et al. 2011), 7 kb upstream of the distal boundary of the inv-en Polycomb domain (Figure 2A, “tou-MiMIC”). tou-MiMIC flies are homozygous viable and fertile; however, the stocks are not as robust as wild-type flies. MiMIC contains an SV40 transcription terminator (Figure 2B), and tou-MIMIC completely stops extension of all tou transcripts (Figure 2C). Using qRT-PCR (Figure 2C, left panel) and RNA in situ hybridization (RISH, Figure 2C, right panel) with probes to detect tou transcripts downstream of the tou-MiMIC insertion site, no transcripts were detected, while transcripts upstream of the MiMIC site were detected by RT-PCR (Figure 2C, middle panel). We were somewhat surprised by the complete loss of transcripts downstream of tou-MiMIC, because the start site for the tou-RB transcript is downstream of the tou-MIMIC insertion site. Nonetheless, it is clear that tou-RB is not detectably transcribed in tou-MiMIC larval brains and discs. This is curious because RNA-seq data from wild-type larval brains and discs suggests that a low level of tou-RB transcript is normally made (Figure S1A) (Brown et al. 2018).
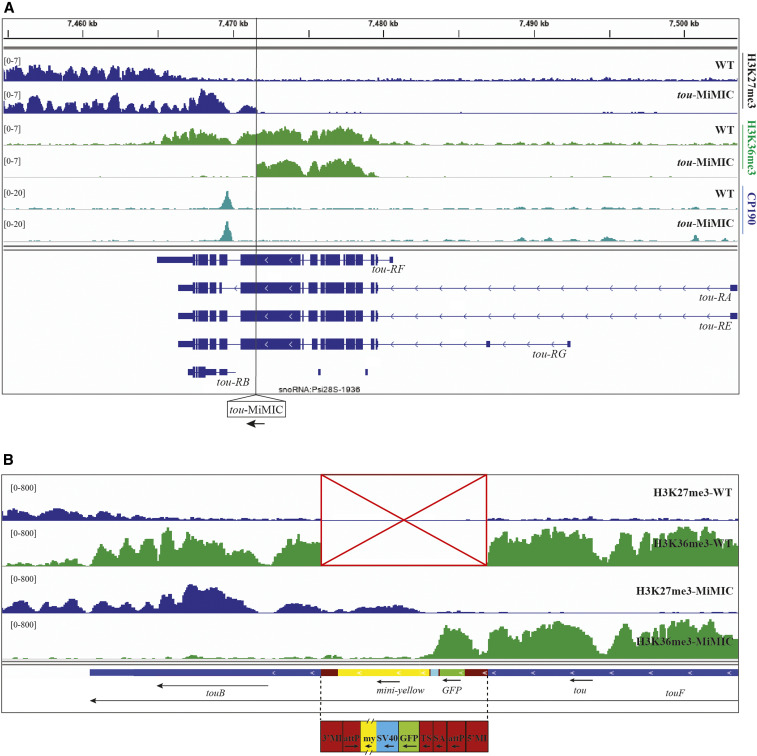
We also examined H3K27me3 and H3K36me3 in tou-MiMIC larvae (Figure 3, A and B). Instead of stopping where the tou gene transcript normally ends, H3K27me3 spreads over the tou-RB transcription unit, past a CP190 binding site, and ends at the SV40 site in the MiMIC transgene (Figure 3, A and B). These data suggest that transcription of tou normally inhibits the activity of PRC2 and stops the spreading of H3K27me3, defining the end of this type 1 Polycomb domain boundary. In addition, we suggest that the H3K27me3 that covers the tou-RB transcription unit in tou-MiMIC larvae inhibits tou-RB expression.
Figure 3.
Transcription termination causes extension of the H3K27me3 domain at tou. (A) ChIP-seq distributions of H3K27me3 (blue), H3K36me3 (green), and CP190 (aqua) in WT and tou-MiMIC. The tou gene isoforms are shown at the bottom (navy blue). The transgene insertion site in tou-MiMIC is indicated with a vertical black line. (B) ChIP-seq profiles of H3K27me3 (rows 1 and 3) and H3K36me3 (rows 2 and 4) in WT and tou-MiMIC in a custom gene track that includes the MiMIC transgene sequences. The transgene is not present in WT (red box with an X). The locations of the tou gene and transcription units are shown at the bottom along with the MiMIC transgene. The scale is different in (B) (0–800) because it is reads per million in only a 400 kb region including the tou-MiMIC transgene and surrounding sequences, rather than the entire genome, as it is in both (A) and other figures in this paper.
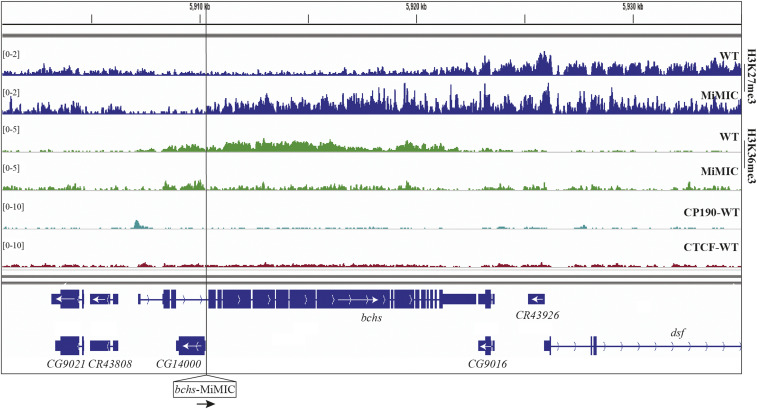
We next asked whether MiMIC could cause a similar effect in another gene. We looked for MiMIC elements inserted in the correct orientation to stop transcription in genes transcribed toward H3K27me3 domains, as in type 1 Polycomb domain boundaries (Figure 1). The bchs gene is expressed at a low level in our samples (derived from larval brains and discs), likely reflecting its expression in a subset of cells in the nervous system (Thurmond et al. 2019). While bchs is not normally covered by H3K27me3 in larvae, it is next to the dsf gene, which is covered by a low level of H3K27me3 (note the difference in scale between Figure 3A and Figure 4). The dsf H3K27me3 domain is not one identified in our bioinformatic analysis because the level of H3K27me3 is beneath the twofold enrichment threshold. Nevertheless, insertion of MiMIC into bchs causes a loss of H3K36me3 at the bchs-MiMIC insertion site and an extension of H3K27me3 from dsf into the bchs gene for ∼10 kb (Figure 4).
Figure 4.
Transcription termination causes extension of the H3K27me3 domain at bchs. ChIP-seq profiles of H3K27me3 (rows 1 and 2) and H3K36me3 (rows 3 and 4) in both WT and bchs-MiMIC, and of CP190 and CTCF distribution in WT (rows 5 and 6). Locations of genes are shown at the bottom in navy blue. The bchs gene harbors another small gene CG14000 that is not transcribed in larval brains and discs (Thurmond et al. 2019). The position of the MiMIC transgene is indicated with a vertical black line. Note that the insertion causes a decrease in H3K36me3 and an increase in H3K27me3 downstream (to the right) of the bchs-MiMIC insertion site.
The inv-en “ON” state extends to the MiMIC-tou insertion site
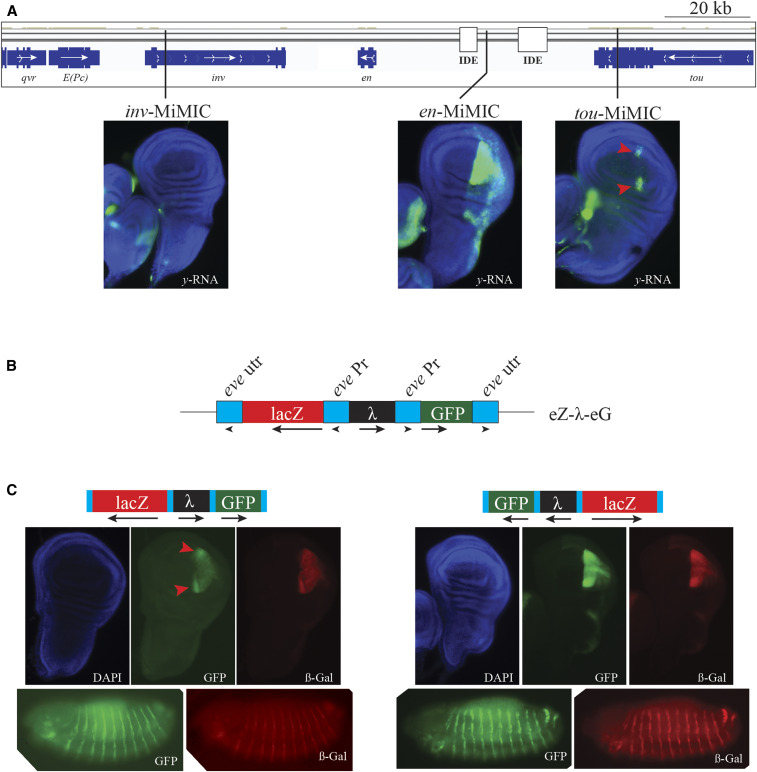
Our data show that H3K27me3 extends over the yellow (y) mini-gene present in MiMIC, suggesting that y is now part of the inv-en regulatory domain (Figure 3B). Is the mini-y gene in tou-MiMIC expressed like inv and en? The inv and en genes are co-expressed in embryos and imaginal discs, driven by at least 15 different enhancers (Cheng et al. 2014). Two of these enhancers drive expression in a stripe at the anterior–posterior boundary in imaginal discs. y RNA from tou-MiMIC is expressed in a subset of the stripe at the anterior–posterior boundary (Figure 5A); however, no other enhancers appear to be able to stimulate y expression at the tou-MiMIC insertion site. Some en enhancers exhibit promoter specificity, especially when acting at a distance (Kwon et al. 2009). In order to test whether inv-en enhancers can activate the y promoter, we examined y expression from two more MiMIC transgenes inserted in the inv-en domain (Figure 2A). In one (inv-MiMIC), we did not observe any y expression (Figure 5A). However, in the other, y expression from en-MiMIC mimicked endogenous en expression in wing imaginal discs, demonstrating that the y promoter can be driven by inv-en enhancers. The lack of expression in inv-MiMIC could be due to at least two different reasons. First, enhancer-promoter proximity could play a role. en-MiMIC is inserted adjacent to the en imaginal disc enhancers, while inv-MiMIC is not (Cheng et al. 2014). Second, inv-MiMIC is inserted inside the inv transcription unit and is transcribed in the opposite orientation to inv, so transcriptional interference may play a role.
Figure 5.
The inv-en “ON” state extends to the tou-MiMIC insertion site. (A) Top panel: inv-en region and two imaginal disc enhancers [IDEs, coordinates- 2R:7435274-7439183 and 2R:7448808-7455476, dm5]. MiMIC insertion sites are indicated with black lines. Bottom panel: yellow gene expression from the MiMIC transgenes in wing imaginal discs by RNA FISH with y probe. Regions where the yellow gene is expressed in tou-MiMIC are indicated with red arrowheads. (B) Schematic of the eZ-λ-eG construct that replaced the MiMIC element in tou. Blue boxes: eve promoter (eve pr) and eve 3′ untranslated region and poly(A) addition signal (eve utr). Black box: ∼0.5 kb bacteriophage λ DNA insert. (C) Immunostaining of GFP and β-galactosidase (β-gal) proteins in wing imaginal discs (anterior to the left) and embryos (bottom) (stage 13, anterior left, dorsal up). eZ-λ-eG was inserted in both orientations into the tou-MiMIC site (orientation shown above). Note that the GFP and β-gal expression in wing discs is stronger in the same cells that express y in tou-MiMIC (red arrowheads).
We have previously found that inv-en enhancers exhibit promoter specificity, especially when acting at a distance (Kwon et al. 2009). Like the en promoter, the eve promoter has paused RNA polymerase II, and eve is a Polycomb-regulated gene (Fujioka et al. 2008, 2013). Thus, we reasoned that the eve promoter would be able to respond to inv-en enhancers if inserted within the inv-en domain. MiMIC elements are flanked by phi-C31 attP integration sites and can be replaced with any DNA using recombinase-mediated cassette exchange (RMCE). We used an attB-vector previously used to test the pairing activity of the eve insulators homie and nhomie (Fujioka et al. 2016). This vector contains two divergently transcribed reporter genes, lacZ and GFP, both driven by the eve promoter, with a bacteriophage λ DNA “spacer” between them (eZ-λ-eG, Figure 5B). eZ-λ-eG was inserted in both orientations via RMCE, replacing the MiMIC transposon in tou. Indeed, when eZ-λ-eG is inserted in either direction in the tou attP-site, both β-galactosidase and GFP are expressed like inv and en in both embryos and larval imaginal discs (Figure 5C). This suggests that extending the inv-en Polycomb domain extends the corresponding regulatory domain.
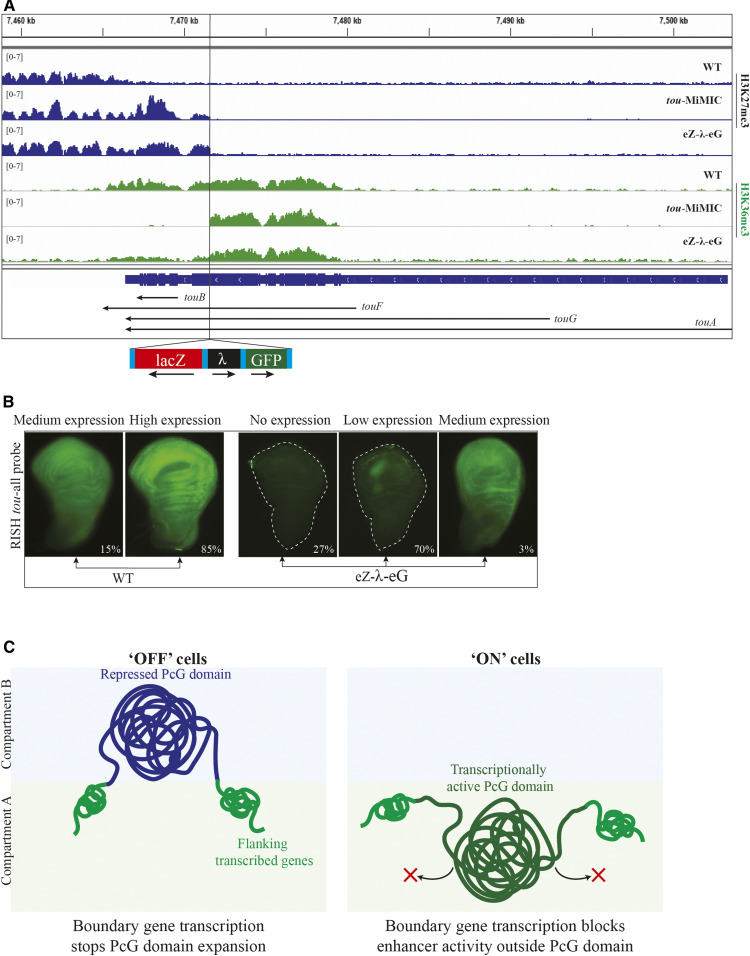
We also examined H3K27me3 and H3K36me3 levels over tou in an eZ-λ-eG transgenic line. Like in tou-MiMIC, H3K27me3 was present over the 3′ regions of the tou gene, extending from inv-en up to the transgene insertion site (Figure 6A). eZ-λ-eG has the eve transcriptional stop, and we expected H3K36me3 to be absent downstream of the transgene insertion site, like for tou-MiMIC. Instead, there is a low level of H3K36me3 overlapping the extended H3K27me3 domain in eZ-λ-eG larval brains and discs (Figure 6A). Using RISH with a probe to the 3′ region of tou, we observed that some wing discs have a low level of tou transcripts (Figure 6B). Unexpectedly, this expression was ubiquitous, not confined to the posterior compartment like en. We also examined expression of tou using a probe (tou-Big) upstream of the tou-MiMIC site that should detect all tou transcripts except tou-RB. Expression of tou was ubiquitous in both wild-type and eZ-λ-eG discs (Figure S1B). These data suggest either that a low level of tou transcription can proceed through the eZ-λ-eG transgene, or that tou-RB is expressed in eZ-λ-eG in a small subset of wing discs, leading to the low level of H3K36me3 we detected.
Figure 6.
eZ-λ-eG transgene disrupts tou transcription. (A) ChIP-seq distribution of H3K27me3 (rows 1–3) and H3K36me3 (rows 4–6) in WT, tou-MiMIC, and eZ-λ-eG inserted in tou. The tou gene is diagrammed at the bottom (navy blue). Arrows indicate the direction and extent of transcription of each tou isoform. The transgene insertion site is indicated with a black vertical line, and the structure of the eZ-λ-eG transgene is shown below. (B) RNA FISH with probe targeting both tou-RB and the 3′-end of all other tou isoforms in WT and eZ-λ-eG larval wing discs. Two representative wing discs (medium expression, high expression) from WT and three wing discs (no expression, low expression and medium expression) from eZ-λ-eG are shown. Percentages of wing discs in each category are shown; total counted: 34 discs in WT and 30 discs in eZ-λ-eG. (C) Cartoon of a PcG domain in transcriptionally “OFF” vs. “ON” cells. PcG domain in “OFF” cells is depicted as a dark blue line and in “ON” as a dark green line. Actively transcribed boundary genes of the PcG domain are depicted as green lines. Compartment A, which is transcriptionally active, is represented as a green-shaded box, while compartment B, which is transcriptionally repressed, is represented as a blue-shaded box.
Discussion
Overall, our data support the hypothesis that actively transcribed genes form the boundaries of most Polycomb domains in Drosophila (Ulianov et al. 2016; Rowley et al. 2017). For domain boundaries formed by actively transcribed genes facing a Polycomb domain, interfering with transcription can cause an extension of the Polycomb domain into the flanking gene. Such an extension of the Polycomb domain could alter the structure of the domain and cause changes in the transcription pattern of genes within that domain.
What about the “ON” transcriptional state of PcG-regulated genes? Our data show that in the absence of tou transcription, H3K27me3 spreads into the tou gene in the tou-MiMIC line. We suggest that this represents an expansion of the functional domain in both the “ON” and “OFF” transcriptional states. Although H3K27me3 is not bound to actively transcribed genes of the Bithorax complex in tissue culture cells (Orlando and Paro 1993) or embryos (Bowman et al. 2014), H3K27me3 is bound to regulatory DNA upstream of the Ubx transcription unit in third instar haltere imaginal discs, where Ubx is actively transcribed (Papp and Muller 2006). Similarly, a low level of H3K27me3 was shown to be bound to the inv-en domain in the posterior compartment of wing imaginal discs, where inv and en are transcribed (Schaaf et al. 2013). H3K27me3 was also found on the inv-en domain in BG3 cells, where these two genes are transcribed (Schaaf et al. 2009). These data support the view that H3K27me3 could be associated with the “ON” transcriptional state in genes that are Polycomb-regulated.
Further evidence that tou transcription forms the boundary of the inv-en domain in the “ON” state comes from our previous work studying the expression of a transgene, P(en3) [a.k.a. P(2.6-en-lacZ)] inserted in tou, 9.8 kb upstream of tou-MiMIC (2R:7481428) (DeVido et al. 2008; Kwon et al. 2009). This transgene is inserted upstream of the tou-RF transcription unit and the H3K36me3 associated with it (Figure S2). P(en3) contains en PREs and forms a small H3K27me3 domain that is confined to the transgene; i.e., it does not spread over tou to the inv-en gene [(De et al. 2016), Figure S2]. Because P(en3) is inserted near the inv-en domain, P(en3) is expressed like en (DeVido et al. 2008). In P(en3), the en PREs are flanked by FRT and loxP sites. When the en PREs are removed, the H3K27me3 domain is lost (De et al. 2016), and the transgene is no longer expressed like en (DeVido et al. 2008). The P(en3) transgene is clearly inserted outside of the inv-en domain. This suggests that the touF transcription unit, and associated H3K36me3 mark, forms the boundary of the inv-en domain. In contrast, the tou-MiMIC insertion site is located within the inv-en domain. This is somewhat surprising since this insertion is past a CP190 site that is designated as the TAD boundary in Hi-C datasets (Ramírez et al. 2018) (http://chorogenome.ie-freiburg.mpg.de:5001/#browser/tou). We note that this CP190 site does not stop the spreading of H3K27me3 in either eZ-λ-eG or tou-MiMIC larvae (Figure 3A and Figure 6A). We also note that, based on our H3K36me3 data, it appears that tou transcription is not hindered by this CP190 binding site. In addition, our data show that inv-en enhancers can act on a transgene inserted past the CP190 binding site in tou, suggesting that CP190 does not block the activity of inv-en enhancers. In summary, several lines of evidence support the hypothesis that active transcription forms the boundary of some Polycomb domains in Drosophila, both in “ON” and “OFF” cells (Figure 6C).
These results bring to mind studies of the boundary between the eve Polycomb domain and TER94. This is a type 3 boundary, where the homie insulator prevents the spreading of the Polycomb domain into TER94, which is transcribed away from the boundary (Fujioka et al. 2013). When homie is deleted, H3K27me3 expands to cover the TER94 promoter, repressing its normally ubiquitous expression. At the same time, the TER94 promoter is activated tissue-specifically by eve enhancers. This activation seems to depend on the expansion of the Polycomb domain, as both are lost when an eve PRE, present next to homie, is also deleted (Fujioka et al. 2013). Without this PRE, the eve Polycomb domain is still present, yet H3K27me3 no longer spreads over TER94 in the absence of homie (Fujioka et al. 2013). This can be explained by the existence of a dynamic balance between H3K27me3 spreading and active transcription (or H3K36me3), so that, in some cases, transcription is sufficient to prevent spreading, while in others, an insulator is also needed. At the inv-en type 1 boundary, an insulator is not needed to prevent the spreading of H3K27me3, and tou promoters are also not activated by inv-en enhancers.
Acknowledgments
We thank Elissa Lei, Yuzhong Cheng, Anna Horacek, and Lesley Brown for comments on this manuscript; Harold Smith [National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)] for next generation sequencing; Ming-an Sun for a bioinformatics analysis that served as the basis for Figure 1; and the Bloomington Stock center for stocks. This work was supported by the Intramural Research Program of the National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH) (S.D., N.D.G., F.W.C., and J.A.K.) and NIH grant number R01GM117458 to J.B.J. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Supplemental material available at figshare: https://doi.org/10.25386/genetics.12890870.
Communicating editor: P. Geyer
Literature Cited
- Bateman J. R., Lee A. M., and Wu C. T., 2006. Site-specific transformation of Drosophila via phiC31 integrase-mediated cassette exchange. Genetics 173: 769–777. 10.1534/genetics.106.056945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman S. K., Deaton A. M., Domingues H., Wang P. I., Sadreyev R. I. et al. , 2014. H3K27 modifications define segmental regulatory domains in the Drosophila bithorax complex. eLife 3: e02833 10.7554/eLife.02833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. L., Sun M. A., and Kassis J. A., 2018. Global changes of H3K27me3 domains and Polycomb group protein distribution in the absence of recruiters Spps or Pho. Proc. Natl. Acad. Sci. USA 115: E1839–E1848. 10.1073/pnas.1716299115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R., Wang L., Wang H., Xia L., Erdjument-Bromage H. et al. , 2002. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 298: 1039–1043. 10.1126/science.1076997 [DOI] [PubMed] [Google Scholar]
- Cheng Y., Brunner A. L., Kremer S., DeVido S. K., Stefaniuk C. M. et al. , 2014. Co-regulation of invected and engrailed by a complex array of regulatory sequences in Drosophila. Dev. Biol. 395: 131–143. 10.1016/j.ydbio.2014.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De S., Mitra A., Cheng Y., Pfeifer K., and Kassis J. A., 2016. Formation of a polycomb-domain in the absence of strong polycomb response elements. PLoS Genet. 12: e1006200 10.1371/journal.pgen.1006200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De S., Cheng Y., Sun M. A., Gehred N. D. and Kassis J. A., 2019. Structure and function of an ectopic Polycomb chromatin domain. Sci. Adv. 5: eaau9739 10.1126/sciadv.aau9739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVido S. K., Kwon D., Brown J. L., and Kassis J. A., 2008. The role of Polycomb-group response elements in regulation of engrailed transcription in Drosophila. Development 135: 669–676. 10.1242/dev.014779 [DOI] [PubMed] [Google Scholar]
- Dorighi K. M., and Tamkun J. W., 2013. The trithorax group proteins Kismet and ASH1 promote H3K36 dimethylation to counteract Polycomb group repression in Drosophila. Development 140: 4182–4192. 10.1242/dev.095786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis N. J., Kingston R. E., and Woodcock C. L., 2004. Chromatin compaction by a polycomb group protein complex. Science 306: 1574–1577. 10.1126/science.1100576 [DOI] [PubMed] [Google Scholar]
- Frey F., Sheahan T., Finkl K., Stoehr G., Mann M. et al. , 2016. Molecular basis of PRC1 targeting to Polycomb response elements by PhoRC. Genes Dev. 30: 1116–1127. 10.1101/gad.279141.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka M., Yusibova G. L., Zhou J., and Jaynes J. B., 2008. The DNA-binding Polycomb-group protein Pleiohomeotic maintains both active and repressed transcriptional states through a single site. Development 135: 4131–4139. 10.1242/dev.024554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka M., Sun G., and Jaynes J. B., 2013. The Drosophila eve insulator Homie promotes eve expression and protects the adjacent gene from repression by polycomb spreading. PLoS Genet. 9: e1003883 10.1371/journal.pgen.1003883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka M., Mistry H., Schedl P., and Jaynes J. B., 2016. Determinants of chromosome architecture: insulator pairing in cis and in trans. PLoS Genet. 12: e1005889 10.1371/journal.pgen.1005889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaydos L. J., Rechtsteiner A., Egelhofer T. A., Carroll C. R., and Strome S., 2012. Antagonism between MES-4 and Polycomb repressive complex 2 promotes appropriate gene expression in C. elegans germ cells. Cell Rep. 2: 1169–1177. 10.1016/j.celrep.2012.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnisz D., Weintraub A. S., Day D. S., Valton A. L., Bak R. O. et al. , 2016. Activation of proto-oncogenes by disruption of chromosome neighborhoods. Science 351: 1454–1458. 10.1126/science.aad9024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H., McElroy K. A., Jung Y. L., Alekseyenko A. A., Zee B. M. et al. , 2015. Sex comb on midleg (Scm) is a functional link between PcG-repressive complexes in Drosophila. Genes Dev. 29: 1136–1150. 10.1101/gad.260562.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassis J. A., Kennison J. A., and Tamkun J. W., 2017. Polycomb and trithorax group genes in Drosophila. Genetics 206: 1699–1725. 10.1534/genetics.115.185116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon D., Mucci D., Langlais K. K., Americo J. L., DeVido S. K. et al. , 2009. Enhancer-promoter communication at the Drosophila engrailed locus. Development 136: 3067–3075. 10.1242/dev.036426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. Y., Harrison M. M., Villalta J. E., Kaplan T., and Eisen M. B., 2014. Establishment of regions of genomic activity during the Drosophila maternal to zygotic transition. eLife 3: e03737 10.7554/eLife.03737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margueron R., Justin N., Ohno K., Sharpe M. L., Son J. et al. , 2009. Role of the polycomb protein EED in the propagation of repressive histone marks. Nature 461: 762–767. 10.1038/nature08398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra V., Rocha P. P., An D., Raviram R., Skok J. A. et al. , 2015. CTCF establishes discrete functional chromatin domains at the Hox clusters during differentiation. Science 347: 1017–1021. 10.1126/science.1262088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlando V., and Paro R., 1993. Mapping Polycomb-repressed domains in the bithorax complex using in vivo formaldehyde cross-linked chromatin. Cell 75: 1187–1198. 10.1016/0092-8674(93)90328-N [DOI] [PubMed] [Google Scholar]
- Papp B., and Muller J., 2006. Histone trimethylation and the maintenance of transcriptional ON and OFF states by trxG and PcG proteins. Genes Dev. 20: 2041–2054. 10.1101/gad.388706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S. Y., Schwartz Y. B., Kahn T. G., Asker D., and Pirrotta V., 2012. Regulation of polycomb group genes Psc and su(z)2 in Drosophila melanogaster. Mech. Dev. 128: 536–547 [corrigenda: Mech. Dev. 149: 53 (2018)]. 10.1016/j.mod.2012.01.004 [DOI] [PubMed] [Google Scholar]
- Ramírez F., Bhardwaj V., Arrigoni L., Lam K. C., Gruning B. A. et al. , 2018. High-resolution TADs reveal DNA sequences underlying genome organization in flies. Nat. Commun. 9: 189 10.1038/s41467-017-02525-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley M. J., Nichols M. H., Lyu X., Ando-Kuri M., Rivera I. S. M. et al. , 2017. Evolutionarily conserved principles predict 3D chromatin organization. Mol. Cell 67: 837–852.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaf C. A., Misulovin Z., Sahota G., Siddiqui A. M., Schwartz Y. B. et al. , 2009. Regulation of the Drosophila Enhancer of split and invected-engrailed gene complexes by sister chromatid cohesion proteins. PLoS One 4: e6202 10.1371/journal.pone.0006202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaf C. A., Misulovin Z., Gause M., Koenig A., Gohara D. W. et al. , 2013. Cohesin and Polycomb proteins functionally interact to control transcription at silenced and active genes. PLoS Genet. 9: e1003560 10.1371/journal.pgen.1003560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitges F. W., Prusty A. B., Faty M., Stutzer A., Lingaraju G. M. et al. , 2011. Histone methylation by PRC2 is inhibited by active chromatin marks. Mol. Cell 42: 330–341. 10.1016/j.molcel.2011.03.025 [DOI] [PubMed] [Google Scholar]
- Schuettengruber B., and Cavalli G., 2013. Polycomb domain formation depends on short and long distance regulatory cues. PLoS One 8: e56531 10.1371/journal.pone.0056531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S., Dorighi K. M., and Tamkun J. W., 2008. Drosophila Kismet regulates histone H3 lysine 27 methylation and early elongation by RNA polymerase II. PLoS Genet. 4: e1000217 10.1371/journal.pgen.1000217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler M. R., Haines J. E., and Eisen M. B., 2017. Convergence of topological domain boundaries, insulators, and polytene interbands revealed by high-resolution mapping of chromatin contacts in the early Drosophila melanogaster embryo. eLife 6: e29550 10.7554/eLife.29550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streubel G., Watson A., Jammula S. G., Scelfo A., Fitzpatrick D. J. et al. , 2018. The H3K36me2 methyltransferase Nsd1 demarcates PRC2-mediated H3K27me2 and H3K27me3 domains in embryonic stem cells. Mol. Cell 70: 371–379.e5. 10.1016/j.molcel.2018.02.027 [DOI] [PubMed] [Google Scholar]
- Thurmond J., Goodman J. L., Strelets V. B., Attrill H., Gramates L. S. et al. , 2019. FlyBase 2.0: the next generation. Nucleic Acids Res. 47: D759–D765. 10.1093/nar/gky1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian K., Henderson R. E., Parker R., Brown A., Johnson J. E. et al. , 2019. Two modes of transvection at the eyes absent gene of Drosophila demonstrate plasticity in transcriptional regulatory interactions in cis and in trans. PLoS Genet. 15: e1008152 10.1371/journal.pgen.1008152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulianov S. V., Khrameeva E. E., Gavrilov A. A., Flyamer I. M., Kos P. et al. , 2016. Active chromatin and transcription play a key role in chromosome partitioning into topologically associating domains. Genome Res. 26: 70–84. 10.1101/gr.196006.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanolst L., Fromental-Ramain C., and Ramain P., 2005. Toutatis, a TIP5-related protein, positively regulates Pannier function during Drosophila neural development. Development 132: 4327–4338. 10.1242/dev.02014 [DOI] [PubMed] [Google Scholar]
- Venken K. J., Schulze K. L., Haelterman N. A., Pan H., He Y. et al. , 2011. MiMIC: a highly versatile transposon insertion resource for engineering Drosophila melanogaster genes. Nat. Methods 8: 737–743. 10.1038/nmeth.1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W., Xu M., Huang C., Liu N., Chen S. et al. , 2011. H3K36 methylation antagonizes PRC2-mediated H3K27 methylation. J. Biol. Chem. 286: 7983–7989. 10.1074/jbc.M110.194027 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article. Sequencing data generated in this study (CTCF, CP190, Pol II from WT and H3K27me3 and H3K36me3 from the transgenic stocks) is deposited in the NCBI BioProject database- PRJNA564118. We also used our previously published ChIP-seq data: WT H3K27me3- GSE77342 and WT H3K36me3- PRJNA494709 (De et al. 2019). All other data and materials are available on request. Supplemental material available at figshare: https://doi.org/10.25386/genetics.12890870.