Abstract
Hepatocellular carcinoma (HCC) surveillance is associated with early tumor detection and improved survival; however, it is often underused in clinical practice. We aimed to characterize surveillance utilization among patients with cirrhosis and the efficacy of interventions to increase surveillance. We performed a systematic literature review using the MEDLINE database from January 2010 through August 2018 to identify cohort studies evaluating HCC surveillance receipt, or interventions to increase surveillance, in patients with cirrhosis. A pooled estimate for surveillance receipt with 95% confidence intervals was calculated. Correlates of surveillance utilization were defined from each study and pre-specified subgroup analyses. Twenty-nine studies, with a total of 118,799 patients, met inclusion criteria, with a pooled estimate for surveillance utilization of 24.0% (95%CI 18.4 – 30.1). In subgroup analyses, the highest surveillance receipt was reported in studies with patients enrolled from subspecialty Gastroenterology/Hepatology clinics and lowest in studies characterizing surveillance in population-based cohorts (73.7% vs. 8.8%, p<0.001). Commonly reported correlates of surveillance included higher receipt among patients followed by subspecialists and lower receipt among those with alcohol- or NASH-related cirrhosis. All eight studies (n=5229) evaluating interventions including patient/provider education, inreach (e.g. reminder and recall systems), and population health outreach strategies reported significant increases (range 9.4% – 63.6%) in surveillance receipt.
Conclusion:
HCC surveillance continues to remain underused in clinical practice, particularly among patients with alcohol- or NASH-related cirrhosis and those not followed in subspecialty gastroenterology clinics. Interventions such as provider education, inreach including reminder systems and population health outreach efforts can significantly increase HCC surveillance.
Keywords: liver cancer, screening, early detection, ultrasound, interventions
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fourth leading cause of cancer-related death worldwide and one of the fastest increasing causes of cancer-related mortality in the United States.1-3 Patients with cirrhosis are the primary at-risk cohort for HCC in the Western world, with an annual incidence of 2-4%, and HCC is a leading cause of death in patients with compensated cirrhosis.3,4 The primary driver of prognosis in HCC patients is tumor stage at diagnosis, with curative options affording 5-year survival exceeding 70% if patients are detected at an early stage. Despite improvements over time, most patients with HCC continue to be detected beyond an early stage and are therefore only eligible for palliative therapies.3
Professional societies including the American Association for the Study of Liver Diseases (AASLD) and European Association for the Study of the Liver (EASL) recommend HCC surveillance in patients with cirrhosis to promote early HCC detection and curative treatment receipt.5,6 Several cohort studies have demonstrated an association between receipt of HCC surveillance and improved survival, even after adjusting for lead time and length time biases.7 However, effectiveness of HCC surveillance to reduce mortality in clinical practice relies on test effectiveness and surveillance utilization. Current surveillance tools, ultrasound and alpha fetoprotein (AFP), have a sensitivity of only ~63% for early HCC detection, with novel imaging and blood-based tests potentially years away from implementation in clinical practice. These data highlight the need for optimizing HCC surveillance utilization.
Implementation of HCC surveillance in clinical practice can be affected by suboptimal patient and provider adherence with surveillance recommendations. Prior studies have suggested many primary care providers have suboptimal knowledge about benefits of HCC surveillance, which can lead to providers not ordering surveillance in at-risk patients.8,9 Patients also report barriers to surveillance completion, such as difficulty with the scheduling process, costs of surveillance testing, and concerns about transportation.10 Accordingly, prior studies have demonstrated that only a minority of patients with cirrhosis undergo HCC surveillance, with even lower rates when considering consistent surveillance every 6 months. Studies have also suggested racial/ethnic and socioeconomic disparities, with lower surveillance rates among racial/ethnic minorities and patients of low socioeconomic status.11
Given increasing data highlighting the underuse of surveillance in clinical practice, there is a clear need for interventions to increase HCC surveillance. Interventions have included system-level e.g. mailed outreach, provider-level such as a best practice advisory, and patient-level such as patient navigation; however, no study has summarized this literature to inform which interventions may be most effective.12-17
The aims of our study were to: 1) quantify utilization of HCC surveillance among patients with cirrhosis, 2) examine socio-demographic correlates of HCC surveillance, and 3) summarize the efficacy of intervention efforts to increase HCC surveillance receipt.
METHODS
Literature Search
We conducted a computer-assisted search with the Ovid interface to Medline to identify relevant published articles. We searched the Medline database from January 1st, 2010 through August 7th, 2018 with the following keyword combinations: [screen$ or surveillance or detect$ or diagnosis] AND [liver ca$ or hepatocellular ca$ or hcc or hepatoma]. Given our focus on current utilization of surveillance within the United States, our search updated a prior systematic review and was limited to human studies published in English after 2010.18 Abstracts from the Digestive Disease Week (DDW), AASLD and EASL conferences from 2017 and 2018 were manually searched for relevant studies. We performed manual searches of references from relevant articles to identify studies that were missed by our computer-assisted search. Finally, we consulted expert hepatologists to identify additional references or unpublished data.
One investigator (E.W.) reviewed all publication titles of citations identified by the search strategy. Potentially relevant studies were retrieved, and selection criteria were applied. The articles were independently checked for inclusion and any uncertainties were resolved through discussion with another author (A.S.). Inclusion criteria included: (i) cohort studies that described receipt of HCC surveillance in patients with cirrhosis and ii) studies published after 2010 so as to be representative of current delivery of care. We excluded studies which characterized receipt of one-time screening and survey studies describing self-reported surveillance utilization, given a bias to over-estimating surveillance receipt. Additional exclusion criteria included non-English language, non-human data, and lack of original data. If publications used the same patient cohort, data from the most recent manuscript were included. The study was conducted in accordance with PRISMA guidelines.
Data Extraction
We independently extracted required information from eligible studies using standardized forms. We collected data regarding the study period, population of interest (patients with cirrhosis vs. patients with HCC), surveillance definition and interval, and duration of follow-up. Data were collected on potential correlates of surveillance receipt including patient age, gender, race/ethnicity, socioeconomic status, and receipt of hepatology care. For the subset of studies assessing interventions to increase surveillance receipt, we recorded a description of the intervention and surveillance receipt in the intervention and control groups. Finally, data were collected on study design, geographic location and date of the study, and number of patients in each study. We assessed the risk of bias for each study using a modified Newcastle-Ottawa scale, which assesses selection of the patient cohort, comparability of study groups, and adequacy of assessing the outcome of interest. Specifically, we assessed: 1) selection of patients (population-based vs. recruited from academic centers), 2) exclusion of patients in whom surveillance is not recommended, e.g. Child C cirrhosis, 3) methods for ascertainment of surveillance receipt, 4) inclusion of cross-sectional imaging toward satisfying need for surveillance imaging, 5) length of follow-up, and 6) reporting of lost to follow-up or death.
Statistical Analysis
Our primary study outcome was HCC surveillance rates among patients with cirrhosis. Surveillance receipt was defined as the proportion of patients who underwent evaluation with repeated imaging and/or AFP prior to HCC diagnosis. The proportion of patients who received surveillance was derived for each study, and 95% confidence intervals were calculated using the adjusted Wald method. A weighed pooled estimate of surveillance rates was computed by multiplying the surveillance rate point estimate for each study by the proportion of individuals with cirrhosis in that study relative to the number of individuals in all included studies. Subset analyses were planned for the following predefined subsets of studies: 1) study location, 2) at-risk population, 3) definition of surveillance, 4) duration of follow-up, and 5) clinical setting including access to subspecialty care. All data analysis was performed using Stata 11 (StataCorp, College Station, TX).
RESULTS
Study Selection
The computer-assisted search yielded 12,728 potentially relevant articles. After initial review, 855 titles were potentially appropriate, and these abstracts were reviewed. Among 69 publications that underwent full-text review, the most common reasons for exclusion were evaluation of one-time screening, duplicate patient cohorts, and non-original data. The remaining 24 studies met all inclusion criteria (Supplemental Figure 1). Recursive literature searches identified 1 additional article and 4 conference abstracts that met inclusion criteria, producing a total of 29 studies (n=118,799 patients) for inclusion in this meta-analysis (Table 1).11,19-43 We also identified 8 studies (n=5,229) evaluating interventions to increase HCC surveillance (Table 2).12-17
Table 1.
Characteristics of Studies
| Author, year | Study Period |
Study Setting | Population (% cirrhosis) | Surveillance Definition | FolLow-Up | No. of patients |
|---|---|---|---|---|---|---|
| Sanyal 2010 | 2002 - 2008 | MarketScan Database, USA | HCV related HCC (100) | q6-12 US ± AFP | NR | 751 |
| NASH related HCC (100) | q6-12 US ± AFP | NR | 1186 | |||
| Davila 2010 | 1994 - 2002 | SEER-Medicare Database, USA | HCC (100) | q12 US ± AFP 2 of 3 years for screening intent |
3 years prior | 1873 |
| Kuo 2010 | 2002 - 2004 | Chang Gung Memorial Hospital, Taiwan | HCC (100) | 2 US ± AFP within 1 year | 1 year prior | 1436 |
| Davila 2011 | 1998 - 2005 | Veterans Affairs System, USA | HCV cirrhosis (100) | US or AFP 2 consecutive years for screening intent | 4 years | 9369 |
| Patwardhan 2011 | 1996 -2010 | Partners Healthcare, USA | Cirrhosis | q12 abdominal imaging | Mean 3.6 (0.3-12.5) | 156 |
| Stroffolini 2011 | 2008 - 2009 | 23 hospitals, Italy | HCC (94.7) | q6 US ± AFP | 1 year prior | 401 |
| Yang 2011 | 2007 - 2009 | Mayo Clinic, USA | HCC (100) | Q6 abdominal imaging | 1 year prior | 368 |
| Singal 2012 | 2005 - 2011 | Parkland Health & Hospital System, USA | HCC (100) | q12 US | 2 years prior | 149 |
| Fenoglio 2013 | 2000 - 2010 | S Croce Hospital, Italy | HCC (91.4) | q6 US | 1 year prior | 256 |
| Singal 2013 | 2000 - 2009 | HALT-C Cohort: USA | HCV cirrhosis (100) | q12 US and AFP | 6.1 years | 408 |
| Palmer 2013 | 2006 - 2007 | North Carolina Medicaid, USA | Cirrhosis | ≥2 abdominal imaging | 1.25 years | 5061 |
| Hasani 2014 | 2010 - 2011 | University of Bern, Switzerland | HCC (100) | q6 US | 1 year prior | 71 |
| Edenvik 2015 | 2005 - 2012 | Karolinska University, Sweden | HCC (82) | ≥67% max interval q8 US | NR | 616 |
| Singal 2015 | 2008 - 2011 | Parkland Health & Hospital System, USA | Cirrhosis | q6 US | 3 years | 786 |
| Thein 2015 | 2000 - 2010 | Ontario Cancer Registry, Canada | Viral-related HCC (51.4) | Q12 US | 2 years prior | 1483 |
| Van Meer 2015 | 2005 - 2012 | 5 academic centers, Netherlands | HCC (100) | 2 tests (imaging ± AFP) in 3 years, with last <18 months prior | 3 years prior | 756 |
| Mittal 2016 | 2004 - 2011 | Veterans Affairs System, USA | HCC (100) | q6-12 imaging | 2 years prior | 556 |
| Signorelli 2016 | 2012 - 2014 | 2 health systems, Brazil | Cirrhosis | q6 US | 4.1 years | 253 |
| Wang 2016 | 1996 - 2013 | 4 California community/academic centers, USA | HBV cirrhosis (100) | q6 imaging | 5.9 (1-15.7) | 164 |
| Aby 2017 | 2009 - 2016 | U California Los Angeles, USA | NASH-related HCC (100) | q6 imaging and AFP | 2 prior | 101 |
| Bucci 2017 | 2000 - 2004 | ITA.LI.CA cohort, Italy | HCC (94.3) | q7 US | ≥1 year prior | 1147 |
| 2010 - 2014 | HCC (90.4) | q7 US | ≥1 year prior | 2421 | ||
| Goldberg 2017 | 2008 - 2010 | Veterans Affairs System, USA | Cirrhosis | >75% PTC imaging | 4.7 (IQR 3.1-6) years | 26577 |
| Mancebo 2017 | 1992 - 2013 | University of Oviedo, Spain | Cirrhosis | q6 imaging ± AFP | 3.5 (IQR 5) years | 770 |
| Nam 2017 | 2007 - 2012 | Seoul National University, Korea | HBV-related HCC (90) | >80% q6, US or CT | 2 years prior | 401 |
| Robinson 2017 | 2014-2017 | Alameda Health System, USA | Cirrhosis | q6-12 US | ≥2 years | 235 |
| Singal 2017 | 2010 - 2012 | Group Health Cooperative, USA | Cirrhosis | q6 US | 2 | 1053 |
| Tran 2018 | 2001-2015 | Stanford University, USA | HCV cirrhosis (100) | q6 imaging | 2.97 (1.83- 5.17) | 2366 |
| Yeo 2018 | 2007-2014 | MarketScan Database, USA | Cirrhosis | q6 imaging | 114,070 person-years | 43,915 |
| Choi 2019 | 2003 - 2013 | SEER-Medicare Database | HCC | q12 US | 3 prior | 13714 |
AFP— Alpha Fetoprotein; HCC— hepatocellular carcinoma; HCV – hepatitis C virus; NR— not reported; US - ultrasound
Table 2.
Implemented Interventions and Subsequent Outcomes
| Author, year | Study Setting | Study Period |
Intervention | Outcome | Pre-Intervention [n (%)] |
Post-Intervention [n (%)] |
Absolute Difference |
Relative Difference |
|---|---|---|---|---|---|---|---|---|
| Aberra 2013a | U. Michigan, USA | 2008-2011 | Nurse base protocol | One-time abdominal imaging | 119/160b (74.4) | 331/355 (93.2) | 18.8% | 25.3% |
| Kennedy, 2013 | Flinders Medical Center, Australia | 2007-2009 | PCP and patient education, system redesignb | Semi-annual US and AFP for two years | 0/22 (0) | 14/22 (63.6) | 63.6% | - |
| Beste, 2015 | Northwest Veterans Affairs, USA | 2011-2012 | EMR Reminder | ≥2 abdominal imaging within 18 months | 103/564 (18.2) | 218/790 (27.6) | 9.4% | 51.6% |
| Del Poggio, 2015 | 120 PCPs, Italy | 1994-2013 | PCP Education | HCC diagnosed by surveillance | 85/244 (34.8) | 105/190 (55.3) | 20.5% | 58.9% |
| Nazareth 2016 | Royal Perth Hospital, Australia | 2010-2015 | Nurse-led clinic | Semi-annual ultrasound | - | 40/76 (52.6) | - | - |
| Farrell 2017 | Royal Liverpool Hospital, UK | 2009-2013 | Radiology led recall | Semi-annual US | - | 368/804 (45.8) | - | - |
| Bui 2017 | KP Northern California, USA | Not reported | EMR identification and physician extender | 3 abdominal imaging in 2 years | 51/224 (22.8) | 183/224 (81.7) | 58.9% | 258.3% |
| Singal, 2019 | Parkland, Dallas, TX | 2014-2016 | Mailed outreach | Semi-annual US over 18 months | 44/600 (7.3) | 247/1200 (20.6) | 13.3% | 182.2% |
PCP - primary care provider; HCC - hepatocellular carcinoma; US - ultrasound; AFP - alpha fetoprotein; EMR – electronic medical reminder
Singal 2011 provides the comparison cohort for the intervention
System redesign, creation of hepatitis nurse for coordinating surveillance and a patient database with automated recall function
Study Characteristics
Characteristics of included studies are detailed in Tables 1 and 2. Most studies were conducted in the US (n=18), with fewer conducted in Europe (n=7), Asia (n=2), Canada (n=1), and South America (n=1). The majority of studies were cohort studies examining HCC surveillance receipt prior to HCC diagnosis, with 13 characterizing surveillance utilization in patients with cirrhosis. Nearly half of studies evaluated surveillance receipt in academic centers, whereas others were conducted in community practices, the Veterans Affairs system, or using large administrative datasets. Although many early studies used operational definitions for surveillance receipt (e.g. annual ultrasound completed in 2 of 3 years), most studies published after 2013 assessed semi-annual surveillance consistent with AASLD and EASL guideline recommendations.
Surveillance Utilization
Overall, the pooled proportion of patients who underwent surveillance was 24.0% (95%CI 18.4% - 30.1%), although there was a wide range across studies (1.1% - 81.5%) (Figure 1). In subgroup analyses, there was no difference in surveillance receipt between studies conducted among patients with cirrhosis and those with HCC (21.8% vs. 25.8%, p=0.57), studies with duration shorter and longer than 1 year (29.4% vs. 22.0%, p=0.38), or between studies conducted prior to and after 2014 (27.4% vs. 24.0%, p=0.29). However, we found notable geographic variation in surveillance receipt, with the lowest surveillance receipt among studies from the United States compared to those from Europe and Asia (17.8% vs. 43.2% vs. 34.6%, p<0.001) (Supplemental Figure 2B). Similarly, surveillance receipt differed by availability of subspecialty care, with highest surveillance receipt among studies in which patients were enrolled from subspecialty Gastroenterology and Hepatology clinics, intermediate among studies from academic centers including both subspecialty and primary care patients, and lowest among studies reporting population-based cohorts (73.7% vs. 29.5% vs. 8.8%, p<0.001) (Figure 2).
Figure 1.
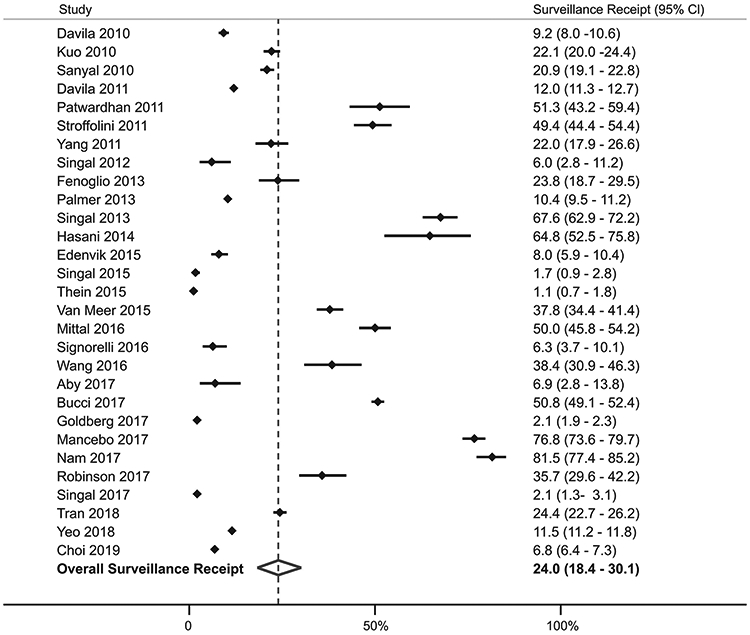
Pooled Surveillance Utilization
Figure 2.
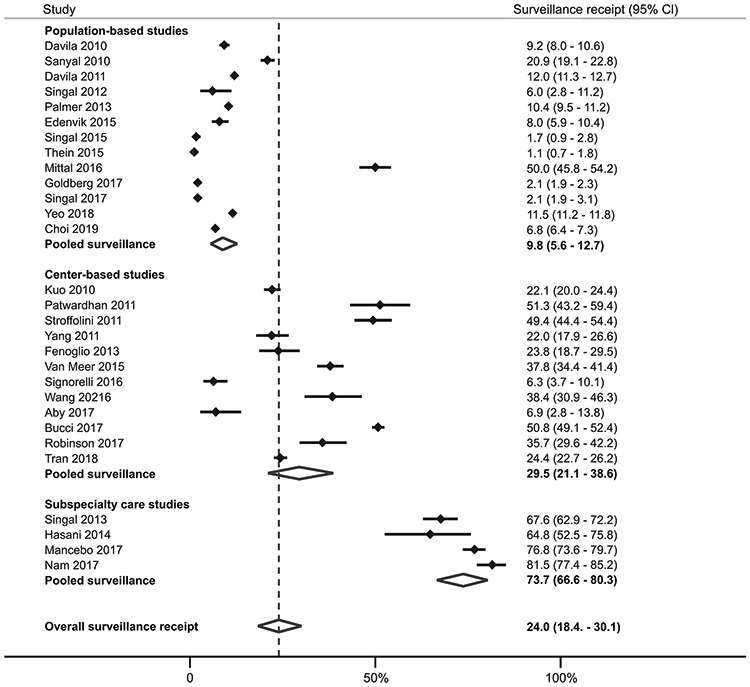
Surveillance utilization, stratified by receipt of subspecialty care
Correlates of Surveillance Utilization
Table 3 describes correlates associated with HCC surveillance utilization. Most studies did not find any significant difference in surveillance receipt by age or sex; however, two studies reported an association between older age with higher surveillance receipt. Similarly, most studies did not report racial/ethnic disparities in HCC surveillance receipt, although two large studies found lower surveillance receipt in Blacks compared to Whites.22,39 Several studies noted differences by liver disease etiology, with lower surveillance in patients with NASH or alcohol-related cirrhosis than other etiologies. Surveillance was less likely in patients with significant medical comorbidities22,29 and those with ongoing alcohol abuse22,26,27,30,31,39,40, likely given perceived lower benefit of HCC surveillance in these subgroups; however many studies found surveillance is more likely in patients decompensated cirrhosis.26,33,40,43 The strongest and most consistent correlates of surveillance receipt across studies were number of clinic visits and receipt of hepatology subspecialty care.
Table 3.
Correlates of Surveillance Utilization for Hepatocellular Carcinoma
| Author, year | Age | Gender | Race | Socioeconomic | Alcohol abuse |
NAFLD/ metabolic syndrome* |
Comorbidities | Liver Decompensation |
Hepatology care |
Number of clinic visits |
|---|---|---|---|---|---|---|---|---|---|---|
| Davila 2010 | NS | + | ||||||||
| Davila 2011 | − (<50) | NS | − (Black) | − | − | + | − | |||
| Patwardhan 2011 | NS | NS | NS | NS | NS | NS | + | |||
| Singal 2012 | NS | NS | NS | NS | − | NS | + | + | NSa | |
| Fenoglio 2013 | − | − | ||||||||
| Palmer 2013 | NS | + (F) | NS | +b | ||||||
| Singal 2013 | NS | NS | NS | NS | NS | NSb | ||||
| Hasani 2014 | NS | NS | + (private insurance) | NS | NS | + | NS | |||
| Edenvik 2015 | NS | NS | − | − | ||||||
| Singal 2015 | NS | + (M) | NS | NS | NS | − | NS | +b | ||
| Thein 2015 | NS | NS | NS | − | NS | NS | NS | +c | ||
| Wang 2016 | NS | NS | NS | NS | +b | |||||
| Goldberg 2017 | +(older) | − (Black) | − | − | +b | |||||
| Mancebo 2017 | NS | NS | − | + | ||||||
| Robinson 2017 | NS | NS | + | + | ||||||
| Singal 2017 | NS | NS | NS | − | − | NS | NS | + | NSa | |
| Tran 2018 | + (>54) | NS | + (Asian) | NS | + | +c |
NAFLD – nonalcoholic fatty liver disease; NS – not significant; ND – not done
Factors with (−) indicate a negative correlation, (+) a positive correlation
considered metabolic syndrome if any component is reported
primary care visits
hepatology visits
unclear specialty visit
Quality Assessment
The quality assessment of individual studies is demonstrated in Table 4. Many of the studies (n=16) assessed surveillance receipt among patients followed at academic centers, with only 13 using population-based registries or cohorts from large integrated health systems. Nearly all studies included patients in whom HCC surveillance is not recommended, such as those with Child Pugh C cirrhosis or significant medical comorbidity, which may have resulted in a lower pooled point estimate for surveillance receipt. Similarly, 14 studies used medical records to determine surveillance utilization and 17 studies did not account for non-ultrasound imaging, both of which may have resulted in ascertainment bias and an underestimation of surveillance receipt. Finally, some studies had high risk of bias related to short duration of follow-up < 1 year (n=7) or not accounting for patients lost to follow-up (n=4).
Table 4.
Quality assessment of studies
| Author and year | Population-based cohort |
Exclusion Child C or comorbidities |
Ascertainment of surveillance receipt |
Accounting for non-US imaging |
Length of follow-up | Accounting for lost to follow-up |
|---|---|---|---|---|---|---|
| Davila 2010 | High | Low | High | Low | High | High |
| Kuo 2010 | Low | Low | Low | Low | Low | High |
| Sanyal 2010 | High | Low | High | Low | Low | High |
| Davila 2011 | High | Low | High | Low | High | High |
| Patwardhan 2011 | Low | Low | Low | High | High | High |
| Stroffolini 2011 | Low | Low | High | Low | Low | High |
| Yang 2011 | Low | Low | Low | High | Low | High |
| Singal 2012 | High | Low | High | Low | High | High |
| Fenoglio 2013 | Low | Low | Low | Low | Low | High |
| Singal 2013 | Low | High | High | Low | High | High |
| Palmer 2013 | High | Low | High | High | High | Low |
| Hasani 2014 | Low | Low | Low | Low | Low | High |
| Edenvik 2015 | High | Low | High | Low | Low | High |
| Singal 2015 | High | Low | High | Low | High | Low |
| Thien 2015 | High | Low | High | Low | High | High |
| Van Meer 2015 | Low | Low | Low | High | High | High |
| Mittal 2016 | High | Low | High | High | High | High |
| Signorelli 2016 | Low | Low | Low | Low | High | Low |
| Wang 2016 | Low | Low | Low | High | High | High |
| Aby 2017 | Low | Low | Low | High | High | High |
| Bucci 2017 | Low | Low | Low | Low | High | High |
| Goldberg 2017 | High | Low | High | High | High | High |
| Mancebo 2017 | Low | High | Low | High | High | High |
| Nam 2017 | Low | Low | Low | High | High | High |
| Robinson 2017 | Low | Low | Low | Low | High | High |
| Singal 2017 | High | Low | High | Low | High | High |
| Tran 2018 | Low | Low | Low | High | High | High |
| Yeo 2018 | High | Low | High | High | High | Low |
| Choi 2019 | High | Low | High | Low | High | High |
Interventions to Increase Surveillance Utilization
We identified eight studies that evaluated the efficacy of interventions to increase HCC surveillance (Table 2). In a study evaluating the efficacy of primary care provider education alone, Del Poggio and colleagues found a significant increase in the proportion of HCC detected by surveillance after the education program in the intervention group (55.3% vs. 34.8%), whereas the proportion of HCC detected by surveillance did not significantly differ in others (39.2% vs. 25.9%). Five studies found significant increases in surveillance utilization using inreach efforts such as electronic medical record (EMR) reminders or nurse-based protocols. Aberra and colleagues found a nurse-based surveillance protocol increased one-time abdominal imaging, despite high baseline surveillance use given all patients were followed by hepatology subspecialists at an academic center (74.4% to 93.2%). Bui and colleagues similarly reported that a dedicated pharmacist-led team increased adequate HCC surveillance (3 imaging studies within 24 months) among patients with cirrhosis followed in a large community practice (22.8% vs. 81.7%), with the largest relative difference in surveillance utilization among all studies. Nazareth et al found a nurse-led clinic yielded semi-annual ultrasound surveillance in 368 (52.6%) of 804 patients. Farrell et al also evaluated a radiology-led recall protocol for patients enrolled in HCC surveillance and found 368 (45.8%) of 804 patients completed semi-annual surveillance imaging. Kennedy and colleagues found an automated reminder system, paired with provider and patient education, increased consistent semi-annual HCC surveillance over two-years from 0% to 63.6% in a small cohort of 22 cirrhosis patients. In the largest study evaluating inreach to date, Beste and colleagues found an EMR reminder alert in the Veterans Affairs system increased adequate HCC surveillance (≥2 imaging studies within 18 months) from 18.2% to 27.6% among cirrhosis patients, whereas control sites without the intervention had no appreciable change in surveillance utilization (16.1% vs. 17.5%). In this study, many patients were followed by primary care providers and surveillance use remained low post-intervention. Finally, Singal and colleagues conducted a large randomized controlled trial evaluating a population health outreach strategy in a safety-net health system among 1800 patients identified as having cirrhosis using ICD-9 codes. In this study, one-time screening within 6 months significantly increased from 24.3% in the usual care visit-based screening arm to 44.5% in the mailed outreach arm; the addition of patient navigation did not significantly increase one-time screening completion (47.2%) compared to outreach alone. In a follow-up study, the team found continued benefits of outreach and navigation over longer periods of time; semi-annual surveillance over an 18-month period was performed in 23.3% of outreach/navigation patients, 17.8% of outreach-alone patients and 7.3% of usual care patients (p<0.001 for both vs. usual care and p=0.02 for outreach ± navigation).
DISCUSSION
Despite the clinical practice guidelines developed by multiple professional societies, our meta-analysis reveals that HCC surveillance utilization continues to be suboptimal in the clinical setting. Surveillance varied widely depending on study setting, with utilization in gastroenterology and hepatology clinics approaching 75% compared to as low as <10% in large population-based cohorts. Consistently observed correlates of surveillance across studies included higher receipt with subspecialty gastroenterology care and lower receipt in patients with alcohol- or NASH-related cirrhosis – increasingly common etiologies of HCC. There have been few studies evaluating interventions to increase surveillance utilization; however, tested interventions appear promising, with relative increases of 60-80%.
We found low receipt of HCC surveillance in this meta-analysis, with a pooled estimate of only 24%. These data highlight minimal improvement over time compared to the 18% pooled estimate reported in a prior systematic review characterizing surveillance receipt in studies through 2010.18 These data highlight HCC surveillance use is substantially lower than that of other cancer screening programs including colorectal, breast, and cervical cancer, with screening rates of approximately 60, 80, and 90% respectively in 2015.44 Lower utilization of HCC surveillance has been attributed to multiple factors including poor provider knowledge of surveillance guidelines, under recognition of cirrhosis or liver disease, and patient-reported barriers. 10,26,50 Survey studies among primary care providers in both safety-net and academic settings found multiple provider-reported barriers including lack of knowledge about surveillance benefits and limited time in clinic with competing clinical concerns.10,50 Prior chart review studies also suggest providers may have difficulty recognizing the at-risk population, with approximately one-third of HCC patients having unrecognized cirrhosis at time of HCC presentation.26 In contrast, unlike the poor patient adherence seen in colorectal cancer screening ranging from 40-50%,45,46 adherence to HCC surveillance has not historically believed to be a major issue.11,26 However, recent data have highlighted that patient-level barriers such as costs of ultrasound and uncertainty where to get testing completed may result in lower surveillance receipt.10
One of the most consistent correlates of HCC surveillance receipt across studies was receipt of subspecialty care. This association was reinforced by subgroup analyses, with the highest surveillance receipt among studies in which patients were enrolled from subspecialty Gastroenterology and Hepatology clinics and lowest among studies reporting population-based cohort, in which many patients were likely followed by primary care providers. Although we also noted variation by geographic location, this was likely driven by type of studies in each area, with most population-based cohort studies from the United States and most studies from Europe being conducted in academic centers. This association may be related to higher provider awareness of HCC surveillance and its potential benefits. Whereas most gastroenterologists strongly believe HCC surveillance is associated with reduced mortality, many primary care providers believe HCC surveillance is associated with early detection but express a desire for more data showing reduced mortality and quantifying possible screening-related harms.50 Studies also noted lower HCC surveillance in patients with alcohol- or NASH-related cirrhosis, which is concerning given these etiologies account for an increasing proportion of HCC cases. Studies have suggested increased difficulty recognizing chronic liver disease or cirrhosis in these patients prior to HCC presentation, compared to chronic hepatitis C cirrhosis; however, further studies should explore other potential barriers such as differential medical comorbidity or patient adherence.
Despite extensive literature highlighting underuse of HCC surveillance, we identified only 7 studies evaluating interventions to increase HCC surveillance. Most evaluated inreach strategies with or without provider education, such as EMR reminders or nurse-led surveillance protocols. Each study reported significant increases in HCC surveillance, although this was only effective for patients who had a clinic visit during the study period. One study evaluating population health outreach reported significant differences in surveillance receipt – both for patients who were actively seen in clinic as well as those without clinic visits. Although each study including patients followed by primary care providers reported improved surveillance receipt, post-intervention surveillance use remained at ~50% or less highlighting the need for more intensive interventions, including potential for multi-level interventions combining inreach and outreach. It is possible that other advances in HCC surveillance, including biomarker-based testing, may also reduce barriers to completion and increase surveillance utilization.
We noted the current literature evaluating HCC surveillance utilization has several limitations. First, studies used varying definitions for HCC surveillance with some using a guideline-concordant definition of semi-annual surveillance but others using operational definitions, e.g. receipt of two imaging studies over an 18-24 month period. Clear and standardized surveillance definitions across studies should be used to provide an accurate interpretation and analysis of surveillance rates. Defining surveillance using a time interval of every six months would only count patients of perfect adherence towards surveillance rates. One potential measure that incorporates frequency and number of tests during a period of interest is the proportion of time up to date with screening, which gives a more continuous measure of screening adherence. Second, there was wide variation of enrollment periods and follow-up intervals between studies, and studies have shown that adherence decreases dramatically over time.49 Although we attempted to reduce the effect of short follow up times by excluding studies that included one-time screening events, some studies encompassed a follow-up time of over ten years, while others limited the follow-up period to 1 year. Third, few studies described reasons for surveillance underuse, which is an important step to inform effective intervention strategies. It is possible that surveillance “underuse” may have been appropriate in some cases if patients had comorbid conditions or liver dysfunction and surveillance was not recommended. Finally, most studies evaluating interventions have been conducted in single-center settings with unclear generalizability, have short durations of follow-up with unclear long-term sustainability of intervention effect, and there are no comparative effectiveness data, so optimal intervention strategies have not been defined.
In summary, this systematic review and meta-analysis highlights that HCC surveillance continued to be underutilized, with only 1 in 4 patients with cirrhosis receiving surveillance. HCC surveillance underuse appears particularly problematic among patients with non-viral liver disease and those followed by primary care providers or outside academic centers. It is clear interventions are needed to increase HCC surveillance. The current literature evaluating such intervention strategies is limited, although each strategy significantly improved surveillance utilization and provides a blueprint to improve early tumor detection and reduce HCC-related mortality.
Supplementary Material
Acknowledgments
Financial Source: This study was conducted with support from National Institute of Health U01 CA230694 and U01 CA230669. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding agency had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation of the manuscript
Footnotes
Conflicts of Interest:
Neehar Parikh has served as a consultant or on advisory boards for Bayer, Eisai, Bristol-Myers Squibb, Exelixis, Eli Lilly, Wako Diagnostics, Exact Sciences, and Freenome. He has received research funding from Exact Sciences, Glycotest, Bayer, and Target Pharmasolutions.
Jorge Marrero has served as a consultant for Glycotest.
Amit Singal has served as a consultant or on advisory boards for Bayer, Eisai, BMS, Exelixis, Wako Diagnostics, Exact Sciences, Roche, Glycotest, Freenome, and TARGET.
None of the other authors have any relevant conflicts of interest.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913–21. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011;365:1118–27. [DOI] [PubMed] [Google Scholar]
- 4.Benvegnu L, Gios M, Boccato S, Alberti A. Natural history of compensated viral cirrhosis: a prospective study on the incidence and hierarchy of major complications. Gut 2004;53:744–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358–80. [DOI] [PubMed] [Google Scholar]
- 6.European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182–236. [DOI] [PubMed] [Google Scholar]
- 7.Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med 2014;11:e1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalton-Fitzgerald E, Tiro J, Kandunoori P, Halm EA, Yopp A, Singal AG. Practice patterns and attitudes of primary care providers and barriers to surveillance of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol 2015;13:791–8 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGowan CE, Edwards TP, Luong MU, Hayashi PH. Suboptimal surveillance for and knowledge of hepatocellular carcinoma among primary care providers. Clin Gastroenterol Hepatol 2015;13:799–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farvardin S, Patel J, Khambaty M, et al. Patient-reported barriers are associated with lower hepatocellular carcinoma surveillance rates in patients with cirrhosis. Hepatology 2017;65:875–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singal AG, Li X, Tiro J, et al. Racial, social, and clinical determinants of hepatocellular carcinoma surveillance. Am J Med 2015;128:90 e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aberra FB, Essenmacher M, Fisher N, Volk ML. Quality improvement measures lead to higher surveillance rates for hepatocellular carcinoma in patients with cirrhosis. Dig Dis Sci 2013;58:1157–60. [DOI] [PubMed] [Google Scholar]
- 13.Beste LA, Ioannou GN, Yang Y, Chang MF, Ross D, Dominitz JA. Improved surveillance for hepatocellular carcinoma with a primary care-oriented clinical reminder. Clin Gastroenterol Hepatol 2015;13:172–9. [DOI] [PubMed] [Google Scholar]
- 14.Del Poggio P, Olmi S, Ciccarese F, et al. A training program for primary care physicians improves the effectiveness of ultrasound surveillance of hepatocellular carcinoma. Eur J Gastroenterol Hepatol 2015;27:1103–8. [DOI] [PubMed] [Google Scholar]
- 15.Farrell C, Halpen A, Cross TJ, Richardson PD, Johnson P, Joekes EC. Ultrasound surveillance for hepatocellular carcinoma: service evaluation of a radiology-led recall system in a tertiary-referral centre for liver diseases in the UK. Clin Radiol 2017;72:338 e11–e17. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy NA, Rodgers A, Altus R, McCormick R, Wundke R, Wigg AJ. Optimisation of hepatocellular carcinoma surveillance in patients with viral hepatitis: a quality improvement study. Intern Med J 2013;43:772–7. [DOI] [PubMed] [Google Scholar]
- 17.Singal AG, Tiro JA, Marrero JA, et al. Mailed Outreach Program Increases Ultrasound Screening of Patients With Cirrhosis for Hepatocellular Carcinoma. Gastroenterology 2017;152:608–15 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singal AG, Yopp A, C SS, Packer M, Lee WM, Tiro JA. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med 2012;27:861–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davila JA, Morgan RO, Richardson PA, Du XL, McGlynn KA, El-Serag HB. Use of surveillance for hepatocellular carcinoma among patients with cirrhosis in the United States. Hepatology 2010;52:132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuo YH, Lu SN, Chen CL, et al. Hepatocellular carcinoma surveillance and appropriate treatment options improve survival for patients with liver cirrhosis. Eur J Cancer 2010;46:744–51. [DOI] [PubMed] [Google Scholar]
- 21.Sanyal A, Poklepovic A, Moyneur E, Barghout V. Population-based risk factors and resource utilization for HCC: US perspective. Curr Med Res Opin 2010;26:2183–91. [DOI] [PubMed] [Google Scholar]
- 22.Davila JA, Henderson L, Kramer JR, et al. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Intern Med 2011;154:85–93. [DOI] [PubMed] [Google Scholar]
- 23.Patwardhan V, Paul S, Corey KE, et al. Hepatocellular carcinoma screening rates vary by etiology of cirrhosis and involvement of gastrointestinal sub-specialists. Dig Dis Sci 2011;56:3316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stroffolini T, Trevisani F, Pinzello G, et al. Changing aetiological factors of hepatocellular carcinoma and their potential impact on the effectiveness of surveillance. Dig Liver Dis 2011;43:875–80. [DOI] [PubMed] [Google Scholar]
- 25.Yang JD, Harmsen WS, Slettedahl SW, et al. Factors that affect risk for hepatocellular carcinoma and effects of surveillance. Clin Gastroenterol Hepatol 2011;9:617–23 e1. [DOI] [PubMed] [Google Scholar]
- 26.Singal AG, Yopp AC, Gupta S, et al. Failure rates in the hepatocellular carcinoma surveillance process. Cancer Prev Res (Phila) 2012;5:1124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fenoglio L, Serraino C, Castagna E, et al. Epidemiology, clinical-treatment patterns and outcome in 256 hepatocellular carcinoma cases. World J Gastroenterol 2013;19:3207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singal AG, Nehra M, Adams-Huet B, et al. Detection of hepatocellular carcinoma at advanced stages among patients in the HALT-C trial: where did surveillance fail? Am J Gastroenterol 2013;108:425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al Hasani F, Knoepfli M, Gemperli A, et al. Factors affecting screening for hepatocellular carcinoma. Ann Hepatol 2014;13:204–10. [PubMed] [Google Scholar]
- 30.Edenvik P, Davidsdottir L, Oksanen A, Isaksson B, Hultcrantz R, Stal P. Application of hepatocellular carcinoma surveillance in a European setting. What can we learn from clinical practice? Liver Int 2015;35:1862–71. [DOI] [PubMed] [Google Scholar]
- 31.Thein HH, Campitelli MA, Yeung LT, Zaheen A, Yoshida EM, Earle CC. Improved Survival in Patients with Viral Hepatitis-Induced Hepatocellular Carcinoma Undergoing Recommended Abdominal Ultrasound Surveillance in Ontario: A Population-Based Retrospective Cohort Study. PLoS One 2015;10:e0138907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Meer S, de Man RA, Coenraad MJ, et al. Surveillance for hepatocellular carcinoma is associated with increased survival: Results from a large cohort in the Netherlands. J Hepatol 2015;63:1156–63. [DOI] [PubMed] [Google Scholar]
- 33.Goldberg DS, Valderrama A, Kamalakar R, Sansgiry SS, Babajanyan S, Lewis JD. Hepatocellular Carcinoma Surveillance Among Cirrhotic Patients With Commercial Health Insurance. J Clin Gastroenterol 2016;50:258–65. [DOI] [PubMed] [Google Scholar]
- 34.Mittal S, Kanwal F, Ying J, et al. Effectiveness of surveillance for hepatocellular carcinoma in clinical practice: A United States cohort. J Hepatol 2016;65:1148–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nazareth S, Leembruggen N, Tuma R, et al. Nurse-led hepatocellular carcinoma surveillance clinic provides an effective method of monitoring patients with cirrhosis. Int J Nurs Pract 2016;22 Suppl 2:3–11. [DOI] [PubMed] [Google Scholar]
- 36.Signorelli IV, Goncalves PL, Goncalves LL, et al. Socioeconomic disparities in access to a hepatocellular carcinoma screening program in Brazil. Clinics (Sao Paulo) 2016;71:361–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang C, Chen V, Vu V, et al. Poor adherence and low persistency rates for hepatocellular carcinoma surveillance in patients with chronic hepatitis B. Medicine (Baltimore) 2016;95:e4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bucci L, Garuti F, Lenzi B, et al. The evolutionary scenario of hepatocellular carcinoma in Italy: an update. Liver Int 2017;37:259–70. [DOI] [PubMed] [Google Scholar]
- 39.Goldberg DS, Taddei TH, Serper M, et al. Identifying barriers to hepatocellular carcinoma surveillance in a national sample of patients with cirrhosis. Hepatology 2017;65:864–74. [DOI] [PubMed] [Google Scholar]
- 40.Mancebo A, Gonzalez-Dieguez ML, Navascues CA, et al. Adherence to a Semiannual Surveillance Program for Hepatocellular Carcinoma in Patients With Liver Cirrhosis. J Clin Gastroenterol 2017;51:557–63. [DOI] [PubMed] [Google Scholar]
- 41.Nam JY, Lee JH, Kim HY, et al. Oral Medications Enhance Adherence to Surveillance for Hepatocellular Carcinoma and Survival in Chronic Hepatitis B Patients. PLoS One 2017;12:e0166188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singal AG, Tiro J, Li X, Adams-Huet B, Chubak J. Hepatocellular Carcinoma Surveillance Among Patients With Cirrhosis in a Population-based Integrated Health Care Delivery System. J Clin Gastroenterol 2017;51:650–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tran SA, Le A, Zhao C, et al. Rate of hepatocellular carcinoma surveillance remains low for a large, real-life cohort of patients with hepatitis C cirrhosis. BMJ Open Gastroenterol 2018;5:e000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.White A, Thompson TD, White MC, et al. Cancer Screening Test Use - United States, 2015. MMWR Morb Mortal Wkly Rep 2017;66:201–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turner BJ, Weiner M, Yang C, TenHave T. Predicting adherence to colonoscopy or flexible sigmoidoscopy on the basis of physician appointment-keeping behavior. Ann Intern Med 2004;140:528–32. [DOI] [PubMed] [Google Scholar]
- 46.Baker DW, Brown T, Buchanan DR, et al. Comparative effectiveness of a multifaceted intervention to improve adherence to annual colorectal cancer screening in community health centers: a randomized clinical trial. JAMA Intern Med 2014;174:1235–41. [DOI] [PubMed] [Google Scholar]
- 47.Walker M, El-Serag HB, Sada Y, et al. Cirrhosis is under-recognised in patients subsequently diagnosed with hepatocellular cancer. Aliment Pharmacol Ther 2016;43:621–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tan D, Yopp A, Beg MS, Gopal P, Singal AG. Meta-analysis: underutilisation and disparities of treatment among patients with hepatocellular carcinoma in the United States. Aliment Pharmacol Ther 2013;38:703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robinson A, Tavakoli H, Cheung R, Liu B, Bhuket T, Wong RJ. Low Rates of Retention Into Hepatocellular Carcinoma (HCC) Surveillance Program After Initial HCC Screening. J Clin Gastroenterol 2019;53:65–70. [DOI] [PubMed] [Google Scholar]
- 50.Simmons OL, Feng Y, Parikh ND, Singal AG. Primary Care Provider Practice Patterns and Barriers to Hepatocellular Carcinoma Surveillance. Clinical Gastroenterology Hepatology 2019; 17(4): 766–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


