Abstract
Feltia subterranea (Fabricius), commonly known as the granulate cutworm, is a common species of owlet moths (Noctuidae) of major agricultural importance, widely distributed in Nearctic and Neotropical regions. This study was conducted to determine the species biological parameters, gather information about its larval host plants, and assess the agricultural significance of this species in the Americas. The viability of the egg, larval, pupal stages, and prepupal period was 98, 98, and 100%, respectively, under laboratory conditions. The average duration of the egg, larval, pupal stages, and prepupal period was 3, 17, 4, and 13 d, respectively. All laboratory-reared larvae developed through five instars. The growth ratio was 1.93 for females and 1.85 for males. The duration of the larval stage was significantly longer in females than in males from the fourth instar. The duration of the pupal stage was significantly shorter in females than in males. When larval and pupal stage durations were combined, there were no significant differences in total development time as a function of sex. In total, 159 botanical taxa belonging to 41 families were recorded as host species for F. subterranea. The families with the greatest number of host species were Fabaceae (22), Poaceae (19), Asteraceae (16), Brassicaceae (13), Solanaceae (12), Amaranthaceae (7), Cucurbitaceae (7), and Malvaceae (5). It is noteworthy that the large number of native weeds used by F. subterranea as host plants could represent a significant source of infestation of crops in the agricultural landscape.
Keywords: biotic potential, immature development, life tables, pest management, reproductive biology
Feltia subterranea (Fabricius, 1794), commonly known as the granulate cutworm (Figs 1–9), is a common species of owlet moths (Noctuidae) of major agricultural importance, widely distributed in Nearctic and Neotropical regions (Lafontaine 2004) (Fig. 10). Early in its original description, the species was readily recognized by the subterranean habits and voracity of its larvae (Fabricius 1794). Even though authors recognized the presence of the species in Central and North America, F. subterranea is vaguely acknowledged as ‘America meridionalis’ in its original description (Fabricius 1794). The intraspecific variability of F. subterranea throughout its range of distribution can be inferred by the rather large number of species-level names, now recognized as synonyms, described from all through the Americas (Poole 1989, Lafontaine 2004): Agrotis annexa Treitschke (1825) from North America, Agrotis decernens Walker ([1857]) from Santo Domingo, Distrito Nacional, Dominican Republic, Agrotis interferens Walker (1858) from Rio de Janeiro, Rio de Janeiro state, Brazil, Xylina lytaea Druce (1889) from Xalapa, Veracruz, Mexico, and Agrotis interposita Maassen (in: Weymer and Maassen 1890) from Puracé, Cauca, Colombia.
Figs. 1–5.
Habitus of Feltia subterranea (Fabricius, 1794). 1–3. Last instar larvae: 1. Dorsal. 2. Lateral. 3. Curled. 4–5. Adult: 4. Male (inset: detail of the antennae). 5. Female. Scale bar = 1 cm.
Figure 10.
Distribution of Feltia subterranea (Fabricius, 1794). Black circles—data taken from examined specimens deposited in collections; white stars—data taken from literature; gray stars—uncertain records taken from literature.
The distribution of F. subterranea ranges from about the 40°N parallel in Nova Scotia, Canada (Ferguson 1953) to the 30°S parallel in Pelotas, Rio Grande do Sul, Brazil (Specht et al. 2004), including the Bermuda and Caribbean islands.
Feltia subterranea common names vary in English-, Portuguese-, and Spanish-speaking countries, but usually refers to the size of its larvae relative to other cutworms, the slow-moving behavior, subterranean habits, its typical curled posture (Fig. 3), or the leathery, granulated skin of the larvae (Figs. 1–3): ‘granulate cutworm’ (Lafontaine 2004), ‘subterranean dart’ (Wagner et al. 2011) in the United States and Canada, ‘lagarta-rosca’ (i.e., ‘curled caterpillar’) in Brazil (Silva et al. 1968), and ‘cortador pequeño’ (i.e., ‘small cutworm’), ‘gusano cuerudo’ (i.e., ‘thick-skinned caterpillar’), and ‘gusano cachazudo’ (i.e., ‘slowly caterpillar’) in Spanish-speaking Latin America (King and Saunders 1984, Coto et al. 1995, Lafontaine 2004). Although F. subterranea is widely known as an important pest of several crops in the Americas, the available information about its biology is patchy and occurs in several publications with different purposes, mainly focusing in North American populations. Feltia subterranea is still frequently misidentified in entomological collections and scientific publications with superficially similar species, especially in the southern part of its distribution, complicating the development of pest management strategies. At least three superficially similar species that until recently were recognized as synonyms of F. subterranea: Agrotis anteposita Guenée, 1852, Agrotis blanchardii Berg, 1882 and Noctua lutescens Blanchard, 1952, replace F. subterranea, in Chile, Uruguay, and southern Argentina, respectively (Lafontaine 2004). In the referred countries, the distribution of these similar cutworms overlaps with that of F. subterranea, making misidentifications frequent (cf. Artigas 1974, Biezanko et al. 1974, Klein and Waterhouse 2000, Angulo et al. 2008, Dias et al. 2019).
The plant injuries caused by F. subterranea include mainly seedling stand reduction, defoliation, and fruit and stem boring. During the day, the larvae have the behavior to move beneath the soil surface, which provides shelter from natural enemies and foliar spray applications compromising the effectiveness of granulate cutworm management (Deitz et al. 1992).
Due to larval voracity, the economic impact of the species is acknowledged in several continents and countries, especially in North America (Howard 1897, 1900; Forbes 1903; Jones 1918; Crumb 1915, 1929, 1956; Whelan 1935; Chamberlin and Allen 1957; Eden et al. 1964; Lee and Bass 1969, 1970; Tietz 1972; Adlerz 1975; Deitz et al. 1992; Rings et al. 1992; Heppner 2007; Wagner et al. 2011; Prestes 2014; Capinera 2019), including Central America (Calderon 1931, King and Saunders 1984, Coto et al. 1995), the Caribbean islands, such as Puerto Rico (Wollcot 1941, 1948), and in several South American countries, such as Colombia (Gallego 1969, Posada Ochoa 1989), Venezuela (Guagliumi 1967), Peru (Valencia and Valdivia 1973), and Brazil (Fonseca 1934, 1937, 1939; Costa 1954, 1959; Mariconi 1954; Gallo and Flechman 1962; Silva et al. 1968; Zikán and Zikán 1968; Vendramim et al. 1982; Pereira et al. 2012).
For these reasons, the objectives of this study were to: 1) conduct life table studies under controlled conditions to describe the biology of F. subterranea; 2) compile host plant data from literature and new records through larvae collection conducted in agricultural regions in central and southern Brazil; and 3) compile the distribution of the species from literature and specimens deposited in entomological collections. In addition, illustrations of F. subterranea were prepared to provide distinctive morphological characters.
Materials and Methods
Species Identification
Specimens and their genitalia preparations were compared with illustrations of the species provided by Lafontaine (2004) and illustrations of the female type deposited at the Zoological Museum, University of Copenhagen, Copenhagen, Denmark (ZMUC). Dissections of the genitalia were conducted as shown in Dias et al. (2017, 2019) and San Blas et al. (2019).
Distribution
The distribution map was based on extrapolated label data of specimens deposited at the following collections: CEUCS: Coleção Entomológica da Universidade de Caxias do Sul, Caxias do Sul, Rio Grande do Sul, Brazil; CLAM: Coleção Alfred Moser, São Leopoldo, Rio Grande do Sul, Brazil; DZUP: Coleção Entomológica Padre Jesus Santiago Moure, Curitiba, Paraná, Brazil; Embrapa: Coleção Entomológica da Embrapa Cerrados, Planaltina, Distrito Federal, Brazil; HT: Coleção Hubert Thöny, Camacan, Bahia, Brazil; IOC: Coleção Entomológica do Instituto Oswaldo Cruz, Rio de Janeiro, Brazil; MCTP: Museu de Ciência e Tecnologia da Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre, Rio Grande do Sul, Brazil; MZUSP: Museu de Zoologia da Universidade de São Paulo, São Paulo, Brazil; UFPel: Coleção Entomológica do Museu Ceslau Biezanko, Universidade Federal de Pelotas, Pelotas, Rio Grande do Sul, Brazil; VOB: Coleção Vitor Osmar Becker, Camacan, Bahia, Brazil; CNC: Canadian National Collection of Insects, Ottawa, Canada; IFML: Instituto y Fundación Miguel Lillo, Tucumán, Argentina; USNM: National Museum of Natural History, Washington D.C., USA; ZMUC: Zoological Museum, University of Copenhagen, Copenhagen, Denmark, and from the following literature data: Audant (1935), Ferguson (1953), Salinas (1967), Zikán and Zikán (1968), Specht (1972), Valencia and Valdivia (1973), Tarragó et al. (1975), Silveira-Neto et al. (1977), Maes and Tellez (1988), Schotman (1989), Specht and Corseuil (2002), Lafontaine (2004), Specht et al. (2004, 2005), Zagatti et al. (2006), Zenker et al. (2010), Prestes (2014), and Torretta et al. (2009). Maps were prepared using SimpleMappr (Shorthouse 2010). Figured and dissected specimens are deposited at the DZUP.
Biological Parameters
Biological parameters were obtained under laboratory conditions at the Laboratório de Entomologia, Embrapa Cerrados, Brasília, Distrito Federal, Brazil. Larvae were kept in a controlled rearing room (25 ± 1°C, 70 ± 10% RH, and a 14-h photophase) and were fed on an artificial diet (Montezano et al. 2013a). Females collected in the field were kept individually in cylindrical plastic cages (10 cm Ø and 15 cm high); the tops of the cages were closed with voile and the bottom with Petri dishes (10.5 cm Ø) lined with filter paper. Adults were fed with 10% honey solution. Filter papers with eggs were maintained inside polystyrene containers (11.5 cm × 11.5 cm × 3 cm) with wet paper towel until hatching. The experiment started with 168 larvae that hatched on the same day, obtained from eggs laid by three females collected in the field (54, 64, and 50 larvae from each female).
Egg
The embryonic survival and incubation period were estimated from the 168 eggs from the three females collected in the field, and 16,883 eggs from females of the first generation of moths reared in laboratory. Because granulate cutworm females usually lay individual eggs, rectangular pieces of craft paper or voile (80mm × 60mm) containing different numbers of eggs were cut out. After the eggs were counted, each piece of paper or voile was placed in a polystyrene container with a moist cotton pad (57 mm Ø) with autoclaved water until hatching.
Larva
The newly hatched larvae were individually transferred using a fine brush to a white PVC container with transparent plastic cover (38 mm Ø × 27 mm height) 12 h after hatching. Each plastic container has a coin-shaped portion of the artificial diet (25 mm Ø × 5 mm) cut with a stainless-steel cutter. Daily observations were made between 8 and 10 a.m. to verify survival and instar change by a collection of the molted head capsules. Every 48 h, larvae were transferred to new containers with a fresh portion of the artificial diet, which allowed for greater asepsis. The head capsules were individually stored in microcentrifuge tubes labeled by larva and measured with a micrometer under a microscope. In cases where the head capsule and exuviae were not recovered (presumed to have been consumed by the larva), instar changes were noted by comparing the size with other larvae and by the presence of pieces of the head capsules in the fecal pellets. The growth ratio was determined by head capsule size, measuring the distance between genae (mm) of each instar from 50 randomly sampled larvae that did not feed on head capsules (25 females and 25 males). The mean growth ratio was calculated by dividing the mean head capsule width of each instar by the mean head capsule width of the previous instar. The prepupal period is characterized by the interruption of feeding and decrease in size. Larvae that did not feed for 24 h were considered prepupae and were transferred into a transparent plastic container (10 cm Ø × 5cm height) containing autoclaved expanded vermiculite moistened with autoclaved water. The prepupae always built the pupal chamber attached to the bottom of the container, allowing detection when the pupal metamorphosis has occurred.
Pupa
Pupae were kept in the same container and conditions as in the prepupal period and checked daily to note adult emergence and to maintain moisture with a few drops of autoclaved water. Two days after pupation, the pupae were removed from pupal chambers for sex determination (Madruga et al. 2019) and weighed with a high precision (1 mg) semianalytical scale. Considering that sex determination is only possible during the pupal stage, the identity of each larva was preserved throughout the study, allowing backtracking the development and sex of each individual larvae from hatching to adult.
Adult
The experiment involving adults used 27 female–male pairs formed with adults that emerged on the same day from the first generation of moths reared in the laboratory. Each pair was maintained within cylindrical plastic containers (10 cm Ø × 15 cm high). The top of the containers was closed with brown voile fastened with a rubber band, facilitating the visualization of eggs (which are white when laid). The bottoms were closed with Petri dishes (10.5 cm Ø). Both the bottom and the walls were lined with craft paper. The roughness of craft paper makes it easier for the moths to stick to the wall and the brown color facilitates the counting of newly laid eggs. Every day, each pair was collected in a glass test tube (2 cm Ø × 20 cm high) and transferred to a newly prepared container. The voile and the craft papers were stored in plastic bags properly identified, and eggs were counted under a stereomicroscope. When it was not possible to count all the eggs on the same day, the plastic bags with the samples were frozen (−17°C) to be counted later. Each container with a pair of moths received daily two Petri dishes (50 mm Ø) filled with cotton wool, one containing an artificial diet and the other with autoclaved mineral water. The artificial diet was prepared with honey (10 g), sorbic acid (1 g), methylparaben (1 g), sucrose (60 g), and distilled water (1,000 ml). All components were dissolved in distilled water and the resulting solution was kept under refrigeration (7°C). To stimulate the feeding of moths, Pilsen beer was daily added to the solution at a proportion of 1:4 beer to the diet and made available to the insects (Hoffmann-Campo et al. 1985). To evaluate the effect of pupal weight on reproductive parameters (Tisdale and Sappington 2001, Specht et al. 2016), records made on the second day after metamorphosis were kept and the fecundity was correlated with pupal weight. Mortality was recorded during the daily changes of moths to new cages; dead moths were kept in 2.5 ml microtubes with ethyl alcohol (96° GL). Dead females were dissected to determine the number of matings by counting the number of spermatophores received during copulation. Fecundity (the number of eggs per female), longevity, and the duration of the preoviposition, postoviposition, and oviposition periods were determined. As the number of pairs in a cage interfere with fertility (Milano et al. 2008, Specht et al. 2016), and as the moths have free access to each other in the field, a cage with five pairs emerged on the same day were kept under the same conditions as the isolated couples mentioned above. Eggs for the study were randomly selected from the 16,883 eggs laid by these couples between the first and last oviposition.
Host Plants
New records of F. subterranea host plants in Brazil were obtained by asystematic surveys conducted from June 2003 to February 2011 in Caxias do Sul, Rio Grande do Sul (by A.S. and D.G.M.), and from June 2013 to August 2017 at the Estação Experimental da Embrapa Cerrados, Planaltina, Distrito Federal, Brazil (by A.S., F.A.D.B., and P.V.M.V.). During these surveys, all larvae found near to the ground feeding on any plant in the field were collected and reared in the laboratory until the emergence of adults. Emerged adults were identified as F. subterranea by comparison with type specimens (deposited at ZMUC) and figures provided by Lafontaine (2004). Plants used as hosts by F. subterranea were collected and identified by a botanist, Dr. Ronaldo A.Wasum, from the Herbarium of the Universidade de Caxias do Sul by comparison with herbarium specimens and literature. An extensive list of F. subterranea host plants was compiled from a variety of databases (Robinson et al. 2010), literature data (Snow and Callahan 1968, Tietz 1972, Heppner 2007, Capinera 2019 [United States], Silva et al. 1968 [Brazil], Maes and Tellez Robleto 1988 [Nicaragua], Posada Ochoa 1989 [Colombia], Coto et al. 1995 [Central America]), and scientific reports (Ingram et al. 1938, Jefferson et al. 1959, Raulston et al. 1972, Wilfret 1980, Smith et al. 1996, Drezner 2014, Gilligan et al. 2019, McCartney et al. 2019). Host plant taxonomy follows the taxonomy used by the Commonwealth Agricultural Bureaux International (CABI 2019), and the United States Department of Agriculture (USDA 2020). Host plants were organized according to the family, genus, species, common name (when available), and references. New records are explicitly indicated.
Data Analysis
All biological parameters were analyzed using descriptive statistics. The fecundity, longevity of both sexes, and the duration of pre- and oviposition periods were correlated with the number of copulations for each pair: unmated females (n = 5 pairs), females that mated once (n = 11 pairs), twice (n = 8 pairs), and three times (n =3 pairs). Shapiro–Wilk was used to confirm normality of data, and Levene’s test to assess the equality of variances. Analysis of Kruskal–Wallis was used to verify the significance of the treatments and χ 2 test was used for the comparison of the means at a 5% probability level (α = 0.05). Pearson’s linear correlation method was used to verify possible association between larval duration on pupal weight. Likewise, we assessed whether there was any effect of the pupal weight on fecundity. To verify the significance of the coefficients of the model (linear coefficient and coefficient of determination), a t-test was used. To verify the quality of the adjusted model, the coefficient of determination (R2) was used. All statistical procedures were performed in IBM SPSS Statistics for Windows, version 19 (IBM Corp., Armonk, NY). Biotic potential (BP) was calculated using the equation described in Silveira-Neto et al. (1976). The life table data of age-specific survival (lx) and the number of offspring per day (mx) were graphically presented by plotting the probability of values at the midpoint of each time interval. Using the life table, the values of F. subterranea reproductive parameters were calculated. The net reproductive rate (R0), given by the ratio between the number of females in two successive generations and the mean generation time (T), which is the mean number of days from the birth of the parents to the birth of offspring; the intrinsic rate of increase (rm), and the finite rate of increase (λ) were calculated as in Silveira-Neto et al. (1976).
Results
Species Identification
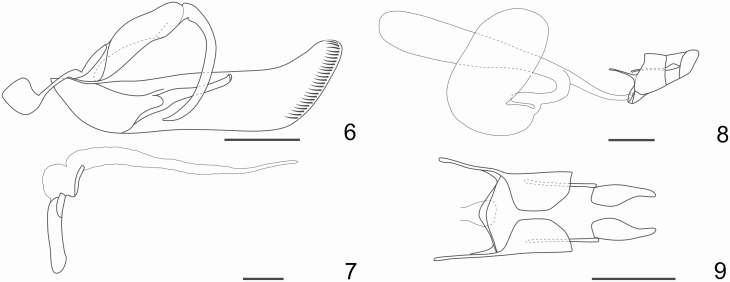
All development stages of F. subterranea are remarkably similar to several other species of cutworms. However, adults can be distinguished by the male doubly serrated antennae, the small and round orbicular spot and the reniform spot connected by a narrow bar and the translucent pearly white hind wing in both sexes (Figs. 4–5). Additionally, the long and posterior truncated valvae of the male genitalia (Figs 6–7) and the female genitalia (Figs 8–9) are decisive to distinguish the species from all other species in the genus.
Figs. 6–9.
Male and female genitalia of Feltia subterranea (Fabricius, 1794). 6–7. Male genitalia. 6. Genital capsule with left valva and aedeagus removed, lateral. 7. Aedeagus with everted vesica, lateral. 8–9. Female genitalia. 8. Lateral. 9. Ventral: ductus, corpus, and appendix bursae hidden. Scale bar = 1mm.
Distribution
The compilation of literature data indicates that F. subterranea is widely distributed in the Americas (Fig. 10). The examined material deposited in entomological collections greatly extends the reported distribution of F. subterranea in South America, significantly extending its range in Colombia, Ecuador, Peru, all regions of Brazil (with most records in the southeastern and southern regions), and to Argentina, in the provinces of Salta, Tucumán and La Rioja from Northwestern Argentina and Misiones and Santa Fe from Eastern Argentina (Fig. 10, black circles). Records from Córdoba and Buenos Aires, provided by Torretta et al. (2009), and the record for Uruguay, provided by Biezanko et al. (1974), are uncertain and need further confirmation (Fig. 10, gray stars).
Biological Parameters
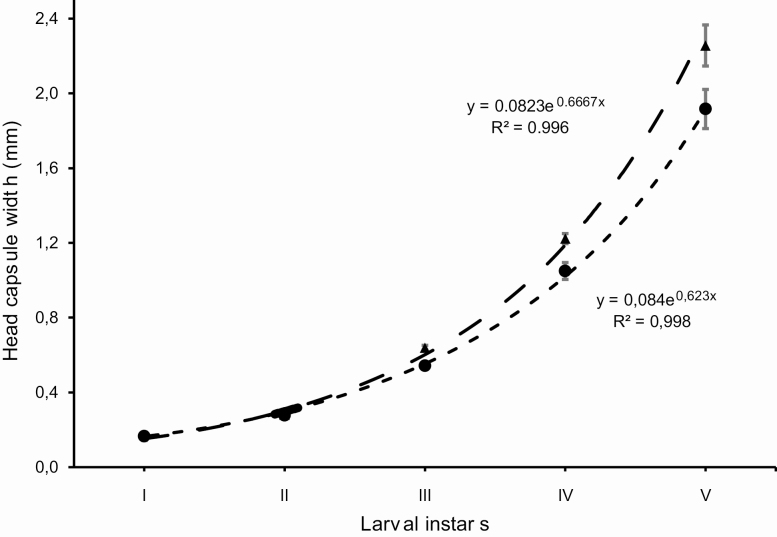
Feltia subterranea survival rates are high in all development stages (95.68%) (Table 1). There was low variation in the duration of each stage among individuals, confirmed by the standard deviation and range values (Table 1). Egg, larval, pupal, and adult stages took 6.08%, 44.2%, 27.00%, and 22.73% of the total development time, respectively (Table 1). Therefore, more than three-quarters of the development time corresponds to the immature stages. All larvae developed through five instars, and there were significant differences between sexes for head capsule size (Table 2; Fig. 11), instar, and stage durations for the fourth and fifth instar (Table 3). The growth of the head capsule size between instars forms a geometric progression, with a growth ratio of 1.93 for females and 1.85 for males (Table 2, Fig. 11). The duration of the larval stage is significantly longer in females than in males from the fourth instar on (including prepupae) (Table 3). In contrast, the duration of the pupal stage is significantly shorter in females than in males. However, when larval and pupal stage durations are combined, there are no significant differences on total development time as a function of sex (Table 3).
Table 1.
Developmental stage survival and duration of Feltia subterranea (Fabricius, 1794) reared under controlled conditions (25 ± 1°C, 70 ± 10% RH, and 14 h photophase) on an artificial diet
| Stage | N initial–final | Survival (%) | Duration (d) | Range (d) |
|---|---|---|---|---|
| Egg | 16,883–16,649 | 98.614 | 3.000 ± 0.000 | — |
| Larval | 168–164 | 97.619 | 17.458 ± 0.619 | 17–24 |
| Prepupal | 164–164 | 100.000 | 4.367 ± 0.543 | 3–6 |
| Pupal | 164–163 | 99.390 | 13.331 ± 0.673 | 12–16 |
| Adult (pairs) | 27– | — | 11.222 ± 2.361 | 6–18 |
| Overall | — | 95.679 | 49.378 |
Table 2.
Feltia subterranea (Fabricius, 1794) head capsule width (mm) (n = 25 for each sex) at each instar and respective growth ratios
| Instar | Female | Male | Significance | ||
|---|---|---|---|---|---|
| Mean ± SE | Growth ratio | Mean ± SE | Growth ratio | ||
| I | 0.168 ± 0.022 | 0.165 ± 0.010 | ns | ||
| II | 0.280 ± 0.020 | 1.669 | 0.278 ± 0.014 | 1.680 | ns |
| III | 0.639 ± 0.019 | 2.279 | 0.543 ± 0.013 | 1.955 | ** |
| IV | 1.223 ± 0.045 | 1.913 | 1.050 ± 0.027 | 1.935 | ** |
| V | 2.257 ± 0.131 | 1.845 | 1.917 ± 0.063 | 1.826 | ** |
| Mean | — | 1.927 | — | 1.849 | — |
Larvae reared under controlled conditions (25 ± 1°C, 70 ± 10% RH, and 14 h photophase) on an artificial diet. Sig.: Comparisons between means of females and males using a Student t-test, considering different variances, at a significance level of 95% (ns—P > 0.05; *—P < 0.01).
Figure 11.
Head capsule sizes of females (triangles and dashed line) and males (circles and dotted line) of Feltia subterranea (Fabricius, 1794) instars. Insectsreared under controlled conditions (25 ± 1°C, 70 ± 10% RH, and 14 h photophase) and larvae reared on an artificial diet.
Table 3.
Mean larval duration (days) and standard deviation (SD) of instars and pupae of Feltia subterranea (Fabricius, 1794)
| Duration | Females (87) | Males (76) | Both (163) | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Sig. | Mean ± SD | |
| Larval instar I | 3.089 ± 0.286 | 3.039 ± 0.196 | ns | 3.066 ± 0.249 |
| Larval instar II | 3.011 ± 0.105 | 3.000 ± 0.000 | ns | 3.006 ± 0.078 |
| Larval instar III | 2.978 ± 0.148 | 2.987 ± 0.115 | ns | 2.982 ± 0.134 |
| Larval instar IV | 4.189 ± 0.538 | 4.013 ± 0.258 | ** | 4.108 ± 0.441 |
| Larval instar V (active feeding) | 4.444 ± 0.705 | 4.118 ± 0.325 | ** | 4.295 ± 0.585 |
| PP (prepupae) | 4.500 ± 0.604 | 4.211 ± 0.410 | ** | 4.368 ± 0.543 |
| Total - PP | 17.711 ± 0.675 | 17.158 ± 0.367 | ** | 19.054 ± 2.372 |
| Total + PP | 22.211 ± 0.868 | 21.368 ± 0.512 | ** | 21.825 ± 0.838 |
| Pupae | 13.078 ± 0.622 | 13.632 ± 0.608 | ** | 13.331 ± 0.673 |
| Total larvae + pupae | 35.289 ± 1.201 | 35.000 ± 0.712 | ns | 35.157 ± 1.015 |
Insects reared under controlled conditions (25 ± 1°C, 70 ± 10% RH, and 14 h photophase) and larvae reared on an artificial diet.
Comparisons between means of females and males using a Student t-test, considering different variances, at a significance level of 95% (Ns—P > 0.05; **—P < 0.001).
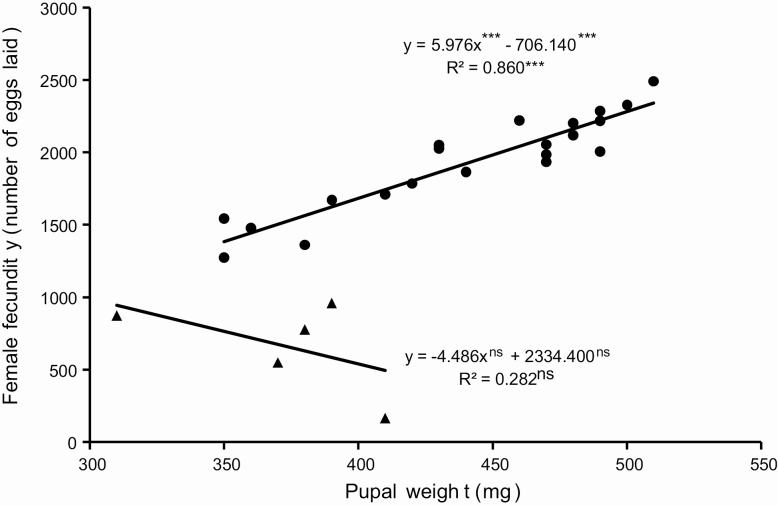
The sex ratio calculated from 163 pupae, 87 females and 76 males, was 0.534, which does not differ significantly from a 1:1 ratio (χ 2 = 0.742; P = 0.389). The head capsule size (even though there is a large variation, especially in females), and pupal weights are significantly larger in females than in males (Table 4). Regression analysis does not identify a relationship between larval stage duration and pupal weight for males and females (Fig. 12). There is no significant difference between males and females in adult longevity (Table 5). However, some individuals died after the sixth day, while others lived for over 2 wk. Females lay eggs from the third day of the adult stage, laying up to 2,494 eggs. From the female moths kept in pairs (n = 27), 5 did not mate, 11 mated once, 8 mated twice, and 3 mated three times. Regression analysis including females that mated at least once indicates a positive correlation between pupal weight and fecundity, which is not observed between unmated females (Fig. 13). Moreover, the preoviposition period of unmated females was significantly longer than females that mated at least once (χ 2 = 16.623; df = 3; P < 0.001). There was no significant difference (χ 2 = 5.295; df = 3; P = 0.151) between the oviposition period between unmated females and females that mated one or more times (Fig. 14). Although there is no significant relationship between pupal weight and number of matings (χ 2 = 7.300; df= 3; P = 0.063), there is a close relationship between the number of matings and the fecundity (χ 2 = 23.273; df = 3; P < 0.001), i.e., the number of laid eggs by female (Fig. 15). The net reproductive rate (Ro) was 799.983 times per generation, and the mean generation time (T) was 43.777 d. The intrinsic rate of increase (rm) was 0.153, with a finite increase rate (λ), meaning that the number of females added to the population per female that will generate another female is 1.165. The maximum rates of population increase occurred between days 42 and 43, during the sixth week of development (Fig. 16). Each female laid, on average, 1,696.963 eggs, with a sex ratio (sr) of 0.534; the overall egg survival is 95.679%, yielding 1,623.638 viable individuals per female (d). The average duration of the life cycle (43.78 d) corresponds to 8.34 generations per year (n). Thus, considering the environmental resistance (er) as null, we obtained the following result for the equation BP = (sr * d)n – er BP = (0.534 × 1,623.638)8.338 – 0 = 3.124 × 1024 individuals per female. In other words, each female could generate more than a septillion offspring per year.
Table 4.
Mean pupal weight (mg) with the number of weighed pupae (n) and standard error (SE) of Feltia subterranea (Fabricius, 1794)
| Sex | n | Mean ± SE | Range |
|---|---|---|---|
| Female | 87 | 415.444 ± 49.744 | 291–650 |
| Male | 76 | 311.842 ± 23.364 | 232–371 |
| Significance | * | - |
Insects reared under controlled conditions (25 ± 1°C, 70 ± 10% RH, and 14 h photophase) and larvae reared on an artificial diet. Comparison of means using a Student t-test, considering different variances, at a significance level of 95% (*P < 0.001).
Figure 12.
The relation between larval duration (days) and pupal weight (mg) of females (full line and circles) and males (dashed line and triangles) of Feltia subterranea (Fabricius, 1794). Insects reared under controlled conditions (25 ± 1°C, 70 ± 10% RH, and 14 h photophase) and larvae reared on an artificial diet.
Table 5.
Means, standard deviation (SD) and range of longevity, pre-, post- and oviposition periods and fecundity of 27 couples of Feltia subterranea (Fabricius, 1794)
| Sex | Biological parameter | Mean ± SD | Range |
|---|---|---|---|
| Female | Longevity (days) | 10.889 ± 2.40 | 6 −15 |
| Pre-oviposition (days) | 2.423 ± 0.694 | 2–4 | |
| Post-oviposition (days) | 0.269 ± 0.555 | 0–2 | |
| Oviposition (days) | 8.077 ± 2.107 | 4–12 | |
| Fecundity (eggs) | 1,696.963 ± 591.874 | 166–2,494 | |
| Male | Longevity (days) | 11.556 ± 2.309 | 6 −18 |
Insects reared under controlled conditions (25 ± 1°C, 70 ± 10% RH, and 14 h photophase) and larvae reared on an artificial diet. Comparisons of male and female mean longevity using a Student t test, considering different variances, at 5% level of significance (ns—P = 0.304).
Figure 13.
Relation between pupal weight (mg) and fecundity of mated (circles) and unmated (triangles) females of Feltia subterranea (Fabricius, 1794). Insects reared under controlled conditions (25 ± 1°C, 70 ± 10% RH, and 14 h photophase) and larvae reared on an artificial diet.
Figure 14.
Average and standard deviation of preoviposition (dark gray) and oviposition periods (light gray) of Feltia subterranea (Fabricius, 1794) unmated females (n = 5) and females that mated once (n = 11), twice (n = 8), or three times (n = 3).
Figure 15.
Average and standard deviation observed fecundity of Feltia subterranea (Fabricius, 1794) for unmated females (n = 5), and females that mated once (n = 11), twice (n = 8) or three times (n = 3).
Figure 16.
Relationship between age-specific survival (lx—full line) and the number of offspring per day (mx—dashed line) of Feltia subterranea (Fabricius, 1794).
Host Plants
In total, 159 botanical taxa belonging to 41 families are recorded as F. subterranea host plants. The compilation of literature data records 100 taxa being used as hosts, and further 35 and 24 taxa are newly recorded as hosts to the species in Distrito Federal and Rio Grande do Sul, respectively (Table 5). The families with the greatest number species used as hosts are: Fabaceae (22), Poaceae (19), Asteraceae (16), Brassicaceae (13), Solanaceae (12), Amaranthaceae (7), Cucurbitaceae (7), and Malvaceae (5). It is noteworthy the large number of native weeds used as host plants could represent a source of infestation of crops in the agricultural landscape.
Discussion
Specific Identity
Misidentification of F. subterranea with other species, such as F. submontana (Köhler, 1961) in Brazil (Dias et al. 2019), and F. lutescens (Blanchard, 1852) and Pseudoleucania anteposita (Guenée, 1852) in Argentina and Chile are reported. Feltia lutescens and P. anteposita were considered synonyms of F. subterranea, corresponding to some of the southern most records assigned to the species in the literature. Lafontaine (2004) revised the species, synonyms, and type material of F. subterranea and concluded that F. lutescens and P. anteposita were valid species, and the latter was combined with Pseudoleucania Staudinger, 1899. Feltia lutescens differs from F. subterranea by its deeply serrated antenna, with a double tuft of setae on each serration, and longer and more coiled vesica of aedeagus in males and ductus bursae and apophyses much longer in females. Furthermore, F. lutescens is restricted to the extreme south of South America, with its northern most records in Santiago, Chile and Neuquén, Argentina (Jana-Sáenz 1989), farther south than all known records of F. subterranea. Feltia subterranea is comprehensively distinguished from F. submontana by Dias et al. (2019).
Distribution
Feltia subterranea is widely distributed throughout the Americas (Fig. 10), and literature data reveal a strong bias for records in North and Central American countries and the Caribbean islands, with very few records from South America. Records from Córdoba and Buenos Aires, provided by Tarretta et al. (2019), are uncertain. All specimens identified as ‘F. subterranea’ from these provinces deposited in the IFML correspond to its former synonym, F. lutescens (most similar to the type of Euxoa bosqi Köhler, 1945). Similarly, all specimens from Chile identified as ‘F. subterranea’ also correspond to F. lutescens (most similar to the type of Noctua lutescens Blanchard, 1852). Thus, the occurrence of F. subterranea in Chile is unlikely. Biezanko et al. (1974) report the occurrence of the species in Uruguay, no specific location given (Fig. 10, gray star). However, Uruguayan specimens of F. subterranea were not located at UFPel, where Ceslau Biezanko usually deposited his specimens. The presence of F. subterranea in Uruguay cannot be ruled out entirely, as the southern most record for the species is from Pelotas, Rio Grande do Sul, Brazil, which is very close to the Uruguayan border.
Biological Parameters
Feltia subterranea development duration is similar to that observed by Vendramim et al. (1982) using the same temperature conditions with both kale leaves and a similar, but different formulated pinto bean-based artificial diet as larval food. The development duration is also similar to other cutworm species that do not go through diapause, especially regarding the relative durations of each development stage, as Agrotis ipsilon (Hufnagel, 1767) (black cutworm) (Bento et al. 2007); Peridroma saucia (Hübner, 1808) (variegated cutworm) (Moreno Farjado and Serna Cardona 2006); and Anicla infecta (Ochsenheimer, 1816) (green cutworm) (Teston et al. 2001). Preliminary data of pupal recovery from digging in fallow fields in the Florida Panhandle, Jay, FL, which is a transition zone between temperate and tropical areas, indicated no diapause condition and adults emerge approximately in 1 week (S.V.P.M.).
The high survival rate obtained for F. subterranea in this study was ¼ superior obtained for a São Paulo population (Vendramim et al. 1982) whose larvae were fed on an artificial diet (61.74%) and kale leaves (60.99%). The survival was also higher than observed for several other owlet moths and cutworms reared in nearly identical conditions by some of the authors (A.S., D.G.M., V.F.R.-S., S.V.P.M., and I.S.B.) (e.g., Montezano et al. 2013a,b, 2014a,b, 2015a,b, 2019a,b; Specht and Roque-Specht 2016, 2019; Silva et al. 2018a, b). Considering that the adequacy of different formulations of owlet moths artificial diets modify the developmental paramenters, (e.g., Bavaresco et al. 2004) the lower survival rates of F. subterranea obtained by Vendramim et al. (1982) on artificial diet, may be partly accounted to the differences between the artificial diet formulations used by those authors and in the present study.
Eggs
The embryonic duration of F. subterranea (3 d without variation) are in line with previous studies, such as Snow and Callahan (1968) at room temperature, and Vendramim et al. (1982) at a controlled temperature, with the same average temperature used in this study, but with more variation (±2°C). It is important to note that Lee and Bass (1969) observed a large average embryonic duration of 4.8 d using the same average temperature used in this study. Similarly, Walkden (1950) acknowledged 5 d of embryonic duration in summer generations and 6 d in winter generations. There is no significant variation between embryonic durations of F. subterranea (Vendramim et al. 1982) and other owlet moths (Specht et al. 2019) of the same population reared with different diets. This discrepancy in embryonic duration in different studies can be linked to genetic and/or latitudinal variations among different populations (Brito et al. 2019). Studies conducted in North America collected specimens in latitudes over 30°N parallel, while Vendramim et al. (1982) and the current study collected specimens at the 22°S and 15°S parallel, respectively. Owlet moths are generally larger in higher latitudes, and larger moths have longer embryonic durations.
The fertility of F. subterranea in this study (Table 1) is considerably higher than in other studies. The average fertility reported by Vendramim et al. (1982) was 76% for larvae fed on kale leaves and 83% for larvae fed on an artificial diet, adults of both treatments fed on a 10% honey solution. The average fertility reported by Snow and Callahan (1968) for larvae that fed on peanut leaves was 49%, ranging from 0% to 89.72%, adult diet was not reported. To maximize mating (see Snow and Callahan 1968, Kehat and Gordon 1975, Ellis and Steele 1982, Rogers and Marti Jr. 1997, Specht et al. 2016), multiple pairs confined in a cage resulted in a higher mating number, observed fecundity, and fertility rate. Moreover, the artificial diet used to feed adults in this study (Hoffmann-Campo et al. 1985) may have positively influenced the observed fecundity and fertility.
Larvae
In this study, all F. subterranea larvae developed through five instars (Tables 2 and 3). Other studies indicate that F. subterranea usually develops through six instars. Although Snow and Callahan (1968) indicate that the species develop through five (26%), six (65%), and seven (8%) instars for individuals from the same population, other studies indicate that the species develop through six instars (e.g., Crumb 1929, Walkden 1950, Vendramim et al. 1982, Capinera 2019). It was expected that the marked sexual dimorphism in the size of the larva, pupa (Tables 2 and 3; Fig. 12), and adult (Lafontaine 2004) would cause the larger females to develop through an additional instar, as observed in other Lepidoptera (Esperk and Tammaru 2006, 2010; Esperk et al. 2007a,b), including several other species of owlet moths such as representatives of Spodoptera Gueneé, 1852 (Montezano et al. 2013a, 2014b, 2015a; Specht and Roque-Specht 2016).
Vendramim et al. (1982) did not find significant differences in biological parameters (weight, duration, fecundity, etc.) between individuals of F. subterranea reared on natural and artificial diet. However, the results of the present study, using a different formulation of artificial diet, but otherwise somewhat similar rearing conditions, indicate a reduction in development duration, reduction in the number of instars, and an increase in fecundity and survival. These differences are most likely related to the artificial diet used in this study. The artificial diet used in this study is probably more appropriate for F. subterranea than the artificial diet used by Vendramim et al. (1982). Other studies including cutworms (e.g., Santos and Shields 1998, Esperk et al. 2007b, Cohen 2003, Schneider 2009) indicate that a more appropriate diet is related to faster development durations and a reduction in the number of instars.
The sexual dimorphism of F. subterranea was described only for adults of the species (Lafontaine 2004), and differences between sexes of larval stages were widely disregarded (e.g., Walkden 1950, Snow and Callahan 1968, Lee and Bass 1969, Vendramim et al. 1982). Nevertheless, from the third instar on the size of the head capsule is different among sexes (Table 2, Fig. 11), and, from the fourth instar on, the duration of stages is also different (Table 3).
The significantly larger larval stage and prepupal period duration in females corroborates with the hypothesis that larger insects need more time to development to reach the pupal stage (Esperk and Tammaru 2006, 2010, Esperk et al. 2007b). However, the relation between larval duration and pupal weight of each sex separately demonstrates that larval stage duration is not related to the pupal weight (Fig. 12). Thus, the hypothesis that larger pupal weight is related to larger development duration only holds between individuals of different sexes, but not between individuals of the same sex.
The head capsule growth rate of F. subterranea (1.8) (Table 2) is larger than the growth rate of 1.5 ± 2 estimated by Dyar (1890) for Lepidoptera. The higher growth rate in F. subterranea may be related to instars and to the morphology of the cutworms (Agrotini), in which the head capsule is retractable and unusually small in relation to the body size. For example, the average head capsule size of the last instar (fifth) of F. subterranea is 2.26 mm and the pupal weight is 415.44 mg, while in S. frugiperda (J.E. Smith, 1797) (growth ratio = 1.52) reared in similar conditions, the average head capsule size of the last instar (sixth) is 2.80 mm and the pupal weight is only 230.53 mg (Montezano et al. 2019a). This indicates that the head capsule growth rate is linked not only to effects listed by Esperk et al. (2007a), but also to the morphology associated with feeding habits and relationships with the environment, such as the subterranean habits of most cutworms.
Pupae
The average pupal weight of F. subterranea (0.368 ± 0.065mg) was similar to the weight measured by Vendramim et al. (1982). As expected, there is a significant difference (of about 100 mg) between the average weight of the male and female pupae (Table 4). The difference is probably a manifestation of the sexual dimorphism (Lafontaine 2004) observed in some Noctuinae, including in species of Feltia (Specht et al. 2013, Dias et al. 2017, 2019, San Blas and Agrain 2017). It is interesting to note the variation in pupal weight between individuals, especially females, where the heaviest pupa is twice the weight of the lightest one (Table 4). This variation was not expected since the individuals were reared under identical conditions. However, major weight variations were also observed in species of Spodoptera reared under similar conditions (Montezano et al. 2013a, 2014b, 2015a, 2019a; Specht and Roque-Specht 2016).
It is interesting to note the significantly longer pupal stage duration of males in relation to females (Table 3), also reported to other species of Noctuinae (Montezano et al. 2013a, 2014b, 2015a, 2019a; Specht and Roque-Specht 2016). The longer duration of the pupal stage in males balanced the longer duration of the larval stage in females, and therefore, the emergence of the males and females happened at roughly the same time. Even though several studies report both protogyny and protandry in Lepidoptera (see Degen et al. 2015), the simultaneous emergence of males and females of F. subterranea was similarly reported by Cline and Habeck (1977) in Florida, USA.
Adult
The different number of matings (unmated, n = 5; mated once, n = 11; mated twice, n = 8; mated three times, n = 3) is similar to that observed by Snow and Callahan (1968) and Cline and Habeck (1977). The majority of females will mate at least once, to a maximum of four matings under laboratory conditions (Snow and Callahan 1968). The oviposition period of F. subterranea, starting in the third night after adult emergence, is similar to previous observations (Cline and Habeck 1977) and is also significantly linked to matings (Fig.14). The longer preoviposition period for unmated females is related to the necessity of fertilization to stimulate the beginning of the oviposition period, as previously observed in F. subterranea (Cline and Habeck 1977) and other species of Noctuidae (Montezano et al. 2013b, 2014a, 2015b, 2019b; Specht and Roque-Specht 2019). Conversely, in the present study, the oviposition period was similar among unmated females and females that mated once, twice, or three times (Fig. 14). In other studies, the oviposition period lasted longer for unmated females (Montezano et al. 2013b, 2014a, 2015b, 2019b; Specht and Roque-Specht 2019). The fecundity was much larger in the present study (Table 5) than other F. subterranea studies (Jones (1918): 529.4 laid eggs and 264.8 eggs retained on the abdomen of dissected females, 794.2 eggs in total (average of 10 moths); Crumb (1929): 403 eggs (one moth), and 325 eggs (average of three moths); Walkden (1950): 970 eggs retained on the abdomen of a dissected female; Snow and Callahan (1968): 1,142 eggs (average of nine moths), Lee and Bass (1969): 647 eggs; Cline and Habeck (1977): 746 eggs (average of mated females) and 286 eggs (average of unmated females); Vendramim et al. (1982): 1,035.15 eggs (average of individuals fed on natural diet as a larvae) and 1,390.30 eggs (average of individuals fed on artificial diet as a larvae, adults fed on 10% honey solution). The dissimilarity may be related to the adequacy of the larval diet (Scheider 2009), pupal weights, and/or the adequacy of the adult diet. However, the high fecundity of F. subterranea in the present study is similar to the fecundity of other species of cutworms that can lay an average of 2,000 eggs (e.g., Archer et al. 1980, Bento et al. 2007, Specht et al. 2013).
The fecundity of mated females is higher than the fecundity of unmated females, as previously observed by Cline and Habeck (1977) (Figs. 13 and 15). While the fecundity of unmated females is unrelated to pupal weights, the fecundity of mated females is significantly related to pupal weights (Fig. 13). Larger pupae produce females with higher fecundity, as observed in S. eridania (Cramer, 1782) (Specht et al. 2016) and S. frugiperda (Montezano et al. 2019b). This observation is directly related to the greater investment in female size to maximize fecundity (Tammaru et al. 2002). Although there is no significant relationship between the number of matings and pupal weight in the present study (χ 2 = 7.300; df = 3; P = 0.063), the increase in the number of matings significantly increases the fecundity of F. subterranea (Fig. 15). The significant positive relationship between fecundity and number of matings is observed in other species of owlet moths (e.g., Snow et al. 1970; Ellis and Steele 1982; Chu and Yang 1991; Rogers and Marti Jr 1994, 1996; Ward and Landolt 1995; Landolt 1997; Hou and Sheng 1999; Sadek and Anderson 2007; Montezano et al. 2013b, 2014a, 2015b, 2019b; Specht and Roque-Specht 2019). Thus, several factors may be related to these observations, such as the specific vigor of the individuals used in the experiment, hormonal effects in multiple mating females (Zeng et al. 1997), and the availability of nutrimental material obtained from spermatophores in ‘re-mating’ received by females during mating in some Lepidoptera (but not in Noctuidae) (Boggs and Watt 1981, Greenfield 1982).
Life Table
The life table parameters presented for F. subterranea are similar to other polyphagous owlet moth pests that fed on an artificial diet (Barrionuevo et al. 2012; Montezano et al. 2013a, 2014b, 2015b, 2019b; Silva et al. 2018a,b; Specht and Roque-Specht 2019) or on their preferred host plants (Greenberg et al. 2001, Santos et al. 2005, Farahani et al. 2011, Bortoli et al. 2012, Specht et al. 2019). These parameters are characterized by a high net reproductive rate (Ro) and short mean generation time (T). The other high reproductive parameters values (intrinsic rate of increase [rm] and finite rate of increase [λ]) are as a function of R0 and T combined with a high survival. For example, some pest species of Spodoptera, such as S. albula (Walker, 1857) and S. eridania, reared under similar controlled conditions, present relatively lower R0 values (353.90 and 560.53) linked to similar low T values (37.19 and 35.81 d). Conversely, S. cosmioides (Walker, 1858) and S. dolichos (Fabricius, 1794) present higher R0 values (1,711.98 and 2,191.77) linked to similarly higher T values (46.41 and 56.19 d). In these cases, all rm and λ values ranged between 0.135 and 0.177 and 1.13 and 1.18, respectively. Spodoptera frugiperda, the most important pest species of the genus, presents higher R0 value (1,079.73) linked to lower T value (32.00), resulting in markedly higher rm (0.22) and λ (1.24) values. Therefore, it is important to note that the reproductive parameters presented here for F. subterranea (R0 = 799.983; T = 43.777; rm = 0.153 and λ = 1.165) indicate that under favorable conditions the species has the potential to increase its population rapidly, owing to the species and individual polyphagy, potentially causing crop injury and damage at the beginning of the growing season. This potential is further supported by many cases in which human involvement was required to protect crops from losses caused by outbreaks of F. subterranea (Jones 1918, Crumb 1929, Chamberlin and Madden 1942, Walkden 1950, Snow and Callahan 1968, Lee and Bass 1969, Morgan and French 1971, Adlerz 1975, Bass and Johnson 1978).
Biotic Potential
The biotic potential of F. subterranea (3.124 × 1024 individuals per female) is particularly high, and similar to the biotic potential of some major pest species of owlet moths and loopers (Specht et al. 2019), Helicoverpa armigera (Hübner) (old world cotton bollworm) (Silva et al. 2018a) and species of Spodoptera (e.g., Montezano et al. 2013a, 2014b, 2019b).
The association of F. subterranea high biotic potential, high larval polyphagy, wide distribution, and high dispersal capacity may explain the reports of outbreaks of the species in North America (e.g., Cook and Horne 1905, Jones 1918, Chamberlin and Madden 1942), Central America (Cook and Horne 1905, Wolcott 1941, Maes and Tellez Robleto 1988, Coto et al. 1995, Saunders et al. 1998) and South America (Mariconi 1954, Costa 1959, Salinas 1967, Vendramim et al. 1982, Posada Ochoa 1989) in the past. Feltia subterranea occurs simultaneously with other species of cutworms throughout its range; its populations are usually larger in warmer climates (Cook and Horne 1905, Jones 1918, Audant 1935, Wolcott 1948, Salinas 1967, Snow and Callahan 1968, Bass and Johnson 1978, Maes and Tellez Robleto 1988, Posada Ochoa 1989, Coto et al. 1995, Saunders et al. 1998) and smaller in colder climates (Jones 1918, Walkden and Whelan 1942, Snow and Callahan 1968, Specht 1972, Tarragó et al. 1975, Bass and Johnson 1978, Specht and Corseuil 2002, Zenker et al. 2010) in relation to other cutworm species.
Host Plants
The record of 159 plants from 41 families reported in this study (Table 6) represents a contribution to the list of F. subterranea host plants, but possibly represents just a fraction of the number of plants used as hosts. The great majority of host plants recorded in the literature are linked to economic crops; the relatively small number of weeds usually corresponds to species most commonly found in agricultural ecosystems, possibly playing a role as a source of infestation of this species in the landscape. Besides the economic crops and weeds in cultivated systems listed among the 59 new records of host plants in this study, plant species in natural systems are also included. Similar to other species of agricultural importance, such as the black cutworm (Crumb 1929; Rings et al. 1975; Busching and Turpin 1976, 1977; Costa and Link 1984; Link and Costa 1984; Link and Pedrolo 1987) and the variegated cutworm (Crumb 1929, Rings et al. 1976), F. subterranea is highly polyphagous, both as a species (i.e., larvae of the species are able to feed in several species of plants) and as individuals (i.e., an individual larvae are able to feed in several species throughout its development) (Crumb 1929, Chamberlin and Madden 1942, Link and Knies 1973, Santos and Nakano 1982, Link and Costa 1984, Leonard et al. 1993, Allen et al. 2018). The host plant range of this species indicates its potential to establish and cause outbreaks in cover crops and conservation-tillage systems (e.g., Oliver and Chapin 1981, Gaylor and Foster 1987, Leonard et al. 1993, Allen et al. 2018).
Table 6.
Host plants of Feltia subterranea (Fabricius, 1794) compiled from the literature, with new records from Brazil
| Family | Scientific name and authority | Common name | References | |
|---|---|---|---|---|
| 1. | Alismataceae | Echinodorus grandiflorus (Cham. & Schltdl.) Micheli. | Chapéu-de-couro | ** |
| 2. | Amaranthaceae | Amaranthus sp. | 7, 14 | |
| 3. | Amaranthus cruentus L. | Red amaranth | * | |
| 4. | Amaranthus deflexus L. | Large fruit amaranth | * | |
| 5. | Amaranthus dubius Mart. ex Thell. | Spleen amaranth | * | |
| 6. | Amaranthus hybridus L. | Slim amaranth | * | |
| 7. | Amaranthus spinosus L. | Spiny amaranth | 5, 16 | |
| 8. | Celosia cristata L. | Cockscomb | * | |
| 9. | Amaryllidaceae | Allium cepa L. | Onion | 3, 5,9, 11, 12, 16 |
| 10. | Allium sativum L. | Garlic | 3, 9, 11 | |
| 11. | Coriandrum sativum L. | Coriander | ** | |
| 12. | Apiaceae | Apium graveolens L. | Wild celery | 5, 16, 17 |
| 13. | Daucus carota L. | Carrot | 5, 9, 11,13,14,16 | |
| 14. | Petroselinum crispum (Mill.) Nyman ex A.W. Hill | Parsley | 13 | |
| 15. | Aquifoliaceae | Ilex crenata Thunb. | Japanese holly | 13 |
| 16. | Araceae | Caladium sp. | Caladium | 13 |
| 17. | Asparagaceae | Asparagus officinalis L. | Asparagus | 7, 11,13, 14 |
| 18. | Asteraceae | Arctium lappa L. | Greater burdock | 7,13, 14 |
| 19. | Bidens pilosa L. | Beggar-ticks | * | |
| 20. | Calendulaofficinalis L. | Pot marigold | ** | |
| 21. | Chrysanthemum morifolium Ramat | Florist’s daisy | ** | |
| 22. | Cichorium endivia L. | Endive | 13 | |
| 23. | Conyza bonariensis (L.) Cronquist | Asthmaweed | * | |
| 24. | Dahlia pinnata Cav. | Margarita | ** | |
| 25. | Gerbera jamesonii Bolus ex Hook. F. | Barberton daisy | ** | |
| 26. | Helianthus annuus L. | Sunflower | 11, 14 | |
| 27. | Lactuca sativa L. | Lettuce | 4, 5,7, 9, 11, 13, 14, 16 | |
| 28. | Lactuca serriola L. | Prickly lettuce | 14 | |
| 29. | Senecio brasiliensis (Spreng.) Less. | Brazilian ragwort | * | |
| 30. | Stevia rebaudiana (Bertoni) Bertoni | Candyleaf | * | |
| 31. | Tagetes L. | marigold | 13 | |
| 32. | Taraxacum officinale F.H. Wigg. | Dandelion | 5, 7, 13, 14 | |
| 33. | Xanthium strumarium L. var. canadense (Mill.) Torr. & A. Gray | Canada cocklebur | 5, 7, 13, 14 | |
| 34. | Boraginaceae | Symphytum officinale L. | Common comfrey | ** |
| 35. | Brassicaceae | Brassica napus L. var. napus L. | Rape | ** |
| 36. | Brassica oleracea var. acephala DC. | Kale | 5,16 | |
| 37. | Brassica oleracea var. botrytis L. | Cauliflower | 3, 5, 16 | |
| 38. | Brassica oleracea var. capitata L. | Cabbage | 3, 5, 7, 11, 9, 16 | |
| 39. | Brassica oleracea gemmifera DC | Brussels sprouts | 5, 71316 | |
| 40. | Brassica oleracea var. italica Plenk | Broccoli | 5, 7, 1316 | |
| 41. | Brassica rapa L. var. rapa L. | Turnip | 5, 713, 14, 16 | |
| 42. | Brassica rapa L. var. amplexicaulis Tanaka & Ono | Field mustard | 3 | |
| 43. | Capsella bursa-pastoris (L.) Medik. | Shepherd’s purse | 5, 713, 14, 16 | |
| 44. | Eruca vesicaria (L.) Cav. ssp. sativa (Mill.) Thell. | Rocket salad | * | |
| 45. | Lepidium sativum L. | Gardencress pepperweed | * | |
| 46. | Lepidium virginicum L. | Virginia pepperweed | 7, 13, 14 | |
| 47. | Raphanus sativus L. | Cultivated radish | 5, 13,16 | |
| 48. | Cactaceae | Carnegiea gigantea (Engelm.) Britton & Rose | Saguaro | 15 |
| 49. | Hylocereus undatus (Haw.) Britton & Rose | Pitaya | * | |
| 50. | Caryophyllaceae | Dianthus caryophyllus L. | Carnation | 12 |
| 51. | Gypsophila paniculata L. | Baby’s breath | 6 | |
| 52. | Chenopodiaceae | Chenopodium quinoa Willd. | Quinoa | 18 |
| 53. | Beta vulgaris L. ssp. cicla (L.) W.D.J. Koch | Chard | 3 | |
| 54. | Beta vulgaris ssp. vulgaris var. conditiva Alef. | Beet | 3, 5, 7, 11, 9, 13, 14, 16 | |
| 55. | Beta vulgaris var. saccharifera Alef. | Sugar beet | * | |
| 56. | Spinacia oleracea L. | Spinach | 5, 16 | |
| 57. | Commelinaceae | Commelina diffusa Burm. F. | Climbing dayflower | ** |
| 58. | Tradescantia zebrina hort. ex Bosse | Inch plant | ** | |
| 59. | Convolvulaceae | Dichondra J.R. Forst. & G. Forst. | Ponysfoot | 2, 5 |
| 60. | Ipomoea batatas (L.) Lam. | Sweet potato | 5, 7,9,11, 13, 14,16 | |
| 61. | Ipomoea purpurea (L.) Roth | Tall morning-glory | * | |
| 62. | Cucurbitaceae | Citrullus lanatus (Thunb.) Matsum. & Nakai var. lanatus | Watermelon | 3, 45, 9, 11, 14, 16 |
| 63. | Cucumis melo L. | Melon | 3, 4, 9, 10, 11,13, 14 | |
| 64. | Cucumis sativus L. | Cucumber | 3, 9, 11, 14 | |
| 65. | Cucurbita moschata Duchesne var. toonas (Makino) Makino | Kabocha | 11 | |
| 66. | Cucurbita pepo L. | Marrow | 3, 9 | |
| 67. | Fevillea cordifolia L. | Antidote vine | * | |
| 68. | Sechium edule (Jacq.) Sw. | Chayote | 9, 11 | |
| 69. | Euphorbiaceae | Chamaesyce prostrata (Aiton) Small | Prostrate sandmat | ** |
| 70. | Codiaeum variegatum (L.) A. Juss. | Garden croton | * | |
| 71. | Manihot esculenta Crantz | Cassava | 9, 11 | |
| 72. | Ricinus communis L. | Castor bean | * | |
| 73. | Fabaceae | Acacia mearnsii De Willd. | Black wattle | 4, 14 |
| 74. | Arachis hypogaea L. | Peanut | 5, 11, 12, 13,1416 | |
| 75. | Arachis pintoi Krapov. & Gregory | Pinto peanut | * | |
| 76. | Cajanus cajan (L.) Millsp. | Pigeonpea | * | |
| 77. | Cicer arietinum L. | Chickpea | 11 | |
| 78. | Crotalaria breviflora DC. | Short flower rattlebox | * | |
| 79. | Desmodium adscendens (Sw.) DC. | Zarzabacoa galana | * | |
| 80. | Desmodium tortuosum (Sw.) DC. | Dixie ticktrefoil | 5 | |
| 81. | Glycine max (L.) Merr. | Soybean | 5, 9, 16 | |
| 82. | Lathyrus latifolius L. | Perennial pea | 5 | |
| 83. | Lespedeza Michx. | Lespedeza | 5, 16 | |
| 84. | Medicago lupulina L. | Black medick | 5, | |
| 85. | Medicago sativa L. | Alfalfa | 5, 13, 14,16 | |
| 86. | Mucuna pruriens var. utilis (Wall. ex Wight) Baker ex Burck | Velvet bean | 14 | |
| 87. | Phaseolus lunatus L. | Sieva bean | 13, 14 | |
| 88. | Phaseolus vulgaris L. | Kidney bean | 3, 45, 7, 9, 10, 11, 12, 13, 14, 16 | |
| 89. | Pisum sativum L. | Pea | 5, 7, 11, 12, 13, 14,16 | |
| 90. | Pueraria montana (Lour.) Merr. | Kudzu | * | |
| 91. | Trifolium sp. | Clover | 5, 7, 13,14,16 | |
| 92. | Trifolium repens L. | White clover | ||
| 93. | Vicia faba L. | Fava bean | 5, 11, 16 | |
| 94. | Vigna unguiculata (L.) Walp | Cow pea | 5, 9, 11, 13, 14, 16 | |
| 95. | Geraniaceae | Geranium traversii Hook. f | Cranesbill | ** |
| 96. | Iridaceae | Cipura campanulata Ravenna | Cipura | 5 |
| 97. | Gladiolus sp. | Gladiolus | 8 | |
| 98. | Lamiaceae | Melissa officinalis L. | Bee balm | ** |
| 99. | Mentha spicata L. | Spearmint | ** | |
| 100. | Ocimum basilicum L. | Sweet basil | ** | |
| 101. | Origanum majorana L. | Sweet marjoram | ** | |
| 102. | Lauraceae | Persea americana Mill | Avocado | 5, 11, 13 |
| 103. | Liliaceae | Lilium candidum L. | Madonna lily | ** |
| 104. | Malvaceae | Abelmoschus esculentus (L.) Moench | Chimbinvoy | 4, 14 |
| 105. | Gossypium barbadense L. | Creole cotton | 14 | |
| 106. | Gossypium hirsutum L. | Cotton | 4, 5, 7, 9, 11, 12,13, 14,16 | |
| 107. | Hibiscus cannabinus L. | Bimli-jute | 13 | |
| 108. | Sida rhombifolia Linn. | Arrow-leaf | * | |
| 109. | Onagraceae | Fuchsia regia (Vand Ex Vell) Munz | Fuchsia | ** |
| 110. | Ludwigia peruviana (L.) H. Hara | Peruvian primrose-willow | * | |
| 111. | Oxalidaceae | Oxalis articulata Savigny | Azedinha | ** |
| 112. | Passifloraceae | Passiflora incarnata L. | Purple passionflower | 5, 7,13, 14,16 |
| 113. | Pedaliaceae | Sesamum indicum L. | Sesame | 9, 11 |
| 114. | Piperaceae | Piper sp. | Pepper | 5, 13 |
| 115. | Plantaginaceae | Plantago sp. | Plantain | 5, 7, 13, 14,16 |
| 116. | Poaceae | Avena sativa L. | Cultivated oat | 4, 11, 14 |
| 117. | Cynodon dactylon (L.) Pers. | Bermuda grass | 2, 5 | |
| 118. | Cynodon nlemfuensis Vanderyst | African Bermuda grass | * | |
| 119. | Digitaria sanguinalis (L.) Scop. | Crabgrass | * | |
| 120. | Eremochloa ophiuroides (Munro) Hack. | Centipede grass | 13 | |
| 121. | Hordeum vulgare L. | Barley | 5 | |
| 122. | Lolium perene L. ssp. multiflorum Lam. Husnot | Annual ryegrass | 2 | |
| 123. | Oryza sativa L. | Rice | 4, 9, 10, 11,14 | |
| 124. | Panicum miliaceum L. | Proso millet | * | |
| 125. | Paspalum distichum L. | Knotgrass | 5 | |
| 126. | Pennisetum purpureum Schum. | Elephant grass | * | |
| 127. | Saccharum officinarum L. | Sugarcane | 1, 9 | |
| 128. | Sorghum bicolor L. Moench ssp. bicolor | Grain sorghum | 9,16 | |
| 129. | Stenotaphrum secundatum (Walter) Kuntze | St. Augustine grass | 5, 13 | |
| 130. | Triticum aestivum L. | Wheat | 4,5, 7, 13, 14,16 | |
| 131. | Urochloa brizantha (Hochst. Ex A. Rich. R. Webster | Palisade grass | * | |
| 132. | Urochloa maxima (Jacq.) R. Webster | Guinea grass | * | |
| 133. | Urochloa plantaginea (Link) R. Webster | Plantain signalgrass | * | |
| 134. | Zea mays L. | Corn | 4,5, 7, 9, 11, 12, 13,14,16 | |
| 135. | Polygoniaceae | Polygonum aviculare L. | Prostrate knotweed | 7, 13,14 |
| 136. | Rumex crispus Linn | Curled dock | ** | |
| 137. | Portulacaceae | Portulaca grandiflora Hook. | Rose moss | * |
| 138. | Phytolaccaceae | Phytolacca dodecandra L’Her. | Pokeweed | * |
| 139. | Rosaceae | Fragaria ananassa Duchesne | Cultivated strawberry | 5, 11,12, 16 |
| 140. | Prunus sp. | Peach | 5, 13,16 | |
| 141. | Rubiaceae | Coffea arabica L. | Coffee | 9, 11,14 |
| 142. | Rutaceae | Citrus sp. | Citrus | 4,11,14 |
| 143. | Ruta graveolens L. | Common rue | ** | |
| 144. | Solanaceae | Capsicum annuum L. | Bell pepper | 3, 5, 7, 1113, 14, 16 |
| 145. | Nicandra physalodes (L.) Scop. | Apple of Peru | 5, 7,13,14 | |
| 146. | Nicotiana tabacum L. | Tobacco | 4,5, 7,9, 10, 11, 13,14,16 | |
| 147. | Petunia sp. | Petunia | 13 | |
| 148. | Physalis peruviana L. | Peruvian groundcherry | * | |
| 149. | Physalis philadelphica Lam. | Mexican groundcherry | 17 | |
| 150. | Solanum aethiopicum. L | Jiló | 4,14 | |
| 151. | Solanum lycopersicum Mill | Tomato | 4,5, 7,9,11, 13, 14,16 | |
| 152. | Solanum melongena L | Eggplant | 3, 4,5,7, 9, 13,14,16 | |
| 153. | Solanum seaforthianum Andrews | Brazilian nightshade | 14 | |
| 154. | Solanum sisymbriifolium Lam. | Sticky nightshade | ** | |
| 155. | Solanum tuberosum L. | Potato | 4,5, 7, 9, 11, 12, 13,14,16 | |
| 156. | Tropaeolaceae | Tropaeolum majus L. | Nasturtium | * |
| 157. | Verbenaceae | Lantana camara L. | Lantana | ** |
| 158. | Phyla nodiflora (L.) Greene | Turkey tangle fogfruit | 13 | |
| 159. | Violaceae | Viola tricolor L. | Johnny jumpup | ** |
References: 1. Ingram et al. (1938), 2. Jefferson et al. (1959), 3. Salinas (1967), 4. Silva et al. (1968), 5. Snow and Callahan (1968), 6. Raulston et al. (1972), 7. Tietz (1972), 8. Wilfret (1980), 9. Maes and Tellez (1988), 10. Posada Ochoa (1989), 11. Coto et al. (1995), 12. Smith et al. (1996), 13. Heppner (2007), 14. Robinson et al. (2010), 15. Drezner (2014), 16. Capinera (2019), 17. Gilligan et al. (2019), 18. McCartney et al. (2019). New records to Brazil: Distrito Federal (*) and Rio Grande do Sul State (**).
Feltia subterranea larvae feed on debris and can survive without food sources for weeks, and disperse in search of suitable host plants (Crumb 1929, Chamberlin and Madden 1942). This larval behavior is relevant to IPM, since the application of herbicides should be considered 4 to 6 wk before planting the crop to prevent outbreaks of this pest (Leonard et al. 1993). In cases where postemergence weed control with herbicides is not possible, pyrethroid insecticide application in a narrow band behind the planter is recommended (Allen et al. 2018).
The available data about the immature stages of cutworm species (e.g., Specht 1972, Link and Kies 1973, Angulo and Weigert 1975, Bryan et al. 2000, Baudino 2004, Corró Molas et al. 2017) and adults collected by light traps (e.g., Hills 1968, Tarragó et al. 1975, Lara et al. 1977, Lara and Silveira Neto 1977, Silveira Neto et al. 1977, Specht and Corseuil 2002, Specht et al. 2005, Zenker et al. 2010, Bernardi et al. 2011) indicate spatial and temporal variations of the species as a function of climate conditions, available crops, and insect management. Therefore, a local assessment of cutworm species and their host plants is recommended to avoid yield loss, particularly due to the stand reduction. The proper management of F. subterranea should consider soil and weed management strategies (Leonard et al. 1993) and natural biological control, such as the preservation of natural enemies, thus reducing operational costs and preserving the environment and protecting human health. Previous studies list several natural enemy organisms of cutworms, including F.subterranea. These organisms include microorganisms (Jones 1918, Crumb 1929, Seaver and Waterston 1946, Adlerz 1975, Hamm and Lynch 1982, Hamm et al. 1986), predators and parasitoids (Jones 1918, Crumb 1929, Sauer 1947, Lima 1949, Arnaud 1957, Bravo 1958, Silva et al. 1968, Adlerz 1975, Guimarães 1977, Saunders et al. 1998, Fernandes et al. 2014, Amiune and Valverde 2017, Capinera 2019), bats (Dood et al. 2015, Pinzari et al. 2019), and birds (Genung and Green Jr. 1974). Supplementary long-term studies should be conducted in the field to assess the importance of these organisms to the population dynamics of F. subterranea under natural conditions (see Baudino 2005, Pereira et al. 2018). Feltia subterranea also likely plays a role as a pollinator of native plants and commercial crops (Torretta et al. 2009, Benning 2015), and are a food source for other invertebrate and vertebrate animals in natural, anthropized, and agricultural ecosystems (Jones 1918, Crumb 1929, Arnaud 1957, Silva et al. 1968, Genung and Green Jr. 1974, Adlertz 1975, Hamm and Lynch 1982, Hamm et al. 1986, Saunders et al. 1998, Baudino 2005, Dood et al. 2015, Pinzari et al. 2019). Feltia subterranea eggs and first instars are present in natural systems, on weeds, and other covers (e.g., Crumb 1929, Chamberlin and Madden 1942, Link and Knies 1973, Santos and Nakano 1982, Link and Costa 1984, Leonard et al. 1993, Allen et al. 2018), and share several natural enemies with others owlet moths (e.g., Silva et al. 1968). The presence of F. subterranea may be important to maintain natural biological control in cultivated systems, especially off-season, when the preferred hosts of other owlet moths are not available or when the conditions for its development are limited by other edaphoclimatic factors.
Acknowledgments
We thank the Drs. Wilson Sampaio de Azevedo-Filho (CEUCS), Emilia Constanza Perez (IFML), Jerson Vanderlei Carús Guedes and Ervandil Corrêa Costa (UFSM), Alfred Moser (CLAM), Vitor Osmar Becker (VOB), Hubert Thöny (HT), Amabílio José Aires de Camargo (EMBRAPA), Marcelo Duarte (MZUSP), Gervásio Carvalho (MCTP), Donald Lafontaine (CNC), Thomas Pape (ZMUC), Eduardo Ely e Silva (UFPel), Jane Margaret Costa von Sydow and Aline Vieira Miranda (IOC), and Paul Goldstein (USNM) for allowing access to specimens under their care. To all of those involved with field collections of specimens used in this study: Adriano Q. Mesquita, Américo I. Ciociola Júnior, André L. Filipiake, Antônio C.S. Araújo, Balbino A. Evangelista, Brenda M. Moreira, Daniel B. Fragoso, Daniel R. Sosa-Gomez, Denivaldo C. Castro, Dirceu Pratissoli, Elisângela G.F. Morais, Erivaldo A. Santos, Felipe O. Mateus, Harry Ebert, João B.G. Santos Filho, Jorge U. P. Corrêa, José A. Teston, José R. Carvalho, José S. Zanúncio Júnior, Leonardo Mardgan, Maicon Coradini, Marco A.P. Silva, Marcos R.O. Serpa, Murilo Fazolin, Naylor B. Perez, Oriverto Tonon, Paulo R.V.S. Pereira, Rafael M. Pitta, Rinaldo J. Silva Júnior, Rodison N. Sisti, Sandra M.M. Rodrigues, Tiago C. C. Lima, Vander C.M. Claudino and Wilson Pozenato.To Fabiano Bastos—Embrapa Cerrados, for the last instar larvae pictures (Figs. 1–3). We also thanks to Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq: AS (processo 306601/2016-8); MMC (processo 30284/2017-7); OHHM (processo 304849/2019-7); for fellowships and research funds (processo 403376/2013-0), and Empresa Brasileira de Pesquisa Agropecuária - Embrapa (SEG MP2 02.13.14.006.00.00) partially funded this study. To Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES (Edital Capes-Embrapa 15/2014 - Proposta 92) provided fellowships to FMSD. We are also grateful to ICMBio and MMA for the Authorizations for Scientific Activities (SISBIO 48218-3 and 38547/6).
References Cited
- Adlerz W C. 1975. Natural control of three rindworm species and chemical control of the granulate cutworm, Feltia subterranea on watermelon. Proc. Fla State Hort. Soc. 88: 204–207. [Google Scholar]
- Allen K C, Luttrell R G, Sappington T W, Hesler L S, and Papiernik S K. . 2018. Frequency and abundance of selected early-season insect pests of cotton. J. Integr. Pest Manag. 9; 1–11 [Google Scholar]
- Amiune M J, and Valverde L. . 2017. Population dynamics of Encarsia portieri (Hymenoptera: Aphelinidae), egg parasitoid of soybean pests (Lepidoptera: Noctuidae) in Northern Argentina. Mun. Ent. Zool. 12: 603–607. [Google Scholar]
- Angulo A O, and Olivares T S. . 2005. Un inventario global y bibliográfica de la Subfamilia Noctuinae de Chile (Lepidoptera: Noctuidae). SHILAP Revta. Lepid. 33: 131–166. [Google Scholar]
- Angulo A O, and Weigert G T. . 1975. Noctuidae (Lepidoptera) de interes enonomico del Valle de Ica, Peru: clave para estados inmaduros. Rev. Per. Entomol. 18: 98–103. [Google Scholar]
- Angulo A O, Olivares T S, and Weigert G T. . 2008. Estados inmaduros de lepidópteros nóctuidos de importancia agrícola y forestal en Chile y claves para su identificación (Lepidoptera: Noctuidae). 3rd ed., Concepción, Universidad de Concepción, Chile. [Google Scholar]
- Archer T L, Musick G L, and Murray R L. . 1980. Influence of temperature and moisture on black cutworm (Lepidoptera: Noctuidae) development and reproduction. Can. Entomol. 112: 665–673. [Google Scholar]
- Arnaud P H. 1957. The occurrence of Salmacia longipulvilli in the Hawaiian Islands (Diptera: Larvaevoridae). Entomol. News. 68: 259–263. [Google Scholar]
- Artigas J N. 1974. Ensayo sobre simulación del daño ocasionado por un insecto en cultivo de soya. Bol. Soc. Biol. Concepcion. 47: 63–69. [Google Scholar]
- Audant A. 1935. Insect conditions in Haiti for March and April 1935. Insect Pest Surv. Bull. 15: 101. [Google Scholar]
- Barrionuevo M J, Murúa M G, Goane L, Meagher R, and Navarro F. . 2012. Life table studies of Rachiplusia nu (Guenée) and Chrysodeixis (=Pseudoplusia) includens (Walker) (Lepidoptera: Noctuidae) on artificial diet. Fla. Entomol. 95: 944–951. [Google Scholar]
- Bass M H, and Johnson S J. . 1978. Granulate cutworm: Evaluation of insecticides for control. Auburn Univ. (Ala.) Agric. Exp. Sta. Leaflet 95: 1–3. [Google Scholar]
- Baudino E. 2004. Presencia y distribución temporal del complejo de orugas cortadoras (Lepidoptera: Noctuidae) en pasturas de alfafa (Medicago sativa L.) del área fisiográfica Oriental de la provincia de La Pampa, Argentina. Rev. Fac. Agron. UNLPam. 15: 31–42. [Google Scholar]
- Baudino E. 2005. Ichneumonoideos (Hymenoptera) parasitoides del complejo de orugas cortadoras en pasturas de alfalfa (Medicago sativa L.) en la Argentina Central. Neotrop. Entomol. 34: 407–414. [Google Scholar]
- Bavaresco A, Garcia M S, Grützmacher AD, Ringenberg R, and Foresti J. . 2004. Adequação de uma dieta artificial para a criação de Spodoptera cosmioides (Walk.) (Lepidoptera: Noctuidae) em laboratório. Neotrop. Entomol. 33: 155–161. [Google Scholar]
- Benning J W. 2015. Odd for an Ericad: Nocturnal pollination of Lyonia lucida (Ericaceae). Amer. Midl. Nat. 174: 204–217. [Google Scholar]
- Bento F M M, Magro S R, Fortes P, Zério N G, and Parra J R P. . 2007. Biologia e tabela de vida de fertilidade de Agrotis ipsilon em dieta artificial. Pesq. Agropec. Bras. 42: 1369–1372. [Google Scholar]
- Berg C. 1882. Analecta Lepidopterologica. Contribuciones al estudio de la fauna de la República Argentina y otros países americanos. An. Soc. Cient. Argent. 14: 275–288 [Google Scholar]
- Bernardi O, Garcia M S, Ely E J, Zazycki L C F, Bernardi D, and Finkenauer E. . 2011. Levantamento populacional e análise faunística de Lepidoptera em Eucalypitus spp. no município de Pinheiro Machado, RS. Ci. Fl. 21: 735–744. [Google Scholar]
- Biezanko C M, Ruffinelli A, and Link D. . 1974. Plantas y otras sustancias alimenticias de las orugas de los lepidopteros uruguayos. Rev. Centro Ciências Rurais 4: 107–148. [Google Scholar]
- Boggs C L, and Watt W B. . 1981. Population structure of pierid butterflies IV. Genetic and physiological investment in offspring by male Colias. Oecologia. 50: 320–324. [DOI] [PubMed] [Google Scholar]
- Boisduval J B A D, and Guenée A. . 1852. Historie naturelle des insectes. Species général des lépidoptéres. Tome premier, Paris Roret. France. [Google Scholar]
- Bortoli L C, Bertin A, Efrom C F S, and Botton M. . 2012. Biologia e tabela de vida de fertilidade de Spodoptera eridania (Cramer) (Lepidoptera: Noctuidae) em morangueiro e videira. Rev. Bras. Frutic. 34: 1068–1073. [Google Scholar]
- Bravo G V. 1958. Breve studio biologico del predator Coleomejilla maculata De Geer (Coleoptera - Coccinellidae) en El Valle de Medellin. Rev. Fac. Agronomia. 18: 1–36. [Google Scholar]
- Brito R, Specht A, Gonçalves G L, Moreira G R P, Carneiro E, Santos F L, Roque-Specht V F, Mielke O H H, and Casagrande M M. . 2019. Spodoptera marima: a New Synonym of Spodoptera ornithogalli (Lepidoptera: Noctuidae), with notes on adult morphology, host plant use and genetic variation along its geographic range. Neotrop. Entomol. 48: 433–448. [DOI] [PubMed] [Google Scholar]
- Bryan W W, Sutula-Agdia C L, Adamczyk J J Jr., Adams L C, Hardee D D, Brown R L, Davis F M, Harris A F, Robbins J T, Price B Jr., et al. 2000. Incidence of the granulate cutworm, Feltia subterranea (F.) (Lepidoptera: Noctuidae) in early season Mississippi cotton: an example of the utility of a Heliothine egg identification system (Hel-ID) in IPM. Proc. Beltwide Cotton Conf. 2: 1004–1006. [Google Scholar]
- Busching M K, and Turpin F T. . 1976. Oviposition preferences of black cutworm moths among various crop plants, weeds, and plant debris. J. Econ. Entomol. 69: 587–590. [Google Scholar]
- Busching M K, and Turpin F T. . 1977. Survival and development of black cutworm (Agrotis ipsilon) larvae on various species of crop plants and weeds. Environ. Entomol. 6: 63–65. [Google Scholar]
- CABI. 2019. Commonwealth Agricultural Bureaux International—Invasive species compendium datasheets—Feltia subterranea (granulate cutworm). https://www.cabi.org/isc/datasheet/23954 [Google Scholar]
- Calderon S. 1931. Insect conditions in Salvador, Central America. Insect Pest Survey Bull. 11: 686–688. [Google Scholar]
- Capinera J L. 2019. Granulate Cutworm, Feltia subterranea (Fabricius) (Insecta: Lepidoptera: Noctuidae). UF/IFAS Extension. EENY559. https://edis.ifas.ufl.edu [Google Scholar]
- Chamberlin F S, and Allen N. . 1957. Tobacco cutworms how to control them. USDA Leaflet 417: 1–8. [Google Scholar]
- Chamberlin F S, and Madden A H. . 1942. Insect pests of cigar-type tobaccos in the southern districts. USDA Circ. 639: 1–54. [Google Scholar]
- Chu Y I, and Yang S C O. . 1991. Ovipositional biology of the tobacco cutworm (Spodoptera litura F.). Chin. J. Entomol. 11: 188–196. [Google Scholar]
- Cline L D, and Habeck D H. . 1977. Reproductive biology of the Granulate Cutworm. J. Ga. Entomol. Soc. 12: 35–41. [Google Scholar]
- Cohen A C. 2003. Insect diets: science and technology, 2nd ed., CRC Press, New York, USA. [Google Scholar]
- Cook M T, and Horne W T. . 1905. Insectos y enfermedades del tabaco. Estación Central Agronómica de Cuba, La Habana, Cuba [Boletin n°1]. [Google Scholar]
- Corró Molas A, Baudino E, Vilches J, Guillot Giraudo W, Babinec F, Vergara G, Niveyro S, Ghironi E, and Ferrero C. . 2017. Estudio comparativo de la densidad del complejo de orugas cortadoras (Lepidoptera: Noctuidae) en diferentes ambientes y cultivos antecesores en la región subhumeda pampeana central. Rev. Fac. Agronomia - UNLPam 27: 29–35. [Google Scholar]
- Costa J M. 1954. Algumas pragas do fumo e seus meios de combate. Bol. Téc. Inst. Agron. Leste, Ba. 1: 27–58. [Google Scholar]
- Costa J M. 1959. Controle da lagarta ‘rosca’ do fumo Feltia subterranea (Fabricius, 1794) com modernos inseticidas orgânicos sintéticos. Bol. Téc. Inst. Agron. Leste, Ba. 5: 37–51. [Google Scholar]
- Costa E C, and Link D. . 1984. Estimativa de danos e estudo sobre o comportamento de Agrotis ipsilon (Hufnagel, 1767) em feijoeiro. Rev. Centro Ciências Rurais 14: 9–17. [Google Scholar]
- Coto D, Saunders J L, Vargas C L S, and King A B S. . 1995. Plagas invertebradas de cultivos tropicales con énfasis en América Central. CATIE, Turrialba, Costa Rica. [Google Scholar]
- Crumb S E. 1915. A key to the cutworms affecting tobacco. J. Econ. Entomol. 8: 392–396. [Google Scholar]
- Crumb S E. 1929. Tobacco cutworms. USDA. Tech. Bull. 88: 1–178. [Google Scholar]
- Crumb S E. 1956. The larvae of the Phalaenidae. USDA, Tech. Bull. 1135: 1–356. [Google Scholar]
- Degen T, Hovestadt T, Mitesser O, and Hölker F. . 2015. High female survival promotes evolution of protogyny and sexual conflict. PLoS One. 10: e0118354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitz S S, Chapin J W, and Adler P H, . 1992. Feeding-site preference of fall armyworm, corn earworm, and granulate cutworm (Lepidoptera: Noctuidae) on Florunner Peanut. Peanut Sci. 19: 63–68. [Google Scholar]
- Dias F M S, Specht A, Blas G S, Casagrande M M, and Mielke O H H. . 2017. Resurgence of a forgotten Southern Brazil endemic species: taxonomic position, redescription, and spatio-temporal distribution of Porosagrotis carolia Schaus, 1929 (Lepidoptera: Noctuidae: Noctuinae). Zootaxa. 4363: 421–433. [DOI] [PubMed] [Google Scholar]
- Dias F M S, Specht A, Roque-Specht V F, San Blas G, Casagrande M M, and Mielke O H H. . 2019. Feltia submontana (Noctuidae, Noctuinae): redescription, taxonomy, life cycle, and spatial distribution of a neglected South American potential pest species. Neotrop. Entomol. 48: 98–110. [DOI] [PubMed] [Google Scholar]
- Dodd L E, Lacki M J, Johnson J S, and Rieske L K. . 2015. Prey size and dietary niche of Rafinesque’s Big-Eared Bat (Corynorhinus rafinesquii). Southeast. Nat. 14: 685–696. [Google Scholar]
- Drezner T D. 2014. The keystone saguaro (Carnegiea gigantea, Cactaceae): a review of its ecology, associations, reproduction, limits, and demographics. Plant Ecol. 215: 581–595. [Google Scholar]
- Druce H. 1889. Descriptions of new species of Lepidoptera, chiefly from Central America. Ann. Mag. Nat. Hist. (Ser. 6). 4: 77–94. [Google Scholar]
- Dyar H G. 1890. The number of molts of lepidopterous larvae. Psyche 5: 420–422. [Google Scholar]
- Eden W G, Brogden C A, and Sconyers M. . 1964. The granulate cutworm in peanuts and its control. Highlights Agr. Res. 11: 13. [Google Scholar]
- Ellis P E, and Steele G. . 1982. The effect of delayed mating on the fecundity of females of Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae). Bull. Entomol. Res. 72: 295–302. [Google Scholar]
- Esperk T, and Tammaru T. 2006. Determination of female-biased sexual size dimorphism in moths with a variable instar number: the role of additional instars. Eur. J. Entomol. 103: 575–586. [Google Scholar]
- Esperk T, and Tammaru T. . 2010. Size compensation in moth larvae: attention to larval instars. Physiol. Entomol. 35: 222–230. [Google Scholar]
- Esperk T, Tammaru T, and Nylin S. . 2007a. Intraspecific variability in number of larval instars in insects. J. Econ. Entomol. 100: 627–645. [DOI] [PubMed] [Google Scholar]
- Esperk T, Tammaru T, Nylin S, and Teder T. . 2007b. Achieving high sexual size dimorphism in insects: females add instars. Ecol. Entomol. 32: 243–256. [Google Scholar]
- Fabricius J C. 1794. Entomologiae systematica emendata et aucta. Secundum classes, ordines, genera, species adjectis synonimis, locis, observationibus, descriptionibus. Vol. 3, Part 2. Proft et Storch, Hafniae [=Copenhagen], Denmark. [Google Scholar]
- Farahani S, Naseri B, and Talebi A A. . 2011. Comparative life table parameters of the beet armyworm, Spodoptera exigua (Hübner) (Lepidoptera, Noctuidae) on five host plants. J. Entomol. Res. Soc. 13: 91–101. [Google Scholar]
- Ferguson D C. 1953. The Lepidoptera of Nova Scotia. Proc. N. S. Inst. Sci., 23: 161–375. [Google Scholar]
- Fernandes D R R, Onody H C, Lara R I R, and Perioto N W. . 2014. Annotated checklist of Brazilian Ophininae (Hymenoptera: Ichneumonidae). EntomoBrasilis 7: 124–133. [Google Scholar]
- Fonseca J P. 1934. Relação das principais pragas observadas nos anos de 1931, 1932 e 1933, nas plantas de maior cultivo no estado de S. Paulo. Arq. Inst. Biol. 5: 263–289. [Google Scholar]
- Fonseca J P. 1937. Lagartas nocivas aos milharais, capinzais, alfafais e algodoais. Biológico 3: 45–50. [Google Scholar]
- Fonseca J P. 1939. Contra a lagarta ‘rosca’ que ataca as hortaliças. Campo. 10: 61. [Google Scholar]
- Forbes S A. 1903. Twenty-second report of the state entomologist: On the noxious and beneficial insects of the State of Illinois. The Gazette Press, Champaign, USA. [Google Scholar]
- Gallego F L M. 1969. Lista preliminar de insectos de importancia economica y secundarios, que afectan los principales cultivos, animales domésticos y al hombre, en Colombia. Rev. Fac. Nac. Agron. Medellín. 26: 32–66. [Google Scholar]
- Gallo D, and Flechtmann C H W. . 1962. As mais importantes pragas das grandes culturas. Bol. Didát. 3: 1–144. [Google Scholar]
- Gay C. 1852. História física y política de Chile. Zoologia. Vol. 7 Maulde Y Renou, Paris. France. [Google Scholar]
- Gaylor M J, and Foster M A. . 1987. Cotton pest management in the southeastern United States as influenced by conservation tillage practices, pp. 29–54. In: House G J and Stinner B R (eds.). Arthropods in conservation tillage systems. Entomological Society of America, Misc. Publ. 65, Lanham, MD. [Google Scholar]
- Genung W G, and Green V E Jr. 1974. Food habits of the meadowlark in the Everglades in relation to agriculture. Environ. Entomol. 3: 39–42. [Google Scholar]
- Gilligan T M, Goldstein P Z, Timm A E, Farris R, Ledezma L, and Cunningham A P. . 2019. Identification of Heliothine (Lepidoptera: Noctuidae) larvae intercepted at U.S. ports of entry from the New World. J. Econ. Entomol. 112: 603–615. [DOI] [PubMed] [Google Scholar]
- Greenberg S M, Sappington T W, Legaspi B C Jr., Liu T-X, and Sétamou M. . 2001. Feeding and life history of Spodoptera exigua (Lepidoptera: Noctuidae) on different host plants. Ann. Entomol. Soc. Am. 94: 566–575. [Google Scholar]
- Greenfield D M. 1982. The question of paternal nutrient investment in Lepidoptera: male contributed proteins in Plodia interpunctella. Int. J. Invertebr. Reprod. 5: 323–330. [Google Scholar]
- Guagliumi P. 1967. Insects and arachnids of the common plants of Venezuela reported in the period 1938–63. Relaz. Monogr. Agrario-Subtrop. Trop. (N.S.). 86: 1–391. [Google Scholar]
- Guimarães J H. 1977. Host-Parasite and parasite-host catalogue of south american Tachinidae (Diptera). Arq. Zool. 28: 1–131. [Google Scholar]
- Hamm J J, and Lynch R E. . 1982. Comparative susceptibility of the granulate cutworm, fall armyworm and corn earworm to some entomopathogens. J. Georgia Entomol. Soc. 17: 363–369. [Google Scholar]
- Hamm J J, Pair S D, and Marti O G Jr. 1986. Incidence and host range of a new Ascovirus isolated from fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Fla. Entomol. 69: 524–531. [Google Scholar]
- Harris C R, Mazurek J H, and White G V. . 1962. The life history of the black cutworm, Agrotis ipsilon (Hufnagel), under controlled conditions. Can. Entomol. 94: 1183–1187. [Google Scholar]
- Heppner J B. 2007. Lepidoptera of Florida, part 1: introduction and catalog. Arthropods Fla. Neighboring Land Areas. 17: 1–670. [Google Scholar]
- Hills O A. 1968. Seasonal populations and flight patterns of several noctuid moths in South-Central Arizona. USDA Product. Res. Rep. 100: 1–14. [Google Scholar]
- Hoffmann-Campo C B, Oliveira E B, and Moscardi F. . 1985. Criação massal da lagarta da soja (Anticarsia gemmatalis). Embrapa Soja Doc. 10: 1–23. [Google Scholar]
- Hou M L, and Sheng C F. . 1999. Fecundity and longevity of Helicoverpa armigera (Lepidoptera: Noctuidae): effects of multiple mating. J. Econ. Entomol. 92: 569–573. [Google Scholar]
- Howard L O. 1897. Insects affecting the cotton plant. Farmers’ Bull. 47: 01–32. [Google Scholar]
- Howard L O. 1900. The principal insects affecting the tobacco plant. Farmers’ Bull. 197: 01–32. [Google Scholar]
- Ingram J W, Jaynes H A, and Lobdell R N. . 1938. Sugarcane pests in Florida. Int. Soc. Sugar Cane Tech. Proc. 6: 89–98. [Google Scholar]
- Jana-Sáenz C. 1989. Las especies del género Agrotis Ochsenheimer (Lepidoptera: Noctuidae) de importancia agrícola en Chile. Gayana Zool. 53: 63–71. [Google Scholar]
- Jefferson R N, Deal A S, and Sher S A. . 1959. Turfgrass and Dichondra pests in Southern California. South. Calif. Turfgrass Cult. 9: 9–15. [Google Scholar]
- Jones T H. 1918. Miscellaneous truck-crop insects in Louisiana. II - Granulated cutworm important enemy of vegetable crops in Louisiana. USDA Bull. 703: 7–14. [Google Scholar]
- Kehat M, and Gordon D. . 1975. Mating, longevity, fertility, and fecundity of the cotton leaf-worm, Spodoptera littoralis (Boisd.) (Lepidoptera: Noctuidae). Phytoparasitica 3: 87–102. [Google Scholar]
- King A B S, and Saunders J L. . 1984. The invertebrate pests of annual food crops in Central America. Overseas Development Administration, London, UK. [Google Scholar]
- Klein C, and Waterhouse D F. . 2000. The distribution and importance of arthropods associated with agriculture and forestry in Chile. ACIAR Monogr. 68: 1–234. [Google Scholar]
- Lafontaine J D. 2004. The moths of America North of Mexico. Fascicle 27.1: Noctuidae (part) - Agrotini. The Wedge Entomological Research Foundation, Washington, D.C., USA. [Google Scholar]
- Landolt P J. 1997. Cabbage looper (Lepidoptera: Noctuidae) fecundity maximized by a combination of access to water and food, and remating. Ann. Entomol. Soc. Am. 90: 783–789. [Google Scholar]
- Lara F M, and Silveira Neto S. . 1977. Flutuações populacionais de noctuídeos pragas, na região de Jaboticabal, SP. Científica 5: 262–270. [Google Scholar]
- Lara F M, Silveira Neto S, and Forti L C. . 1977. Constância e diversidade de espécies de noctuídeos coletados em Jaboticabal e Piracicaba com auxílio de armadilhas luminosas. Científica 5: 144–151. [Google Scholar]
- Lee B L, and Bass M H. . 1969. Rearing technique for the granulate cutworm and some effects of temperate on its life cycles. An. Entomol. Soc. Am. 62: 1216–1217. [Google Scholar]
- Lee B L, and Bass M H. . 1970. Controlling granulate cutworms in peanuts. Auburn Univ. (Ala.) Agric. Exp. Sta. Highlights Agricult. Res. 17: 5. [Google Scholar]
- Leonard B R, Clay P A, Hutchinson R L, and Graves J B. . 1993. Cultural management of cutworm spp. in conservation tillage systems for cotton, pp. 108–113. In. Proc. Southern Conservation Tillage conf. for sustainable agriculture. LA State Univ, Baton Rouge, LA. [Google Scholar]
- Lima A M C. 1949. Entomófagos Sul Americanos (Parasitos e Predadores) de Insetos Nocivos à Agricultura. Bol. Soc. Bras. Agron. 11: 1–32. [Google Scholar]
- Link D, and Costa E C. . 1984. Comportamento larval da lagarta-rosca Agrotis ipsilon (Hufnagel, 1767). Rev. Centro Ciências Rurais 14: 191–199. [Google Scholar]
- Link D, and Knies G. . 1973. Aspectos bionômicos sobre as lagartas roscas que ocorrem em Santa maria, RS. An. Soc. Entomol. Brasil 2: 66–73. [Google Scholar]
- Link D, and Predolo S S. . 1987. Aspectos biológicos de Agrotis ipsilon (Hufnagel, 1767). Rev. Centro Ciências Rurais. 17: 309–317. [Google Scholar]
- Madruga J, Specht A, Salik L M G, and Casagrande M M. . 2019. The External Morphology of Mythimna (Pseudaletia) sequax (Lepidoptera: Noctuidae). Neotrop. Entomol. 48: 834–852. [DOI] [PubMed] [Google Scholar]
- Maes J M, and Tellez Robleto J S. . 1988. Catálogo de los insectos e artrópodos terrestres asociados a las principales plantas de importancia económica en Nicaragua. Rev. Nica. Ent. 5: 1–95. [Google Scholar]
- Mariconi F A M. 1954. As lagartas-rosca: Pragas das plantas hortícolas. Biológico 20: 41–46. [Google Scholar]
- McCartney B N, Ahumada M I, Muñoz M P, Rosales M, Fierro A M, and Chorbadjian R A. . 2019. Effects of saponin-rich quinoa (Chenopodium quinoa Wild.) bran and bran extract in diets of adapted and non-adapted quinoa pests in laboratory bioassays. Cien. Inv. Agr. 46: 125–136. [Google Scholar]
- Milano P, Berti Filho E, Parra J R, and Cônsoli F L. . 2008. [Temperature effects on the mating frequency of Anticarsia gemmatalis Hüebner and Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae)]. Neotrop. Entomol. 37: 528–535. [DOI] [PubMed] [Google Scholar]
- Montezano D G, Specht A, Sosa-Gómez D R, Roque-Specht V F, Bortolin T M, Fronza E, Pezzi P, Luz P C, and Barros N M. . 2014a. Biotic potential, fertility and life table of Spodoptera albula (Walker) (Lepidoptera: Noctuidae), under controlled conditions. An. Acad. Bras. Cienc. 86: 723–732. [PubMed] [Google Scholar]
- Montezano D G, Specht A, Sosa-Gómez D R, Roque-Specht V F, and Barros N M. . 2014b. Immature stages of the armyworm, Spodoptera eridania: Developmental parameters and host plants. J. Insect Sci. 14: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montezano D G, Specht A, Sosa-Gómez D R, Roque-Specht V F, Paula-Moraes S V, Peterson J A, and Hunt T E. . 2019a. Developmental parameters of Spodoptera frugiperda (Lepidoptera: Noctuidae) immature stages under controlled and standardized conditions. J. Agric. Sci. 11: 76–89 [Google Scholar]
- Montezano D G, Specht A, Sosa-Gómez D R, Roque-Specht V F, Malaquias J V, Paula-Moraes S V, Peterson J A, and Hunt T E. . 2019b. Biotic Potential and Reproductive Parameters of Spodoptera frugiperda (J. E. Smith, 1797) (Lepidoptera: Noctuidae) J. Agric. Sci. 11: 240–252. [Google Scholar]
- Montezano D G, Specht A, Bortolin T M, Fronza E, Sosa-Gómez D R, Roque-Specht V F, Pezzi P, Luz P C, and Barros N M. . 2013a. Immature stages of Spodoptera albula (Walker) (Lepidoptera: Noctuidae): developmental parameters and host plants. An. Acad. Bras. Cienc. 85: 271–284. [DOI] [PubMed] [Google Scholar]
- Montezano D G, Specht A, Sosa-Gómez D R, Roque-Specht V F, and Barros N M. . 2013b. Biotic potential and reproductive parameters of Spodoptera eridania (Stoll) (Lepidoptera, Noctuidae) in the laboratory. Rev. Bras. Entomol. 57: 340–345. [DOI] [PubMed] [Google Scholar]
- Montezano D G, Sosa-Gómez D R, Paula-Moraes S V, Roque-Specht V F, Fronza E, Barros N M, and Specht A. . 2015a. Immature development of Spodoptera dolichos (Fabricius) (Lepidoptera: Noctuidae). Neotrop. Entomol. 45: 22–27. [DOI] [PubMed] [Google Scholar]
- Montezano D G, Sosa-Gómez D R, Paula-Moraes S V, Roque-Specht V F, Fronza E, Barros N M, and Specht A. . 2015b. Biotic potential and reproductive parameters of Spodoptera dolichos (Fabricius, 1794) (Lepidoptera: Noctuidae), in the laboratory. Zoologia 32: 485–491. [Google Scholar]
- Moreno Fajardo M L, and Serna Cardona F J. . 2006. Biología de Peridroma saucia (Lepidoptera: Noctuidae: Noctuinae) en flores cultivadas del híbrido comercial de Alstroemeria spp. Rev. Fac. Nal. Agr. Medellín 59: 3435–3448. [Google Scholar]
- Morgan L W, and French J C. . 1971. Granulate cutworm control in peanuts in Georgia. J. Econ. Entomol. 64: 937–939. [Google Scholar]
- Oliver A D, and Chapin J B. . 1981. Biology and illustrated key for the identification of twenty species of economically important noctuid pests. La. Agric. Exp. Stn. Bull. 733: 1–26. [Google Scholar]
- Pastrana J A. 2004. Los lepidópteros argentinos: sus plantas hospedadoras y otros sustratos alimenticios. Sociedad Entomológica Argentina, Buenos Aires, Argentina. [Google Scholar]
- Pereira R B, Pinheiro J B, Anderson J, and Reis A. . 2012. Doenças e pragas do jiloeiro. Circ. Téc. 106: 1–13. [Google Scholar]
- Pereira R R, Neves D V C, Campos J N, Santana Júnior P A, Hunt T E, and Picanço M C. . 2018. Natural biological control of Chrysodeixis includens. Bull. Entomol. Res. 108: 831–842. [DOI] [PubMed] [Google Scholar]
- Pinzari C A, Peck R W, Zinn T, Gross D, Montoya-Aiona K, Brinck K W, Gorresen P M, and Bonaccorso F J. . 2019. Hawaiian hoary bat (Lasiurus cinereus semotus) activity, diet and prey availability at the Waihou Mitigation Area, Maui. Hawaii Coop. Stud. Unit Tech. Rep. 90: 1–68. [Google Scholar]
- Poole R W. 1989. Lepidopterorum Catalogus. 118 - Noctuidae. Brill-Flora & Fauna Publ., New York, USA. [Google Scholar]
- Posada Ochoa L. 1989. Lista de insectos dañinos y otras plagas en Colombia. 4th Ed Instituto Colombiano Agropecuario, Boletin Técnico, Bogotá. 43: 1–662. [Google Scholar]
- Prestes A S. 2014. A new exotic noctuid for the Hawaiian archipelago Feltia subterranea (Fabricius) (Lepidoptera): Noctuidae: Noctuinae. J. Lepid. Soc. 68: 220–221. [Google Scholar]
- Raulston J C, Poe S L, and Maronsky F I. . 1972. Cultural concepts of Gypsophila paniculata L. production in Florida. Proc. Fla. State Hort. Soc. 85: 423–428. [Google Scholar]
- Rings R W, Johnson B A, and Arnold F J. . 1976. Host range of the variegated cutworm on vegetables: a bibliography. Bull. Entomol. Soc. Am. 22: 409–415. [Google Scholar]
- Rings R W, Metzler E H, Arnold F J, and Harris D H. . 1992. Owlet moths of Ohio: Order Lepidoptera, Family Noctuidae. Ohio State Univ. Res. Rep. Ser. 4: 1–219. [Google Scholar]
- Rings R W, Arnold F J, and Johnson B A. . 1975. Host range of the black cutworm on vegetables: a bibliography. Bull. Entomol. Soc. Am. 21: 229–234. [Google Scholar]
- Robinson G S, Ackery P R, Kitching I J, Beccaloni G W, and Hernández L M. . 2010. HOSTS - A Database of the World’s Lepidopteran Hostplants. Natural History Museum, London: http://www.nhm.ac.uk/hosts. [Google Scholar]
- Rogers C E, and Marti O G Jr. 1994. Reproductive potential of once-mated moths of the fall armyworm (Lepidoptera: Noctuidae). Fla. Entomol. 77: 402–410. [Google Scholar]
- Rogers C E, and Marti O G Jr. 1996. Beet armyworm (Lepidoptera: Noctuidae): effects of age at first mating on reproductive potential. Fla. Entomol. 79: 343–352. [Google Scholar]
- Rogers C E, and Marti O G Jr. 1997. Once-mated beet armyworm (Lepidoptera: Noctuidae): effects of age at mating on fecundity, fertility, and longevity. Environ. Entomol. 26: 585–590. [Google Scholar]
- Sadek M M, and Anderson P. . 2007. Modulation of reproductive behavior of Spodoptera littoralis by host and nonhost plant leaves. Basic Appl. Ecol. 8: 444–452. [Google Scholar]
- Salinas P J. 1967. Lista de los insectos observados en algunas hortalizas en Venezuela. Serviço Shell para el agricultor, Fundação Shell, Caracas, Venezuela. [Google Scholar]
- San Blas G, and Agraín F. . 2017. Revalidation and redescription of Feltia deprivata (Walker) (= bilitura of authors) (Lepidoptera: Noctuidae), a pest species on South America. Zootaxa 4323: 250–260. [Google Scholar]
- San Blas G, Silva Dias F M, Specht A, Martins Casagrande M, and Hendrik Mielke O H. . 2019. Overlooked South American noctuid species: revalidation of Feltia llanoi stat. rev. and redescription of Feltia brachystria (Lepidoptera: Noctuidae). Neotrop. Entomol. 48: 614–627. [DOI] [PubMed] [Google Scholar]
- Santos H R, and Nakano O. . 1982. Dados biológicos sobre a lagarta-rosca Agrotis ipsilon (Hufnagel, 1767) (Lepidoptera, Noctuidae). An. Soc. Entomol. Brasil 11: 33–48. [Google Scholar]
- Santos L, and Shields E J. . 1998. Temperature and diet effect on black cutworm (Lepidoptera: Noctuidae) larval development. J. Econ. Entomol. 91: 267–273. [Google Scholar]
- Santos K B, Meneguim A M, and Neves P M O J. . 2005. Biologia de Spodoptera eridania (Cramer) (Lepidoptera: Noctuidae) em diferentes hospedeiros. Neotrop. Entomol. 34: 903–910. [Google Scholar]
- Sauer H F G. 1947. Constatação de Himenópteros e Dípteros Entomófagos no Estados de S. Paulo. Bol. Fitossanit. 3: 7–23. [Google Scholar]
- Saunders J L, Coto D T, and King A B S. (Eds). 1998. Plagas invertebradas de cultivos anuales alimenticios en América Central. CATIE Ser. Téc. Manual Téc. 29: 1–305. [Google Scholar]
- Schneider J. 2009. Principles and procedures for rearing high quality insects. Mississippi State University, Starkville, USA. [Google Scholar]
- Schotman C Y L. 1989. Plant pests of quarantine importance to the Caribbean. RLAC-PROVEG, 21: 1–80. [Google Scholar]
- Seaver F J, and Waterston J M. . 1946. Contributions to the Mycoflora of Bermuda. Mycologia. 38: 180–201. [Google Scholar]
- Shorthouse D P. 2010. SimpleMappr, an online tool to produce publication-quality point maps http://www.simplemappr.net. Accessed 12 June 2020. [Google Scholar]
- Silva A G A, Gonçalves C R, Galvão D M, Gonçalves A J L, Gomes J, Silva M M, and Simoni L. . 1968. Quarto Catálogo dos insetos que vivem nas plantas do Brasil: seus parasitos e predadores. Parte II, 1º Tomo. Insetos, hospedeiros e inimigos naturais. Ministério da Agricultura, Rio de Janeiro, Brazil. [Google Scholar]
- Silva I F, Baldin E L L, Specht A, Sosa-Gómez D R, Roque-Specht V F, Morando R, and Paula-Moraes S V. . 2018a. Biotic potential and life table of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) from three Brazilian regions. Neotrop. Entomol. 47: 344–351. [DOI] [PubMed] [Google Scholar]
- Silva I F, Baldin E L L, Specht A, Sosa-Gómez D R, Roque-Specht V F, Morando R, Paula-Moraes S V. . 2018b. Biological and molecular characterization of the postinvasion immature stages of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Fla. Entomol. 101: 25–32. [Google Scholar]
- Silveira Neto S, Nakano O, Barbin D, and Villa Nova N A. . 1976. Manual de ecologia dos insetos. Ed. Agronômica Ceres, Piracicaba, Brazil. [Google Scholar]
- Silveira Neto S, Lara F M, and Fazolin M. . 1977. Quociente e porcentagem de similaridade entre as comunidades de noctuídeos amostradas em Jaboticabal e Piracicaba, S.P. Científica 5: 257–261. [Google Scholar]
- Smith R A, Zuloaga J C, and Navarrete B F. . 1996. Resolución 419 - Inventario subregional de plagas y enfermedades de los vegetables de importancia económica para el Área Andina. Gaceta Oficial del Acuerdo de Cartagena 13: 1–115. [Lima, Peru]. [Google Scholar]
- Snow J W, and Callahan P S. . 1968. Biological and morphological studies of the Granulate Curtworm, Feltia subterranea (F.) in Georgia and Louisiana. Ga. Agric. Exp. Stn. Tech. Bull. 42: 1–23. [Google Scholar]
- Snow J W, Young J R, and Jones R L. . 1970. Competitiveness of sperm in female fall army worms mating with normal and chemosterilized males. J. Econ. Entomol. 63: 1799–1802. [Google Scholar]
- Specht H B. 1972. Cutworms of tobacco in Nova Scotia. I. Species complex and infestation. Can. Ent. 104: 1855–1864. [Google Scholar]
- Specht A, and Corseuil E. . 2002. Diversidade dos noctuídeos (Lepidoptera, Noctuidae) em Salvador do Sul, Rio Grande do Sul, Brasil. Rev. Bras. Zool. 19: 281–298. [Google Scholar]
- Specht A, and Roque-Specht V F. . 2016. Immature stages of Spodoptera cosmioides (Lepidoptera: Noctuidae): developmental parameters and host plants. Zoologia, 33: e20160053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specht A, and Roque-Specht V F. . 2019. Biotic potential and reproductive parameters of Spodoptera cosmioides (Walker) (Lepidoptera: Noctuidae) in the laboratory. Braz. J. Biol. 79: 488–494. [DOI] [PubMed] [Google Scholar]
- Specht A, Silva E J E, and Link D. . 2004. Noctuídeos (Lepidoptera, Noctuidae) do Museu Entomológico Ceslau Biezanko, Departamento de Fitossanidade, Faculdade de Agronomia ‘Eliseu Maciel’, Universidade Federal de Pelotas, RS. R. Bras. Agrociência 4: 389–409. [Google Scholar]
- Specht A, Teston J A, Di Mare R A, and Corseuil E. . 2005. Noctuídeos (Lepidoptera, Noctuidae) coletados em quatro Áreas Estaduais de Conservação do Rio Grande do Sul, Brasil. Rev. Bras. Entomol. 49: 130–140 [Google Scholar]
- Specht A, Angulo A O, Olivares T S, Fronza E, Roque-Specht V F, Valduga E, Albrecht F, Poletto G, and Barros N M. . 2013. Life cycle of Agrotis malefida (Lepidoptera: Noctuidae): a diapausing cutworm. Zoologia 30: 371–378 [Google Scholar]
- Specht A, Montezano D G, Sosa-Gómez D R, Paula-Moraes S V, Roque-Specht V F, and Barros N M. . 2016. Reproductive potential of Spodoptera eridania (Stoll) (Lepidoptera: Noctuidae) in the laboratory: effect of multiple couples and the size. Braz. J. Biol. 76: 526–530. [DOI] [PubMed] [Google Scholar]
- Specht A, Sosa-Gómez D R, Roque-Specht V F, Valduga E, Gonzatti F, Schuh S M, and Carneiro E. . 2019. Biotic potential and life tables of Chrysodeixis includens (Lepidoptera: Noctuidae), Rachiplusia nu, and Trichoplusia ni on soybean and forage turnip. J. Insect Sci. 19: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammaru T, Esperk T, and Castellanos I. . 2002. No evidence for costs of being large in females of Orgyia spp. (Lepidoptera, Lymantriidae): larger is always better. Oecologia. 133: 430–438. [DOI] [PubMed] [Google Scholar]
- Tarragó M F S, Carvalho S, and Link D. . 1975. Levantamento da família Noctuidae, através de armadilhas luminosas, em Santa Maria, RS. Rev. Centro Ciênc. Rurais 5: 125–130. [Google Scholar]
- Teston J A, Specht A, and Corseuil E. . 2001. Biology of Anicla infecta (Ochsenheimer, 1816) (Lepidoptera, Noctuidae, Noctuinae), under laboratory conditions. Braz. J. Biol. 61: 661–666. [DOI] [PubMed] [Google Scholar]
- Tietz H M. 1972. An index to the described life histories, early stages and hosts of the Macrolepidoptera of the continental United States and Canada. The Allyn Museum of Entomology, Sarasota, FL. [Google Scholar]
- Tisdale R A, and Sappington T W. . 2001. Realized and potential fecundity, egg fertility, and longevity of laboratory-reared female beet armyworm (Lepidoptera: Noctuidae) under different adult diet regimes. An. Entomol. Soc. Am. 94: 415–419. [Google Scholar]
- Torretta J P, Navarro F, and Medan D, . 2009. Visitantes florales nocturnos del girasol (Helianthus annuus, Asterales: Asteraceae) en la Argentina. Rev. Soc. Entomol. Arg. 68: 339–350. [Google Scholar]
- Treitschke F. 1825. Die Schmeterlinge von Europa. Vol. 5 Abtheilung 1. Gerhard Fleischer, Leipzig, Germany. [Google Scholar]
- USDA, NRCS. 2020. The PLANTS database. National Plant Data Team, Greensboro, NC 27401-4901 USA: http://plants.usda.gov [Google Scholar]
- Valencia-V L, and Valdivia-M. R. 1973. Noctuideos del Valle de Ica, sus plantas hospedeiras y inimigos naturales. Rev. Peru. Entomol. 16: 94–101. [Google Scholar]
- Vendramim J D, Ferraz N C V D, and Parra J R P. . 1982. Biologia comparada de Agrotis subterranea em meios natural e artificial. O Solo 74: 76–80. [Google Scholar]
- Wagner D L, Schweitzer D F, Sullivan J B, and Reardon R C. . 2011. Owlet caterpillars of Eastern North America. Princeton University Press, Princeton, NJ. [Google Scholar]
- Walkden H H. 1950. Cutworms, armyworms and related species attacking cereal and forage crops in the Central Great Plains. USDA Circ. 849: 1–52. [Google Scholar]
- Walkden H H, and Whelan D B. . 1942. Owlet moths (Phalaenidae) taken at light traps in Kansas and Nebraska. USDA Circ. 643: 1–25. [Google Scholar]
- Walker F. [1857] 1856. List of the specimens of lepidopterous insects in the collection of the British Museum. Vol. 10 pp. 253–491. Edward Newman, London, UK. [Google Scholar]
- Walker F. 1858. List of the specimens of lepidopterous insects in the collection of the British Museum. pp. 1521–1888. Vol. 15 Edward Newman, London, UK. [Google Scholar]
- Ward K E, and Landolt P J. . 1995. Influence of multiple mating on fecundity and longevity off female cabbage looper moths (Lepidoptera: Noctuidae). Ann. Entomol. Soc. Am. 88: 768–772. [Google Scholar]
- Weymer G, and Maassen P. . 1890. Lepidopteren gesammelt auf einer reise durch Colombia, Ecuador, Peru, Brasilien, Argentinien and Bolivien in den Jarhren 1868–1877 von Alphons Stübel. Asher and Co., Berlin. Germany. [Google Scholar]
- Whelan D B. 1935. A key to Nebraska cutworms and armyworms that attack corn. Nebraska Agr. Exp. Sta., Res. Bull. 81: 1–27 [Google Scholar]
- Wilfret G J. 1980. 6 - Gladiolus. pp. 165–181. In Larson R A. (Ed.). Introduction to floriculture. Academic Press, New York, USA. [Google Scholar]
- Wolcott G N. 1941. A supplement to ‘Insectae borinquenses’. J. Agr. Univ. Puerto Rico. 25: 33–158. [Google Scholar]
- Wolcott G N. 1948. The insects of Puerto Rico - Lepidoptera - Rhopalocera: Butterflies. J. Agr. Univ. Puerto Rico 32: 537–748. [Google Scholar]
- Zagatti P, Lalanne-Cassou B, and d’Aubigny J D. . 2006. Catalogue of the Lepidoptera of the French Antilles. Institut National de la Recherche Agronomique. http://www.inra.fr/papillon/ [Google Scholar]
- Zeng F, Shu S, Park Y I, and Ramasway S B. . 1997. Vitellogenin and egg production in the moth, Heliothis virescens. Arch. Insect Biochem. Physiol 34: 287–300. [Google Scholar]
- Zenker M M, Botton M, Teston J A, and Specht A. . 2010. Noctuidae moths occurring in grape orchards in Serra Gaúcha, Brazil and their relation to fruit-piercing. Rev. Bras. Entomol. 54: 288–297. [Google Scholar]
- Zikán J F, and Zikán W. . 1968. Inseto-fauna do Itatiaia e da Mantiqueira. Lepidoptera. Pesq. Agropec. Bras. 3: 45–109. [Google Scholar]