Abstract
Introduction
Tension-type headache (TTH) is the most prevalent primary headache. Every year, about 2–3% of patients with TTH progress to chronic TTH with daily or near-daily headache, warranting preventive treatment. The treatment of chronic TTH is complex and very often associated with significant tolerability issues. To date, melatonin has been studied in only a few small uncontrolled trials. The aim of this surveillance program was to evaluate the efficacy of melatonin (Melaxen®) in patients with TTH and disruption of circadian rhythms in real-world practice.
Methods
Sixty-one patients with chronic TTH were enrolled. After the 30-day baseline period, patients took 3 mg of melatonin at bedtime for 30 days with a follow-up period of another 30 days. VAS pain intensity assessments, Hamilton Anxiety Rating Scale (HAM-A), Hamilton Depression Rating Scale (HAM-D), HIT-6 and Levin sleep quality scores were obtained at the baseline visit, at month 1, and month 2.
Results
A significant decrease in the number of headache days per month, VAS pain intensity, HAM-A, HAM-D and HIT-6 scores, and an improvement in sleep quality were observed throughout the study. No treatment-emergent adverse events were reported.
Conclusions
Melatonin is an effective and safe alternative for the treatment of chronic TTH.
Keywords: Headache, Melatonin, Tension-type headache
Key Summary Points
| Why carry out this study? |
| Tension-type headache (TTH) is the most prevalent primary headache. |
| The treatment of chronic TTH is complex and very often associated with significant tolerability issues. |
| The aim of this surveillance program was to evaluate the efficacy of melatonin in patients with TTH and disruption of circadian rhythms in real-world practice. |
| What was learned from the study? |
| Sixty-one patients with chronic TTH were treated with melatonin 3 mg before bedtime for 30 days with a follow-up of another 30 days. |
| A significant decrease in headache frequency and an improvement in multiple secondary endpoints were observed across the treatment and follow-up phases. |
| Melatonin is a useful treatment for patients with chronic TTH and sleep disorders. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13055717.
Introduction
Tension-type headache (TTH) is the most prevalent primary headache [1]. Large-scale epidemiological studies have shown TTH prevalence in the general population to vary between 42 and 78% [2, 3]. In most cases, TTH keeps its episodic pattern and the pain intensity is low. However, in about 2–3% of patients, TTH progresses to chronic TTH with daily or near-daily headache. This type of TTH warrants prompt preventive treatment [2, 3].
Chronic TTH is the second most prevalent chronic headache after chronic migraine [4]. Moreover, the treatment of chronic TTH is complex. The efficacy of approved medications often does not exceed 38%, and the number of medications with evidence-based efficacy is very low [5]. All medications approved for the preventive treatment of TTH (especially, amitriptyline) are associated with significant tolerability issues [5], and TTH prophylaxis warrants further research. TTH is also very closely associated with psychiatric conditions, primarily affective and anxiety disorders [2, 6].
In addition to high levels of anxiety and depression, chronic TTH is frequently associated with disruption of circadian rhythms and sleep [7]. The relationships between chronic pain and sleep disorders have been shown to be bidirectional. In their meta-analysis, Smith et al. selected 17 well-designed studies, including three large longitudinal studies (1–12 years in length) and showed that elevated insomnia symptoms increase the risk of headache [8]. Moreover, the authors concluded that sleep problems increase the risk of chronic pain in pain-free individuals, worsen the long-term prognosis of existing headache and chronic musculoskeletal pain, and influence daily fluctuations in clinical pain. Conversely, good sleep appeared to improve the long-term prognosis of individuals with chronic pain conditions.
Melatonin may play an important role in pain perception and modulation. It has been extensively studied in the prevention of migraine headache and is very likely to be a promising alternative for migraine prophylaxis [9].
At the same time, data on melatonin treatment for TTH are scarce. Some patients with TTH have been included only in small uncontrolled studies [10, 11]. However, in the recent consensus article published in 2019 by the European Headache Federation, it is clearly stated that in the absence of registered prophylactic drugs for chronic TTH, a broad range of drugs can be attempted resting on individual clinical responsibility [12]. As reported in the current literature melatonin appears to be the safest option.
The aim of this surveillance program was to evaluate the efficacy of melatonin (Melaxen®) in patients with TTH and disruption of circadian rhythms in real-world practice.
Methods
This study was a post-marketing surveillance program to assess the real-world safety and efficacy of melatonin (Melaxen®) in the treatment of patients with chronic TTH and disruption of circadian rhythms in accordance with the local product registration.
The study was approved by the Institute of Interdisciplinary Medicine ethical committee. All subjects signed a written informed consent in accordance with the Declaration of Helsinki.
The trial ran between October 2019 and March 2020 at three trial sites: Volgograd State Medical University, Volgograd (OVK), Rostov-on-Don Regional Headache Center, Rostov-on-Don (EAS), and S.M. Kirov Military Medical Academy, Saint-Petersburg (SAZ).
Eligible subjects were aged 18–65 and diagnosed with chronic TTH, according to the International Classification of Headache Disorders, version 3 [13]. Twenty-seven patients had chronic TTH associated with pericranial tenderness and 34 patients had chronic TTH not associated with pericranial tenderness. All patients had normal self-reported quality and duration of night sleep during the month before enrollment but experienced a discrepancy between the desired and required time of sleep onset. At baseline, subjects rated their pain intensity as over 40 mm at a 100-mm Visual Analogue Scale (VAS) and had no history of intolerance or hypersensitivity to the study drug.
Key exclusion criteria were other neurologic or psychiatric diseases, headaches other than chronic TTH, medication overuse, use of antidepressants, antiepileptics, opiates, or hypnotics at least 30 days before inclusion, pregnancy, and breastfeeding. For headache control, only simple non-steroidal analgesics were allowed.
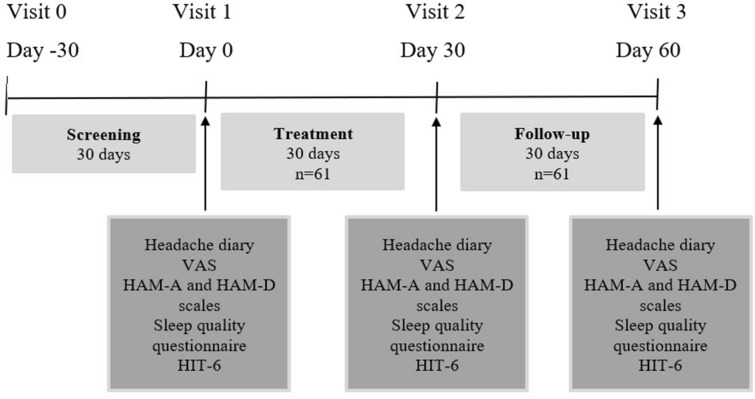
To confirm headache frequency, all subjects completed a headache diary during the 30-day baseline period. After inclusion, patients took 3 mg of melatonin 30 min before going to sleep (not later than 10:30 p.m.) for 30 days with a follow-up period of another 30 days (Fig. 1). VAS pain intensity assessments, Hamilton Anxiety Rating Scale (HAM-A), Hamilton Depression Rating Scale (HAM-D), HIT-6 and Levin sleep quality scores were obtained at the baseline visit, at month 1 and month 2.
Fig. 1.
Study design. VAS Visual Analogue Scale, HAM-A Hamilton Anxiety Scale, HAM-D Hamilton Depression Scale
The primary endpoint was the change in the monthly number of headache days at month 1 (treatment phase) and month 2 (follow-up) compared to the baseline headache frequency. Secondary endpoints included the change in headache intensity, levels of depression, anxiety and HIT-6 disability, and sleep quality at day 30 and day 60 compared to the corresponding baseline values. Change in the analgesic intake during month 1 and month 2 of the study compared to the baseline period was also included into the preplanned analysis. None of the participants dropped out during the 30-day trial and 30-day follow-up period.
The Shapiro–Wilk normality test was performed to evaluate the distribution of clinical, demographic, and behavioral variables. Basic statistics on parameters with normal distribution is presented as mean ± standard deviation, other continuous variables are presented as median and interquartile range (Q25; Q75). Nominal variables are presented in relative (%) frequencies. The comparison between baseline values and treatment results was performed by means of Wilcoxon test for dependent samples. Nominal variables were compared with the two-tailed McNemar test. Significant difference was set at a two-tailed p value of < 0.05. All analyses were performed using IBM SPSS® Statistics.
Results
Patient Demographics
Sixty-one patients with chronic TTH were enrolled. The group consisted of 37 females and 24 males with a mean age of 42.1 ± 13.7 years. Of the patients, 44.2% had associated pericranial tenderness. Enrolled patients had a mean height of 170.9 ± 6.9 cm and mean weight of 75.7 ± 13.6 kg, with the mean BMI averaging 25.3 ± 4.1 kg/cm2. The median systolic blood pressure at the time of inclusion was 130 (Q25 120; Q75 137) mmHg, diastolic blood pressure—80 (Q25 77; Q75 88) mmHg. The baseline median depression level was 7 (Q26 6; Q75 10) points. 50.8% of patients had no HAM-D-defined depression, and 32.8% of patients had mild depression. The median level of anxiety before treatment was 12 (Q25 8; Q75 16) points, corresponding to mild HAM-A defined anxiety.
Treatment Efficacy
Primary Endpoint
After 30 days of melatonin treatment, headache frequency decreased significantly from a median of 20 to 14 days per month and further to 12 days per month after 30 days of follow-up (Fig. 2, Table 1). A median decrease in headache frequency over 33% was observed in 93.4% of patients. In 6.6% of patients, no change in headache frequency was observed. At 30 days after the end of the study (on day 60), a significant decrease in headache frequency was still observed in 29/61 (47.5%) patients.
Fig. 2.
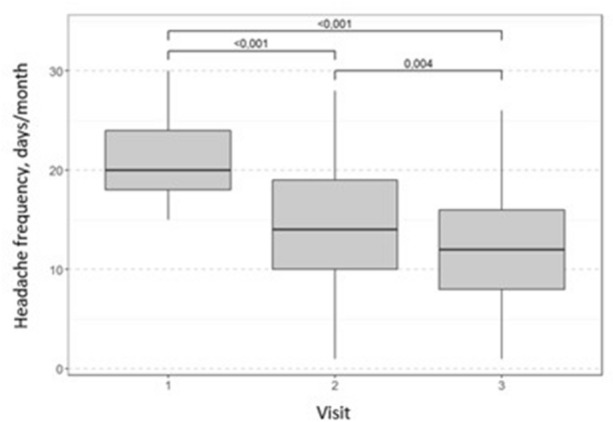
Change from baseline in the median headache frequency throughout the study. Wilcoxon test with Bonferroni correction for multiple comparisons
Table 1.
Change from baseline in the median headache frequency throughout the study
| Visit | Median change (days) | Wilcoxon test, p |
|---|---|---|
| 1–2 | − 6 | < 0.001 |
| 2–3 | − 2 | 0.004 |
| 1–3 | − 8 | < 0.001 |
Secondary Endpoints
Headache intensity decreased significantly from visit 1 to visit 2 at a median of 31% (Fig. 3). This decrease was observed in 56/61 (92%) patients and was stable during the 30-day follow-up period.
Fig. 3.
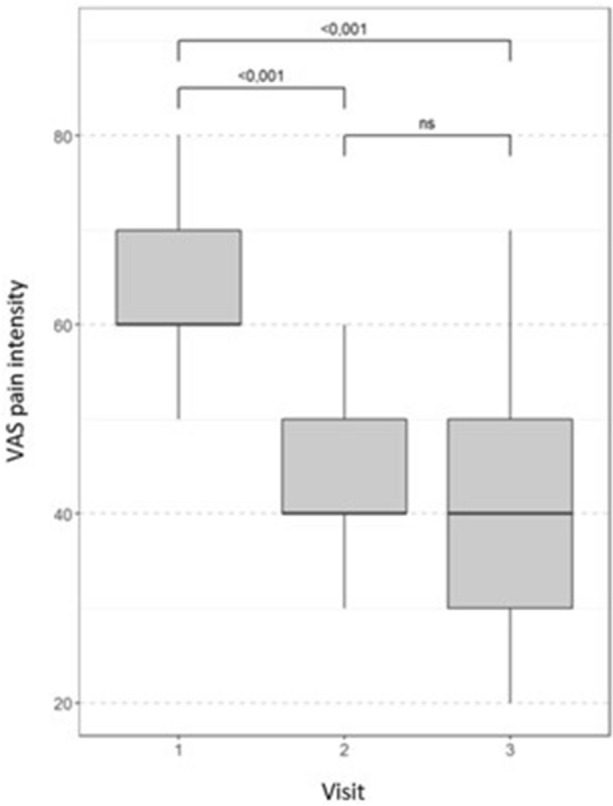
Change from baseline in the median pain intensity throughout the study. Wilcoxon test with Bonferroni correction for multiple comparisons, ns non-significant (p > 0.05), VAS Visual Analogue Scale
The depression level decreased significantly by visit 2 and remained stable during month 2 (Table 2). Anxiety levels also decreased significantly during the treatment period, and remained significant at visit 3, despite the tendency to rise again during the follow-up period.
Table 2.
Change from baseline in the median depression and anxiety levels throughout the study
| Parameter | Visit | Median change (points) | Change (%) | Wilcoxon test, p |
|---|---|---|---|---|
| HAM-D | 1–2 | − 1 | 21.6 | < 0.001 |
| 2–3 | 0 | 0 | 1 | |
| 1–3 | − 1 | 21.6 | < 0.001 | |
| HAM-A | 1–2 | − 4 | 37.3 | < 0.001 |
| 2–3 | 0 | 13 | 0.002 | |
| 1–3 | − 4 | 29.2 | < 0.001 |
After melatonin treatment, the HIT-6 disability score decreased significantly at a median of 13.4%. A significant improvement was observed in 53/61 (87%) patients (Fig. 4). The index remained stable during the follow-up period.
Fig. 4.
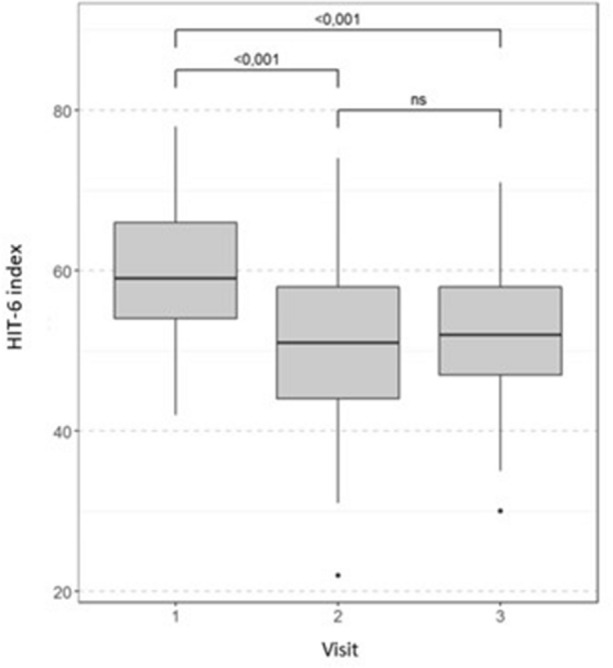
Change from baseline in the median HIT-6 score throughout the study. Wilcoxon test with Bonferroni correction for multiple comparisons, ns non-significant (p > 0.05)
After 30 days of treatment, a significant increase in sleep quality was observed, with a median of 44.6%. This parameter remained stable during the 30-day follow-up period (at day 60) (Fig. 5).
Fig. 5.
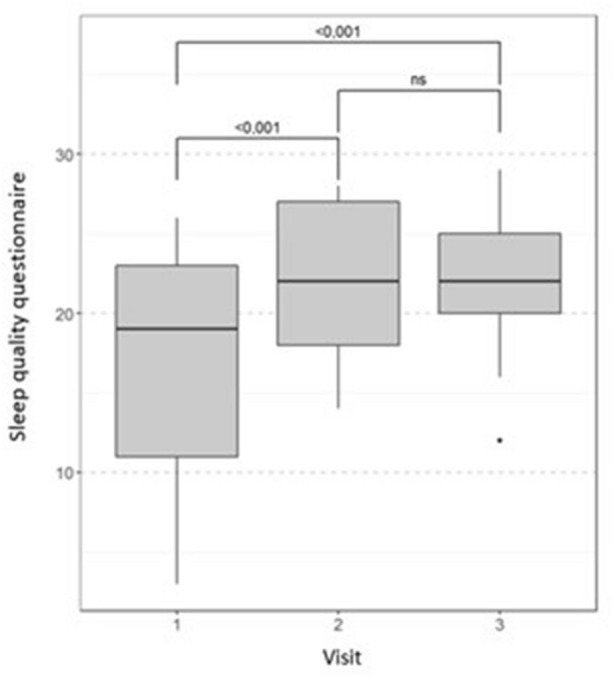
Change from baseline in the median sleep quality throughout the study. Wilcoxon test with Bonferroni correction for multiple comparisons
The prevalence of pericranial tenderness decreased significantly by 48% after 30 days of treatment (Table 3) and was stable during the follow-up period.
Table 3.
Change from baseline in the prevalence of pericranial tenderness
| Pericranial tenderness | Visit | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Yes | 27 | 14 | 13 |
| No | 34 | 47 | 48 |
| McNemar test (p) | 0.0003 | ||
| 1 | |||
| 0.0015 | |||
During the screening period, the median frequency of analgesic intake was 15 (Q25 10; Q75 19) doses per month. During treatment with melatonin, the monthly number of analgesic doses decreased significantly by a median of 42.4% in 52/61 (85%) patients (Table 4). Three (4.9%) patients continued taking the same amount of analgesics, and six (9.8%) patients increased their analgesic use. No further changes in analgesic intake were observed during the 30-day follow-up period.
Table 4.
Change from baseline in the median number of analgesic doses throughout the study
| Visit | Median change (doses) | Change (%) | Wilcoxon test, p |
|---|---|---|---|
| 1–2 | − 7 | − 42.4 | < 0.001 |
| 2–3 | 0 | − 16.2 | 1 |
| 1–3 | − 7 | − 51.7 | < 0.001 |
Treatment Safety
All patients completed the study and the follow-up period. No changes in systolic and diastolic blood pressure or heart rate were observed (Wilcoxon test, p > 0.05) (Table 5). No treatment-emergent adverse events were reported throughout the study.
Table 5.
Cardiovascular parameters
| Parameter | Visit | N | Median | Q25 | Q75 |
|---|---|---|---|---|---|
| Systolic blood pressure, mmHg | 1 | 61 | 130 | 120 | 137 |
| 2 | 61 | 129 | 120 | 134 | |
| 3 | 61 | 129 | 120 | 136 | |
| Diastolic blood pressure, mmHg | 1 | 61 | 80 | 75 | 89 |
| 2 | 61 | 80 | 77 | 88 | |
| 3 | 61 | 83 | 78 | 89 | |
| Heart rate, beats per minute | 1 | 61 | 75 | 70 | 78 |
| 2 | 61 | 73 | 70 | 78 | |
| 3 | 61 | 74 | 69 | 78 |
Discussion
To date, the rationale and perspectives of melatonin use in primary headaches, especially TTH, are understudied. This study aimed to investigate the efficacy and safety of melatonin 3 mg in the treatment of chronic TTH. After 30 days of treatment, patients reported a significant decrease in headache frequency. Secondary endpoints directly affecting the quality of life were also met. A decrease in VAS-defined headache intensity, HAM-D depression and HAM-A anxiety levels, HIT-6 disability score, and an improvement in sleep quality were observed. Additionally, a decrease in the prevalence of pericranial tenderness was reported.
The effect of melatonin was easily observable—the majority of patients reported an over 30% decrease in headache frequency. According to the current recommendations, a decrease in headache frequency of 30% or more in clinical trials is considered sufficient to translate to a clinically meaningful improvement for patients [14]. In a large proportion of patients, this effect continued to be observed throughout the 30-day follow-up period, resulting in lower consumption of analgesics and reduced chance of medication overuse headache.
Another important finding of this study is the stability of the observed improvements in most studied endpoints, including headache intensity, HIT-6 score, and depression severity, during the 30-day follow-up period. However, the anxiety level tended to increase after the end of the treatment phase. We can conclude that long-term improvements in the studied parameters may warrant longer treatment with melatonin, similarly to other medications used for headache prevention.
Antinociceptive effects of melatonin have been demonstrated in various animal pain models including acute, inflammatory, and neuropathic pain [15, 16]. Melatonin as adjunctive therapy has been reported to improve sleep, severity of pain, and tender point count in patients suffering from fibromyalgia [17]. Additionally, patients with temporomandibular disorder taking 5 mg of melatonin for 28 days experienced significant improvements in pain scores (− 44%), pressure pain threshold (+ 39%), and sleep quality; the effect on pain was independent from that on sleep [18]. The antinociceptive effect of melatonin has also been demonstrated in patients suffering from chronic low back pain [19] and irritable bowel syndrome [20].
Melatonin efficacy has also been reported in various primary headaches [21]. Its effect in migraine prevention has been studied most extensively [9, 21, 22], and several authors have documented melatonin effect in cluster headache [21, 23, 24]. However, its efficacy in TTH has been studied in only a single small trial on adult patients. The study by Bougea et al. [10] included 12 patients with chronic TTH who received melatonin 4 mg. The authors report a statistically significant decrease in both headache frequency and HIT-6 score. The effect of melatonin on depression levels was not documented.
The mechanisms of the antinociceptive effect of melatonin remain unclear. Most probably, melatonin acts via a large range of antinociceptive pathways. Firstly, it restores circadian rhythms, thus enhancing adaptive capabilities, which may play an important role in chronic pain [8]. The anxiolytic effect of melatonin documented in a variety of studies may also be of special importance in the treatment of chronic pain [16, 25]. Finally, there is an independent analgesic effect of melatonin on the melatonin receptors in the areas of the brain responsible for pain perception and control and on several other neurotransmitter systems (GABA, opioids, l-arginine/NO pathway, etc.) [24, 26, 27]. Melatonin may also act via GABA, muscarinic, serotonin, α1- and α2-adrenoreceptors located in the brain and dorsal horn of the spinal cord [26]. Additionally, melatonin increases the release of beta-endorphins, blocks calcium channels, reduces NMDA-dependent neuronal activation, and acts as a free radical scavenger [24, 27, 28].
In headache pathophysiology, melatonin has been related to both GABA and glutamate neurotransmission, its antagonistic effects on glutamate release and neurotoxicity in the cerebral cortex have also been reported [29]. Melatonin is also involved in cerebrovascular regulation, where it generally decreases vascular reactivity and the modulation of 5-HT neurotransmission. Melatonin receptors are present in various areas of the brain and spinal cord, including the thalamus, hypothalamus, spinal trigeminal tract, and trigeminal nucleus caudalis, and play an important role in headache and orofacial pain processing [30].
The absolute safety of melatonin opens up important treatment perspectives. In our study, all patients completed the treatment and follow-up phases, and no treatment-related adverse events were reported. This is in line with the reports of other authors who studied the effect of melatonin in the treatment of various diseases and pain syndromes [17, 26], while in controlled studies melatonin has demonstrated a safety profile comparable with that of placebo [9]. Due to its favorable safety profile, melatonin may become an especially useful adjunctive treatment in patients with multiple somatic comorbidities and sleep disorders.
There are certain limitations to our study that could be accounted for in future studies. The main one is the open-label design and the lack of a control group. Other issues to address are the relatively small sample size and short treatment period. On the other hand, to date, this is the largest study of melatonin efficacy in chronic TTH, which also evaluated the dynamics of depression and anxiety levels and headache impact on day-to-day activities.
Future studies with higher doses of melatonin to investigate the dose–response relationship in TTH are warranted. Prospective trials with longer follow-up are also warranted to study the potential length of effect and required treatment duration.
Conclusions
We can conclude that this post-marketing real-world surveillance program has shown the rationale and perspectives for melatonin use in chronic TTH.
Acknowledgements
We thank the participants of the study for their time and willingness to participate in this study.
Funding
The study was funded by Unipharm. No funding or sponsorship was received for the publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Andrei B. Danilov and Alexey B. Danilov are members of the journal’s Editorial Board. Olga V. Kurushina, Elena A. Shestel, Sergey A. Zhivolupov and Nina V. Latysheva declare no conflicts of interest pertaining to this study.
Compliance with Ethics Guidelines
The study was approved by the Institute of Interdisciplinary Medicine ethical committee. All subjects signed a written informed consent in accordance with the Declaration of Helsinki.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Ferrante T, Manzoni GC, Russo M, Camarda C, Taga A, Veronesi L, Pasquarella C, Sansebastiano G, Torelli P. Prevalence of tension-type headache in adult general population: the PACE study and review of the literature. Neurol Sci. 2013;34(Suppl 1):S137–138. doi: 10.1007/s10072-013-1370-4. [DOI] [PubMed] [Google Scholar]
- 2.Crystal SC, Robbins MS. Epidemiology of tension-type headache. Curr Pain Headache Rep. 2010;14(6):449–454. doi: 10.1007/s11916-010-0146-2. [DOI] [PubMed] [Google Scholar]
- 3.Jensen R, Stovner LJ. Epidemiology and comorbidity of headache. Lancet Neurol. 2008;7:354. doi: 10.1016/S1474-4422(08)70062-0. [DOI] [PubMed] [Google Scholar]
- 4.Senthil C, Gunasekaran N. Clinical profile of patients with chronic headache in a tertiary care hospital. Int J Adv Med. 2016;3(3):721–726. [Google Scholar]
- 5.Bendtsen L. Drug and nondrug treatment in tension-type headache. Ther Adv Neurol Disord. 2009;2(3):155–161. doi: 10.1177/1756285609102328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.da Silva Jr A, Costa EC, Gomes JB, et al. Chronic headache and comorbidities: a two-phase, population-based, cross-sectional study. Headache. 2010;50(8):1306–1312. doi: 10.1111/j.1526-4610.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- 7.Rains JC, Davis RE, Smitherman TA. Tension-type headache and sleep. Curr Neurol Neurosci Rep. 2015;15(2):520. doi: 10.1007/s11910-014-0520-2. [DOI] [PubMed] [Google Scholar]
- 8.Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev. 2004;8(2):119–132. doi: 10.1016/S1087-0792(03)00044-3. [DOI] [PubMed] [Google Scholar]
- 9.Long R, Zhu Y, Zhou S. Therapeutic role of melatonin in migraine prophylaxis: a systematic review. Medicine (Baltimore) 2019;98(3):e14099. doi: 10.1097/MD.0000000000014099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bougea A, Spantideas N, Lyras V, Avramidis T, Thomaidis T. Melatonin 4 mg as prophylactic therapy for primary headaches: a pilot study. Funct Neurol. 2016;31(1):33–37. doi: 10.11138/FNeur/2016.31.1.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miano S, Parisi P, Pelliccia A, Luchetti A, Paolino MC, Villa MP. Melatonin to prevent migraine or tension-type headache in children. Neurol Sci. 2008;29(4):285–287. doi: 10.1007/s10072-008-0983-5. [DOI] [PubMed] [Google Scholar]
- 12.Steiner TJ, Jensen R, Katsarava Z, et al. Aids to management of headache disorders in primary care (2nd edition) J Headache Pain. 2019;20:57. doi: 10.1186/s10194-018-0899-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnold M. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211. doi: 10.1177/0333102417738202. [DOI] [PubMed] [Google Scholar]
- 14.Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–121. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Esposito E, Paterniti I, Mazzon E, Bramanti P, Cuzzocrea S. Melatonin reduces hyperalgesia associated with inflammation. J Pineal Res. 2010;49:321–331. doi: 10.1111/j.1600-079X.2010.00796.x. [DOI] [PubMed] [Google Scholar]
- 16.Stefani LC, Muller S, Torres IL, et al. A phase II, randomized, double-blind, placebo controlled, dose-response trial of the melatonin effect on pain threshold of healthy subjects. PLoS ONE. 2013;8(10):e74107. doi: 10.1371/journal.pone.0074107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hussain SA, Al-Khalifa II, Jasim NA, Gorial FI. Adjuvant use of melatonin for treatment of fibromyalgia. J Pineal Res. 2011;50(3):267–271. doi: 10.1111/j.1600-079X.2010.00836.x. [DOI] [PubMed] [Google Scholar]
- 18.Vidor LP, Torres IL, Custódio de Souza IC, Fregni F, Caumo W. Analgesic and sedative effects of melatonin in temporomandibular disorders: a double-blind, randomized, parallel-group, placebo-controlled study. J Pain Symptom Manage. 2013;46(3):422–432. doi: 10.1016/j.jpainsymman.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 19.Kurganova JM, Danilov AB. A role of melatonin in the treatment of low back pain. Zh Nevrol Psikhiatr Im S S Korsakova. 2015;4:30–35. doi: 10.17116/jnevro20151154130-35. [DOI] [PubMed] [Google Scholar]
- 20.Mozaffari S, Rahimi R, Abdollahi M. Implications of melatonin therapy in irritable bowel syndrome: a systematic review. Curr Pharm Des. 2010;16(33):3646–3655. doi: 10.2174/138161210794079254. [DOI] [PubMed] [Google Scholar]
- 21.Gelfand AA, Goadsby PJ. The role of melatonin in the treatment of primary headache disorders. Headache. 2016;56(8):1257–1266. doi: 10.1111/head.12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonçalves AL, Martini Ferreira A, Ribeiro RT, Zukerman E, Cipolla-Neto J, Peres MF. Randomized clinical trial comparing melatonin 3 mg, amitriptyline 25 mg and placebo for migraine prevention. J Neurol Neurosurg Psychiatry. 2016;87(10):1127–1132. doi: 10.1136/jnnp-2016-313458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leone M, D'Amico D, Moschiano F, Fraschini F, Bussone G. Melatonin versus placebo in the prophylaxis of cluster headache: a double-blind pilot study with parallel groups. Cephalalgia. 1996;16(7):494–496. doi: 10.1046/j.1468-2982.1996.1607494.x. [DOI] [PubMed] [Google Scholar]
- 24.Danilov A, Kurganova J. Melatonin in chronic pain syndromes. Pain Ther. 2016;5(1):1–17. doi: 10.1007/s40122-016-0049-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mistraletti G, Umbrello M, Sabbatini G, et al. Melatonin reduces the need for sedation in ICU patients: a randomized controlled trial. Minerva Anestesiol. 2015;81(12):1298–1310. [PubMed] [Google Scholar]
- 26.Wilhelmsen M, Amirian I, Reiter RJ, Rosenberg J, Gögenur I. Analgesic effects of melatonin: a review of current evidence from experimental and clinical studies. J Pineal Res. 2011;51(3):270–277. doi: 10.1111/j.1600-079X.2011.00895.x. [DOI] [PubMed] [Google Scholar]
- 27.Shavali S, Ho B, Govitrapong P, et al. Melatonin exerts its analgesic actions not by binding to opioid receptor subtypes but by increasing the release of beta-endorphin an endogenous opioid. Brain Res Bull. 2005;64:471–479. doi: 10.1016/j.brainresbull.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Ambriz-Tututi M, Rocha-González HI, Cruz SL, Granados-Soto V. Melatonin: a hormone that modulates pain. Life Sci. 2009;84(15–16):489–498. doi: 10.1016/j.lfs.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 29.Peres M, Masruha M, Zukerman E, Moreira-Filho C, Cavalheiro E. Potential therapeutic use of melatonin in migraine and other headache disorders. Expert Opin Investig Drugs. 2006;15(4):367–375. doi: 10.1517/13543784.15.4.367. [DOI] [PubMed] [Google Scholar]
- 30.He Y, Fan W, Xu Y, Liu YL, He H, Huang F. Distribution and colocalization of melatonin 1a-receptor and NADPH-d in the trigeminal system of rat. Peer J. 2019;7:e6877. doi: 10.7717/peerj.6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.