Abstract
Coronavirus disease 2019 (COVID-19) has substantially disrupted many processes of care related to emergency cardiac conditions, while there has been no clinical guidance regarding the management of type A aortic dissection. A retrospective multicenter study involving 52 consecutive patients (mean age 52.3, 28.9% women) with type A aortic dissection during COVID-19 pandemic was conducted at tertiary aortic centers in Michigan, Wuhan and Changsha (China). Twenty-four (46.2%) were considered clinically suspicious for COVID-19 based on radiographic lung lesions (70.8%) followed by dyspnea (25.0%), cough (12.5%), and fever (12.5%). Overall, 47 (90.4%) underwent an operation and 5 (9.6%) managed nonoperatively. All suspected patients underwent a reverse-transcriptase–polymerase-chain-reaction at arrival, whereas 82.1% in the nonsuspected (P = 0.054). Among the 24 patients either nonoperatively managed or whose operation was delayed for >24 hours, only 1 (4.2%) died. A total of 3 (6.4%) operated patients had a positive reverse-transcriptase–polymerase-chain-reaction at various timings, including 1 nonsuspected patient preoperatively and 2 with very recent COVID-19 infection. The first patient died of respiratory failure despite uneventful surgical repair and maximal medical management. The postoperative course of both patients with recent COVID-19 was characterized by severe coagulopathy requiring massive transfusions and prolonged ICU stay. However, both survived to hospital discharge. In light of the possible dismal outcomes associated with dual diagnoses of type A aortic dissection/COVID-19 and the higher-than-expected number of asymptomatic carriers, all type A dissection patients should be immediately tested for COVID-19. Surgical interventions in patients recovered from recent COVID-19 may be safe.
Keywords: Acute type A aortic dissection, COVID-19, Acute respiratory distress syndrome
Abbreviations: COVID-19, Coronavirus disease 2019; RT-PCR, reverse-transcriptase–polymerase-chain-reaction; IRB, institutional review board; CTA, computed tomography angiography; COPD, chronic obstructive lung disease; eGFR, estimated glomerular filtration rate; ACP, antegrade cerebral perfusion; RCP, retrograde cerebral perfusion; POD, postoperative day; ICU, intensive care unit; N/A, not applicable
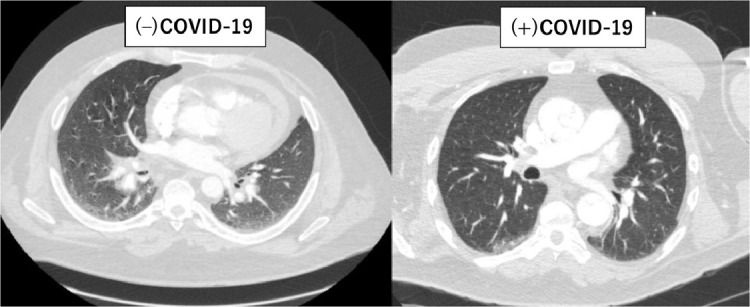
Ground-glass opacity in (–) COVID patient and normal CT in (+) COVID patient at arrival.
Alt-text: Unlabelled box
Central Message.
All type A dissection should be immediately tested for COVID-19. Operating on patients with COVID-19 may be associated with fatal outcomes, while it may be safe in patients recovered from COVID-19.
Alt-text: Unlabelled box
Perspective Statement.
In light of the continued pandemic, possible upcoming second surge and the higher-than-expected number of asymptomatic carriers, all acute type A dissection patients should be immediately tested for COVID-19. Surgical intervention in patients with dual diagnoses of type A aortic dissection/COVID-19 may be associated with fatal outcomes, while it may be safe in patients recovered from recent COVID-19.
Alt-text: Unlabelled box
INTRODUCTION
On December 31, 2019, a novel corona virus (SARS-CoV-2) disease (COVID-19) was first reported in Wuhan City, Hubei Province, China and it has rapidly spread worldwide.1 On January 19, 2020, the first case of COVID-19 was confirmed in Snohomish Country, Washington, United States.2 As of May 27, 2020, approximately 5,500,000 cases and 350,000 deaths related to COVID-19 have been reported in > 200 countries/territories.3 Healthcare infrastructures and resources, particularly as it relate to the care of the most critically ill patients, remains strained globally. In this context, the American College of Surgeons and the Centers for Medicare & Medicaid Services released recommendations for the management of surgical procedures,4 , 5 suggesting delaying all elective procedures without high acuity in order to focus on more urgent cases, preserve valuable resources and prevent the spread of COVID-19 among healthcare providers, patient families and the public.
Acute type A aortic dissection is considered as one of the most lethal conditions, requiring urgent surgical intervention. In contrast, there is a universal concern regarding compromised surgical outcomes in the setting of substantially affected cardiac surgery care. Furthermore, the optimal management of patients with dual diagnoses of type A aortic dissection and COVID-19 is unknown. This study was undertaken to review and share our experience with type A aortic dissection at major aortic centers located in significantly affected geographic regions by COVID-19 in the United States and China in order to ultimately help guide the management of type A aortic dissection during this world-wide outbreak, which is expected to persist for the foreseeable future. Supplemental Video 1 summarizing the present study is available.
METHODS
Study Design and Patients
This is a retrospective multicenter study conducted at University of Michigan, Tongji Hospital, and the Second Xiangya Hospital of Central South University Hospital. Each site is located within or closes proximity to one of the epicenters of COVID-19 epidemic in the United States and China. We reviewed 52 consecutive patients who presented with acute type A aortic dissection during COVID-19 outbreak at University of Michigan (between March 10 and May 15, 2020; n = 16), Wuhan (between December 31, 2019 and March 31, 2020; n = 6) and Changsha (between February 1 and March 31, 2020; n = 30). The study cohort is summarized in Supplementary Figure 1. The study period was determined from the time of the first confirmed COVID-19 case in each area. Each site represents the tertiary aortic center in the respective geographic region. Investigators at each site collected clinical data through medical records including demographics, imaging studies, clinical variables, perioperative, and short-term follow-up outcomes.
This study was approved by the institutional review boards (IRB) of the 3 institutions. The approval included a waiver of informed consent, and investigators analyzed only deidentified data.
Diagnosis and Management
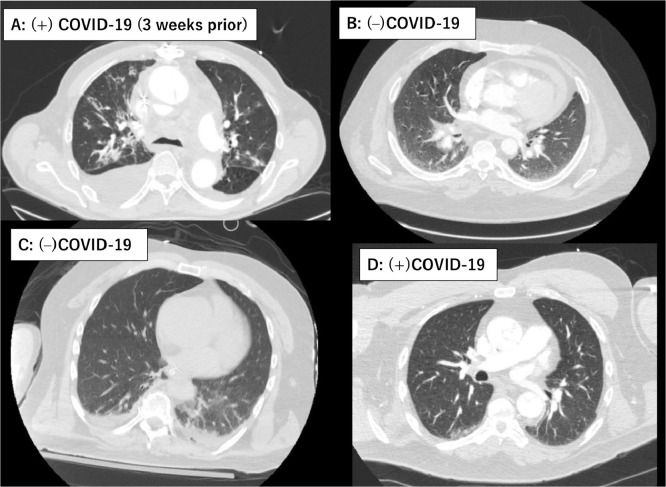
The diagnosis of acute type A aortic dissection was established radiographically by computed tomography (CT) angiography in all patients. CT findings of the lungs were also simultaneously evaluated (Fig. 1 A–D). All patients were considered for surgical repair except for 5 (9.6%) medically managed patients with various clinical reasons. In selected patients with malperfusion syndrome of other vital organs, the most compromised organ system was treated by upfront endovascular reperfusion therapy followed by surgery at resolution of organ failure. Details describing the endovascular reperfusion procedure have been previously published.6
Figure 1.
Representative chest computed tomography (CT) images of concurrent lung lesions at the time of the acute type A aortic dissection occurrence. (A) Multifocal consolidation/ground-glass attenuation in both lungs with pleural effusion in a 66-year-old male with COVID-19 infection 3 weeks prior to the occurrence of the acute type A aortic dissection. (B) Bilateral basilar ground-glass attenuation and septal thickening. There was wall thickening tracking along the main pulmonary artery and bilateral pulmonary arteries in a 42-year-old made (COVID-19 negative). (C) Dependent atelectasis bilateral lower lobes and left lower lobe infiltrates in a 53-year-old male (COVID-19 negative). (D) Unremarkable CT in a 53-year-old male (COVID-19 positive). COVID-19, coronavirus disease 2019.
Nasopharyngeal swab samples were immediately collected and sent for COVID-19 detection except for 5 (9.6%) nonsuspected patients at the very start of the pandemic period.
Study Definitions
A confirmed case of COVID-19 was defined by a positive result on a reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay. Clinically suspected COVID-19 was defined as a condition meeting at least one of the following criteria at presentation; fever ≥ 38°C, cough and/or dyspnea without a clear alternative cause, recent domestic/international travel, employment as a healthcare worker or contact with a known COVID-19 patient or positive CT pulmonary findings. Positive CT was defined as presence of any pulmonary lesions, which include typical, indeterminate and atypical findings for COVID-19 (ground-glass opacity, consolidation, reticulation/thickened interlobular septa, nodules), which are often times nonspecific findings.7
Immediate surgical repair was defined as a repair conducted within 24 hours after arrival. Prolonged ventilation was defined as mechanical ventilation for > 48 hours, which included time from exit from the operating room to extubation.
Surgical Technique
All surgical repairs were through sternotomy with cardiopulmonary bypass and hypothermic circulatory arrest with antegrade and/or retrograde cerebral perfusion. Cardiopulmonary bypass was established with direct aortic, right subclavian, innominate artery, or femoral arterial cannulation depending on the aortic pathology at the discretion of the operating surgeon. Myocardial protection was achieved through cold blood cardioplegia delivered antegrade and/or retrograde. Cerebral oximetry as well as bladder and nasopharyngeal temperatures were routinely monitored. The extent of the proximal or distal aortic repair with or without frozen elephant trunk was individualized based on the aortic pathology.
Statistical Analysis
Continuous variables are expressed as mean ± standard deviation for normally distributed variables and median with interquartile range (IQR) for non-normally distributed variables. Normality was tested with the Shapiro-Wilk test. Categorical variables are presented as proportion and absolute number. Differences between groups were detected using the Chi-sqaure test or Fisher's exact test for categorical variables and Student's t test or Mann-Whitney U test for continuous variables. All P-values were results of 2-tailed tests. The statistical analysis was performed using SPSS 25.0 (IBM, Armonk, NY).
RESULTS
Patient Demographics
Patient demographic data are shown in Table 1 . The mean age was 53.4 ± 12.8 years and 15 (28.9%) were woman. Most patients had a DeBakey type I dissection. Two (3.9%) presented with cardiac tamponade and shock state. Twenty-one (38.5%) demonstrated end-organ malperfusion with leg malperfusion being the most common (35.0%) followed by mesenteric (20.0%).
Table 1.
Patient Demographics
| Variables | Entire Cohort (n = 52) | Suspected COVID (n = 24) | Nonsuspected COVID (n = 28) | P Value |
|---|---|---|---|---|
| Age (Y) | 52.3 ± 12.8 | 54.0 ± 10.2 | 50.6 ± 14.9 | 0.34 |
| Female | 15 (28.9) | 9 (37.5) | 6 (21.4) | 0.20 |
| Diabetes | 10 (19.2) | 2 (8.3) | 8 (28.6) | 0.086 |
| Hypertension | 32 (61.5) | 18 (75.0) | 14 (50.0) | 0.065 |
| Dyslipidemia | 14 (26.9) | 6 (25.0) | 8 (28.6) | 0.77 |
| Renal function at arrival | ||||
| eGFR* | ||||
| ≥ 90 | 31 (59.6) | 13 (54.2) | 18 (64.3) | 0.46 |
| 60-89 | 10 (19.2) | 6 (25.0) | 4 (14.3) | 0.33 |
| 30-59 | 7 (13.5) | 3 (12.5) | 4 (14.3) | 1.00 |
| 15-29 | 3 (5.8) | 1 (4.2) | 2 (7.1) | 1.00 |
| < 15 | 1 (1.9) | 1 (4.2) | 0 | 0.46 |
| Dialysis | 1 (1.9) | 1 (4.2) | 0 | 0.49 |
| COPD | 2 (3.9) | 2 (8.3) | 0 | 1.00 |
| Body mass index | 27.4 ± 6.2 | 26.5 ± 4.6 | 28.3 ± 7.4 | 0.35 |
| Current smoker | 19 (36.5) | 9 (37.5) | 10 (35.7) | 0.89 |
| Left ventricular ejection fraction | 58.2 ± 17.4 | 55.9 ± 23.5 | 60.6 ± 6.8 | 0.39 |
| Aortic insufficiency ≥ moderate | 11 (21.2) | 5 (20.8) | 6 (21.4) | 1.00 |
| Previous cardiovascular surgery | 5 (9.6) | 2 (8.3) | 3 (10.7) | 1.00 |
| DeBakey Classification | 0.77 | |||
| Type I | 47 (90.4) | 22 (91.7) | 25 (89.3) | |
| Type II | 5 (9.6) | 2 (8.3) | 3 (10.7) | |
| Cardiac tamponade | 2 (3.9) | 0 | 2 (7.1) | 0.49 |
| End-organ malperfusion | 20 (38.5) | 7 (29.2) | 13 (46.4) | 0.20 |
| Culprit organ | ||||
| Mesenteric | 4 (20.0) | 1 (14.3) | 3 (23.1) | 1.00 |
| Renal | 2 (10.0) | 1 (14.3) | 1 (7.7) | 1.00 |
| Legs | 7 (35.0) | 4 (57.1) | 3 (23.1) | 0.17 |
| Cerebral | 3 (15.0) | 1 (14.3) | 2 (15.4) | 1.00 |
| Coronary | 2 (10.0) | 0 | 2 (15.4) | 0.52 |
| Spinal cord | 1 (5.0) | 0 | 1 (7.7) | 1.00 |
| Others | 1 (5.0) | 0 | 1 (7.7) | 1.00 |
Estimated using the Cockcroft-Gault formula, male: ([140 − age] × weight in kg)/(serum creatinine × 72), female: multiplied by 0.85. COVID-19, coronavirus disease 2019; COPD, chronic obstructive lung disease; eGFR, estimated glomerular filtration rate.
Due to the pandemic situation, 24 (46.2%) patients were considered clinically suspicious for COVID-19 based on the aforementioned criteria. Of note, there were two patients (3.9%) who sustained a recent COVID-19 infection prior to the occurrence of the aortic dissection. Both patients were categorized in the suspected group due to the presence of symptoms (fever, dyspnea, cough, positive CT (Fig. 1A)) and the possibility of persistent or recurrent COVID-19. There was no significant difference regarding the clinical characteristics between nonsuspicious and suspicious groups.
COVID-19 Relevant Clinical Data at Presentation and Aortic Dissection Management
Relevant clinical data related to COVID-19 and patient management are shown in Table 2 . Among suspected patients, the leading reason for suspected COVID-19 was the presence of lung lesions on CT scan (70.8%) followed by dyspnea (25.0%), cough (12.5%) and fever ≥ 38.0°C (12.5%). Representative CT lung findings are demonstrated in Figure 1A–D.
Table 2.
Relevant Clinical Data Associated With COVID-19
| Variables | Entire Cohort (n = 52) | Suspected COVID (n = 24) | Nonsuspected COVID (n = 28) | P Value |
|---|---|---|---|---|
| Body temperature (°C) | 36.8 ± 0.6 | 37.0 ± 0.6 | 36.7 ± 0.4 | 0.12 |
| Body temperature ≥ 38.0°C | 3 (5.8) | 3 (12.5) | 0 | N/A |
| Dyspnea | 6 (11.5) | 6 (25.0) | 0 | N/A |
| Cough | 3 (5.8) | 3 (12.5) | 0 | N/A |
| Unexplained hypoxia | 1 (1.9) | 1 (4.2) | 0 | N/A |
| Pulmonary lesions on computed tomography scan | 17 (32.7) | 17 (70.8) | 0 | N/A |
| Recent travel to COVID-19 affected area | 1 (1.9) | 1 (4.2) | 0 | N/A |
| Recent history of COVID-19 infection | 2 (3.9) | 2 (8.3) | 0 | 0.21 |
| COVID-19 testing at arrival | 47 (90.4) | 24 (100) | 23 (82.1) | 0.054 |
| Positive results | 0 | 0 | 0 | N/A |
| Management | ||||
| Open surgical repair | 47 (90.4) | 23 (95.8) | 24 (85.7) | 1.00 |
| Immediate repair | 28 (59.6) | 13 (59.1) | 15 (60.0) | 0.78 |
| Delayed repair | 19 (40.4) | 10 (45.5) | 9 (36.0) | 0.56 |
| Ruling out COVID-19 | 13 (68.4) | 10 (100) | 3 (33.3) | 0.003 |
| Controlled pericardiocentesis | 1 (5.3) | 0 | 1 (11.1) | 0.47 |
| Endovascular malperfusion therapy | 3 (15.8) | 1 (10.0) | 2 (22.2) | 0.58 |
| Subacute aortic dissection not needing emergent repair | 3 (15.8) | 0 | 3 (33.3) | 0.23 |
| Novel oral anticoagulants | 2 (10.5) | 1 (10.0) | 1 (11.1) | 1.00 |
| Initial medical management due to spinal cord injury | 1 (5.3) | 0 | 1 (11.1) | 0.47 |
| Nonoperative management | 5 (9.6) | 1 (4.2) | 4 (14.3) | 0.37 |
| Reason for nonoperative management | ||||
| Advanced age without suitable endovascular aortic repair anatomy | 1 (20.0) | 0 | 1 (25.0) | 1.00 |
| Intramural hematoma | 1 (20.0) | 0 | 1 (25.0) | 1.00 |
| Refusal of surgery | 2 (40.0) | 0 | 2 (50.0) | 1.00 |
| Free rupture | 1 (20.0) | 1 (100) | 0 | 0.20 |
Bold indicates statistically significant (P < 0.05).
COVID-19, coronavirus disease 2019; N/A, not applicable.
All patients in the suspected COVID group underwent RT-PCR at arrival, while 82.1% in the nonsuspected COVID group (P = 0.054). There were no positive RT-PCRs at arrival. Overall, 47 (90.4%) underwent a surgical repair. Among operated patients, 59.6% were immediate and 40.4% were delayed. The leading reason for the delayed operation was the waiting time ruling out COVID-19 (68.4%), which was applicable to all suspected COVID patients who underwent a delayed operation (100%), whereas 33.3% in nonsuspected COVID patients (P = 0.003). Some patients had more than one reasons for the delay. One nonsuspected patient with hemodynamic instability went into cardiac arrest in the emergency room and underwent a controlled pericardiocentesis, yielding 40 mL bloody effusion drained. This patient was stabilized afterwards and safely underwent a definitive surgical repair after confirming the negative COVID-19 test.
In contrast, 5 (9.6%) were managed nonoperatively. The reasons for nonoperative management comprised patient refusal for surgical intervention (40.0%), resolved intramural hematoma (20.0%) and advanced age (89-year-old) without suitable endovascular aortic repair anatomy (20.0%). No patients were managed nonoperatively because of COVID-19-related reasons.
Operative Data
Operative data are shown in Supplementary Table 1. The median time between the arrival to the operating room was 20.5 hours (IQR 7.0–44.0) and 23.8 hours (IQR 13.1–75.3) in the suspected and non-suspected COVID groups, respectively (P = 0.89). There was no difference with respect to the cerebral protection strategy, extent of aortic repair or cardiopulmonary bypass/aortic cross-clamp/circulatory arrest times between groups. The majority of patients received blood transfusions.
In-hospital Outcomes
In-hospital outcomes are shown in Table 3 . The mortality was 7.8% and 6.4% in the entire cohort and surgically managed cohort, respectively. One (1.9%) suspected patient for COVID-19 died of aortic rupture while waiting for the RT-PCR results. A total of 8 (17.0%) underwent a COVID-19 testing postoperatively and 1 (12.5%) came back positive. Among the 3 deaths in the surgically managed cohort, 1 (33.3%) died of COVID-19 related respiratory failure and 2 (66.6%) died of severe neurological sequela.
Table 3.
In-hospital Outcomes
| Variables | Medical Management (n = 5) | Delayed Surgery + Medical Management (n = 24) | Surgery (n = 47) |
|---|---|---|---|
| Postoperative COVID-19 testing | N/A | N/A | 8 (17.0) |
| Positive COVID-19 | N/A | N/A | 1 (12.5) |
| In-hospital mortality | 1 (20.0) | 1 (4.2) | 3 (6.4) |
| Cause of death | |||
| Respiratory failure related to COVID-19 | 0 | 0 | 1 (33.3) |
| Neurological | 0 | 0 | 2 (66.7) |
| Aortic rupture | 1 (20.0) | 1 (4.2) | 0 |
| Disabling stroke | 0 | 0 | 3 (6.4) |
| Spinal cord injury | 0 | 0 | 2 (4.3) |
| Tracheostomy | 0 | 2 (8.0) | 3 (10.0) |
| Reintubation | 1 (4.2) | 2 (8.0) | 2 (4.3) |
| Pneumonia | 0 | 6 (24.0) | 10 (21.3) |
| Prolonged mechanical ventilation | 1 (20.0) | 5 (20.0) | 14 (29.8) |
| Nitric oxide | 0 | 0 | 2 (4.3) |
| Mechanical ventilation duration (h) | N/A | N/A | 41 (18.5–66.2) |
| Renal failure | 1 (20.0) | 3 (12.5) | 7 (14.9) |
| Dialysis* | 0 | 1 (4.2) | 3 (6.4) |
| ICU length of stay (h) | N/A | N/A | 112 (68–165) |
| Postoperative length of stay (d) | N/A | N/A | 16 (11.6–22.0) |
Among patients without end-stage renal disease on dialysis; COVID-19, coronavirus disease 2019; N/A, not applicable.
In contrast, 80% (4 out of 5) of nonoperative managed patients survived to hospital discharge and are alive to date. The composite mortality of nonoperatively managed patients (n = 5; 9.6%) and patients whose surgical intervention was delayed more than 24 hours (n = 19; 36.5%) was 4.2%, suggesting the safety of delayed surgical intervention or nonoperative management in selected patients. These 4 nonoperatively managed survivors remain alive as of 2 months following the hospital discharge.
Clinical Course of Patients With Positive COVID-19
A total of 3 (5.8%) surgically managed patients had a positive COVID-19 test at various timings. The clinical course of these patients is summarized in Table 4 . All patients had an extremely complicated postoperative course.
Table 4.
Clinical Characteristics in Patients With Positive COVID-19 and Acute type A Aortic Dissection
| Age Sex |
Body Temperature (°C) | Symptoms/Recent COVID-19 Exposure | Pulmonary Lesions on CT Scan | Preoperative COVID-19 Test | Postoperative COVID-19 Test | Timing of COVID-19 Infection | Hospital Course | Outcomes |
|---|---|---|---|---|---|---|---|---|
| (1) 53M | 37.5 | None | Negative | Not performed | Positive (RT-PCR) | Preoperative | Unexplained hypoxia from postoperative day (POD) #1. Extubated on the second postoperative day. Reintubation on POD#4. COVID-19 confirmed on POD#5. Expired on POD#11 due to respiratory failure. | Death |
| (2) 40F | 38.0 | Cough | Positive | Positive remotely (RT-PCR + antibody titers) | Negative | 6 weeks prior | Extubated on POD#3, ICU stay for 2 weeks. Discharged to an extended medical facility on POD#19. | Survival to hospital discharge |
| (3) 66 M | 37.4 | Cough | Positive | Positive remotely (RT-PCR) | Negative | 3 weeks prior | Severe coagulopathy requiring delayed sternal closure and prolonged ICU course. Discharged to home on POD#18. | Survival to hospital discharge |
COVID-19, coronavirus disease 2019; CT, computed tomography; F, female; ICU, intensive care unit; M, male; RT-PCR, reverse transcriptase polymerase chain reaction.
Patient 1, a previously healthy nonsmoking 52-year-old Michigan resident, did not demonstrate any clinical signs suspicious for COVID-19 at arrival, not meeting any of the institutional criteria for RT-PCR. There were only 33 COVID-19 cases in the entire State of Michigan and no case in our county (Washtenaw County) at that time.8 Therefore, this patient was not even tested preoperatively. However, he developed fever and severe acute respiratory distress syndrome on the third postoperative day after the uneventful operation and initial ICU course. He died of severe respiratory failure despite maximum medical therapy. Several providers involved in his care were immediately quarantined on the fifth postoperative day when the RT-PCR came back positive. Two ICU staffs involved in his care acquired COVID-19 infection afterward. While it was unclear at what time point this patient acquired COVID-19, we suspect it was preoperatively, given the time course of his clinical deterioration.
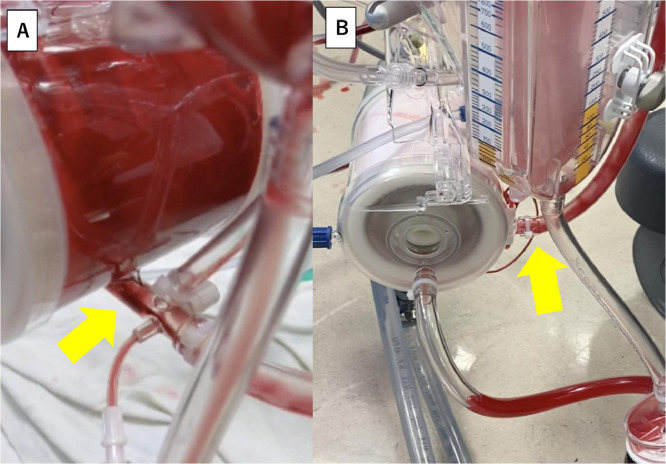
Patients 2 and 3 were both preoperatively deconditioned due to the recent COVID-19 infection, but considered convalescent based on the negative RT-PCR at the time of type A aortic dissection presentation. Patient 2 was a 40-year-old male with end-stage renal disease on dialysis who was tested positive for COVID-19 and was hospitalized for 6 weeks prior to the aortic dissection occurrence. The patient was febrile (38.0°C) and dyspneic at arrival, however, the RT-PCR was negative. Patient 3 was a 66-year-old male with positive RT-PCR 3 weeks prior. He was still not ambulatory. At arrival, he demonstrated cough, dyspnea and bilateral pulmonary infiltrates with moderate size right pleural effusion on the CT (Fig. 1A), whereas RT-PCR was negative. Both operations were characterized by extremely severe coagulopathy requiring massive blood transfusions. Case 2 was taken back to the operating room to address the bleeding. During the procedure of Case 3, obvious thrombus formation was noted in the oxygenator of the cardiopulmonary bypass circuit (Fig. 2 A) at the end of the case without protamine introduction into the circuit. Although the association between the recent COVID infection and severe bleeding or thrombus formation in the oxygenator remains unclear, this is an exceedingly unusual finding (Fig. 2B demonstrates a normal appearing oxygenator for comparison from a patient without recent COVID-19 infection). Despite the compromised preoperative functional status, complicated procedures and postoperative prolonged ICU course, both patients survived.
Figure 2.
Obvious thrombus (yellow arrow) appeared in the oxygenator of the cardiopulmonary bypass circuit at the end of the procedure in Case 3. Association between severe bleeding and this thrombus formation remains unclear (A). Normal appearance of an oxygenator without thrombus (yellow arrow) at the same timing in another patient with type A aortic dissection repair for comparison (B). (Color version of figure is available online at http://www.semthorcardiovascsurg.com.)
As for pulmonary CT findings, it is noteworthy to mention that Case 1 did not demonstrate any pulmonary lesions at arrival despite the preoperative COVID-19 acquirement (Fig. 1D). In contrast, Case 2–3 demonstrated pulmonary infiltrates on the CT scan (Fig. 1A). However, preoperative RT-PCRs at arrival were both negative.
DISCUSSION
This study represents the first investigation describing the management and outcomes of type A aortic dissection during the pandemic. In the context of the persistent pandemic situation or possible second surge, a thoughtful discussion regarding optimized management is of paramount importance.
The primary findings of interest in this study are (1) nearly half of patients with type A aortic dissection demonstrated clinical characteristics suspicious for COVID-19; (2) eliciting of COVID-19 clinical signs in the setting of type A aortic dissection severe symptoms was challenging; (3) the overall surgical outcomes remained acceptable despite the severe COVID-19 burden to the healthcare system. However, in light of complicated postoperative course and potential COVID-19 spread to healthcare providers, strict preoperative COVID-19 testing prior to surgical interventions, regardless of hemodynamic instability or presence of symptoms, is mandatory.
Furthermore, personal communications with investigators at another major hospital in Wuhan and 11 New York City hospitals,9 3 of 6 (50%) patients with dual diagnoses of COVID-19 and type A aortic dissection died of severe pulmonary complications and survivors had an extremely complicated ICU course.
The positive COVID-19 case presented in this report was seen in the initial phase of COVID-19 outbreak when the recognition of COVID-19 infection was low. The same clinical scenario may occur in any geographic locations including where relaxation of COVID-19 lockdown is currently underway.
Did Type A Aortic Dissection Also Quarantine?
There has been a perception that patients with certain cardiovascular conditions have been observed less frequently than usual during the pandemic. Reports from United States, Spain and Italy demonstrated a similar reduction pattern in the activity of cardiac catheterization laboratory.10, 11, 12 Another data from 11 major New York City hospitals demonstrated a significant drop in the monthly surgical case volume of type A aortic dissection from 12.8 cases/month to 3.0 cases/month, representing a 76.5% decrease in volume during the pandemic.9
While a number of theories that could drive the incidence of urgent cardiac pathologies down, type A aortic dissection, at least at the regional aortic centers located in one of epicenters in the United States and China in the present report, did not appear to be in line with these reported observations. For instance, the number of cases at University of Michigan was the highest (n = 16) and its occurrence was constant during the present study period (March 10–May 15, 2020) compared with the same calendar months in the 5 previous years (2015–2019) (Supplementary Figure 2). Indeed, our case volume alone was 2.7 times higher than the total volume of 11 New York City hospitals during the pandemic. This higher-than-usual case volume despite the heavy COVID burden (As of May 16th, 50,504 cases and 4880 deaths related to COVID-19 in the State of Michigan8), may be explained by the limited capacity for emergency surgery at other local institutions.
Optimal Management of Type A Aortic Dissection During COVID-19 Outbreak
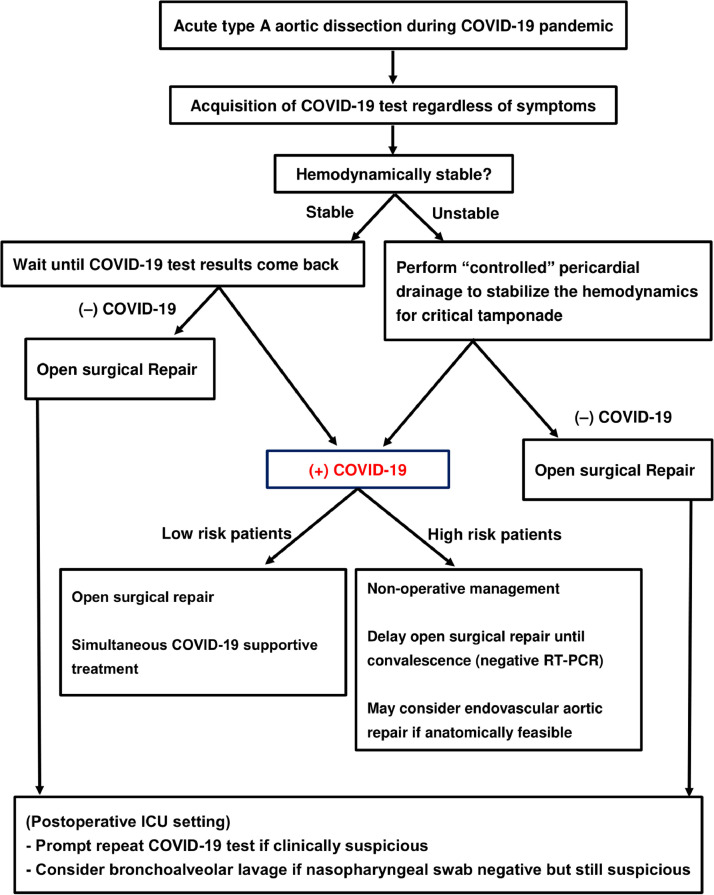
Based on our experience, the following management algorithm has been formulated (Fig. 3 ). While maintaining superb surgical outcomes, protecting healthcare providers and other patients from COVID-19 is an important priority.
Figure 3.
Recommended management algorithm of patients with acute type A aortic dissection in the COVID-19 era. *High risk patients represent patients with at least one of following clinical features including respiratory failure already requiring intubation at arrival, patients aged 65 or older,19 end-organ malperfusion syndrome, shock or critical tamponade, moderate or worse chronic lung disease20 at baseline, chronic kidney disease stage 4 or end-stage renal disease on dialysis, compromised functional status (activities of daily living with assistance), congestive heart failure NYHA II or above at baseline. COVID-19, coronavirus disease 2019; TEVAR, thoracic endovascular aortic repair.
Although consensus for general COVID-19 management has been changing day by day due to the rapidly changing circumstances, we strongly advocate for mandatory testing, regardless of the presence of symptoms suspicious for COVID-19 or hemodynamic instability. There are clearly patients with mild symptoms or even asymptomatic patients who are infected with COVID-1913 that will not be detected by the current screening process such as Case 1 in Table 4. Moreover, patients with type A aortic dissection typically present with severe chest/back pain and sometimes in an unconscious state or already intubated due to cardiogenic shock and/or cerebral malperfusion, raising a concern for masked COVID-19 symptoms. Such patients can then be placed in the appropriate isolation precautions and pre-emptive COVID treatment and providers can wear the appropriate personal protective equipment.
Furthermore, many surgeons might think a negative CT for typical radiographic features suffices as a screening for COVID-19 infection rather than waiting for RT-PCR results. Compared to RT-PCR, CT imaging may be a more reliable and practical method to diagnose COVID-19, especially in the epidemic area.7 However, it did not seem to be helpful in the presence of type A aortic dissection and its positive predictive value for detecting COVID was very low. Those typical COVID-19 pneumonia findings appeared surprisingly common in type A dissection patients. At arrival, 33% demonstrated simultaneous pulmonary lesions on the CT scan. More importantly, among these 3 cases with confirmed perioperative COVID-19, Case 1 who was initially not suspected for, but later diagnosed with COVID-19, presented with normal CT findings (Fig. 1D). Cases 2 and 3 demonstrated obvious pulmonary lesions (Fig. 1A), while the preoperative RT-PCR tests were negative. CT pulmonary findings, some of which mimic COVID-19 CT features, are known to accompany acute aortic dissection.14 Lungs can be affected due to compressive effect of dissecting aneurysm, hematoma spread along with the pulmonary artery (Fig. 1B), perfusion defect in the corresponding lung, pleural effusion, and thickening of the peribronchovascular interstitium.14 , 15
Timely surgical intervention is imperative in the management of type A aortic dissection. On the contrary, the delay associated with upfront COVID-19 testing did not affect the outcomes in the present study. The only mortality was an aortic rupture in a suspected patient during the wait time for the RT-PCR result. This fatality occurred at the very beginning of the pandemic when the processing time for RT-PCR was significantly longer (> 24 hours). The current turnaround time at many institutions including our own is < 2 hours. While waiting strategy in type A aortic dissection is controversial, this fact in combination of aggressive medical management assures safety. Critical tamponade can be safely temporized by controlled pericardial drainage procedures. There has been another historic myth that pericardial drainage might induce cardiac rupture by abruptly elevating blood pressure and dislodging of already formed thrombus from the pericardial space16 based on a series of 10 patients back in 90s. However, more recent reports support controlled pericardial drainage procedures in unstable patients with tamponade with more favorable results.17 In our practice, as performed in 1 patient in the present study, controlled pericardial drainage procedure is routinely performed in the emergency room or ICU setting for critical tamponade with hemodynamic collapse. Many patients with type A aortic dissection can be safely managed nonoperatively short-term at experienced aortic centers.
Surgical Strategy for Dual Diagnoses of Type A Aortic Dissection and COVID-19
Surgical repair in patients with confirmed COVID-19 may result in fatal outcomes based on this report and unpublished personal communication data from Wuhan and New York City. Martens et al reported an otherwise healthy asymptomatic 64-year-old patient with type A dissection in whom COVID-19 was incidentally detected via RT-PCR on the first postoperative day from their postoperative mandatory screening process.18 No preoperative testing was performed. Despite respiratory complications and prolonged hospital course, this patient survived to hospital discharge. We speculate early recognition of COVID-19 and immediate aggressive treatment in Case 1 might have diverted the outcome despite the concurrent COVID-19. In contrast, fatal outcomes may be inevitable in patients with advanced age and/or severe medical comorbidities. Such operation is not only being futile but also risking the provider team for COVID-19 exposure. In such circumstance, optimal medical treatment, rather than surgical repair, may be preferred. Operation may be considered only for those achieved convalescence from COVID-19. The definition of convalescence in patients needing open heart surgery is unknown. However, we propose acquisition of negative RT-PCR results to be one indicator for the readiness of surgical repair in this unique setting. The fact that 2 very sick patients (Cases 2 and 3 in Table 4) who had a recent COVID-19 prior to the aortic dissection survived to hospital discharge also supports this management, although postoperative severe coagulopathy was noted in both patients. Alternatively, ascending aortic endovascular repair may be considered if anatomically suitable.
Study Limitations
This study has several limitations inherent to its retrospective nature with small sample size. The difference in surgical and medical management of type A aortic dissection among the 3 institutions may be significant. In view of the ongoing and future COVID-19 burden to the global healthcare system, further investigation involving other institutions using standardized methodology and diagnostic criteria is highly warranted to validate our results.
In summary, in light of the potential high mortality associated with dual diagnoses of type A aortic dissection and COVID-19, the higher-than-expected number of asymptomatic COVID-19 carriers, masked symptoms in type A dissection and unreliability of CT scan as a screening tool, we strongly advocate testing all comers for COVID-19 immediately after arrival and confirming a negative result before performing any surgical repair to avoid fatal outcomes and spread of COVID-19 to caregivers and other patients. Surgical intervention in patients with positive COVID-19 may be considered for low risk patients with minimal symptoms. Otherwise our recommendation is non-operative management until full convalescence, which may be a negative RT-PCR result. We believe lessons learned from this series remains critically important for the management of type A aortic dissection for the upcoming persistent COVID-19 pandemic or second major surge in the post-lockdown era.
Footnotes
Funding Source: None to report.
Conflict of Interest: The authors have no conflicts of interest to report.
QR Code.

Supplementary Material
Summary of the present study presented by one of authors.
REFERENCES
- 1.Available at: https://www.who.int/news-room/detail/08-04-2020-who-timeline—covid-19. Accessed April 17, 2020
- 2.Holshue M.L., DeBolt C., Lindquist S. First case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200527-covid-19-sitrep-128.pdf?sfvrsn=11720c0a_2. Accessed May 28, 2020
- 4.Available at: https://www.facs.org/about-acs/covid-19/information-for-surgeons/elective-surgery. Accessed March 23, 2020
- 5.Available at: https://www.cms.gov/files/document/31820-cms-adult-elective-surgery-and-procedures-recommendations.pdf. Accessed March 23, 2020
- 6.Yang B., Norton E.L., Rosati C.M. Managing patients with acute type A aortic dissection and mesenteric malperfusion syndrome: A 20-year experience. J Thorac Cardiovasc Surg. 2019;158:675–687.e4. doi: 10.1016/j.jtcvs.2018.11.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ai T., Yang Z., Hou H. Correlation of chest CT and RT-PCR testing in Coronavirus Disease 2019 (COVID-19) in China: A report of 1014 cases. Radiology. 2020 doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Available at: https://www.michigan.gov/coronavirus/0,9753,7-406-98163-520743–,00.html. Accessed May 16
- 9.El-Hamamsy I., Brinster D.R., DeRose J.J. The COVID-19 pandemic and acute aortic dissections in New York: A matter of public health. J Am Coll Cardiol. 2020;76:227–229. doi: 10.1016/j.jacc.2020.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia S., Albaghdadi M.S., Meraj P.M. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2871–2872. doi: 10.1016/j.jacc.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodríguez-Leora O., Cid-Álvarez B., Ojeda S. Impacto de la pandemia de COVID-19 sobre la actividad asistencial en cardiología intervencionista en España. REC Interv Cardiol. 2020 doi: 10.24875/RECIC.M20000120. [DOI] [Google Scholar]
- 12.De Filippo O., D'Ascenzo F., Angelini F. Reduced rate of hospital admissions for ACS during Covid-19 outbreak in Northern Italy. N Engl J Med. 2020;383:88–89. doi: 10.1056/NEJMc2009166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bai Y., Yao L., Wei T. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323:1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Semiz-Oysu A., Okur A., Sahin S. Pulmonary multislice computed tomography findings in acute aortic dissection. J Thorac Dis. 2012;4:485–489. doi: 10.3978/j.issn.2072-1439.2012.07.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamasaki A., Kikuchi C., Hirota M. Pulmonary infiltration shadows associated with acute aortic dissection mimicking coronavirus pneumonia. J Card Surg. 2020;35:1106–1107. doi: 10.1111/jocs.14533. [DOI] [PubMed] [Google Scholar]
- 16.Isselbacher E.M., Cigarroa J.E., Eagle K.A. Cardiac tamponade complicating proximal aortic dissection. Is pericardiocentesis harmful? Circulation. 1994;90:2375–2378. doi: 10.1161/01.cir.90.5.2375. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi T., Tsukube T., Yamashita T. Impact of controlled pericardial drainage on critical cardiac tamponade with acute type-A aortic dissection. Circulation. 2012;126:S97–S101. doi: 10.1161/CIRCULATIONAHA.111.082685. [DOI] [PubMed] [Google Scholar]
- 18.Martens T., Vande Weygaerde Y., Vermassen J., Malfait T. Acute Type A Aortic Dissection Complicated by COVID-19 Infection. Ann Thorac Surg. 2020;110:e421–e423. doi: 10.1016/j.athoracsur.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Available at: https://goldcopd.org/wp-content/uploads/2018/02/WMS-GOLD-2018-Feb-Final-to-print-v2.pdf. Accessed June 11, 2020
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of the present study presented by one of authors.