Abstract
This paper explores which traits are correlated with fine-scale (0.25 m2) species persistence patterns in the herb layer of old-growth forests. Four old-growth beech forests representing different climatic contexts (presence or absence of summer drought period) were selected along a north–south gradient in Italy. Eight surveys were conducted in each of the sites during the period spanning 1999–2011. We found that fine-scale species persistence was correlated with different sets of plant functional traits, depending on local ecological context. Seed mass was found to be as important for the fine-scale species persistence in the northern sites, while clonal and bud-bank traits were markedly correlated with the southern sites characterised by summer drought. Leaf traits appeared to correlate with species persistence in the drier and wetter sites. However, we found that different attributes, i.e. helomorphic vs scleromorphic leaves, were correlated to species persistence in the northernmost and southernmost sites, respectively. These differences appear to be dependent on local trait adaptation rather than plant phylogenetic history. Our findings suggest that the persistent species in the old-growth forests might adopt an acquisitive resource-use strategy (i.e. helomorphic leaves with high SLA) with higher seed mass in sites without summer drought, while under water-stressed conditions persistent species have a conservative resource-use strategy (i.e. scleromorphic leaves with low SLA) with an increased importance of clonal and resprouting ability.
Subject terms: Forest ecology, Population dynamics
Introduction
Vegetation dynamics are complex phenomena involving many processes underpinning changes of vegetation patterns, such as directional succession of plant assemblages, cyclic patterns emerging as consequence of recurrent disturbance events1, or fluctuating population-dynamic trajectories ensuring community stability responding to, for instance, interannual variability of the weather2. While successional and cyclic dynamics have received much attention, this is less the case for fluctuations, especially in forest ecosystems3–5.
Recent studies of forest dynamics have considered the importance of clonal mobility (i.e. spatio-temporal patterns of plant individuals) for vegetation patterns6, examined temporal species turnover7,8, and explored the impact of forest management on forest floor vegetation9. While it has been strongly suggested that long-term studies should pursue multiple essential objectives to understand critical ecological mechanisms10, very few studies focusing on fine-scale species persistence have used long-term datasets covering at least a decade and different observation targets over time. Fine-scale observations are essential, as they can provide crucial information on the genesis and maintenance of diversity11 and seem fundamental for understanding large-scale spatial and temporal processes and their underpinning mechanisms5,12. For example, Nygaard & Ødegaard4 and Økland & Eilertsen3 found changes in species composition due to variation in soil pH and water availability in boreal forests. Økland13, who re-analysed permanent plots annually from 1988 to 1993 in boreal forests and related persistence to some clonal traits, emphasised the importance of clonal growth, ramet longevity and mobility for species persistence. However, more studies are needed, especially those employing traits related to various plant functions and different environmental and/or climatic contexts. It has been demonstrated that species dynamics strongly depend on the environmental context8,14–16. In seeking to understand the complexity of vegetation dynamics, a crucial factor in analysing is species persistence—the tendency of a species to maintain the spatial original position12,13. Likely, the patterns in the relationships between plant persistence and traits would change as an adaptive response, possibly dependent on locally differentiated plant communities (species composition) and plant traits abundance distribution.
The herb layer of old-growth forests (in this context, those older than 100 years) is characterised by ecological continuity as the relatively stable ecological conditions may persist for a long time. This continuity can activate environmental filters favouring the colonization and persistence of specialist species17,18. It has been demonstrated that well-adapted understory species can influence the long-term stability of the ecosystem19; they also affect the microbiological processes and nutrient cycling of the organic layer19. Thus, these species are widely considered to be of high conservation value14,18,20. Therefore, it is vital focus on a question asking which functional traits are essential for the persistence of these species. Plant functional traits are a very useful tool in understanding mechanisms that shape species patterns within ecosystems21. In detail, Weiher et al.22 identified seed, leaf, and clonal traits as those traits informing about the significant challenges of plant persistence. The seed and leaf traits provide information about resource acquisition and use strategies (e.g. Leaf–Height–Seed scheme23; Leaf Economic Spectrum24). Nevertheless, other key plant functions related to different ecological dimensions, such as space occupancy and recovery after damage, remained largely neglected22. Traits that can effectively capture these understudied functions are those associated with clonality (traits linked to vegetative reproduction and clonal spreading) and bud bank (traits related to the occurrence of stem- and root-derived buds)25.
Beech forest specialist species are usually clonal species15, characterised by larger specific leaf area (SLA), higher multiplication rates, and higher seed mass14. However, the crucial traits related to their persistence are still unknown, especially for cold temperate and Mediterranean forests. Further, it is not clear if the sharing of similar traits and the preference for the same (stable and shady) environment is better reflected by community phylogenetics or by functional adaptations26.
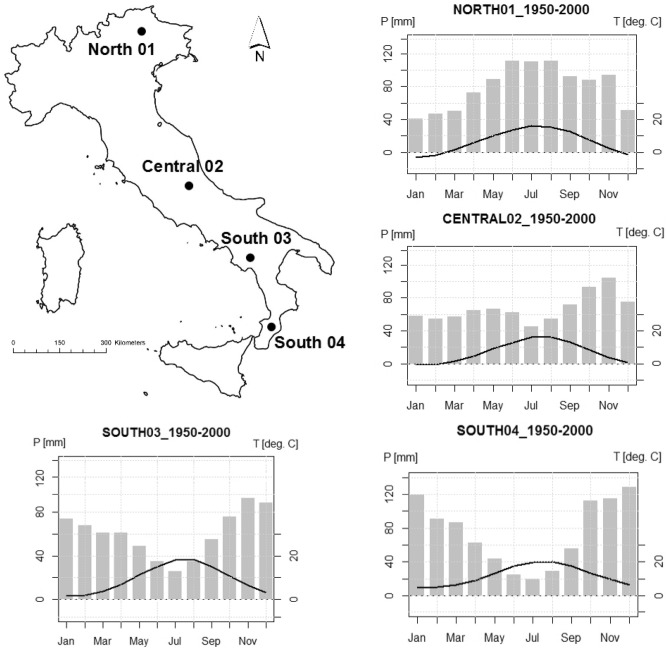
A unique opportunity to examine the traits-driven species persistence is provided by the Italian Forest Ecosystem Monitoring Network. This Network involves Permanent Monitoring Plots (hereafter, ‘sites’), where the ground vegetation assessment was performed repeatedly at a fine scale (0.25 m2) for longer than a decade. We selected four mature old-growth beech (Fagus sylvatica L.) forests representing different climatic contexts (presence or absence of summer drought period; Fig. 1) along a north–south gradient in Italy. We used the data of the monitoring of those forests to explore the trait-based mechanisms of species persistence in forest understory.
Figure 1.
Location of the four sites and Bagnouls-Gaussen climate diagrams (based on WorldClim 50 years meteorological data51).
Firstly, we verified whether the local ecological conditions of each site were so profoundly site-specific that they significantly differentiated not only the understory species composition but also the plant traits pools and relative abundance distribution. Secondly, we assessed if the local functional patterns were correlated with phylogenetic signals. Then, we devised an index of species persistence by using both presence/absence and abundance data. This combined approach was designed to reveal the potential different behaviour of dominant with respect to subordinate species. As the final step, we ascertained which traits were correlated with fine-scale species persistence, and hence formulated the following hypotheses:
H1:
Seed mass is related to seedling survival and is usually larger in plants in old-growth forests9,27 characterised by closed canopies and shaded understories. Therefore, we hypothesise that larger seed mass would favour species persistence across all four old-growth beech forest stands.
H2:
Among the leaf traits, SLA is related to water-use efficiency, photosynthetic capacity, and relative growth rate that, in turn, is under control of water, light, and nutrient availability28,29. Leaf anatomy30 is related to both water and nutrient conservation and prevention of mechanical damage31. Therefore, we expect lower SLA and presence of scleromorphic leaves to favour species persistence in forests experiencing marked summer drought.
H3:
Clonal traits related to mobility support plant persistence in habitats with more stress-inducing (drought) conditions, increasing the fine-scale spatial resource acquisition by increasing the foraging ability11,32,33. Further, bud bank traits related to regeneration reflect plants ability to re-sprout after disturbance14,15,34. Based on this rationale, we hypothesise that clonal and bud-bank traits would favour species persistence in forests more impacted by summer drought.
Results
Differences in species composition and trait abundance among the sites
PERMANOVA revealed that at the beginning of the monitoring the four sites differed significantly in terms of species composition and traits abundance (species composition R2 = 0.35, p = 0.001, df = 3; traits abundance R2 = 0.48, p = 0.001, df = 3).
Relationship between functional and phylogenetic patterns
The site-level correlations between functional and phylogenetic diversity were low and non-significant (North01, rho = 0.062, p = 0.539; Central02, rho = 0.045, p = 0.844; South03, rho = 0.114, p = 0.271; South04, rho = 0.117, p = 0.252).
Plant functional traits and species persistence
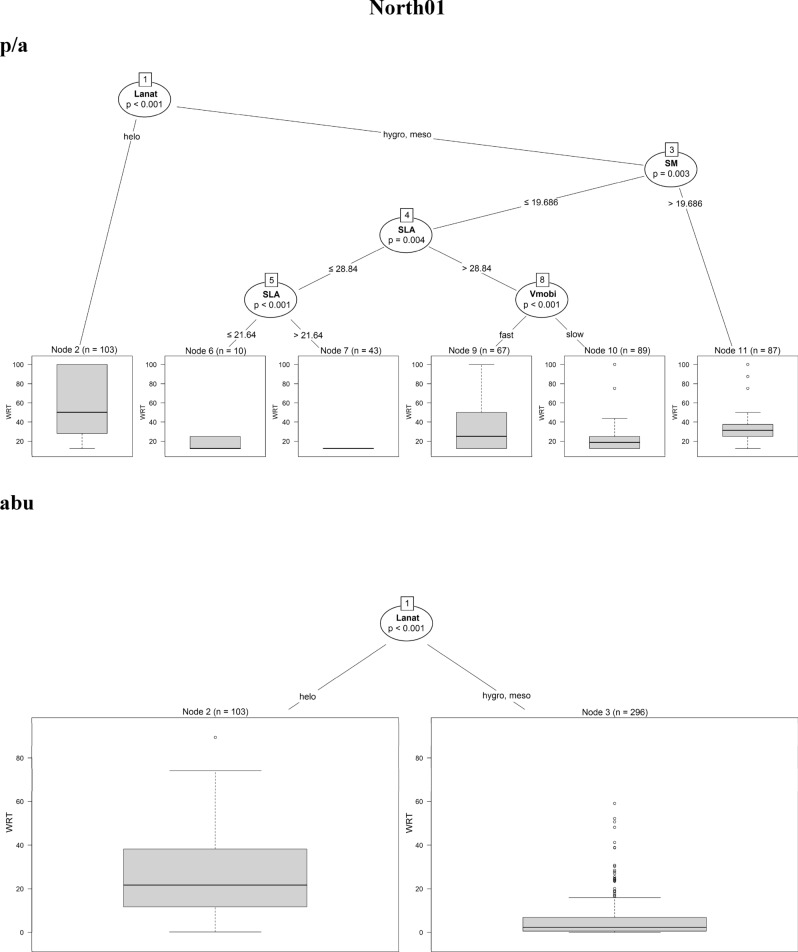
The regression trees for the four sites showed that the selected traits were significantly correlated with the fine-scale species persistence over time. In North01, species with helomorphic leaves were solidly persistent when using both presence/absence and abundance data. In addition, by using presence/absence data, the regression tree for North01 was more complex: species with hygro/mesomorphic leaves with higher seed mass (> 19.7 mg) or higher SLA (> 28.8 mm2.mg−1) and fast vegetative mobility were also persistent. On the contrary, species with lower seed mass (≤ 19.7 mg) and lower SLA (≤ 28.8 mm2.mg−1) were characterised by the lowest value of persistence (Fig. 2a).
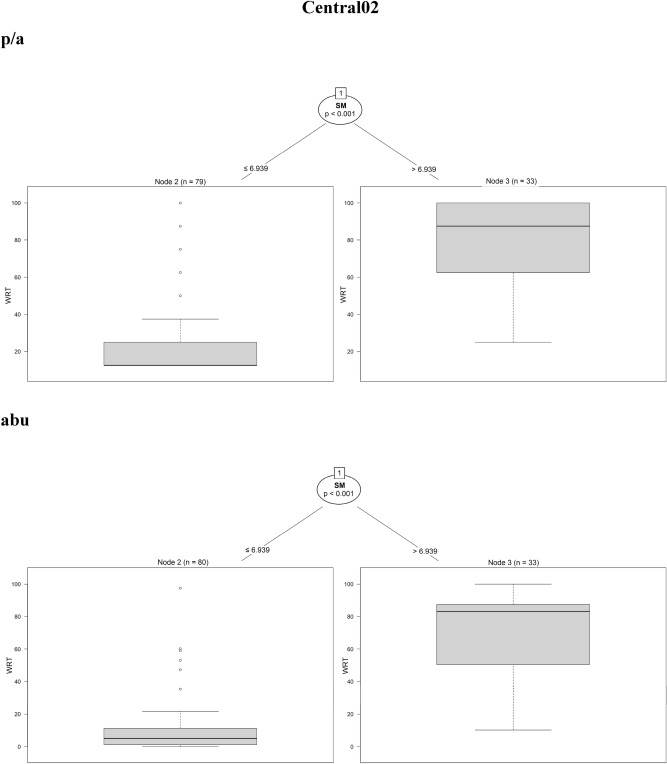
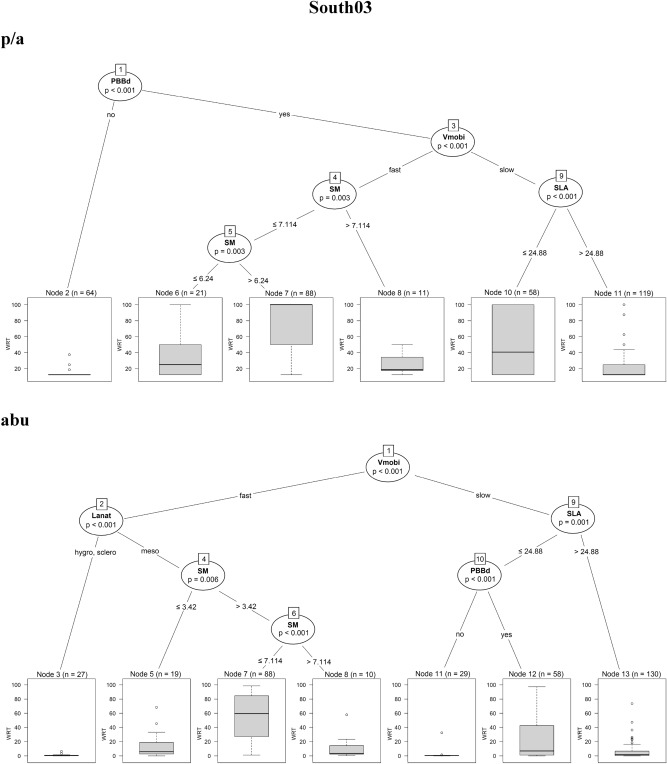
Figure 2.
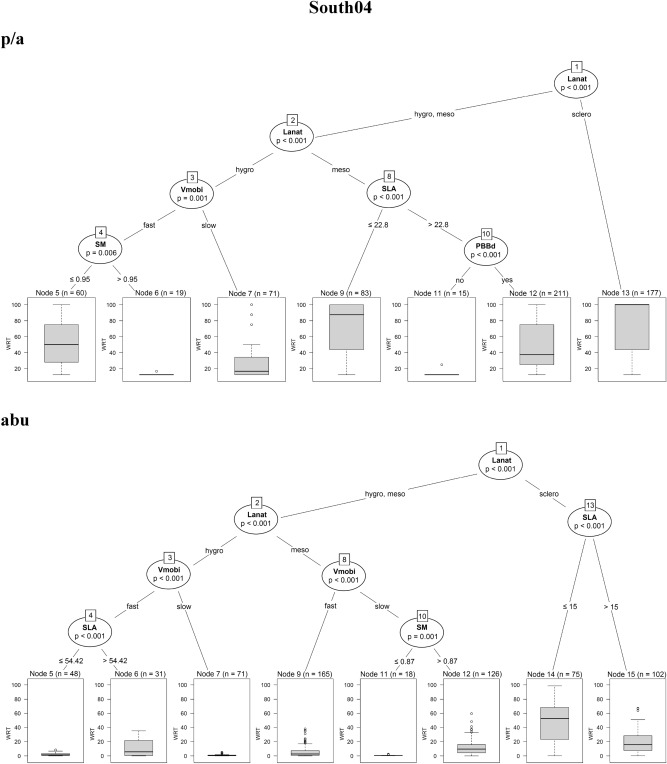
Conditional inference trees for North01, Central02, South03, South04, indicating which plant functional traits are driving species persistence during the time series. Results coming from presence/absence (p/a) and abundance data (abu) are compared. In the split of the trees, all species traits are tested and the trait that best discriminates between homogeneous groups is selected. The response variable (summarised as boxplots) is the weighted residence time (expressed as WRTp/a and WRTabu) along the 8 diachronic observations within the time series of 12 years. The split of the tree is described by the trait used at the split, as well as the permutation-based significance of the split (p-value) and the trait values at which the split occurs. Lanat leaf anatomy, Helo helomorphic leaves, hygro hygromorphic leaves, Meso mesomorphic leaves, Sclero scleromorphic leaves, SM seed mass, SLA specific leaf area, Vmobi vegetative mobility, PBBd perennial bud bank belowground.
In Central02, both methods showed that the only trait driving species persistence was seed mass (Fig. 2b). The most persistent species were those characterised by higher seed mass (> 6.9 mg).
In South03, species persistence was mainly correlated with clonal and bud-bank traits (perennial belowground bud bank and vegetative mobility) (Fig. 2c). In detail, when using presence/absence data, species lacking perennial belowground bud bank did not persist. Species with perennial belowground bud bank, fast vegetative mobility and a seed mass 6.2 < × ≤ 7.1 mg were solidly persistent. In case of slow vegetative mobility, only species with lower SLA (≤ 24.9 mm2.mg−1) were moderately persistent. When using abundance data, species with fast vegetative mobility, mesomorphic leaves and a seed mass 3.4 < × ≤ 7.1 mg were solidly persistent.
In South04, the traits driving species persistence were mainly leaf anatomy, specific leaf area and vegetative mobility by using both presence/absence and abundance data (Fig. 2d). In case of presence/absence data, species characterised by scleromorphic leaves or mesomorphic leaves with lower SLA (≤ 22.8 mm2.mg−1) were solidly persistent, while, species with hygromorphic leaves, fast vegetative mobility, and a very low seed mass (≤ 0.95 mg) were moderately persistent as well. In case of using species abundances, species characterised by scleromorphic leaves and lower SLA (≤ 15.0 mm2.mg−1) were solidly persistent.
Discussion
Our study suggests that fine-scale species persistence is correlated with different plant functional traits in different local ecological contexts. Substantial differences in functional traits driving fine-scale patterns of species persistence were mainly observed between the two Northern and the two Southern sites, which showed different resource use and reproductive strategies of persistent species. We suggest that these differences might appear to be dependent on local trait adaptation rather than plant phylogenetic history.
The different ecological contexts (i.e. climate, soil, and topography; see Table 1) might be underpinning the differences in species composition among the studied sites. Overall, our dataset included 87 species, only one of which (Cardamine bulbifera) occurred in all four sites, and only seven occurred in three out of four sites (e.g. Galium odoratum, Lactuca muralis, Sanicula europaea). The vast majority of species (65%) belonged exclusively to one of the sites. Since we used trait values from the literature (i.e. fixed attributes for species), the compositional differences among sites are mirrored by differences in trait abundance distribution.
Table 1.
The main abiotic features of the studied beech sites.
| Site | Coordinates | Elevation. (m) | Slope (%) | Aspect | Bedrock and soil type | Bare soil and rocky outcrops (%) | Stand age (yrs) | Mean annual P (mm year−1) | Mean annual T (° C) | Summer P–PET ratio (June–July–August) |
|---|---|---|---|---|---|---|---|---|---|---|
| North01 | + 460326N + 120156E | 1100 | 10 | NW | Limestone–Haplic Luvisol | 1 | 135 | 1900 | 5 | 0.91 |
| Central02 | + 415051N + 133523E | 1500 | 30 | SW | Limestone–Aluandic Andosol | 1.8 | 125 | 1300 | 10 | 0.52 |
| South03 | + 402558N + 152610E | 1175 | 30 | W | Limestone–Eutrosilic Andosol | 4.2 | 115 | 1250 | 10 | 0.27 |
| South04 | + 382538N + 161047E | 1100 | 20 | NE | Granites–Haplic Umbrisol | 2.5 | 125 | 1500 | 10 | 0.19 |
Our study highlighted that each of the selected traits related to the challenges to plant persistence was important for the fine-scale species persistence observed in at least one site. Higher values of seed mass were correlated with species persistence mainly in the Northern sites (North01 and Central02). This trait played a secondary role in South03 and a marginal role in South04. Therefore, the hypothesis H1 about the general importance of this trait has been rejected. Graae & Sunde9 and Aubin et al.27 found that seed mass is a fundamental trait for the understory layer, with species characterised by heavy seeds being more abundant in ancient forests. Several studies demonstrated that heavy seeds: (i) enable seedlings to tolerate shade by providing a more significant initial energy reserve, and (ii) provide seedlings with the increased height relative to small-seeded species, which can be of an advantage for germination below litter35,36. Our results confirmed these findings mainly for the stands less impacted by summer drought. In summary, seed mass played a crucial role in Central02 (as indicated by both presence/absence and abundance data), where the particular light regime due to the highest density and lowest height of trees (Table 2) can favour species with heavy seeds. Corroborating this pattern, Central02 showed the lowest values of understory species cover and density37.
Table 2.
The main stand structural features of the studied beech sites. Data from Fabbio et al.76.
| Site | Tree density (n ha−1) | Tree species (No.) | Mean dbh (cm) | Basal area (m2) | Mean height (m) | Top height (m) | Canopy depth (m) | LAI (m2/m2) | Leaf litter (Mg ha−1) |
|---|---|---|---|---|---|---|---|---|---|
| North01 | 345 | 1 | 35.8 | 34.64 | 23.90 | 25.20 | 7.40 | 5.25 | 2.318 |
| Central02 | 899 | 1 | 23.7 | 40.09 | 19.50 | 24.60 | 9.30 | 4.67 | 2.969 |
| South03 | 228 | 2 | 51.5 | 47.57 | 26.80 | 28.00 | 14.10 | n.a | 2.295 |
| South04 | 333 | 2 | 39.1 | 39.90 | 24.10 | 28.60 | 14.10 | 4.36 | 4.644 |
Leaf traits demonstrated to be correlated with species persistence mainly at both extremes of the climatic gradient. This pattern is confirmed by both presence/absence and abundance data. Interestingly, in both sites, leaf anatomy seemed to be an essential trait in determining species persistence at a fine scale, but with opposite attributes according to the climatic context. This North vs South pattern in leaf anatomy (i.e. helomorphic vs scleromorphic leaves, respectively) seems to be consistent with our findings of higher SLA of persistent species in the northernmost site and a lower SLA of persistent species in South03. These results confirm H2 addressing the importance of lower SLA and scleromorphic leaves in the southern sites characterised by stronger summer drought. The latter leaf traits are related to water-use strategy and photosynthetic performance22. As reported by Wellstein et al.29, lower levels of SLA enhance water use efficiency in arid environments, as shown for the Mediterranean vegetation38,39. On the contrary, higher levels of SLA and leaf water content led to higher relative growth rates. The significance of such traits in determining species persistence in forest ecosystems was also confirmed by other studies based on more extended time (20 to 50 years40,41) or a long chronosequence (90 years14).
Clonal and bud-bank traits were correlated with species persistence at fine scale mainly in the Southern stands characterised by stronger summer drought (South03, South04), confirming the H3 that clonal and bud-bank traits are crucial for species persistence in forests more impacted by summer drought. Clonality increases fine-scale spatial resource acquisition, assisting plants to persist under stressful conditions15,33,42. Further, having a perennial bud-bank is related to the capacity of regeneration after disturbance43. In fact, they are mainly important in determining species persistence at a fine scale in South03 which is characterised by a combination of steep slopes and rocky soil (Table 1). This site is embedded in pasture-dominated landscape41 where the potential impact of grazing can likely affect the forest understory vegetation. Several studies found clonal and bud-bank traits to be important in disturbed forests, that is, post-harvested and grazed stands34,41, but also in more water stress-prone mature forest stands characterised by steep slopes with diminished soil depth, fertility and moisture14,15. In support of our findings, Campetella et al.14 found a strong correlation between stand slope and the occurrence of a large and perennial bud bank.
Interestingly, the most important traits determining fine-scale species persistence were generally consistent when using presence/absence and abundance data (i.e. Leaf anatomy in North01 and South04; Seed mass in Central02). This result reveals a similar trait-driven persistence behaviour of both dominant and subordinate species. Only in South03 the most important trait correlated with species persistence changes according to the used approach: with presence/absence data, perennial bud bank belowground was hierarchically first and vegetative mobility second. On the contrary, with abundance data, vegetative mobility was hierarchically first and perennial bud bank belowground third. However, these traits reflect the same plant strategy: being clonal and having a bud bank, provides plants with an effective strategy to cope with disturbances and changing environments44.
Some differences were detected between the two approaches (i.e. using presence/absence or abundance data) in terms of structure of the regression trees, number of significant traits, and their relative importance. Particularly, the differences appear in sites characterised by higher values of understory species cover and density37. On the contrary, they were perfectly consistent in Central02, which is featured by the lowest species cover and density37. This clearly reflects the sensitivity of the approach with respect to the community structure. The abundance method properly accounts for aboveground species dominance, thus recognizing the proportionally greater effect of dominant species on the ecosystem properties with respect to subordinate species45. However, the combined use of both methods can reveal different persistence strategies of dominant vs subordinate species in communities with low values of evenness.
Finally, we suggest that the same species abundance value can be reached through different strategies of space occupation (e.g. single and not interconnected ramets, dense tussocks, interconnected ramets with above- or belowground connections). In some species, these strategies can change over time as an adaptive response to environmental conditions46. This aspect can be extremely relevant for plant persistence at fine scale and should deserve particular attention47.
Our study, using permanent monitoring plots in four old-growth beech forest stands, demonstrated how fine-scale species persistence is correlated with different functional strategies, depending on local ecological factors. Local functional strategies were found not to be related with phylogenetic history, but seem to be dependent on trait adaptation. It suggests that, in these old-growth forests characterised by relatively stable ecological conditions (e.g. forest structure and canopy closure), understory dynamics are primarily governed by the fit of species to their abiotic environment (ecological filter)48. The maintenance of ecological continuity is crucial for the persistence, and consequently, for the conservation, of specialist species founding niche in the understory of old-growth forests.
Persistent species in stands characterised by less marked summer water stress showed an acquisitive resource use strategy (helomorphic leaves with high SLA) and higher seed mass. In contrast, in the old-growth beech forests with potential summer soil water depletion, persistent species seemed to adopt a conservative resource-use strategy (scleromorphic leaves with low SLA), supported by clonal and resprouting abilities. We assessed the species persistence through survival of the aboveground organs. Naturally, the disappearance of aboveground organs does not mean the disappearance of a plant individual as it may survive the form of belowground organs. In addition, we acknowledge that the patterns reported in the present study do not account for intraspecific trait variation. Future research should (a) explore the belowground dimension of plant persistence, hence of the persistence of belowground organs, (b) assess the interactions between plant persistence and resident microbial community, and (c) incorporation of intraspecific trait variability into the notion of persistence since the trait variability may play a fundamental role in plant community responses to the environment49.
Materials and methods
Study area
We selected four sites of the Italian forest ecosystem monitoring network (CONECOFOR50) located in old-growth (> 100 years old) beech forests, spanning a latitude of 46°03′ N–38°25′ N, with mean annual precipitation of 1900–1250 mm. The sites are located in the following Italian political regions: Veneto (North01), Abruzzo (Central02), Campania (South03), and Calabria (South04) (Fig. 1; Table 1). Fifty years51 of monthly temperature and precipitation data52 indicate that the North01 and Central02 sites do not experience aridity in summer, while the Southern sites (South03 and South04) are exposed to summer drought (Fig. 1). Analysis of the ratio between Precipitation and Potential Evapotranspiration (P/PET) in the summer months53 (June- to August) indicates that the North01 site experiences a slight potential summer drought (0.92), while the Southern sites (South03 and South04) show marked potential summer drought (0.27 and 0.19, respectively; Table 1). According to the beech distribution54, South04 exemplifies one of the southernmost beech forests in Europe.
Sampling design
Within each 50 × 50 m site, we established a systematic grid of non-contiguous permanent sampling units (0.50 m × 0.50 m each; Appendix S1), separated by at least 5 m. This minimum distance assured that spatial autocorrelation would be rendered non-significant (see Appendix S2). In each sampling unit, presence/absence and abundance (species cover values, %) of the herb layer vascular plant species were recorded over twelve years (1999–2011), during eight surveys (1999, 2000, 2001, 2002, 2005, 2006, 2008, and 2011; Appendix S3). The data collection protocol followed the ICP Forests Manual on Ground Vegetation Assessment55 in order to ensure dataset commensurability56. The sampling was always performed during the optimal growing period in the pertinent regions.
Plant functional traits
Among the different traits related to species persistence22, we selected (i) not correlated traits, and (ii) traits with available attributes to cover our list of species (see Appendix S4 for details). This selection includes five above- and belowground plant traits (Table 3), such as (1) seed mass, pertinent to space/time dispersal ability and seedling establishment; (2) perennial belowground bud bank, reflecting resprouting ability after disturbance; (3) specific leaf area (SLA), related to water use strategy and photosynthetic capacity), (4) leaf anatomy27, related to leaf water and nutrient conservation and prevention of mechanical damage31; and, (5) vegetative mobility, pertinent to fine-scale spatial resource acquisition11,32,33. The traits mentioned above are likely to be useful for predicting plant responses to disturbance and a broad range of environmental factors22. Traits values were obtained from existing database and literature sources14,30,57–61 and partly from our field measurements using international standardised sampling measurement protocols62. Information was available for 96% of the studied species. We did not consider intraspecific trait variability and used fixed trait values for each species.
Table 3.
Summary of the selected plant functional traits. For detailed on the trait selection process see Appendix S4.
| Plant trait | Description | Data type | Data source |
|---|---|---|---|
| Seed mass | Oven-dry mass of an average seed of a species | Quantitative | 14,58,59 |
| Perennial bud bank (belowground) | Bud bearing organs persisting for 2 or more years are classified as perennial | Binary | 14,57 |
| Specific leaf area | One sided area of a fresh leaf divided by its oven-dry mass | Quantitative | 14,60,61 |
| Vegetative mobility | Lateral spread per year | Categorical | 14,57 |
| Leaf anatomy | Leaf structure according to water content and gas exchange | Categorical | 30 |
Data analyses
Differences in species composition and trait abundance among the sites
To ascertain whether species composition and trait abundance significantly vary between the four selected sites at the start of the sampling period, we tested for the significance of compositional change with a permutational analysis of variance (PERMANOVA) based on the Bray–Curtis dissimilarity index63. The PERMANOVA test performs a permutational test (999 permutations) using distance matrices of the species and traits abundance composition, to find significant differences between sites64.
Relationship between functional and phylogenetic patterns
To assess if the trait patterns depended solely on local trait adaptation or if potential evolutionary signal reflected by phylogenetic patterns could be detected, we compared functional and phylogenetic diversity at plot level in each site, using the following approach:
The evolutionary history of the species in our dataset was inferred using the Neighbor-Joining method65. The evolutionary distances were computed using the Maximum Composite Likelihood method66 and are in the units of the number of base substitutions per site. This analysis involved 62 nucleotide sequences. All ambiguous positions were removed for each sequence pair (pairwise deletion option). There were a total of 2145 positions in the final dataset. Evolutionary analyses were conducted in MEGA X67. In case of missing species we used similar species of the same genus. (see Appendix S5 for the phylogenetic tree).
We then calculated both functional (FD) and phylogenetic diversity (PD) for each 0.5 × 0.5 m plot in each site for the first year (i.e. 1999) by using the mean pairwise trait distances based on Gower distance68. Specifically, FD has been calculated using a set of numerical and categorical traits listed in the manuscript.
The FD and PD scores at plot level were correlated in each site (through Spearman's rho correlations) in order to explore if there is a relationship between the two diversity indices.
Species persistence assessment
Different methods have been proposed to assess species persistence, including those based on immigration/extinction rates or other indirect approaches (for instance involving differences with respect to randomly generated patterns)5. We evaluated species persistence starting from the concept of local aboveground survival (i.e. recording directly whether a species remains present aboveground during specific years or a specified interval of observation). We introduced a new index, based on the estimation of the weighted residence time, or mean residence time, that is, the mean period in which a species is observed in the same sampling unit over the surveyed time. We calculated the weighted residence time in each sampling unit of each site using the following formula:
where WRT is the persistence as weighted residence time, Pim is the sum of occurrences of species i in sampling unit m over the surveyed period, Tim are the non-consecutive occurrences of species i in sampling unit m over the surveyed period, and Z is the total number of surveys. Persistence values can be based on both species presence/absence and abundance data (see Table 4 for details).
Table 4.
Concepts for species persistence estimation with the proposed “Weighted residence time” (WRT) method for different species (spn) over time (tn), by using both presence/absence (p/a) and abundance data (abu). To obtain WRT with abundance data, we weighted WRT(p/a) with respect to the mean relative species cover (Rel.cov.) over time (i.e. WRT(abu) = WRT(p/a)*Rel.cov.).
| t1 | t2 | t3 | t4 | t5 | t6 | t7 | t8 | WRT(p/a) | Rel.cov | WRT(abu) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| sp1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 12.5 | 0.5 | 6.25 |
| sp2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 25 | 0.6 | 15 |
| sp3 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 18.6 | 0.3 | 5.58 |
| sp4 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 25 | 0.4 | 10 |
| sp5 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 100 | 0.7 | 70 |
| sp6 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 87.5 | 0.8 | 70 |
| sp7 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 31.3 | 0.5 | 18.7 |
Species persistence related to plant functional traits
To evaluate whether specific traits are correlated with the patterns of species persistence at a fine scale in all the successive periods (i.e. 1999‒2000; 2000‒2001; 2001‒2002, etc.), we constructed conditional inference trees (CITs) based on the relationship between WRT (both with presence/absence and abundance data) of species estimated within each of the 100 quadrates in the grid, and the list of selected traits. A conditional inference tree (also known as classification and regression tree, CART69,70) estimates whether the selected traits determine species partitioning into homogeneous groups concerning changes in species occurrence between the sampling events40. The algorithm selects the input variable with the strongest association with the response, and it stops if the null hypothesis of independence between the input variables and the response cannot be rejected. We used CITs because they (a) account for nonlinear hierarchical relationships, (b) treat categorical, ordinal and quantitative data simultaneously, and (c) deal with missing values69,71. In each split of the tree, all species traits are tested, and the trait that best discriminates between homogeneous groups of species is selected.
All statistical analyses were performed in the R environment, on the incidence matrix with species presence/absence and abundance data. In particular, the following R packages were used: package vegan72 (function adonis) for PERMANOVA, package party (function ctree) for regression trees73, package picante74 for the calculation of FD and PD, package FD75 for the Gower distance matrices of traits.
Supplementary information
Acknowledgements
This work was partially supported by the Thünen Institute (Hamburg) and the Italian Focal Centre of ICP Forests (CUFA, Comando per la Tutela della Biodiversità e dei Parchi—Ufficio Studi e Progetti, Roma). S.B. was supported by the GINOP-2.3.2-15-2016-00019 project. We would like to thank Sheila Beatty for editing the English usage in the manuscript. L.M. acknowledges the logistic support of the Iluka Chair in Vegetation Science and Biogeography at the Murdoch University.
Author contributions
G.C. and R.C. conceived the idea and the sampling design; G.C., S.C. and E.S. and wrote the first version of the manuscript; E.S. and D.G. performed the analysis; C.D. assessed the phylogenetic patterns in order to address an issue raised by a referee; S.B., C.W., N.P., L.M., M.C. edited the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-76289-7.
References
- 1.Watt AS. Pattern and process in plant community. J. Ecol. 1947;35:1–22. doi: 10.2307/2256497. [DOI] [Google Scholar]
- 2.Ozinga W, et al. Local above-ground persistence of vascular plants: Life-history trade-offs and environmental constraints. J. Veg. Sci. 2007;18:489–497. doi: 10.1111/j.1654-1103.2007.tb02563.x. [DOI] [Google Scholar]
- 3.Økland RH, Eilertsen O. Dynamics of understory vegetation in an old-growth boreal coniferous forest, 1988–1993. J. Veg. Sci. 1996;7:747–762. doi: 10.2307/3236386. [DOI] [Google Scholar]
- 4.Nygaard PH, Ødegaard T. Sixty years of vegetation dynamics in a south boreal coniferous forest in southern Norway. J. Veg. Sci. 1999;10:5–16. doi: 10.2307/3237155. [DOI] [Google Scholar]
- 5.Palmer MW, Rusch GM. How fast is the carousel? Direct indices of species mobility with examples from an Oklahoma grassland. J. Veg. Sci. 2001;12:305–318. doi: 10.2307/3236844. [DOI] [Google Scholar]
- 6.Zobel M, Moora M, Herben T. Clonal mobility and its implications for spatio-temporal patterns of plant communities: What do we need to know next? Oikos. 2010;119:802–806. doi: 10.1111/j.1600-0706.2010.18296.x. [DOI] [Google Scholar]
- 7.Chaideftou E, Kallimanis AS, Bergmeier E, Dimopoulos P. How does plant species composition change from year to year? A case study from the herbaceous layer of a submediterranean oak woodland. Comm. Ecol. 2012;13:88–96. doi: 10.1556/ComEc.13.2012.1.11. [DOI] [Google Scholar]
- 8.Chapman JI, McEwan RW. Spatiotemporal dynamics of α-and β-diversity across topographic gradients in the herbaceous layer of an old-growth deciduous forest. Oikos. 2013;122:1679–1686. doi: 10.1111/j.1600-0706.2013.00544.x. [DOI] [Google Scholar]
- 9.Graae BJ, Sunde PB. The impact of forest continuity and management on forest floor vegetation evaluated by species traits. Ecography. 2000;23:720–730. doi: 10.1111/j.1600-0587.2000.tb00315.x. [DOI] [Google Scholar]
- 10.Bakker JP, Olff H, Willems JH, Zobel M. Why do we need permanent plots in the study of long-term vegetation dynamics? J. Veg. Sci. 1996;7:147–156. doi: 10.2307/3236314. [DOI] [Google Scholar]
- 11.Van der Maarel E. Pattern and process in the plant community: fifty years after A.S. Watt. J. Veg. Sci. 1996;7:19–28. doi: 10.2307/3236412. [DOI] [Google Scholar]
- 12.Herben T, Krahulec F, Hadincová V, Skálová H. Small-scale variability as a mechanism for large-scale stability in mountain grasslands. J. Veg. Sci. 1993;4:163–170. doi: 10.2307/3236101. [DOI] [Google Scholar]
- 13.Økland RH. Persistence of vascular plants in a Norwegian boreal coniferous forest. Ecography. 1995;18:3–14. doi: 10.1111/j.1600-0587.1995.tb00114.x. [DOI] [Google Scholar]
- 14.Campetella G, Botta-Dukát Z, Wellstein C, Canullo R, Gatto S, Chelli S, Mucina L, Bartha S. Patterns of plant trait-environment relationship along a forest succession chronosequence. Agric. Ecosyst. Environ. 2011;145:38–48. doi: 10.1016/j.agee.2011.06.025. [DOI] [Google Scholar]
- 15.Canullo R, Campetella G, Mucina L, Chelli S, Wellstein C, Bartha S. Patterns of clonal growth modes along a chronosequence of post-coppice forest regeneration in beech forest of Central Italy. Fol. Geobot. 2011;46:271–288. doi: 10.1007/s12224-010-9087-0. [DOI] [Google Scholar]
- 16.Rūsiņa S, Gavrilova I, Roze I, Šulcs V. Temporal species turnover and plant community changes across different habitats in the lake Engure nature park Latvia. Proc. Latv. Acad. Sci. Sect. B. Nat. Exact Appl. Sci. 2014;68:68–79. [Google Scholar]
- 17.Norden B, Appelqvist T. Conceptual problems of ecological continuity and its bioindicators. Biodivers. Conserv. 2001;10:779–791. doi: 10.1023/A:1016675103935. [DOI] [Google Scholar]
- 18.Bartha S, Canullo R, Chelli S, Campetella G. Unimodal relationships of understory alpha and beta diversity along chronosequence in coppiced and unmanaged beech forests. Diversity. 2020;12:101. doi: 10.3390/d12030101. [DOI] [Google Scholar]
- 19.Gilliam FS. The ecological significance of the herbaceous layer in temperate forest ecosystems. Bioscience. 2007;57:845–857. doi: 10.1641/B571007. [DOI] [Google Scholar]
- 20.Campetella G, et al. Scale dependent effects of coppicing on the species pool of late successional beech forest in the Central Apennines (Italy) Appl. Veg. Sci. 2016;19:474–485. doi: 10.1111/avsc.12235. [DOI] [Google Scholar]
- 21.Lavorel S, Garnier E. Predicting changes in community composition and ecosystem functioning from plant traits: Revisiting the Holy Grail. Funct. Ecol. 2002;16:545–556. doi: 10.1046/j.1365-2435.2002.00664.x. [DOI] [Google Scholar]
- 22.Weiher E, van der Werf A, Thompson K, Roderick M, Garnier E, Eriksson O. Challenging theophrastus: A common core list of plant traits for functional ecology. J. Veg. Sci. 1999;10:609–620. doi: 10.2307/3237076. [DOI] [Google Scholar]
- 23.Westoby M. A Leaf-height-seed (LHS) plant ecology strategy scheme. Plant Soil. 1998;199:213–227. doi: 10.1023/A:1004327224729. [DOI] [Google Scholar]
- 24.Wright IJ, et al. The worldwide leaf economic spectrum. Nature. 2004;428:821–827. doi: 10.1038/nature02403. [DOI] [PubMed] [Google Scholar]
- 25.Klimešová J, Martínková J, Ottaviani G. Belowground plant functional ecology: Towards an integrated perspective. Funct. Ecol. 2018;32:2115–2126. doi: 10.1111/1365-2435.13145. [DOI] [Google Scholar]
- 26.de Bello F, Berg MP, Dias AT, Diniz-Filho JAF, Götzenberger L, Hortal J, Ladle RJ, Lepš J. On the need for phylogenetic ‘corrections’ in functional trait-based approaches. Fol. Geobot. 2015;50:349–357. doi: 10.1007/s12224-015-9228-6. [DOI] [Google Scholar]
- 27.Aubin I, Messier C, Bouchard A. Can plantations develop understory biological and physical attributes of naturally regenerated forests? Biol. Conserv. 2008;141:2462–2476. doi: 10.1016/j.biocon.2008.07.007. [DOI] [Google Scholar]
- 28.Dahlgren JP, Eriksson O, Bolmgren K, Strindell M, Ehrlén J. Specific leaf area as a superior predictor of changes in field layer abundance during forest succession. J. Veg. Sci. 2006;17:577–582. doi: 10.1111/j.1654-1103.2006.tb02481.x. [DOI] [Google Scholar]
- 29.Wellstein C, et al. Effects of extreme drought on specific leaf area of grassland species: A meta-analysis of experimental studies in temperate and sub-Mediterranean systems. Glob. Change Biol. 2017;23:2473–2481. doi: 10.1111/gcb.13662. [DOI] [PubMed] [Google Scholar]
- 30.Lindacher R, Böcker R, Bemmerlein-Lux FA, Kleemann A, Haas S. PHANART Datenbank der Gefäßpflanzen Mitteleuropas, Erklärung der Kennzahlen, Aufbau und Inhalt. Veröff. Geobot. Inst. ETH, Stift. Rübel. 1995;125:1–436. [Google Scholar]
- 31.Turner IM. Sclerophylly: Primarily protective? Funct. Ecol. 1994;8:669–675. doi: 10.2307/2390225. [DOI] [Google Scholar]
- 32.Van Groenendael JM, Klimeš L, Klimešová J, Hendriks RJJ. Comparative ecology of clonal plants. Philos. Trans. Roy. Soc. B. 1996;351:1331–1339. doi: 10.1098/rstb.1996.0116. [DOI] [Google Scholar]
- 33.Sammul M, Kull K, Niitla T, Mols T. A comparison of plant communities on the basis of their clonal growth patterns. Evol. Ecol. 2004;18:443–467. doi: 10.1007/s10682-004-5139-3. [DOI] [Google Scholar]
- 34.Canullo R, Simonetti E, Cervellini M, Chelli S, Bartha S, Wellstein C, Campetella G. Unravelling mechanisms of short-term vegetation dynamics in complex coppice forest systems. Fol. Geobot. 2017;52:71–81. doi: 10.1007/s12224-016-9264-x. [DOI] [Google Scholar]
- 35.Kidson R, Westoby M. Seed mass and seedling dimensions in relation to seedling establishment. Oecologia. 2000;125:11–17. doi: 10.1007/PL00008882. [DOI] [PubMed] [Google Scholar]
- 36.Moles AT, Westoby M. Seed size and plant strategy across the whole life cycle. Oikos. 2006;113:91–105. doi: 10.1111/j.0030-1299.2006.14194.x. [DOI] [Google Scholar]
- 37.Campetella G, Canullo R, Allegrini MC. Status and changes of ground vegetation at the CONECOFOR plots, 1999–2005. Ann. Silvicult. Res. 2008;34:29–48. [Google Scholar]
- 38.Wright IJ, Reich PB, Westoby M. Strategy shifts in leaf physiology, structure and nutrient content between species of high- and low-rainfall and high- and low-nutrient habitats. Funct. Ecol. 2001;15:423–434. doi: 10.1046/j.0269-8463.2001.00542.x. [DOI] [Google Scholar]
- 39.Ackerly DD. Functional traits of chaparral shrubs in relation to seasonal water deficit and disturbance. Ecol. Monogr. 2004;74:25–44. doi: 10.1890/03-4022. [DOI] [Google Scholar]
- 40.Kopecký M, Hédl R, Szabó P. Non-random extinctions dominate plant community changes in abandoned coppices. J. Appl. Ecol. 2013;50:79–87. doi: 10.1111/1365-2664.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naaf T, Wulf M. Traits of winner and loser species indicate drivers of herb layer changes over two decades in forests of NW Germany. J. Veg. Sci. 2011;22:516–527. doi: 10.1111/j.1654-1103.2011.01267.x. [DOI] [Google Scholar]
- 42.Ottaviani G, Martínková J, Herben T, Pausas JG, Klimešová J. On plant modularity traits: Functions and challenges. Trends Plant Sci. 2017;22:648–651. doi: 10.1016/j.tplants.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 43.Klimešová J, Klimeš L. Bud banks and their role in vegetative regeneration—A literature review and proposal for simple classification and assessment. Perspect. Plant Ecol. Evol. Syst. 2007;8:115–129. doi: 10.1016/j.ppees.2006.10.002. [DOI] [Google Scholar]
- 44.Chelli S, et al. Climate is the main driver of clonal and bud bank traits in Italian forest understories. Persp. Plant Ecol. Evol. Syst. 2019;40:125478. doi: 10.1016/j.ppees.2019.125478. [DOI] [Google Scholar]
- 45.Grime JP. Benefits of plant diversity to ecosystems: Immediate, filter and founder effects. J. Ecol. 1998;86:902–910. doi: 10.1046/j.1365-2745.1998.00306.x. [DOI] [Google Scholar]
- 46.Alpert P, Simms EL. The relative advantages of plasticity and fixity in different environments: When is it good for a plant to adjust? Evol. Ecol. 2002;16:285–297. doi: 10.1023/A:1019684612767. [DOI] [Google Scholar]
- 47.Denney DA, Jameel MI, Bemmels JB, Rochford ME, Anderson JT. Small spaces, big impacts: Contributions of micro-environmental variation to population persistence under climate change. AoB Plants. 2020;12:5. doi: 10.1093/aobpla/plaa005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swenson NG, et al. Temporal turnover in the composition of tropical tree communities: Functional determinism and phylogenetic stochasticity. Ecology. 2012;93:490–499. doi: 10.1890/11-1180.1. [DOI] [PubMed] [Google Scholar]
- 49.Kichenin E, Wardle DA, Peltzer DA, Morse CW, Freschet GT. Contrasting effects of plant inter-and intraspecific variation on community-level trait measures along an environmental gradient. Funct. Ecol. 2013;27:1254–1261. doi: 10.1111/1365-2435.12116. [DOI] [Google Scholar]
- 50.Petriccione B, Pompei E. The CONECOFOR programme: general presentation, aims and co-ordination. J. Limnol. 2002;61:3–11. doi: 10.4081/jlimnol.2002.s1.3. [DOI] [Google Scholar]
- 51.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005;25:1965–1978. doi: 10.1002/joc.1276. [DOI] [Google Scholar]
- 52.Bagnouls F, Gaussen H. Les climats biologiques et leur classification. Ann. Geogr. 1957;335:193–220. doi: 10.3406/geo.1957.18273. [DOI] [Google Scholar]
- 53.FAO/UNESCO/WMO. World map of desertification. Food and Agricultural, Organization, Rome (1997).
- 54.EUFORGEN. Distribution map of Beech (Fagus sylvatica), www.euforgen.org (2009).
- 55.Dupouey JL. Manual on methods and criteria for harmonized sampling, assessment, monitoring and analysis of the effects of air pollution on forests Part VIII. Assessment of Ground Vegetation. Hamburg: ICP-Forests; 1998. [Google Scholar]
- 56.Canullo R, Campetella G, Allegrini MC, Smargiassi V. Management of forest vegetation data series: The role of database in the frame of quality assurance procedure. J. Limnol. 2002;61:100–105. doi: 10.4081/jlimnol.2002.s1.100. [DOI] [Google Scholar]
- 57.Klimeš L, Klimešová J, Hendriks R, van Groenendael J. Clonal plant architectures: a comparative analysis of form and function. In: de Kroon H, van Groenendael J, editors. The Ecology and Evolution of Clonal Plants. Leiden: Backhuys Publishers; 1997. pp. 1–29. [Google Scholar]
- 58.Cerabolini B, Ceriani RM, Caccianiga M, De Andreis R, Raimondi B. Seed size, shape and persistence in soil: A test on Italian flora from Alps to Mediterranean coasts. Seed Sci. Res. 2003;13:75–85. doi: 10.1079/SSR2002126. [DOI] [Google Scholar]
- 59.Royal Botanical Gardens Kew. Seed Information Database (SID), Version 7.1. Available from https://data.kew.org/sid/ (2008).
- 60.Kleyer M, et al. The LEDA Traitbase: A database of plant life-history traits of North West European Flora. J. Ecol. 2008;96:1266–1274. doi: 10.1111/j.1365-2745.2008.01430.x. [DOI] [Google Scholar]
- 61.Wellstein C, Kuss P. Diversity and frequency of clonal traits along natural and land-use gradients in grasslands of the Swiss Alps. Fol. Geobot. 2011;46:255–270. doi: 10.1007/s12224-010-9075-4. [DOI] [Google Scholar]
- 62.Pérez-Harguindeguy N, et al. New handbook for standardised measurement of plant functional traits worldwide. Austr. J. Bot. 2013;61:167–234. doi: 10.1071/BT12225. [DOI] [Google Scholar]
- 63.Reinecke J, Klemm G, Heinken T. Vegetation change and homogenization of species composition in temperate nutrient deficient Scots pine forests after 45 yr. J. Veg. Sci. 2014;25:113–121. doi: 10.1111/jvs.12069. [DOI] [Google Scholar]
- 64.Anderson MJ. A new method for non-parametric multivariate analysis of variance. Aust. Ecol. 2001;26:32–46. [Google Scholar]
- 65.Saitou N, Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 66.Tamura K, Nei M, Kumar S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Nat. Acad. Sci. USA. 2004;101:11030–11035. doi: 10.1073/pnas.0404206101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weiher E, Clarke GDP, Keddy PA. Community assembly rules, morphological dispersion, and the coexistence of plant species. Oikos. 1998;81:309. doi: 10.2307/3547051. [DOI] [Google Scholar]
- 69.Breiman L, Friedman JH, Olshen RA, Stone CJ. Classification and regression trees. Belmont: Wadsworth International Group; 1984. [Google Scholar]
- 70.Ryo M, Rillig MC. Statistically reinforced machine learning for nonlinear patterns and variable interactions. Ecosphere. 2017;8(11):e01976. doi: 10.1002/ecs2.1976. [DOI] [Google Scholar]
- 71.De'ath G, Fabricius KE. Classification and regression trees: A powerful yet simple technique for ecological data analysis. Ecology. 2000;81:3178–3192. doi: 10.1890/0012-9658(2000)081[3178:CARTAP]2.0.CO;2. [DOI] [Google Scholar]
- 72.Oksanen, J., Blanchet, F. G., Kindt, R., Legendre, P., Minchin, P.R., O’Hara, R.B., Simpson, G.L., Solymos, P., Stevens, M. H. H. & Wagner, H. Vegan: Community Ecology Package. R package version 2.0–7. (2013) Available at https://CRAN.R-project.org/package=vegan
- 73.Hothorn T, Hornik K, Zeileis A. Unbiased recursive partitioning: A conditional inference framework. J. Comput. Graph. Stat. 2006;15:651–674. doi: 10.1198/106186006X133933. [DOI] [Google Scholar]
- 74.Kembel SW, Cowan PD, Helmus MR, Cornwell WK, Morlon H, Ackerly DD, Blomberg SP, Webb CO. Picante: R tools for integrating phylogenies and ecology. Bioinformatics. 2010;26:1463–1464. doi: 10.1093/bioinformatics/btq166. [DOI] [PubMed] [Google Scholar]
- 75.Laliberté E, Legendre P. A distance-based framework for measuring functional diversity from multiple traits. Ecology. 2010;91:299–305. doi: 10.1890/08-2244.1. [DOI] [PubMed] [Google Scholar]
- 76.Fabbio G, Manetti MC, Bertini G. Aspects of biological diversity in the CONECOFOR plots. I. Structural and species diversity in the tree community. Ann. Silvicul. Res. 2006;30:17–28. [Google Scholar]
- 77.Trabucco, A. & Zomer, R. J. Global aridity index (global-aridity) and global potential evapo-transpiration (global-PET) geospatial database. CGIAR Consortium for Spatial Information (2009).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.