Sir,
D-dimers represent a breakdown product of fibrin formation, and D-dimer testing is a common laboratory procedure in haemostasis laboratories.1 D-dimer testing may be requested in patients as an investigative tool for assessment of venous thromboembolism (VTE), such as deep vein thrombosis (DVT) or pulmonary embolism (PE), typically combined with a pre-test probability score (e.g., Well's score), or else for assessment and potential monitoring of disseminated intravascular coagulation (DIC).1 Of particular relevance to the current report, is that D-dimer testing has found particular utility as a potential prognostic marker for disease severity in coronavirus disease 2019 (COVID-19), which characterises a pandemic produced by severe acute respiratory syndrome virus coronavirus 2 (SARS-CoV-2). At time of writing, COVID-19 comprised over 17 million confirmed cases, causing nearly 700,000 deaths.2 The disease expresses various pathophysiological derangements, including (micro) thrombosis,3, 4, 5 which in turn is associated with various derangements of haemostasis parameters, in particular including D-dimer.6 As noted, D-dimer also potentially serves as a prognostic marker for severe disease and/or mortality.7 Thus, it is anticipated that D-dimer testing will increase substantially as clinicians assess and treat increasing numbers of COVID-19 patients.
Of additional relevance to this correspondence is wide under-recognition of the substantial variation in D-dimer reporting units,8 and thus also the likelihood of misreporting D-dimer data because of poor or incomplete information.9 Although at least 28 potential theoretical combinations of D-dimer units can be identified,8 a summary of the eight most common was recently identified,9 including recognition that different manufacturers of D-dimer reagents report in several different preferential units. Two layers of possible misreporting exist. The first reflects using either D-dimer units (DDU) or fibrinogen equivalent units (FEU), the latter being almost 2× those of DDU. The second is the actual measuring units used: these may be in ng, μg, mg, or g, per mL, L and potentially even μL. This secondary layer creates the possibility of some 1000-fold difference in reporting values,9 which combined with the first layer leads to the possibility of a 2000-fold error in reporting values.9
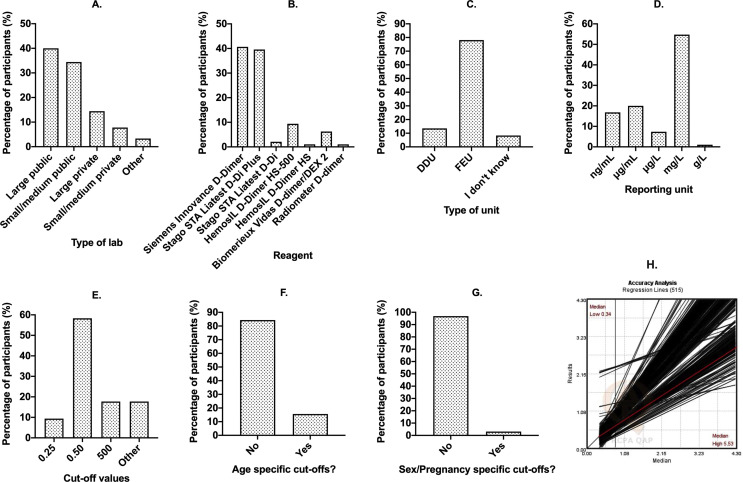
Given the recent assessment showing several errors in D-dimer reporting in the COVID-19 literature,9 and a subsequent call for action by the International Society on Thrombosis and Hemostasis (ISTH) Scientific Standardization Committee (SSC) on Fibrinolysis,10 we thought it worthwhile to investigate current test practice from participants of the Royal College of Pathologists of Australasia Quality Assurance Programs (RCPAQAP). For this purpose, we constructed a simple survey using Survey Monkey that we asked participants to complete voluntarily. The number of D-dimer results being reported to RCPAQAP in 2020 is 515, representing a total of 407 participants (note that many participants report D-dimer values on multiple instruments). After exclusion of duplicate entries, 100 participants (24.6% of active participants) provided answers to the survey questions. Although this is arguably a minor sampling of the total possible pool, it is not unusual for a voluntary survey, and still provides a reasonable snapshot of current status and associated problems. Results are summarised in Fig. 1 . The breakdown of participant laboratory types (as self determined by participants) is shown in Fig. 1A, and indicates respondents as mostly deriving from publicly funded facilities. The breakdown of manufacturer reagents in use by participants (Fig. 1B) indicates two main reagents in use by almost 80% of participants; however, in total, seven different reagents are currently in use. Most participants report in FEU units (Fig. 1C), which also tends to be that recommended by most manufacturers.9 Worryingly, 8% of respondents reported that they did not know whether they reported in FEU or DDU. Most laboratories reported in mg/L (Fig. 1D), which is also the current recommendation from the RCPA.11 However, four other units were alternatively reported, with ng/mL and μg/mL each being reported by 7–20% of participants. Most participants reported use of a normal cut-off value of ‘0.5’ as representing the ‘cross over line’ for abnormal D-dimer values (Fig. 1E). Naturally, these sites were also predominantly reporting in FEU and mg/mL. However, a substantial number of laboratories used either ‘500’ or ‘0.25’ as the cut-off value. Moreover, 15 (15%) participants reported ‘other’ different and quite varied cut-offs (e.g., 0.20, 0.35, 0.40, 0.41, 0.49, 0.51, <0.5, 400, <400). Naturally, responses comprising 0.49, 0.5, 0.51, and <0.5 might represent differing interpretations by participants potentially providing the same ‘answer’ to the question. Very few participants reported age specific D-dimer cut-off values (Fig. 1F) and fewer still reported pregnancy specific D-dimer cut-off values (Fig. 1G).
Fig. 1.
Summary of findings from the 2020 RCPAQAP survey on D-dimer testing. (A–G) Percent of survey respondents (y-axis) giving a specific response as identified on x-axis. (A) Type of laboratory. Other included ‘regional’ and ‘community’. (B) Type of D-dimer reagent. (C) Type of D-dimer unit (DDU, D-dimer unit; FEU, fibrinogen equivalent unit). (D) D-dimer reporting unit. (E) Cut-off value separating normal from abnormal or negative from positive. (F) Use of age specific D-dimer cut-offs? (G) Use of sex/pregnancy specific cut-offs? (H) Linear regression lines for individual participants as reflecting the relationship between their lowest D-dimer to highest D-dimer reported values within the 2019 EQA cycle. There is an extraordinary variation in reported data for D-dimer testing of the same homogeneous samples in different laboratories using different methods.
Experts in the field8, 9, 10 , 12 recognise that all D-dimer assays are not the same; they may use different calibrators, methods, and antibodies. However, this may be under-recognised by laboratories testing and reporting, as well as the clinicians requesting these tests. In one report,12 over 30 different D-dimer assays were identified as being available commercially, and these used more than 20 different kinds of detecting antibodies. Indeed, the sole similarity between methods may be the measuring units, the cut-off value and whether DDU of FEU are employed.9 However, even in the current study of a single external quality assurance provider, we can identify in 2020 the use of seven different reagents (Fig. 1B), at least five different reporting units (Fig. 1D), and at least 10 different cut-off values (Fig. 1E). Despite potential additional utility, age adjusted and pregnancy specific cut-off values are the exception rather than the rule (Fig. 1F,G). Given the above, it is perhaps not surprising that there is great variability in reported D-dimer values between laboratories even when testing the same homogenous sample.9 An example of this variability using data from the RCPAQAP is shown in Fig. 1H.
In conclusion, we show continued variability in D-dimer reporting in 2020. Whilst the standardisation of D-dimer assays may not be truly possible, we would continue to encourage manufacturers to at least standardise D-dimer assays to a common unit of measurement, with the RCPA recommendation of mg/L,11 preferably in FEU, also reflecting our recommendation.
Conflicts of interest and sources of funding
The authors state that there are no conflicts of interest to disclose.
References
- 1.Thachil J., Lippi G., Favaloro E.J. D-dimer testing: laboratory aspects and current issues. Methods Mol Biol. 2017;1646:91–104. doi: 10.1007/978-1-4939-7196-1_7. [DOI] [PubMed] [Google Scholar]
- 2.COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) Global Cases.; Cited 30 Jul 2020. https://www.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 [Google Scholar]
- 3.Schulman S. Coronavirus disease 2019, prothrombotic factors, and venous thromboembolism. Semin Thromb Hemost. 2020;46:772–776. doi: 10.1055/s-0040-1710337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thachil J., Srivastava A. SARS-2 coronavirus associated hemostatic lung abnormality in COVID-19: is it pulmonary thrombosis or pulmonary embolism? Semin Thromb Hemost. 2020;46:777–780. doi: 10.1055/s-0040-1712155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levi M., Thachil J. Coronavirus disease 2019 coagulopathy: disseminated intravascular coagulation and thrombotic microangiopathy—either, neither, or both. Semin Thromb Hemost. 2020;46:781–784. doi: 10.1055/s-0040-1712156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lippi G., Favaloro E.J. D-dimer is associated with severity of coronavirus disease 2019: a pooled analysis. Thromb Haemost. 2020;120:876–878. doi: 10.1055/s-0040-1709650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Favaloro E.J., Lippi G. Recommendations for minimal laboratory testing panels in patients with COVID-19: potential for prognostic monitoring. Semin Thromb Hemost. 2020;46:379–382. doi: 10.1055/s-0040-1709498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lippi G., Tripodi A., Simundic A.M., Favaloro E.J. International survey on D-dimer test reporting: a call for standardization. Semin Thromb Hemost. 2015;41:287–293. doi: 10.1055/s-0035-1549092. [DOI] [PubMed] [Google Scholar]
- 9.Favaloro E.J., Thachil J. Reporting of D-dimer data in COVID-19: some confusion and potential for misinformation. Clin Chem Lab Med. 2020;58:1191–1199. doi: 10.1515/cclm-2020-0573. [DOI] [PubMed] [Google Scholar]
- 10.Thachil C., Longstaff C., Favaloro E.J., Lippi G., Urano T., Kim P.Y. The need for accurate D-dimer reporting in COVID-19: Communication from the ISTH SSC on Fibrinolysis. J Thromb Haemost. 2020;18:2408–2411. doi: 10.1111/jth.14956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Standards for Pathology Informatics in Australia (SPIA). Reporting Terminology and Codes Haematology (v3.0) RCPA; Sydney: 2017. https://www.rcpa.edu.au/getattachment/bb8ce49b-26dd-490f-b544-e35141028080/SPIA-Haematology-Reporting-Terminology-and-Codes.aspx [Google Scholar]
- 12.Longstaff C., Adcock D., Olson J.D., et al. Harmonisation of D-dimer - a call for action. Thromb Res. 2016;137:219–220. doi: 10.1016/j.thromres.2015.11.031. [DOI] [PubMed] [Google Scholar]