Abstract
Glaucoma is a neurodegenerative disorder that leads to the slow degeneration of retinal ganglion cells, and results in damage to the optic nerve and concomitant vision loss. As in other disorders affecting the viability of central nervous system neurons, neurons affected by glaucoma do not have the ability to regenerate after injury. Recent studies indicate a critical role for optic nerve head astrocytes (ONHAs) in this process of retinal ganglion cell degeneration. Cleavage of tau, a microtubule stabilizing protein and constituent of neurofibrillary tangles (NFT), plays a major part in the mechanisms that lead to toxicity in CNS neurons and astrocytes. Here, we tested the hypothesis that estrogen, a pleiotropic neuro- and cytoprotectant with high efficacy in the CNS, prevents tau cleavage, and hence, protects ONHAs against cell damage caused by oxidative stress. Our results indicate that estrogen prevents caspase-3 mediated tau cleavage, and thereby decreases the levels of the resulting form of proteolytically cleaved tau protein, which leads to a decrease in NFT formation, which requires proteolytically cleaved tau protein. Overall, our data propose that by stopping the reduction of estrogen levels involved with aging the sensitivity of the optic nerve to glaucomatous damage might be reduced. Furthermore, our data suggest that therapeutic use of estrogen may be beneficial in slowing or preventing the onset or severity of neurodegenerative diseases such as glaucoma and potentially also other degenerative diseases of the CNS through direct control of posttranslational modifications of tau protein.
Keywords: Brain, Central nervous system, Eye, Glaucoma, Neurofibrillary tangles, Phosphorylation, 17β-estradiol, Retina, Tau cleavage
Introduction
Progressive neurodegenerative disorders, such as Alzheimer’s disease (AD) often show significant sex differences with the incidence rate of AD in women being 2–3 times higher (Pike 2017). In addition, the likelihood of developing dementia over the long term rises with premature menopause (Ryan et al. 2014). A connection between the decline of estrogen during post-menopause and developing AD has been hypothesized (Geerlings et al. 2001). In addition, cognitive impairment in females seems to be higher when compared to males at the same stage of the disease, which also has been attributed to decreased levels of estrogen found in women who are post-menopausal (Laws et al. 2016).
When adequately utilizing therapeutic windows for estrogen replacement therapy, estrogen reduces the likelihood that healthy women will develop AD and improves cognitive function in women who have AD (Tang et al. 1996; Pike et al. 2009; Engler-Chiurazzi et al. 2015, 2016).
Glaucoma, a neurodegenerative disease of the CNS that damages the optic nerve by inducing cell death in retinal ganglion cells, results in the irreversible loss of vision (Munemasa and Kitaoka 2013). Similar to AD, decreased estrogen levels raise the risk for developing glaucoma, while adequate estrogen replacement therapy appears to reduce women’s risk for glaucoma (Dewundara et al. 2016; Newman-Casey et al. 2014), which is supported by strong preclinical evidence (Prokai-Tatrai et al. 2013; Kaja et al. 2003). An early decline in endogenous estrogen levels has been suggested to result in a higher susceptibility of the optic nerve to glaucoma-mediated damage (Vajaranant and Pasquale 2012).
Both AD and glaucoma appear to be linked and potentially share underlying neurodegenerative mechanisms. For example, AD patients are three times more likely to develop glaucoma when compared to the general population (Bayer et al. 2002). In addition, aggregated and missorted tau accumulated in retinal neurons of a rat model for glaucoma, while knocking down tau protein levels protected neurons and axons from degeneration (Chiasseu et al. 2016).
Microglia and astrocytes are involved in mediating several mechanisms of protection against neurotoxicity (Ries and Sastre 2016). In addition, estrogen receptors are expressed on glia cells and are a target for estrogen’s protective properties in a variety of pathologies (Barreto 2016; Arevalo et al. 2010; Dhandapani and Brann 2007). Reduced inflammation and oxidative stress were measured when glial cells were dosed with estrogen prior to exposure to cytotoxic signals (Villa et al. 2016; Acaz-Fonseca et al. 2014). Astrocytes are needed for estrogen to exert its neuroprotective properties on primary neurons after induction of Aβ-mediated neurotoxicity (Sortino et al. 2004). Similarly, glial fibrillary acidic protein (GFAP) expression, a protein critical for the health and proper function of glia cells is regulated by estrogen in neuron-glia co-cultures and in ovariectomized mice that had been dosed with estrogen (Rozovsky et al. 2002). Conversely, increased reactive gliosis is observed when there is a reduction in estrogen levels in female mice independent of age (Struble et al. 2007).
Here, we hypothesized that estrogen acts as a protective agent for optic nerve head astrocytes exposed to chemically induced oxidative stress. Specifically, we determined if estrogen exerts its glioprotective effects by stopping the cleavage of tau. The results indicate that estrogen prevents caspase-3 activation and the subsequent production of the truncated tau form generated by caspase-3, and ultimately the formation of neurofibrillary tangles (NFT). We further determined that estrogen exerts its protective effects by preventing the dephosphorylation of tau at Ser422, thereby reducing access of active caspase-3 to the caspase-3 cleavage site Asp421 in ONHAs. At the same time, treatment with estrogen also decreased apoptosis in ONHAs undergoing oxidative stress. Together, these results provide evidence for a novel mechanism of action underlying the clinical rationale that preventing the age-related decline in estrogen decreases the susceptibility of the optic nerve to oxidative stress, a component of glaucoma pathogenesis.
Materials and Methods
Cell Culture
Rat optic nerve head astrocytes (ONHAs) were isolated (Brown Norway; age 3 months; male) as primary cells and cultured as described previously (Kaja et al. 2015). ONHAs were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 20% Fetal Bovine Serum (FBS) and 100 U/mL penicillin and 100 mg/mL streptomycin and passaged every 3–4 days. The identity and purity of ONHA cultures was validated with immunocytochemistry measuring immunoreactivity of the astrocyte marker GFAP.
Estrogen and tBHP Treatment
Cells were treated with media containing 50–100 μM tert-butyl hydroperoxide (tBHP) overnight as described previously to induce oxidative stress (Means et al. 2017). For protection assays, cells were pretreated with 25 μM estrogen (17β-estradiol; Steraloids, Inc, Newport, RI; E0950-000; or methanol as a control (vehicle)) for 2 h or 18 h prior to tBHP addition with mock defined as receiving vehicle only.
Cell Viability
Trypan blue was used to determine cell viability as described previously (Means et al. 2017; Matsukawa et al. 2009). The number of viable cells were determined by counting 4 fields of view and setting the untreated as 100%. Viability was determined twenty-four hours after tBHP was added.
Measurement of Caspase Activity
To measure caspase activity, the fluorogenic Ac-DEVD-AFC (Ac-Asp-Glu-Val-Asp-7-Amino-4-trifluoromethylcoumarin, Santa Cruz Biotechnology, Dallas, TX, USA; product number, sc-311274) caspase-3 substrate was used as previously described (Means et al. 2017). Eighteen hours after treatment cells were collected and centrifuged at 2000 × g. The cell pellets were re-suspended in buffer (20 mM HEPES, pH 7.5, 50 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM DTT with protease inhibitor cocktail (Complete ULTRA Tablets, Roche Diagnostics, Indianapolis, IN, USA) and lysed by three freeze/thaw cycles. The lysate was incubated for 1 h at 37 °C with caspase-3 fluorogenic substrate (0.5 μM). Caspase activity was based on the fluorescent products generated by caspase-3 activity and determined fluorometrically (excitation 405 nm, emission 535 nm) and plotted as relative arbitrary fluorescence units.
Antibodies
To detect caspase cleaved tau a mouse monoclonal anti-tau (caspase cleaved at Asp421) antibody (MilliporeSigma, Billerica, MA; product number, MAB5430; 1:500) was used. An anti-tau phospho-Ser422 (GenScript, Piscataway, NJ; product number, A00900; 1:1000) rabbit antiserum was used to detect tau phosphorylated at Ser422. A mouse anti-tau monoclonal antibody (Developmental Studies Hybridoma Bank, Iowa City, IA, USA; product number/clone, 5A6; 1:500) was used to detect full-length tau. A mouse anti-GFAP monoclonal antibody (abcam, Eugene, OR; product number ab4648; clone2A5; 1:1000) was used to detect GFAP. For a loading control a mouse anti-Actin monoclonal antibody (MilliporeSigma; product number, MAB1501R; 1:1000) was used to detect actin.
Thioflavin S Staining
Thioflavin S staining was used to detect fibrillary tangles in ONHAs. Cells were rinsed with distilled water followed by a 5 min fixation at room temperature using 3% PFA treatment. The cells were washed 3 × 5 min with Phosphate Buffered Saline (PBS) and permeabilized (3 min) at room temperature using 0.2% Triton-X-100 in PBS. Cells were washed with PBS (3 × 5 min) and incubated with 0.05% Thioflavin S (Sigma-Aldrich, St. Louis, MO, USA; product number, T1892) in distilled water for 5 min. Cells were washed for 5 min in 70% ethanol. Next, several washes with distilled water (10 × 5 min, 1 × overnight) were performed.
Microscopy
Representative images were obtained using a laser-scanning confocal microscope (Leica TCS SP5). A 63X/1.4 oil immersion objective was used. An argon ion laser (excitation 488 nm, barrier 500–555 nm) was used to visualize Thioflavin S-positive cells. Hoechst 33258 (120 ng/μL; Enzo Life Sciences Inc., Farmingdale, NY) was used to label cell nuclei. Cells were mounted using AquaPolymount (Polysciences Inc., Washington, PA).
Statistical Analysis
Prism5 software (GraphPad Inc., La Jolla, CA, USA) was used for statistical analysis. For densitometric analysis of immunoblots to quantify individual bands ImageJ software (Version 1.50i, Developer: Wayne Rasband, National Institutes of Health, Bethesda, MA, USA) was utilized. Student’s t-test for comparisons between two groups or by one-way analysis of variance (ANOVA) was used to statistically compare means. In addition, the Bonferroni post hoc test was used for various comparisons with treatment conditions (mock, or estrogen) and insults (tBHP) as variables was used. Statistical significance was set at p ≤ 0.05. All experiments were performed in triplicate (n = 3).
Results
Estrogen Protects ONHAs from Oxidative Stress
ONHAs exposed to oxidative stress induced by tBHP showed a decrease in cell viability that was significant (Fig. 1). To determine whether estrogen could act as a protective agent, we pretreated cells with estrogen for either 2 h or 18 h prior to inducing oxidative stress. When compared to vehicle controls, estrogen pretreatment attenuated the reduction in cell viability with the longer pretreatment (18 h) being significantly more protective (Fig. 1). There was no significant difference for the 18 h estrogen pretreatment when compared to the mock treatment, indicating full protection from the oxidative stress insult (Fig. 1).
Fig. 1.
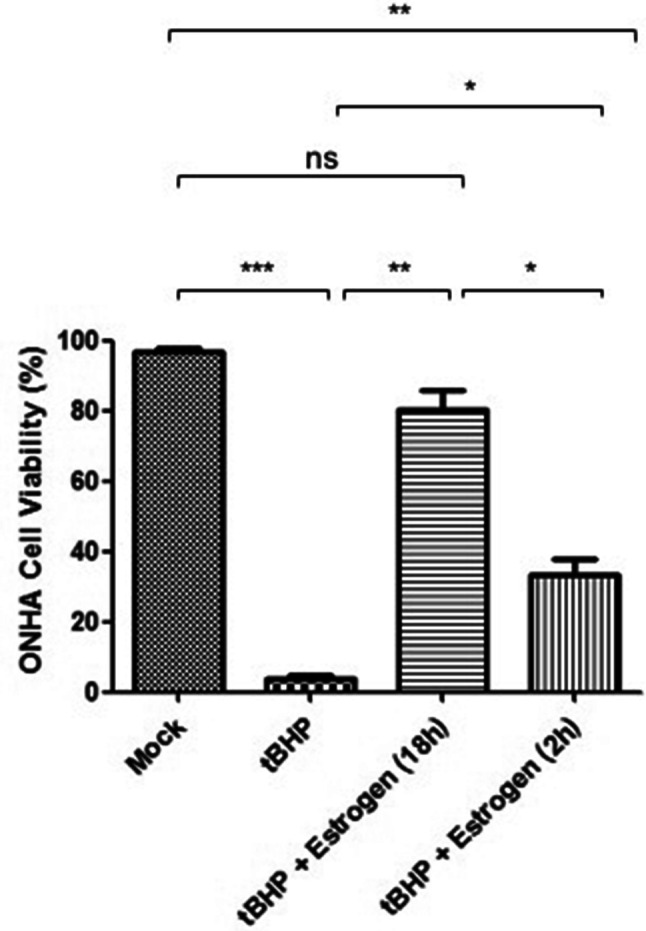
Estrogen preventing tBHP-induced cell death in optic nerve head astrocytes (ONHAs). ONHAs were pretreated with 25 µM estrogen for 2 h or 18 h followed by treatment with tBHP. Twenty-four hours later viability was determined using Trypan Blue. In tBHP-treated cells, there was a significant reduction in cell viability compared to cells that were mock-treated (***p = 0.0002). ONHAs pretreated with estrogen for 2 h (*p = 0.0236) or 18 h (**p = 0.0053) prior to tBHP treatment had a significant increase in viable cells compared to tBHP-treated cells. When compared to mock-treated ONHAs, pretreatment with estrogen for 2 h still showed significantly reduced cell viability (**p = 0.0052), while pretreatment with estrogen for 18 h had no significant difference in viable cells compared to mock-treated (nsp = 0.0982). Estrogen pretreatment for 18 h was significantly more protective than pretreatment for 2 h (*p = 0.0221). Experiments were performed in triplicate (n = 3) and values were depicted as mean ± SEM and analyzed using ANOVA (p = 0.0002). For statistical comparison, the Bonferroni post hoc test and Student’s t-test were used. Statistical significance was set at p ≤ 0.05
Caspase Activation and Tau Cleavage are Induced by Oxidative Stress in ONHAs and Pretreatment with Estrogen Inhibits These Markers of Cell Degeneration
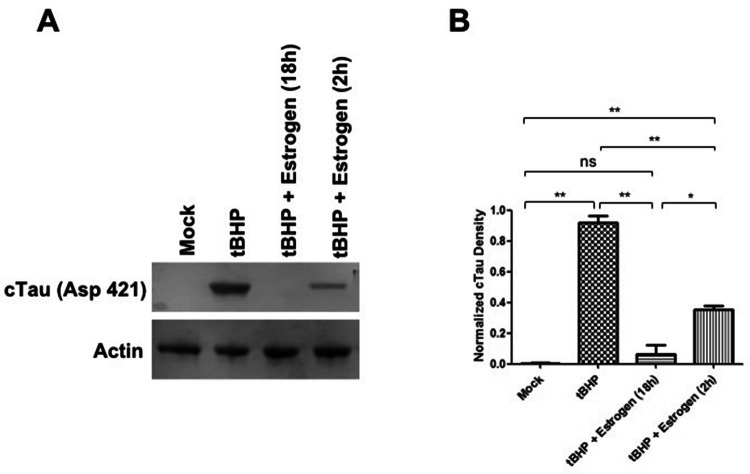
To determine whether oxidative stress leads to caspase activation in ONHAs, we treated cells with tBHP and measured caspase activity. Cells undergoing oxidative stress have high levels of active caspase-3 (Fig. 2). Active caspases during oxidative stress can target tau for proteolytic cleavage, and we detected cleaved tau in ONHAs undergoing oxidative stress (Fig. 3). Next, we wanted to determine whether estrogen is able to attenuate this degenerative process and could act as a neuroprotective agent during oxidative stress. ONHAs were pretreated with estrogen for either 2 h or 18 h followed by tBHP addition. Estrogen pretreatment significantly inhibited caspase activation at either of the two pretreatment times, but the longer pretreatment (18 h) fully inhibited caspase activation when compared to the mock treatment (Fig. 2). Consequently, the reduced caspase activity that resulted from the estrogen treatment also led to a significant reduction in cleaved tau (cTau) for both, the 2 h and the 18 h pretreatment times (Fig. 3) with the longer pretreatment (18 h) reducing cTau production to mock treatment levels (Fig. 3B).
Fig. 2.
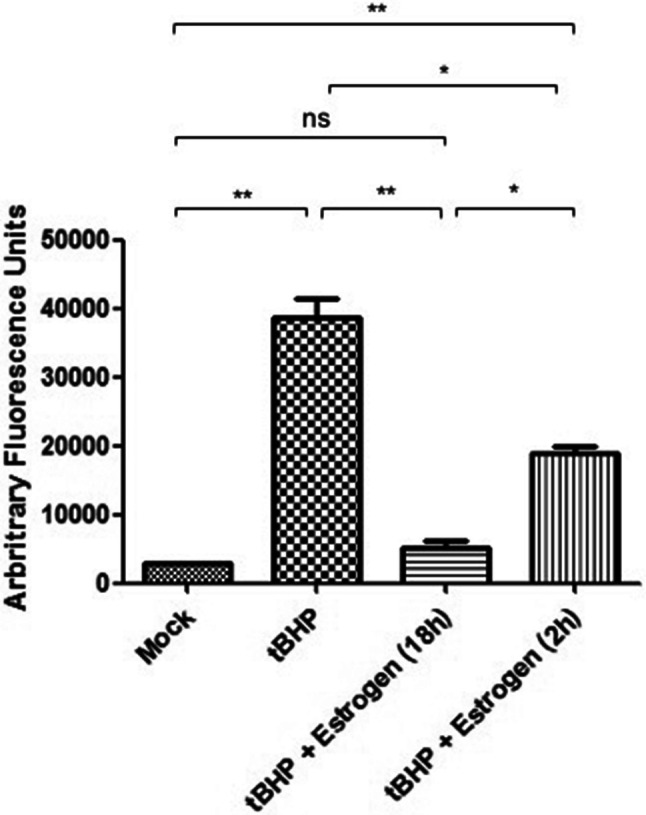
Estrogen inhibiting oxidative stress-induced caspase activation in ONHAs. Oxidative stress in ONHAs were induced using tBHP and caspase activity was measured using Ac-DEVD-AFC. Caspase activity in ONHAs that were treated with tBHP was significantly higher compared to mock-treated (**p = 0.0058). ONHAs pretreated with 25 µM estrogen for 2 h (*p = 0.0208) or 18 h (**p = 0.0077) before tBHP addition had a significant decrease in caspase activity compared to tBHP-treated cells. Estrogen pretreatment for 18 h was significantly more effective at inhibiting caspase activity compared to a 2 h pretreatment (*p = 0.0144). ONHAs pretreated for 2 h with estrogen had a significant level of active caspases versus mock-treated (**p = 0.0035), while pretreatment with estrogen for 18 h showed no significant difference in caspase activity versus mock-treated (nsp = 0.1974). Experiments were done in triplicate (n = 3) and values were depicted as mean ± SEM and analyzed using ANOVA (p = 0.0003). For statistical comparison, the Bonferroni post hoc test and Student’s t-test were used. Statistical significance was set at p ≤ 0.05
Fig. 3.
Estrogen inhibiting oxidative stress-induced tau cleavage in ONHAs. ONHAs were pretreated with 25 µM estrogen followed by induction of oxidative stress with tBHP. a tBHP-treated ONHAs were used in immunoblotting assays to measure cleaved tau (cTau) levels. For a loading control actin was used. b tBHP-treated ONHAs had a significant amount of cTau compared to mock-treated (**p = 0.0025). Estrogen pretreatment for 2 h led to a significant decrease in detectable cTau (**p = 0.0083). Estrogen pretreatment for 18 h further decreased cTau levels significantly (**p = 0.0078). Pretreatment with estrogen for 18 h was more effective than a 2 h pretreatment in attenuating cTau levels (*p = 0.0486). ONHAs pretreated with estrogen for 2 h still showed a significant amount of cTau compared to mock (**p = 0.0055). Pretreatment of ONHAs for 18 h had no significant difference from mock (nsp = 0.4565). Experiments were done in triplicate (n = 3) and values were depicted as mean ± SEM and analyzed using ANOVA (p = 0.0003). For statistical comparison, the Bonferroni post hoc test and Student’s t-test were used. Statistical significance was set at p ≤ 0.05
Tau Dephosphorylation at Ser422 is Prevented by Estrogen
Under normal physiological conditions, tau is phosphorylated at Ser422. This residue is important for tau proteolysis and located immediately adjacent to the caspase cleavage site, Asp421 (Fig. 4). During oxidative stress, Ser422 is dephosphorylated in ONHAs (Fig. 4a). Pretreatment of these cells with estrogen prevents this dephosphorylation at Ser422 with the 18 h pretreatment maintaining tau phosphorylation at mock treatment levels (Fig. 4a, b).
Fig. 4.
Dephosphorylation of tau at Ser422 blocked by estrogen in ONHAs. ONHAs pretreated with 25 µM estrogen were exposed to tBHP to induce oxidative stress. a Phosphorylation of Tau at Ser422 was examined by immunoblotting. ONHAs showed Ser422 tau phosphorylation under control conditions (see mock). b tBHP-treated ONHAs had a significant reduction in tau phosphorylation versus mock (**p = 0.0027). ONHAs pretreated with estrogen for 2 h followed by tBHP treatment had a significant increase in phosphorylated tau versus ONHAs treated with tBHP alone (*p = 0.0482). When estrogen pretreatment was increased to 18 h tau, phosphorylation was increased significantly when compared to tBHP treatment (**p = 0.0037). Estrogen pretreatment for 18 h was more effective than a 2 h pretreatment (**p = 0.0085) in restoring phosphorylation of tau at Ser422. ONHAs pretreated with estrogen for 18 h (nsp = 0.5598) followed by tBHP treatment showed no significant difference from mock-treated ONHAs, while the 2 h pretreatment still showed a significant difference compared to mock-treated (**p = 0.0070). Experiments were done in triplicate (n = 3) and values were depicted as mean ± SEM and analyzed using ANOVA (p = 0.0002). For statistical comparison, the Bonferroni post hoc test and Student’s t-test were used. Statistical significance was set at p ≤ 0.05
Estrogen Reverses Oxidative Stress-Induced NFT Formation in ONHAs
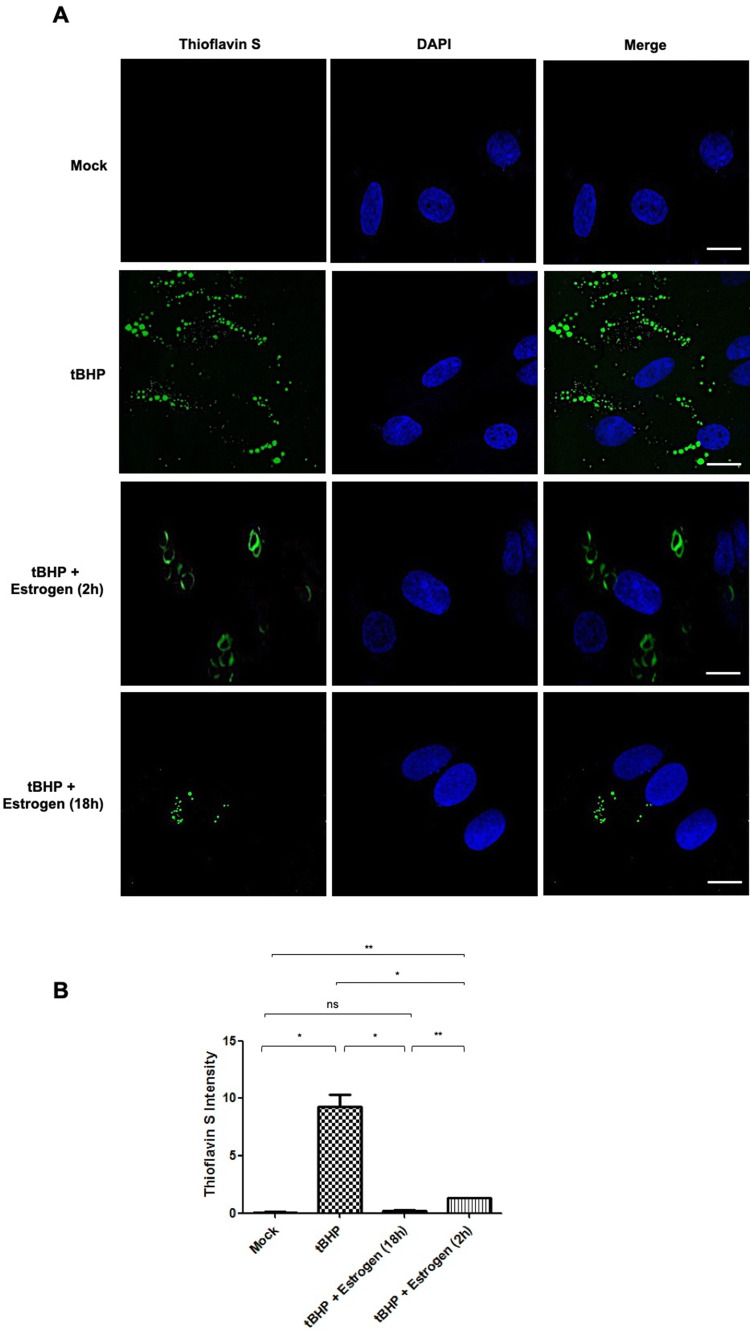
Oxidative stress in ONHAs induced by tBHP treatment leads to a significant amount of Thioflavin S-positive cells, a histochemical marker that labels NFTs (Fig. 5). When ONHAs are pretreated with estrogen the number of Thioflavin S-positive cells was significantly reduced (Fig. 5a, b) with the extent of the reduction dependent on the length of the pretreatment with the longer duration (18 h) being significantly more effective than the shorter period (2 h). The longer pretreatment with estrogen led to a reduction in NFTs that showed no statistical difference to mock treatment (Fig. 5b). Interestingly, Thioflavin S staining in the 2 h estrogen pretreatment condition, while significantly more intense than in the 18 h estrogen pretreatment condition is more diffuse potentially indicating not only a reduction in the total amount of proteolytically cleaved cTau contributing to NFT formation but also a concomitant change in tangle formation itself (Fig. 5a).
Fig. 5.
Estrogen preventing NFT formation induced by tBHP treatment in optic nerve head astrocytes (ONHAs). a ONHAs were pretreated with 25 µM estrogen followed by induction of oxidative stress using tBHP. Production of NFTs was determined by staining with Thioflavin S (green). b tBHP-treated cells showed significant Thioflavin S staining, indicative of NFT formation, compared to mock (*p = 0.0129). Cells pretreated with estrogen for 2 h showed a significant reduction of Thioflavin S staining compared to cells treated with tBHP-treated (*p = 0.0171). In addition, NFTs appeared hallo like and more diffuse for the 2 h pretreatment. Increasing pretreatment time to 18 h with estrogen reduced NFT formation significantly (*p = 0.0134) compared to tBHP-treated cells. Pretreatment with estrogen for 18 h was significantly more effective than the 2 h pretreatment (**p = 0.0024). ONHAs pretreated with estrogen for 2 h still had significant NFT formation compared to mock-treated (**p = 0.0024). Estrogen pretreatment for 18 h was the most effective showing no significant difference in the level of NFT formation compared with mock-treated ONHAs (nsp = 0.1326). Experiments were done in triplicate (n = 3) and values were depicted as mean ± SEM and analyzed using ANOVA (p = 0.0007). For statistical comparison, the Bonferroni post hoc test and Student’s t-test were used. Statistical significance was set at p ≤ 0.05
Estrogen Attenuates Increased GFAP Expression, a Marker of Astroglial Activation, in ONHAs Exposed to Oxidative Stress
ONHAs undergoing oxidative stress display a significant increase in GFAP levels (Fig. 6). When ONHAs are pretreated with estrogen prior to tBHP treatment, the levels of GFAP remain unchanged using the mock treatment for comparison (Fig. 6). While this prevention of astrocyte activation by preincubation with estrogen was highly significant, there was no significant difference with respect to the two estrogen pretreatment times tested (Fig. 6b).
Fig. 6.

During oxidative stress induced by tBHP estrogen stabilizes GFAP levels in optic nerve head astrocytes (ONHAs). a GFAP levels are elevated during oxidative stress as determined by immunoblot assay. b During oxidative stress GFAP levels are significantly upregulated versus mock (**p = 0.0020). 25 µM estrogen pretreatment for 18 h (**p = 0.0022) or 2 h (**p = 0.0074) significantly reduced GFAP levels compared to tBHP-treated ONHAs. When compared to mock-treated ONHAs both 2 h (nsp = 0.3054) and 18 h (nsp = 0.0954) estrogen pretreatment had no significant changes to the levels of GFAP. GFAP levels showed no significant difference when comparing estrogen pretreatment for 2 h or 18 h (nsp = 0.4926). Experiments were done in triplicate (n = 3) and values were depicted as mean ± SEM and analyzed using ANOVA (p = 0.0002). For statistical comparison, the Bonferroni post hoc test and Student’s t-test were used. Statistical significance was set at p ≤ 0.05
Discussion
The development of neurodegenerative disorders such as glaucoma and AD has been linked to decreasing levels of estrogen during aging (Dewundara et al. 2016; Newman-Casey et al. 2014; Moffat et al. 2004; Rosario et al. 2004). In addition, it has been suggested that hormone replacement therapy in women who are post-menopausal can reduce the risk of AD and glaucoma, delay the start of disease, and make cognitive or visual function better, respectively (Newman-Casey et al. 2014; Brinton 2001; Polo-Kantola and Erkkola 2001; Balderechi et al. 1998; Kawas et al. 1997; Paganini-Hill and Henderson 1996).
Mechanisms underlying estrogen’s function as a neuro- and cytoprotective agent have not been fully elucidated. Our results show that estrogen blocks caspase-3 activation (Fig. 2) and regulates the phosphorylation state of tau at Ser422 (Fig. 4), thereby preventing tau cleavage (Fig. 3) and the generation of toxic tau fragments and NFTs (Figs. 3, 5, 6).
Previous work has shown that estrogen has protective effects against various noxious stimuli. For instance, estrogen can protect against apoptosis induced by staurosporine and hydrogen peroxide (Honda et al. 2001; Sur et al. 2003). Potential anti-apoptotic mechanisms of action include the following: estrogen increases the anti-apoptotic protein Bcl-xL levels (Pike 1999). Estrogen attenuates caspase-3 activation, thereby preventing apoptosis (Celsi et al. 2004; Jover et al. 2002). However, neuroprotective properties of estrogen have been associated with a range of signaling pathways and networks including the attenuation of neuroinflammation and oxidative stress, affecting both Aβ and tau protein (Merlo et al. 2017).
Given that tau, a microtubule stabilizing protein, is regulated by phosphorylation at multiple sites that control its function and turnover depending on phosphorylation status, neuroprotective mechanisms involving estrogen signaling mediated effects targeting tau and specifically tau phosphorylation are potentially of high clinical relevance (Arendt et al. 2016; Johnson 2006). Under disease conditions, tau phosphorylation can become dysregulated leading to the buildup of hyperphosphorylated tau, which can aggregate into neurofibrillary tangles. This then can activate multiple signaling cascades that eventually lead to cell death contributing to the pathogenesis of neurodegenerative disorders, such as AD (Arendt et al. 2016; Morris et al. 2011). Estrogen promotes the dephosphorylation of tau in SH-SY5Y neuronal cells and primary rat cortical neurons, where cortical neurons from females are more sensitive to estrogen when compared to the males (Zhang and Simpkins 2010; Alvarez-de-la-Rosa et al. 2005). In the present study, tau phosphorylation at Ser422 is necessary to prevent the proteolytic cleavage of tau. Dephosphorylation of this site allows caspases access to tau leading to the generation of tau fragments that more readily aggregate.
Caspase-3 tau cleavage was deemed an early event that occurs before tau hyperphosphorylation was being detected (Gamblin et al. 2003). In addition, caspase-3 cleaved tau has been detected prior to the onset of apoptosis and NFT formation (Rissman et al. 2004; Ugolini et al. 1997). Tau proteolysis is also mediated by calpain and can be detected before tau hyperphosphorylation occurs (Park and Ferreira 2005).
Estrogen and testosterone can both function individually as neuroprotective signaling molecules through the differential regulation of proteases targeting tau: while testosterone inhibits the activation of calpain, which generates a 17 kDa tau fragment, it does not affect caspase-3 activity resulting in tau truncation at Asp421, the protease signaling pathway attenuated by estrogen (Park et al. 2007). Our results indicate that protective properties of estrogen can also be found in glia, specifically astrocytes: estrogen protects ONHAs from oxidative stress as measured by attenuation of GFAP levels as a surrogate marker of astroglial activation and inhibits caspase-3 activation resulting in reduced tau cleavage by caspase-3, thereby preventing NFT formation. This is in line with previous work indicating estrogen-mediated control of GFAP expression as a protective mechanism (Rozovsky et al. 2002). While both 2 h and 18 h preincubation with estrogen effectively reduced caspase-3 activation and tau proteolytic cleavage, the longer preincubation time showed greater effects. This parallels findings in hippocampal neurons where estrogen-mediated protection from Aβ-induced neurotoxicity was effective not only after 24 h, but also after 2 h preincubation with estrogen prior to insult (Park et al. 2007).
Oxidative stress leads to the buildup of free radicals, which has been connected with numerous neurodegenerative diseases, including AD and glaucoma (Munemasa and Kitaoka 2013; Behl et al. 1995). Antioxidants can be used to safeguard cells against oxidative stress-induced cell damage and death by inhibiting oxidation and thereby preventing free radical formation (Niki and Nakano 1990). Here, we induced oxidative stress using tBHP, thereby modeling the cellular environment encountered during neurodegeneration. Estrogen acting directly as an antioxidant has been shown to protect mouse hippocampal HT22 cells and embryonic rat hippocampal cells from free radical damage and subsequent apoptosis (Behl et al. 1995; Behl 1997; Mooradian 1993), providing an alternate, potentially parallel pathway for the protection of ONHAs identified here.
Conclusion
Our data support the notion that proteolytic cleavage of tau contributes to the degeneration of the neural retina, specifically in ONHAs, during oxidative stress. In addition, we show that this degenerative signaling pathway can be attenuated by estrogen indicating that estrogen-mediated pharmacological control of neurodegeneration in the retina and optic nerve is feasible. At the same time, specific components of these signaling pathways such as caspase-3 and specific tau phosphorylation and cleavage sites represent additional potential therapeutic targets.
Acknowledgements
The authors thank Margaret, Richard and Sara Koulen for generous support and encouragement.
Abbreviations
- Aβ
Amyloid β–peptide
- AD
Alzheimer’s disease
- ANOVA
Analysis of variance
- CNS
Central nervous system
- GFAP
Glial fibrillary acidic protein
- NFT
Neurofibrillary tangle
- ONH
Optic nerve head
- ONHA
Optic nerve head astrocyte
- PBS
Phosphate buffered saline
- PFA
Paraformaldehyde
- tBHP
Tert-butyl hydroperoxide
Author Contributions
JCM and PK conceived and designed the experiments; JCM, AAL and PK performed the experiments; JCM, AAL, and PK analyzed the data and wrote the paper. All authors read and approved the final manuscript.
Funding
Research reported in this publication was supported in part by grants from the National Institutes of Health, National Eye Institute Grants EY014227 and EY022774, National Institute on Aging Grant AG027956, National Center for Research Resources/National Institute of General Medical Sciences grant RR027093 (PK) and a National Institutes of Health Clinical and Translational Science Award Grant (UL1 TR002366) awarded to the University of Kansas. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional support by the Felix and Carmen Sabates Missouri Endowed Chair in Vision Research and a Challenge Grant from Research to Prevent Blindness (PK) is gratefully acknowledged.
Data Availability
The datasets of the present study are available from the corresponding author on reasonable request.
Compliance with Ethical Standards
Conflict of interest
All authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Acaz-Fonseca E, Sanchez-Gonzalez R, Azcoitia I, Arevalo MA, Garcia-Segura LM (2014) Role of astrocytes in the neuroprotective actions of 17β-estradiol and selective estrogen receptor modulators. Mol Cell Endocrinol 389(1–2):48–57. 10.1016/j.mce.2014.01.009 [DOI] [PubMed] [Google Scholar]
- Alvarez-de-la-Rosa M, Silva I, Nilsen J, Perez MM, Garcia-Segura LM, Avila J, Naftolin F (2005) Estradiol prevents neural tau hyperphosphorylation characteristic of Alzheimer's disease. Ann NY Acad Sci 1052:210–224. 10.1196/annals.1347.016 [DOI] [PubMed] [Google Scholar]
- Arendt T, Stieler JT, Holzer M (2016) Tau and tauopathies. Brain Res Bull 126(Pt3):238–292. 10.1016/j.brainresbull.2016.08.018 [DOI] [PubMed] [Google Scholar]
- Arevalo MA, Santos-Galindo M, Bellini MJ, Azcoitia I, Garcia-Segura LM (2010) Actions of estrogens on glial cells: implications for neuroprotection. Biochim Biophys Acta 1800(10):1106–1112. 10.1016/j.bbagen.2009.10.002 [DOI] [PubMed] [Google Scholar]
- Balderechi M, Di Carlo A, Lepore V, Bracco L, Maggi S, Grigoletto F, Scarlato G, Amaducci L (1998) Estrogen-replacement therapy and Alzheimer's disease in the Italian longitudinal study on aging. Neurology 50(4):996–1002 [DOI] [PubMed] [Google Scholar]
- Barreto GE (2016) Targeting astrocytes in brain injuries: a translational research approach. Prog Neurobiol 144:1–4. 10.1016/j.pneurobio.2016.09.001 [DOI] [PubMed] [Google Scholar]
- Bayer AU, Ferrari F, Erb C (2002) High occurrence rate of glaucoma among patients with Alzheimer’s disease. Eur Neurol 47:165–168. 10.1159/000047976 [DOI] [PubMed] [Google Scholar]
- Behl C (1997) Amyloid beta-protein toxicity and oxidative stress in Alzheimer’s disease. Cell Tissue Res 290:471–480 [DOI] [PubMed] [Google Scholar]
- Behl C, Widmann M, Trapp T, Holsboer F (1995) 17-beta estradiol protects neurons from oxidative stress-induced cell death in vitro. Biochem Biophys Res Commun 216:473–482. 10.1006/bbrc.1995.2647 [DOI] [PubMed] [Google Scholar]
- Brinton RD (2001) Cellular and molecular mechanisms of estrogen regulation of memory function and neuroprotection against Alzheimer's disease: recent insights and remaining challenges. Learn Mem 8(3):121–133. 10.1101/lm.39601 [DOI] [PubMed] [Google Scholar]
- Celsi F, Ferri A, Casciati A, D’Ambrosi N, Rotilio G, Costa A, Volonte C, Carri MT (2004) Overexpression of superoxide dismutase 1 protects against beta-amyloid peptide toxicity: effect of estrogen and copper chelators. Neurochem Int 44(1):25–33. 10.1016/S0197-0186(03)00101-3 [DOI] [PubMed] [Google Scholar]
- Chiasseu M, Vargas JLC, Destroismaisons L, Velde CV, Leclerc N, Di Polo A (2016) Tau accumulation, altered phosphorylation, and missorting promote neurodegeneration in glaucoma. J Neurosci 36(21):5785–5798. 10.1523/JNEUROSCI.3986-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewundara SS, Wiggs JL, Sullivan DA, Pasquale LR (2016) Is estrogen a therapeutic target for glaucoma? Sem Ophthal 31(1–2):140–146. 10.3109/08820538.2015.1114845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhandapani KM, Brann DW (2007) Role of astrocytes in estrogen-mediated neuroprotection. Exp Gerontol 42(1–2):70–75. 10.1016/j.exger.2006.06.032 [DOI] [PubMed] [Google Scholar]
- Engler-Chiurazzi EB, Singh M, Simpkins JW (2015) From the 90's to now: a brief historical perspective on more than two decades of estrogen neuroprotection. Brain Res 1633:96–100. 10.1016/j.brainres.2015.12.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler-Chiurazzi EB, Brown CM, Povroznik JM, Simpkins JW (2016) Estrogens as neuroprotectants: estrogenic actions in the context of cognitive aging and brain injury. Prog Neurobiol 157:188–211. 10.1016/j.pneurobio.2015.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamblin TC, Chen F, Zambrano A, Abraha A, Lagalwar S, Guillozet AL, Lu M, Fu Y, Garcia-Sierra F, LaPointe N, Miller R, Berry RW, Binder LI, Cryns VL (2003) Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer's disease. Proc Natl Acad Sci USA 100(17):10032–10037. 10.1073/pnas.1630428100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerlings MI, Ruitenberg A, Witteman JC, van Swieten JC, Hofman A, van Duijn CM, Breteler MM, Launer LJ (2001) Reproductive period and risk of dementia in postmenopausal women. JAMA 285(11):1475–1481. 10.1001/jama.285.11.1475 [DOI] [PubMed] [Google Scholar]
- Honda K, Shimohama S, Sawada H, Kihara T, Nakamizo T, Shibasaki H, Akaike A (2001) Nongenomic antiapoptotic signal transduction by estrogen in cultured cortical neurons. J Neurosci Res 64(5):466–475. 10.1002/jnr.1098 [DOI] [PubMed] [Google Scholar]
- Johnson GV (2006) Tau phosphorylation and proteolysis: insights and perspectives. J Alzheimers Dis 9(3 Suppl):243–250 [DOI] [PubMed] [Google Scholar]
- Jover T, Tanaka H, Calderone A, Oguro K, Bennett MV, Etgen AM, Zukin RS (2002) Estrogen protects against global ischemia-induced neuronal death and prevents activation of apoptotic signaling cascades in the hippocampal CA. J Neurosci 22(6):2115–2124. 10.1523/JNEUROSCI.22-06-02115.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaja S, Yang SH, Wei J, Fujitani K, Liu R, Brun-Zinkernagel AM, Simpkins JW, Inokuchi K, Koulen P (2003) Estrogen protects the inner retina from apoptosis and ischemia-induced loss of Vesl-1L/Homer 1c immunoreactive synaptic connections. Invest Ophthalmol Vis Sci 44(7):3155–3162. 10.1167/iovs.02-1204 [DOI] [PubMed] [Google Scholar]
- Kaja S, Payne AJ, Naumchuk Y, Levy D, Zaidi DH, Altman AM, Nawazish S, Ghuman JK, Gerdes BC, Moore MA, Koulen P (2015) Plate reader-based cell viability assays for glioprotection using primary rat optic nerve head astrocytes. Exp Eye Res 138:159–166. 10.1016/j.exer.2015.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawas C, Resnick S, Morrison A, Brookmeyer R, Corrada M, Zonderman A, Bacal C, Lingle DD, Metter E (1997) A prospective study of estrogen replacement therapy and the risk of developing Alzheimer's disease: the Baltimore longitudinal study of aging. Neurology 48(6):1517–1521 [DOI] [PubMed] [Google Scholar]
- Laws KR, Irvine K, Gale TM (2016) Sex differences in cognitive impairment in Alzheimer's disease. World J Psychiatry 6(1):54–65. 10.5498/wjp.v6.i1.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukawa N, Yasuhara T, Hara K, Xu L, Maki M, Yu G, Kaneko Y, Ojika K, Hess DC, Borlongan CV (2009) Therapeutic targets and limits of minocycline neuroprotection in experimental ischemic stroke. BMC Neurosci 10:126. 10.1186/1471-2202-10-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means JC, Gerdes BC, Koulen P (2017) Distinct mechanisms underlying resveratrol-mediated protection from types of cellular stress in C6 glioma cells. Int J Mol Sci 18(7):1521. 10.3390/ijms18071521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo S, Spampinato SF, Sortino MA (2017) Estrogen and Alzheimer's disease: still an attractive topic despite disappointment from early clinical results. Eur J Pharmacol 817:51–58. 10.1016/j.ejphar.2017.05.059 [DOI] [PubMed] [Google Scholar]
- Moffat SD, Zonderman AB, Metter EJ, Kawas C, Blackman MR, Harman SM, Resnick SM (2004) Free testosterone and risk for Alzheimer disease in older men. Neurology 62(2):188–193. 10.1212/WNL.62.2.188 [DOI] [PubMed] [Google Scholar]
- Mooradian A (1993) Antioxidant properties of steroids. J Steroid Biochem Mol Biol 45:509–511 [DOI] [PubMed] [Google Scholar]
- Morris M, Maeda S, Vossel K, Mucke L (2011) The many faces of tau. Neuron 70(3):410–426. 10.1016/j.neuron.2011.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemasa Y, Kitaoka Y (2013) Molecular mechanisms of retinal ganglion cell degeneration in glaucoma and future prospects for cell body and axonal protection. Front Cell Neurosci 6:60. 10.3389/fncel.2012.00060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman-Casey PA, Talwar N, Nan B, Musch DC, Pasquale LR, Stein JD (2014) The potential association between postmenopausal hormone use and primary open-angle glaucoma. JAMA Ophthal 132(3):298–303. 10.1001/jamaophthalmol.2013.7618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niki E, Nakano M (1990) Estrogens as antioxidants. Methods Enzymol 186:330–333. 10.1016/0076-6879(90)86126-G [DOI] [PubMed] [Google Scholar]
- Paganini-Hill A, Henderson VW (1996) Estrogen replacement therapy and risk of Alzheimer disease. Arch Intern Med 156(19):2213–2217 [PubMed] [Google Scholar]
- Park SY, Ferreira A (2005) The generation of a 17 kDa neurotoxic fragment: an alternative mechanism by which tau mediates beta-amyloid-induced neurodegeneration. J Neurosci 25(22):5365–5375. 10.1523/JNEUROSCI.1125-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Tournell C, Sinjoanu RC, Ferreira A (2007) Caspase-3- and calpain-mediated tau cleavage are differentially prevented by estrogen and testosterone in beta-amyloid-treated hippocampal neurons. Neuroscience 144(1):119–127. 10.1016/j.neuroscience.2007.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike CJ (1999) Estrogen modulates neuronal Bcl-xL expression and beta-amyloid-induced apoptosis: relevance to Alzheimer's disease. J Neurochem 72(4):1552–1563. 10.1046/j.1471-4159.1999.721552.x [DOI] [PubMed] [Google Scholar]
- Pike CJ (2017) Sex and the development of Alzheimer's disease. J Neurosci Res 95(1–2):671–680. 10.1002/jnr.23827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike CJ, Carroll JC, Rosario ER, Barron AM (2009) Protective actions of sex steroid hormones in Alzheimer's disease. Front Neuroendocrinol 30(2):239–258. 10.1016/j.yfrne.2009.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo-Kantola P, Erkkola R (2001) Alzheimer's disease and estrogen replacement therapy—where are we now? Acta Obstet Gynecol Scand 80(8):679–682. 10.1034/j.1600-0412.2001.080008679.x [DOI] [PubMed] [Google Scholar]
- Prokai-Tatrai K, Xin H, Nguyen V, Szarka S, Blazics B, Prokai L, Koulen P (2013) 17β-estradiol eye drops protect the retinal ganglion cell layer and preserve visual function in an in vivo model of glaucoma. Mol Pharm 10(8):3253–3261. 10.1021/mp400313u [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries M, Sastre M (2016) Mechanisms of Aβ clearance and degradation by glial cells. Front Aging Neurosci 8:160. 10.3389/fnagi.2016.00160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman RA, Poon WW, Blurton-Jones M, Oddo S, Torp R, Vitek MP, LaFerla FM, Rohn TT, Cotman CW (2004) Caspase-cleavage of tau is an early event in Alzheimer disease tangle pathology. J Clin Investig 114(1):121–130. 10.1172/JCI20640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario ER, Chang L, Stanczyk FZ, Pike CJ (2004) Age-related testosterone depletion and the development of Alzheimer disease. JAMA 292(12):1431–1432. 10.1001/jama.292.12.1431-b [DOI] [PubMed] [Google Scholar]
- Rozovsky I, Wei M, Stone DJ, Zanjani H, Anderson CVP, Morgan TE, Finch CE (2002) Estradiol (E2) enhances neurite outgrowth by repressing glial fibrillary acidic protein expression and reorganizing laminin. Endocrinology 143(2):636–646. 10.1210/endo.143.2.8615 [DOI] [PubMed] [Google Scholar]
- Ryan J, Scali J, Carrière I, Amieva H, Rouaud O, Berr C, Ritchiea K, Ancelin ML (2014) Impact of a premature menopause on cognitive function in later life. BJOG 121:729–1739. 10.1111/1471-0528 [DOI] [PubMed] [Google Scholar]
- Sortino MA, Chisari M, Merlo S, Vancheri C, Caruso M, Nicoletti F, Canonico PL, Copani A (2004) Glia mediates the neuroprotective action of estradiol on β-amyloid-induced neuronal death. Endocrinology 145(11):5080–5086. 10.1210/en.2004-0973 [DOI] [PubMed] [Google Scholar]
- Struble RG, Nathan BP, Cady C, Cheng X, McAsey M (2007) Estradiol regulation of astroglia and apolipoprotein E: an important role in neuronal regeneration. Exp Gerontol 42(1–2):54–63. 10.1016/j.exger.2006.05.013 [DOI] [PubMed] [Google Scholar]
- Sur P, Sribnick EA, Wingrave JM, Ray SK, Banik NL (2003) Estrogen attenuates oxidative stress-induced apoptosis in C6 glial cells. Brain Res 971(2):178–188. 10.1016/S0006-8993(03)02349-7 [DOI] [PubMed] [Google Scholar]
- Tang MX, Jacobs D, Stern Y, Marder K, Schofield P, Gurland B, Andrews H, Mayeux R (1996) Effect of oestrogen during menopause on risk and age at onset of Alzheimer's disease. Lancet 348(9025):429–432. 10.1016/S0140-6736(96)03356-9 [DOI] [PubMed] [Google Scholar]
- Ugolini G, Cattaneo A, Novak M (1997) Co-localization of truncated tau and DNA fragmentation in Alzheimer's disease neurones. NeuroReport 8(17):3709–3712 [DOI] [PubMed] [Google Scholar]
- Vajaranant TS, Pasquale LR (2012) Estrogen deficiency accelerates aging of the optic nerve. Menopause (New York, NY) 19(8):942–947. 10.1097/gme.0b013e3182443137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa A, Vegeto E, Poletti A, Maggi A (2016) Estrogens, neuroinflammation, and neurodegeneration. Endocr Rev 37(4):372–402. 10.1210/er.2016-1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Simpkins JW (2010) Okadaic acid induces tau phosphorylation in SH-SY5Y cells in an estrogen-preventable manner. Brain Res 1345:176–181. 10.1016/j.brainres.2010.04.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets of the present study are available from the corresponding author on reasonable request.