Abstract
Objectives:
We report the first pledget-assisted suture tricuspid annuloplasty (PASTA) in a patient with torrential tricuspid regurgitation (TR).
Background:
Tricuspid valve regurgitation is a common malignant disease with no commercially available transcatheter therapy. PASTA is a “percutaneous surgical” procedure using pledgeted sutures to create a double-orifice tricuspid valve
Methods:
An 83-year-old man had end-stage TR caused by a defibrillator lead. He consented to undergo PASTA on a compassionate basis. A double-orifice valve was created with pledgeted sutures from percutaneous right ventricular apical access
Results:
TR was reduced from torrential to trace. The vena contracta reduced to from 23 to 1 mm and annular area reduced from 1817 to 782 mm2. However, the annulus dehisced and required closure with a percutaneous nitinol plug. The patient was discharged home and was alive 6 months later but with persistent symptoms.
Conclusions:
The anatomy of a double-orifice valve can eliminate TR but a better solution is required to avoid excessive suture tension on annular tissue.
Keywords: percutaneous tricuspid valve repair, transcatheter electrosurgery, tricuspid valve regurgitation
1 |. INTRODUCTION
Tricuspid regurgitation (TR) is a common progressive disease that increases mortality.1 Isolated surgery for TR is rarely performed and is associated with high mortality.2 There are no commercially available transcatheter repair or replacement therapies available in the United States.
Pledget-assisted suture tricuspid annuloplasty (PASTA) is a “percutaneous surgical” procedure using pledgeted sutures to create a double-orifice tricuspid valve.3 The surgical precedent comes from Hetzer’s double-orifice valve technique.4 In the surgical procedure, after cardiopulmonary bypass is established, cardioplegia administered, and the tricuspid valve exposed via a mid-right atrial incision, pledgeted 3-0 polypropylene sutures are passed from the mid-anterior annulus to the opposite septal annulus to create an anterior and posterior orifice in the tricuspid valve. Good outcomes have been reported in a single-center series.5
The PASTA procedure requires paired bites through the annulus to deliver a spanning pledgeted suture (Figure 1). Targets are chosen at the posteroseptal annulus (thus avoiding septal conduction tissue) and the mid-anterior annulus. The sutures are tied together, bringing two ends of the annulus together to create a double-orifice valve at the annular level. The procedure can be performed via right ventricular apical access or internal jugular access.
FIGURE 1.
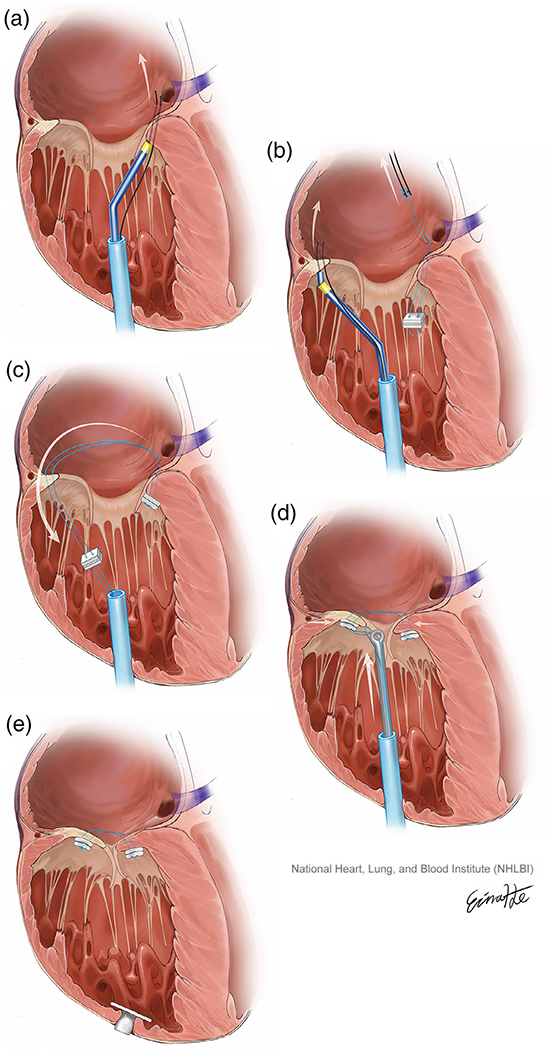
The pledget-assisted suture tricuspid annuloplasty procedure steps. (a) A pair of guidewires are electrified to traverse the septal tricuspid annulus from the right ventricular apex. (b) The guidewires are exchanged for a pledgeted suture loop. Two guidewires then traverse a point on the opposite annulus, beneath the mid-anterior tricuspid valve leaflet. (c) The anterior guidewires guide the sutures down through the anterior annulus. (d) The sutures are tensioned and secured using a knot pusher. (e) The right ventricular apex is closed using a nitinol plug (This is original art from NIH Division of Medical Arts) [Color figure can be viewed at wileyonlinelibrary.com]
This is the first human report of PASTA.
2 |. MATERIALS AND METHODS
2.1 |. Patient
An 83-year-old man presented with recurrent right-sided heart failure secondary to defibrillator-lead associated torrential TR. By echocardiography, the vena contracta measured 22.9 mm (severe >7 mm), septal-lateral diameter 58 mm, and tricuspid annular area 1817 mm2 (Figure 2a,b). The right ventricular function was impaired with a tricuspid annular plane systolic excursion of 7 mm. On preprocedure computed tomography (CT), the right ventricular septal-lateral and anteroposterior dimensions were 72.6 and 83.5 mm, respectively. He was evaluated for surgical valve repair or replacement and felt to be at prohibitive surgical risk.
FIGURE 2.
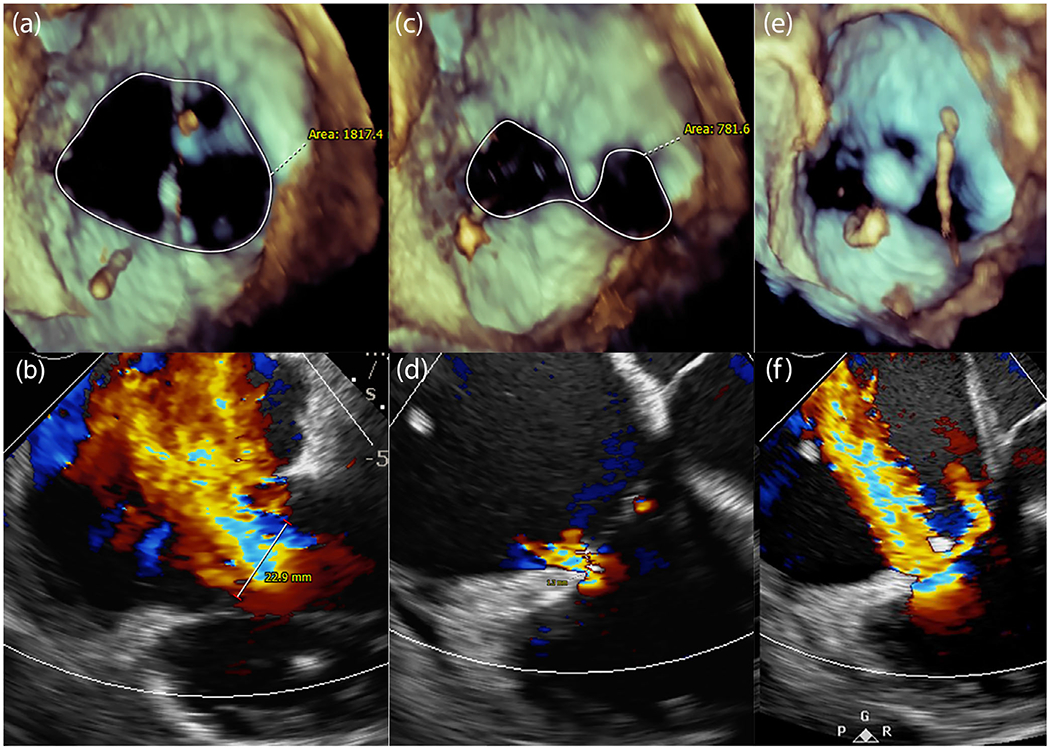
Pledget-assisted suture tricuspid annuloplasty (PASTA) in a patient. (a) Dilated tricuspid annulus with (b) torrential tricuspid regurgitation (TR) at baseline. (c) After PASTA, a double-orifice valve is created, reducing TR to trace (d). (e) However, the septal annulus dehisced adjacent to the pledgeted suture with recurrence of TR (f) [Color figure can be viewed at wileyonlinelibrary.com]
The patient consented to undergo PASTA on a compassionate basis on the understanding that this was an nonproven technique never before attempted, and with the concurrence of the multidisciplinary heart team and the institutional novel procedures committee. The Institutional Review Board at Henry Ford Hospital approved this retrospective communication.
2.2 |. PASTA technique and devices
The right ventricular approach was chosen as the jugular approach had not been fully developed in preclinical studies at the time of the procedure. Percutaneous transthoracic access to the right ventricular apex was planned on CT (Figure 3) and achieved with echocardiography and fluoroscopy-guided micropuncture needle access and secured with a 12-Fr Brite Tip sheath (Cordis). A fractional flow reserve (FFR) wire (Philips Volcano, CA) was positioned in the right coronary artery throughout the case to both delineate the tricuspid annulus and provide physiological assessment of any right coronary artery impingement. A multipurpose guiding catheter was advanced through the apical sheath and positioned at the ventricular surface of the posterior third of the septal annulus. A pair of 0.014″ Confianza guidewires (Asahi Intecc, Japan) sheathed in insulating microcatheters (Piggyback wire convertor; Teleflex, NC) were briefly electrified to traverse the annulus behind the base of the septal tricuspid leaflet and snared in the right atrium at the ostium of the coronary sinus (Figure 1a). Catheters were guided by fluoroscopy and transesophageal and intracardiac echocardiography. The back ends of the guidewires were connected to a pledgeted (Teflon pledget; Ethicon) size 0 braided polyester suture (Ethibond; Ethicon) using crimped low-profile half-hitch knots. The snared guidewires were externalized through the jugular vein, bringing the pledget to the annulus (Figure 1b). Similarly, a pair of guidewires traversed the opposite annulus, behind the mid-anterior tricuspid valve leaflet, directed by a coaxial catheter system comprising an internal mammary catheter inside a multipurpose guiding catheter, and snare-externalized through the jugular vein (Figure 1c). These guidewires were tied to the previously externalized sutures, which were then threaded back down through the anterior annulus and out of the apical sheath (Figure 1d). The sutures were drawn together using a knot pusher from the apical sheath while assessing the reduction of annular dimensions and TR on echocardiography and tied using sliding knots (Figure 1e).
FIGURE 3.
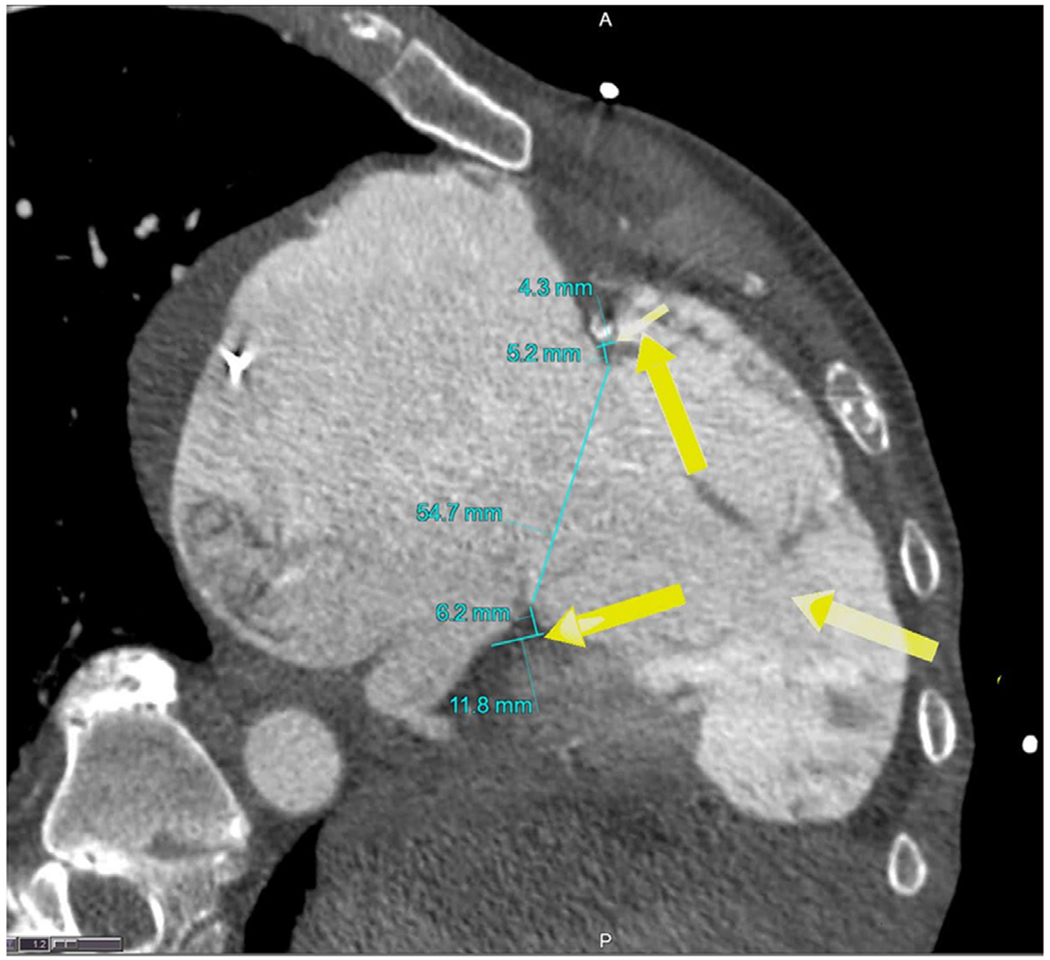
Computed tomography planning for pledget-assisted suture tricuspid annuloplasty. Arrows indicate percutaneous right ventricular access site and trajectory of catheters to achieve transannular suture delivery at the anterior annulus (avoiding the right coronary artery) and posteroseptal annulus [Color figure can be viewed at wileyonlinelibrary.com]
3 |. RESULTS
The result was a double-orifice valve, with only trace TR. The vena contracta was reduced from 23 to 1 mm. The annular area was reduced from 1817 to 782 mm2 (Figure 2c,d). The right coronary artery remained unobstructed with an FFR of 1.0. The cardiac output increased from 3.5 to 4.6 L/min. The apical access site was closed using a 6-mm Amplatzer Ventricular Septal Defect occluder (Abbott Vascular, Chicago, IL).
Unfortunately, the excessive forces on the diseased tricuspid annulus caused septal annular dehiscence in less than 10 min, without pull-through of the pledget anchor resulting in recurrence of severe TR (Figure 2e,f). Closure of the dehisced annulus was attempted with Gore Cardioform devices, with partial seal of the dehiscence. The patient was discharged home and was alive at 6-month follow-up but with persistent symptoms.
4 |. DISCUSSION
This first-in-human case of PASTA failed because of annular dehiscence and provides several learning points for a rapidly developing field of transcatheter tricuspid valve repair therapy. Pull-through is a common problem with novel transcatheter tricuspid repair devices where “single-bite” anchors are used (10% for Trialign, SCOUT trial6; 17% for Tri-Cinch, PREVENT trial NCT02098200). In the PASTA benchtop experiments, the transannular double-bite pledget configuration resisted pull-through compared to a single-bite configuration (40 vs. 8 N, p < .01).3 In fact, at 40 N, the annulus dehisced before pledget pull-through. Annular dehiscence was the mechanism of failure in this patient, although at subjectively much lower forces. This likely reflects the fragile annulus in this end-stage patient, as well as the excessive forces applied focally with a single pledget.
While the animal experiments progressed to a completely jugular technique, we felt that in the absence of dedicated deflectable catheters to reach the targets on this dilated annulus a transapical approach would be more feasible. This case was done via right ventricular (RV) apical access. Although this approach was feasible and both access and closure were performed percutaneously, the transjugular approach is more attractive with less risk of bleeding.
This report demonstrates proof-of-concept that the double-orifice tricuspid annulus technique can eliminate even torrential TR. Anatomically, the anterior tricuspid leaflet is the largest leaflet in humans. A mid-anterior to septal suture creates an anterior orifice with anterior and septal leaflet coaptation, and posterior orifice with anterior and posterior leaflet coaptation. Absent an anatomic septal tricuspid annulus, we created a septal anchor across myocardium into the coronary sinus.
This report also demonstrates that “valve surgery,” using standard sutures, pledgets, and knots, can be performed in a closed chest and beating heart. However, it is likely that the forces on the annulus in this case were too extreme. Redistribution of force with multiple pledgets along the annulus, and choosing more robust annular substrate, may improve the outcome of this procedure in future.
The imaging of the tricuspid valve is often difficult and may require a combination of transesophageal, transthoracic, and intracardiac echocardiography together with fluoroscopic landmarks. In this case, the septal bite was performed from the right ventricle into the coronary sinus ostium, assuring muscular rather than leaflet traversal, and resulting in muscular “annular” dehiscence rather than a leaflet tear.
5 |. CONCLUSION
This case demonstrates that torrential TR can be eliminated by changing the geometry of the tricuspid annulus to form a double-orifice valve and that sutures and pledgets can be delivered percutaneously using off-the-shelf devices. However, the results with a single spanning suture and pledget system in a massively dilated annulus were not durable. Dedicated devices may facilitate multiple spanning sutures via a transjugular approach.
ACKNOWLEDGMENTS
This study is supported by the National Heart Lung and Blood Institute, National Institutes of Health, USA (Z01-HL006040-7).
Funding information
National Heart, Lung, and Blood Institute, Grant/Award Number: Z01-HL006040
Abbreviations:
- PASTA
pledget-assisted suture tricuspid annuloplasty
- TR
tricuspid regurgitation
Footnotes
CONFLICT OF INTEREST
A. B. G. is a proctor for Edwards Lifesciences, Medtronic, and Abbott Vascular. He has an equity interest in transmural systems. J. M. K. is a consultant/proctor for Edwards Lifesciences and Medtronic. T. R. is a consultant/proctor for Edwards Lifesciences and Medtronic. V. C. B. is a consultant for Edwards Lifesciences and Abbott Vascular, and his employer has research contracts for clinical investigation of transcatheter aortic, mitral, and tricuspid devices from Edwards Lifesciences, Abbott Vascular, Medtronic, St Jude Medical, and Boston Scientific. He has an equity interest in transmural systems. D. D. W. is a consultant for Edwards Lifesciences. G. P. is a proctor for Edwards Lifesciences. No other author has a financial conflict of interest related to this research.
REFERENCES
- 1.Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol. 2004;43(3):405–409. [DOI] [PubMed] [Google Scholar]
- 2.Kilic A, Saha-Chaudhuri P, Rankin JS, Conte JV. Trends and outcomes of tricuspid valve surgery in North America: an analysis of more than 50,000 patients from the Society of Thoracic Surgeons database. Ann Thorac Surg. 2013;96(5):1546–1552. discussion 1552. [DOI] [PubMed] [Google Scholar]
- 3.Khan JM, Rogers T, Schenke WH, et al. Transcatheter pledget-assisted suture tricuspid annuloplasty (PASTA) to create a double-orifice valve. Catheter Cardiovasc Interv. 2018;92(3):E175–E184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hetzer R, Komoda T, Delmo Walter EM. How to do the double orifice valve technique to treat tricuspid valve incompetence. Eur J Cardiothorac Surg. 2013;43(3):641–642. [DOI] [PubMed] [Google Scholar]
- 5.Hetzer R, Javier M, Delmo Walter EM. The double-orifice valve technique to treat tricuspid valve incompetence. J Heart Valve Dis. 2016; 25(1):66–71. [PubMed] [Google Scholar]
- 6.Hahn RT, Meduri CU, Davidson CJ, et al. Early feasibility study of a transcatheter tricuspid valve annuloplasty: SCOUT trial 30-day results. J Am Coll Cardiol. 2017;69(14):1795–1806. [DOI] [PubMed] [Google Scholar]


