Abstract
This study was aimed at synthesizing polyethyleneimine-coated magnetic nanoparticles and evaluating their effect on pathogenic bacteria. Polyethyleneimine-coated magnetite (PEIMnF) and nickel ferrite (PEINF) nanoparticles were succesfully synthesized and their surface groups, morphology and chemical structures were characterized using ATR-FTIR (Attenuated Total Reflectance Fourrier Transformed Infra-Red) and SEM (Scanning Electron Microscopy). TGA (Thermogravimetric analysis) was used to analyse the thermal behaviour and stability of synthesized nanomaterials. The minimal inhibitory concentration (MIC) values of the polyethylene imine coated magnetite and nickel ferrite nanomaterials against Staphylococcus aureus, Escherichia coli and Candida albicans was found to be 10 mg/mL. Both nanomaterials (PEIMnF and PEINF) showed very excellent and concentration-dependent biofilm inhibition especially at the highest test concentration of 10 mg/mL at which PEIMnF inhibited biofilm formation on E. coli (89.04 ± 0.50%), S. aureus (82.85 ± 2.42%) and C. albicans (91.37 ± 0.66%). At this concentration, PEINF equally inhibited biofilm formations of E. coli (90.48 ± 2.05%), S. aureus (87.04 ± 1.59%) and C. albicans (90.94 ± 1.03%). Only PEINF showed a concentration-dependent violacein inhibition with highest inhibition of 51.2 ± 3.5% at MIC and quorum sensing with inhibition zones of 16.3 ± 1.0 mm at MIC and 11.5 ± 0.5 mm at MIC/2 which could be attributed to the presence of nickel. The nanomaterials inhibited swimming and swarming motilities in Pseudomonas aeruginosa PA01 and it was found that at the same concentration, swimming inhibition was greater than swarming inhibitions and PEINF showed better inhibition than PEIMnF in both models. Polyethyleneimine-coated magnetite and nickel ferrite nanomaterials could be used in overcoming health problems associated with microbial infections and resistance.
Keywords: Polyethyleneimine-coated magnetic nanoparticles, FTIR, SEM, Antibiofilm activity, Quorum-sensing inhibition
Introduction
Magnetic particles have attracted great attention for their unique properties as controllable particle size, monitoring, non-toxicity, large surface area and easy separation based on their compositions and these are important advantages that these materials offer. The surface properties of magnetic nanoparticle allow functionalizing magnetic nanoparticles by various functional groups for a range of applications. In recent years, studies to synthesize safe and cost-effective magnetic particles in new compositions as antimicrobial agents have increased (Tank et al. 2013; Ezhilarasi et al. 2016). Over the last decade, many studies have been devoted to preparing meta-based nanomaterials that possess antibacterial, antiviral and antifungal activities to combat pathogen-related diseases. It should be noted that the emergence of microbial resistance constitutes a global health problem and therefore it is very necessary to search for synthetic substances which can circumvent this phenomenon. Because of the inability of some existing antibiotics to combat resistant bacterial infections, metallic nanoparticles offer a novel potential means of fighting bacteria through various mechanisms (Sibhghatulla et al. 2019). Nanomaterials are promising candidates for application as novel antimicrobial species attracting much interest since nanoparticles have been shown to possess bactericidal activity against some pathogenic microorganisms such as Escherichia coli, Staphylococcus epidermis, and Bacillus subtilis (Gong et al. 2007; Kim et al. 2007) with the added advantage that their surface could be efficiently used for cleaning and purification of aqueous medium and environmental remediation (Blanco-Esqueda et al. 2015). As a good strategy, antimicrobial nanomaterials can be incoporated into the surface of materials such as catheters, implants, medical delivery systems and other coatings, to prevent microbial adhesion or kill the microorganisms after their attachment to biofilms (Pooyan et al. 2020).
In the area of antibacterial agents metal nanoparticles are of a particular interest because they could be synthesized with high surface area with highly potential active sites and much effort has been devoted over the last decade to the development of organic/nonmetallic inorganic nanocompounds with antimicrobial activities for overcoming human infections and illnesses produced by antibiotic-resistant pathogens (Stoimenov et al. 2002; Chen-Yu et al. 2020). As compared with small molecules antimicrobial agents, polymeric antimicrobials have advantages, such as that they are non-volatile, chemically stable, have long-term antimicrobial activity, and are hard to permeate through the skin and equally exhibit prolonged release of antimicrobial agents (Kenawy, 2001; Jeong et al. 2002; Cakmak et al. 2004; Rezvan et al. 2020). Polyethyleneimine (PEI) is a synthetic polymer prepared from aziridine by cationic polymerization and has in its structure primary, secondary and tertiary amino groups (Yudovin-Farber et al. 2010) which could easily combine with nanoparticles to obtain improved antimicrobial substances.
Surface hydrophilicity, surface roughness of magnetic particles, ionic strength and pH are important factor for the microbial attachment process (Sanpo et al. 2012). The magnetic particles interact directly to the microbial cells and break down to the cell envelope and exchange its metabolism (nucleic acid or protein synthesis) (Prodaan et al. 2013). Magnetite is a mixed iron mineral which has a reverse spinal structure and exhibits ferrimagnetic properties. Magnetite can be obtained by precipitation method (Wu et al. 2007), microemulsion techniques (Chhabra et al. 1996), polyol method (Sun et al. 2011) and sonochemical synthesis (Enomoto et al. 1996). Nickel ferrite is one of the most important members of the spinel ferrite family. Nickel ferrite can be prepared thermal method (Heegn et al. 2000) sol–gel (Vivekanandhan et al. 2004), mechanochemical synthesis (Sepelak et al. 2007), microemulsion (Kale et al. 2004) and electrospinning (Li et al. 2010). The magnetic spinel ferrite nanoparticles are widely used for various areas of biotechnology and bioindustry. They offer many advantages due to its low coercivity, high curie temperature, high electromagnetic performance, high saturation magnetization and chemical stability (Sanpo et al. 2012).
Studies about preparation of new magnetic polymeric materials (MPs) with different magnetic nanoparticles are continued (Ho and Li 2008). MPs have different sizes, different shapes, different roughness and different surface area. MPs comprise of a magnetic centre and polymeric coating. The structure of the polymeric material can be changed due to the aim of the application. A variety of polymers such as pullulan, polystyrene, polyacrylamide, poly(N-isopropylacrylamide), poly(L-lactic-co-glycolic) acid, albumin, cellulose etc. have been used for preparation of these magnetic polymeric materials (Gupta and Gupta 2005; Grodzinski et al. 2006; Osaka et al. 2006; Zintchenko et al. 2006).
In this work, polyethylene imine coated magnetite (PEIMnF) and nickel ferrite (PEINF) nanoparticles were synthesized and evaluated for their quorum sensing mediated processes in pathogenic bacteria.
Materials and methods
Synthesis of magnetite (Fe3O4) nanoparticles
The co-precipitating ferrous and ferric salts method was used for preparing Fe3O4 magnetic particles. 1.4 g of FeSO4.7H2O and 4 g of Fe2(SO4)3.H2O were dissolved in 50 mL deionised water. The solution was mixed with 37.5 mL of 8 M NH4OH solution with stirring at room temperature. The mixture was incubated at 80 °C for 30 min. The Fe3O4 particles were separated and washed several times with deionised water. Then, the particles were dried at incubator at 70 °C (Doğaç et al. 2016).
Synthesis of nickel ferrite (Fe[NiFe]O4) nanoparticles
The co-precipitating nickel and ferric salts method was used for preparing Fe[NiFe]O4 magnetic particles. 1.85 g of Ni(NO3)2.6H2O and 5 g of Fe2(SO4)3.H2O were dissolved in 50 mL deionised water. The solution was mixed with 25 mL of 2 M NaOH solution with stirring at room temperature. The mixture was incubated at 100 °C for three hours. The magnetic particles were separated and washed several times with deionised water. Then, the particles were dried at incubator at 80 °C (Doğaç et al. 2016).
Synthesis of polyethylene imine coated magnetite nanoparticles and polyethylene imine coated nickel ferrite nanoparticles
0.2 mL of citric acid monohydrate and 0.69 g of magnetic nanoparticles (magnetite or nickel ferrite) were added to 20 mL of distilled water and sonicated for 40 min. Then neutralization with 0.5 M NaOH was achieved. The magnetic nanoparticles were separated by centrifugation at 9500 rpm for 20 min. 50 mg of 50% (w / w) polyethyleneimine (PEI-600–1000 kDa) was added to the magnetic nanoparticles (magnetite or nickel ferrite) and mixed for 5 min. The mixture was neutralized with 0.5 M HCl and separated by centrifugation and the particles were washed 3 times with distilled water (Wang et al. 2009).
Characterization of polyethylene imine coated magnetite nanoparticles and polyethylene imine coated nickel ferrite nanoparticles
Surface groups and chemical structure of the magnetic composites were analyze using ATR-FTIR (Thermo Scientific Nicolet iS-5 ATR/FTIR Spectrometer). Thermal analysis of raw magnetic particles and magnetic composites was performed under N2 atmosphere using Perkin Elmer TGA 4000 thermo-gravimetric analyzer in temperature range between 30 and 650 °C with heating rate of 20 °C/min. The surface morphology of nanofibers was studied by SEM using JEOL JSM 7600F model.
Antimicrobial and anti-biofilm activity
Bacterial and fungal strains
Bacterial and fungal strains Staphylococcus aureus (ATCC® 25,923™), Escherichia coli (ATCC® 25,922™) and Candida albicans (ATCC® 10,239™) were selected for the in vitro antimicrobial and anti-biofilm activities, Chromobacterium violaceum CV026 and CV12472 were used as biosensor strains for quorum sensing inhibition and violacein inhibition respectively. Pseudomonas aeroginosa PA01 was used for swarming and swimming motility inhibition assays. The above-mentioned bacteria were grown in Nutrient Broth (NB, Difco) except C. albicans which was grown in Sabouraud dextrose broth (SDB, Difco). The cultures of microorganisms were maintained in their appropriate agar slants at 4 °C throughout the study and used as stock cultures.
Determination of minimal inhibitory concentration (MIC)
MICs were determined by a microtitre broth dilution method as recommended by the Clinical and Laboratory Standards Institute (CLSI) 2006 (CLSI 2006). The MIC was defined as the lowest sample concentration that yielded no visible growth. The test medium was Mueller–Hinton Broth (MHB) and the density of bacteria was 5 × 105 colony-forming units (CFU)/mL. Cell suspensions (100 μL) were inoculated into the wells of 96-well microtitre plates in the presence of samples with different final concentrations (0.25, 0.5, 1, 2.5, 5, 10, 20 mg/mL). The wells containing only MHB and MHB with inoculum were employed as negative and positive controls, respectively. The inoculated microplates were incubated at 37 °C for 24 h. The absorbance was measured at 550 nm. The lowest concentration of the tested samples, which did not show any visual growth of tested organisms after macroscopic evaluation, was determined as MIC, which was expressed in mg/mL. Each assay was performed in triplicate for all bacteria.
Biofilm inhibition assays
The effect of the samples at concentrations of 1, 1/2, 1/4, 1/8, and 1/16 MIC on biofilm-forming ability of the bacterial and fungal strains selected was tested using a microplate biofilm assay (Merritt et al. 2005). Briefly, 1% of overnight cultures of the selected strains was added into 200 μL of fresh Tryptic Soy Broth (TSB) supplemented with 0.25% glucose were diluted in growth medium to 5 × 105 colony-forming units (CFU)/mL and 100 μL and dispensed into each well of 96-well polystyrene flat-bottomed microtitre plates in the presence of 100 μL of sample and incubated without agitation for 48 h at 37 °C. The wells containing TSB + cells were used as control. After incubation, the wells were washed with water to remove planktonic bacteria or yeast cells. The remaining bacteria or yeast were subsequently stained with 0.1% crystal violet solution for 10 min at room temperature. Wells were washed once again to remove the crystal violet solution that had not specifically stained the adherent bacteria. Microplates were inverted and gently tap on paper towels to remove any excess liquid then air dried. 200 μL of 95% ethanol were filled into the plates containing E. coli and C. albicans while 33% glacial acetic acid were filled into the wells of the plates containing S. aureus. Biofilm stains solubilized at room temperature. After shaking and pipetting of the wells, 125 μL of the solution from each well was transferred to a sterile tube and volume made up to 1 mL with distilled water. Finally, the optical density of each well was measured at a wavelength of 550 nm. Each strain was tested for biofilm production in triplicate and the mean deduced. Percentage of inhibition of PEIMnF and PEINF was calculated using the formula given below.
Bioassay for QSI activity using Chromobacterium violaceum CV026
The quorum sensing inhibition potential of samples were performed by following the method specified by Koh and Tham (2011). The limit of detection of activity was also determined by applying serial dilutions of the samples (MIC, MIC/2, MIC/4, MIC/8, and MIC/16 using LB broth as the diluent) against Chromobacterium violaceum CV026. Each experiment was repeated, and the assay plates were incubated at 30 °C for 3 days. The quorum sensing inhibition zones were measured in millimeters and it corresponds to a halo-cream against a violet-coloured lawn on the surface of the media in the test plate.
Violacein inhibition assays using Chromobacterium violaceum CV12472.
Both samples were subjected to qualitative analysis to find their violacein inhibition potentials against Chromobacterium violaceum CV 12,472 (McLean et al. 2004). Overnight culture (10 μL) of C. violaceum (adjusted to 0.4 OD at 600 nm) was added into sterile microtiter plates containing 200 μL of LB broth and incubated in the presence and absence of various concentrations of tested agents (MIC-MIC/16). LB broth containing C. violaceum ATCC 12,472 was used as a positive control. These plates were incubated at 30 °C for 24 h and observed for the reduction in violacein pigment production. Each experiment was performed in triplicate. The absorbance was read at 585 nm. The percentage of violacein inhibition was calculated by following formula:
Swarming and swimming motility assays using Pseudomonas aeruginosa PA01
The swimming and swarming motility assays were performed by following the method described by Packiavathy and co-workers (Packiavathy et al. 2012). In swimming assay, 3 μL overnight culture of the PA01 (0.4 OD at 600 nm) were point inoculated at the center of the swimming agar medium consisting of 1% tryptone, 0.5% NaCl and 0.3% agar with increasing concentrations of polyethyleneimine-coated magnetite ferrite (PEIMnF) and the nickel ferrite (PEINF) nanoparticles (50, 75 and 100 μg/mL). For swarming assays, 5 μL (0.4 OD at 600 nm) overnight culture of PA01 were inoculated at the center of the swarming agar medium consisting of 1% peptone, 0.5% NaCl, 0.5% agar and 0.5% of filter-sterilized d-glucose with increasing concentrations of PEIMnF and PEINF (50, 75 and 100 μg/mL). The plates were then incubated at 30 °C in upright position for 16 h. The reduction in swimming and swarming migration was recorded by measuring the swim and swarm zones of the bacterial cells after 16 h.
Results and discussion
Schematic representation of polyethyleneimine-coated magnetic nanoparticles synthesis
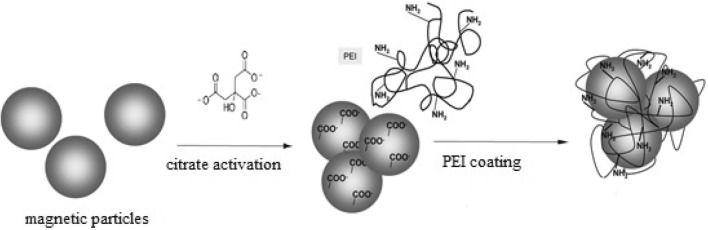
To synthesize polyethylene imine coated magnetite nanoparticles and polyethylene imine coated nickel ferrite nanoparticles, first Fe3O4 and Fe[NiFe]O4 type magnetic nanoparticles were citrate activated and then polyethylene imine coated on the surface. The key steps involved in this synthesis are summarized in Fig. 1 below. The citration step provided suitable reaction sites for the PEI coating to be effected successfully.
Fig. 1.
Schematic representation of the synthesis of polyethyleneimine-coated magnetic nanoparticles
FTIR analysis
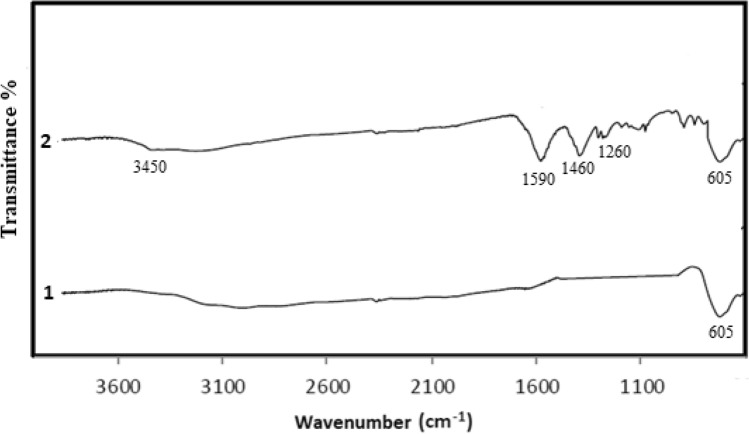
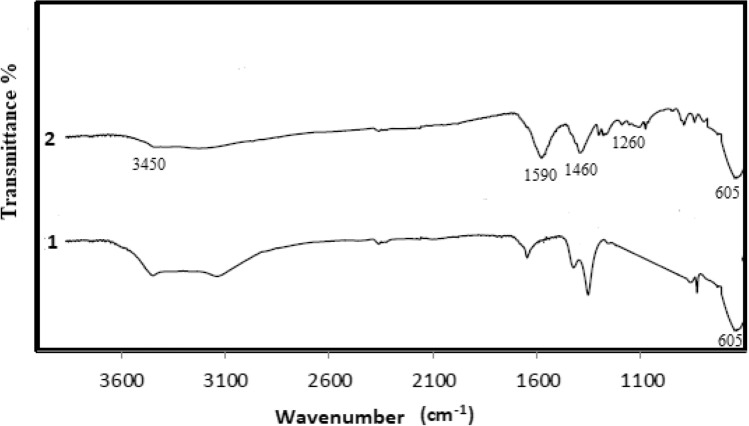
Surface groups and chemical structure of the prepared polyethylene imine coated magnetic nanoparticles were analyzed using ATR-FTIR (Thermo Scientific Nicolet iS-5 ATR/FTIR Spectrometer). The surface groups and functionality of PEIMnF and PEINF nanomaterials are revealed by their FT-IR spectra (Figs. 2 and 3). Absorption peak at 605 cm−1 confirming the presence of a Fe–O bond related to deformation in octahedral and tetrahedral sites of magnetic nanoparticles. The absorption contribution bands appeared at 3450 cm−1 and 1590 cm−1 from (− NH) symmetric and deformation respectively and assignable for polyethylene imine layer. The peaks at 1460 and 1260 cm−1 were attributed to the C–H bending and C–N stretching vibration, respectively, because of the structure of polyethyleneimine.
Fig. 2.
FTIR Spectrum of magnetite nanomaterials: (1) magnetite nanoparticles; (2) polyethylene imine-coated magnetite nanoparticles
Fig. 3.
FTIR Spectrum of nickel ferrite nanomaterials: (1) nickel ferrite nanoparticles; (2) polyethylene imine-coated nickel ferrite nanoparticles
SEM (scanning electron microscopy) analysis
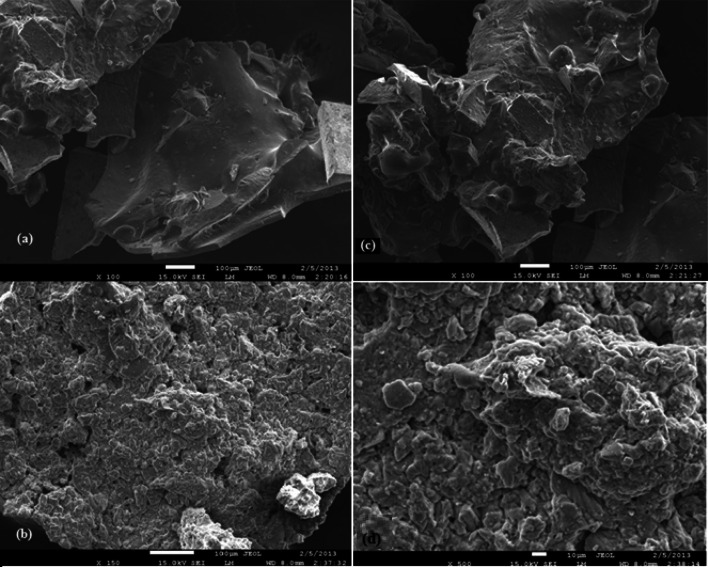
SEM images of polyethylene imine coated magnetic nanoparticles were taken to evaluate their morphological structures. SEM photographs of polyethylene imine coated magnetite and polyethylene imine coated nickel ferrite were given at Fig. 4. SEM patterns appear to clump together due to the magnetic field they create, have an amorphous shape, and the surface has a rough texture and lightens after PEI coating.
Fig. 4.
SEM images of polyethylene imine-coated magnetic nanoparticles a Fe3O4 after citrate activation, b Fe3O4 after PEI coating, c Fe[NiFe]O4 after citrate activation, d Fe[NiFe]O4 after PEI coating
TGA (thermogravimetric analysis)
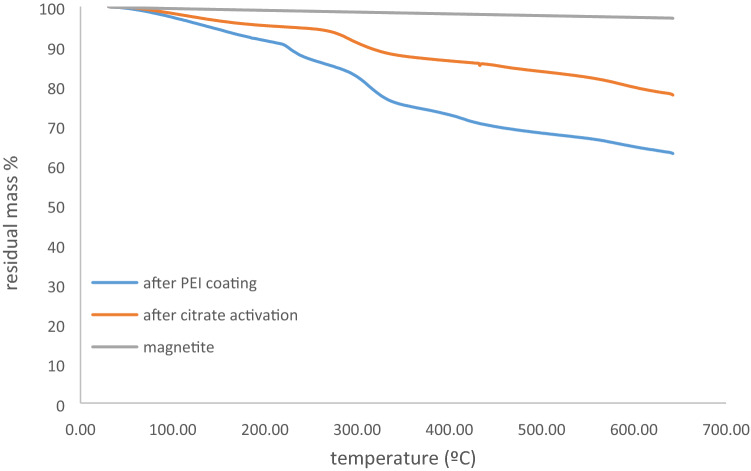
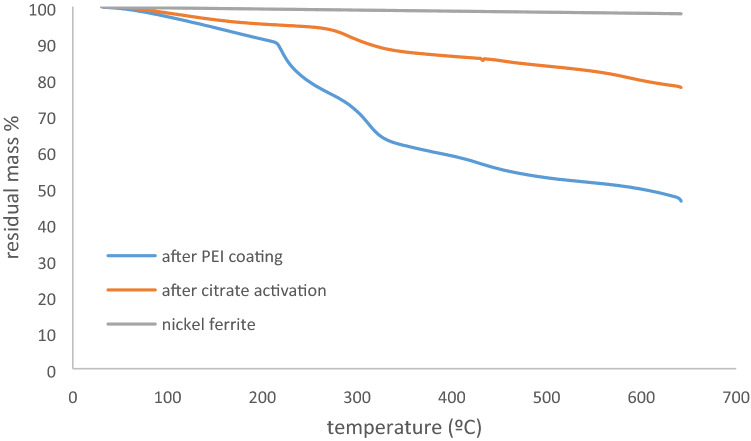
TGA analyzes of the samples were performed crude magnetic particles, after citrate activation and after PEI coating. The analyzes were carried out with a Perkin Elmer TGA 4000 brand thermogravimetric analyzer at a constant heating rate of 20 °C / min between 30 and 650 °C and the results are given in Figs. 5 and 6 as temperature-% residual mass curves.
Fig. 5.
TGA curves a Magnetite material samples
Fig. 6.
TGA curves b Nickel ferrite material samples
Two-step degradation occurred in all samples except the crude samples. As the temperature increased, the mass loss increased gradually and the first sharp degradation steps occurred after 220 °C. This is due to the release of water tightly bound by polar interactions with the amino groups of the polyethyleneimine and the decomposition of the cyclic products followed by the loss of CO2 from the polymer. At higher temperatures, the polyethylene imine backbone is completely destroyed. In thermogravimetric analysis of magnetic particle/ polyethyleneimine composites, while the polymer part is completely burned at certain temperatures, magnetic particles do not burn significantly due to their structural properties. The absence of significant mass loss (~ 2–2.5%) in the crude magnetic particle samples supports this result.
Antimicrobial and Anti-biofilm activities
Prior to antibiofilm activity evaluation, MIC values of newly synthesized PEIMnF and PEINF nanoparticles against test microorganisms were determined and found to be 10 mg / mL on all tested strains. The antibiofim activity of the synthesized PEIMnF and PEINF nanoparticles were evaluated at MIC and sub-MIC (10—0.625 mg/mL) concentrations against Staphylococcus aureus, Escherichia coli and Candida albicans. Due to ability of many resistant bacteria such as MRSA (Methicillin Resistant Staphylococcus aureus) to form biofilms, the treatment against their infections is usually very difficult (Skalickova et al. 2017). Interestingly, the polyethylene imine coated nanomaterials (PEIMnF and PEINF) showed very good biofilm inhibition results which are presented on Table 1. At the highest test concentration of 10 mg/mL, PEIMnF inhibited biofilm formation of E. coli (89.04 ± 0.50%), S. aureus (82.85 ± 2.42%) and C. albicans (91.37 ± 0.66%) as well as PEINF with biofilm inhibitions of E. coli (90.48 ± 2.05%), S. aureus (87.04 ± 1.59%) and C. albicans (90.94 ± 1.03%). This antibiofilm activity decreased in a concentration-dependent manner right down to the low test concentration, for example, at 2.5 mg/mL where PEIMnF inhibited biofilm formation on E. coli (25.07 ± 0.75%), S. aureus (27.98 ± 2.94%) and C. albicans (40.97 ± 1.17%) and PEINF inhibited biofilm formations of E. coli (42.85 ± 1.50%), S. aureus (31.72 ± 1.89%) and C. albicans (48.73 ± 2.00%). At 1.25 mg/mL concentration, PEIMnF inhibited biofilm formation only for C. albicans (13.2 ± 1.22%) while PEINF inhibited for E. coli (14.6 ± 0.41%) and C. albicans (23.89 ± 1.18%). At the concentration of 0.625 mg/mL, both nanoparticles did not show anti-biofilm activities against test microorganisms.
Table 1.
Antibiofilm activity of PEIMnF and PEINF nanomaterials (% inhibition)
| Conc. mg/mL | PEIMnF | PEINF | ||||
|---|---|---|---|---|---|---|
| % Inhibition | ||||||
| E. coli | S. aureus | C. albicans | E. coli | S. aureus | C. albicans | |
| 10 | 89.04 ± 0.50 | 82.85 ± 2.42 | 91.37 ± 0.66 | 90.48 ± 2.05 | 87.04 ± 1.59 | 90.94 ± 1.03 |
| 5 | 73.18 ± 1.20 | 69.47 ± 3.71 | 78.87 ± 2.26 | 72.77 ± 0.50 | 64.23 ± 2.69 | 75.58 ± 2.85 |
| 2.5 | 25.07 ± 0.75 | 27.98 ± 2.94 | 40.97 ± 1.17 | 42.85 ± 1.50 | 31.72 ± 1.89 | 48.73 ± 2.00 |
| 1.25 | – | – | 13.2 ± 1.22 | 14.6 ± 0.41 | – | 23.89 ± 1.18 |
| 0.625 | – | – | – | – | – | – |
– No inhibition
Violacein inhibition on Chromobacterium violaceum CV12472 and anti-quorum sensing activity on Chromobacterium violaceum CV026
Chromobacterium violaceum grows and produces a violet color as an expression of quorum sensing (QS) regulated process and since this is easily observable and quantifiable marker trait, this bacterium is suitable model organism in quorum sensing activity research (Kothari et al. 2017). The minimum inhibitory concentration (MIC) of PEIMnF and PEINF on C. violaceum CV12472 and C. violaceum CV026 was each determined prior to quorum sensing assay and reported together with the anti-quorum sensing activity on Table 2. MIC values were found for PEIMnF to be 10 mg/mL on both C. violaceum CV12472 and C. violaceum CV026 while for PEINF was 10 mg/mL on C. violaceum CV12472 and 5 mg/mL on C. violaceum CV026.
Table 2.
Violacein inhibition percentage on CV12472 and Anti-quorum sensing activity in mm on CV026 of PEIMnF and PEINF
| PEIMnF | PEINF | |||
|---|---|---|---|---|
| CV12472 | CV026 | CV12472 | CV026 | |
| MIC(mg/mL) | 10 | 10 | 10 | 5 |
| Violacein inhibitiona | QSIb | Violacein inhibitiona | QSIb | |
| MIC | – | – | 100 ± 00 | 16.3 ± 2.0 |
| MIC/2 | – | – | 100 ± 00 | 11.5 ± 0.5 |
| MIC/4 | – | – | 43.1 ± 0.5 | – |
| MIC/8 | – | – | 31.98 ± 0.8 | – |
| MIC/16 | – | – | 14.31 ± 0.6 | – |
(–) No effect
aResults are inhibition in the absorbance of violacein (%)
bDiameter of zone of QSI (cream-white halo) in mm
C. violaceum CV 12,472 which grows and produces violacein on its own was used to determine the violacein inhibition potential of the synthesized polyethyleneimine-coated nanoparticles at sub-MIC concentrations (MIC-MIC/8). PEIMnF was found to be inactive while PEINF showed a concentration dependent violacein inhibition of 51.2 ± 3.5% (MIC), 39.8 ± 0.2% (MIC/2), 17.4 ± 0.5% (MIC/4) and 06.2 ± 1.8% (MIC/8). This effect corresponded to a coloration change as shown on Fig. 7.
Fig. 7.
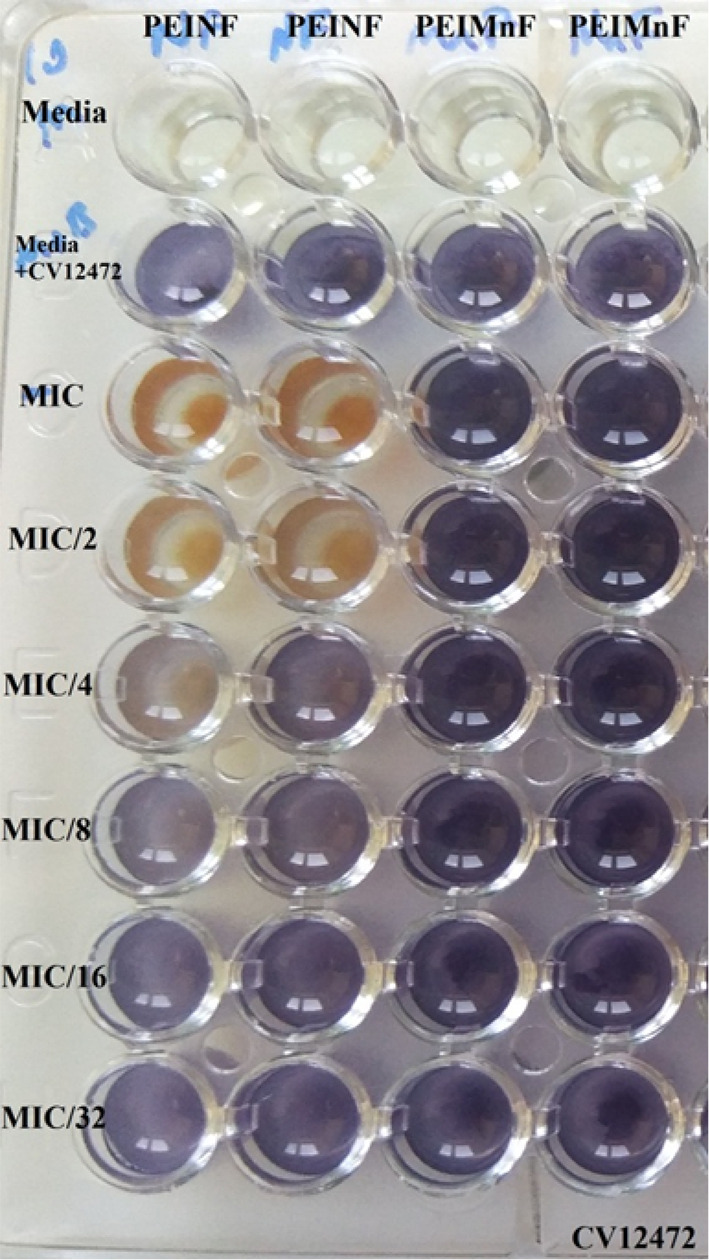
Effect of nanomaterials on violacein formation by C. violaceum CV12472
C. violaceum CV026 is a biosensor mutant strain which only produces violacein when an Acyl Homoserine Lactone (AHL) hormone is supplied to it externally. In this assay, the bacteria growth on the petri as described in material and methods section and the diameter of the quorum sensing inhibition zones recognized as a cream-coloured zone against a violet lawn are measured in mm. The PEIMnF nanoparticles showed no quorum sensing inhibition. However, the PEINF nanoparticles showed quorum sensing with inhibition zones of 16.3 ± 1.0 mm at MIC and 11.5 ± 0.5 mm at MIC/2. The quorum-sensing inhibition zones correspond to the transparent or cream halo as shown on Fig. 8.
Fig. 8.
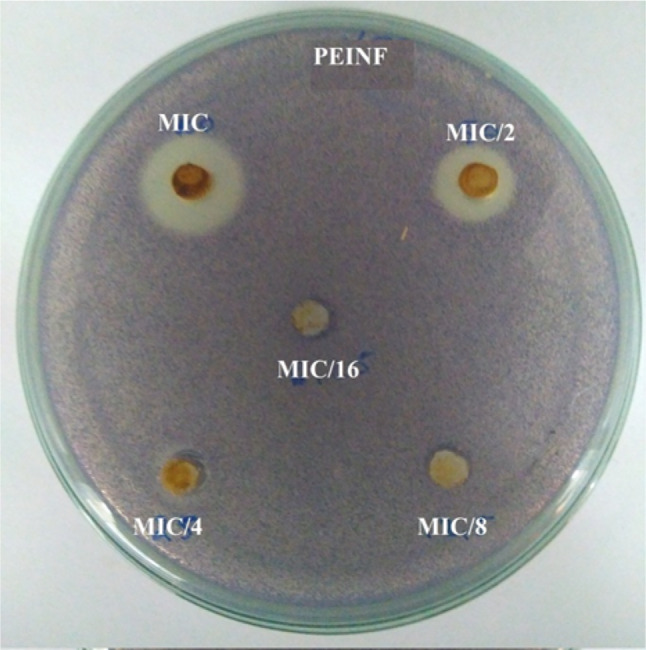
Disruption of quorum sensing of C. violaceum CV026 by nanomaterials
Swimming and swarming motility inhibition on PA01
Bacterial motility plays a key role in the colonization of surfaces by bacteria and the subsequent formation of resistant communities of bacteria called biofilms. Pseudomonas aeruginosa utilizes flagellum-mediated swimming motility to approach a surface, attaches, and then further spreads via the surface through associated motilities such as swarming and twitching (O’May and Tufenkji 2011). PEIMnF inhibited motility in the swimming model at 100 µg/mL (49.64 ± 3.01%) and 75 µg/mL (16.42 ± 0.41%) and in the swarming model at 100 µg/mL (40.86 ± 2.70%) and 75 µg/mL (10.63 ± 1.35%) but showed no inhibition at 50 µg/mL in both models. PEINF inhibited motility in swimming model at 100 µg/mL (52.85 ± 1.60%), 75 µg/mL (42.13 ± 1.00%) and 50 µg/mL (15.12 ± 0.50%). In the swarming model, PEINF showed inhibitions at concentrations of 100 µg/mL (48.49 ± 0.20%), 75 µg/mL (36.98 ± 6.44%) and 50 µg/mL (04.27 ± 0.31%). In all samples, swimming inhibition was greater than swarming inhibitions as shown on Table 3. However, at the same concentrations, PEINF showed better inhibition than PEIMnF in both models.
Table 3.
Swimming and swarming inhibition (% inhibitions) of PEIMnF and PEINF on PA01
| Conc | PEIMnF | PEINF | ||
|---|---|---|---|---|
| P. aeruginosa PA01 | ||||
| Swimming (% Inhibition) | Swarming (% Inhibition) | Swimming (% Inhibition) | Swarming (% Inhibition) | |
| 100 µg/mL | 49.64 ± 3.01 | 40.86 ± 2.70 | 52.85 ± 1.60 | 48.49 ± 0.20 |
| 75 µg/mL | 16.42 ± 0.41 | 10.63 ± 1.35 | 42.13 ± 1.00 | 36.98 ± 6.44 |
| 50 µg/mL | – | – | 15.12 ± 0.50 | 04.27 ± 0.31 |
– No effect
Discussion
Most often, antimicrobial nanoparticles are coated for greater applicability. Polymers are often used as matrix for nanomaterials or attached to their surfaces and this design of antimicrobial nanomaterials has added advantages. Firstly in that there is excellent affinity between them and bacteria at the interface which increases antimicrobial efficacy and polymer matrix improves tensile strength, biodegradability, environmental safety and provides a barrier properties towards gases and water vapor (Yadollahi et al. 2015; Rasoulzadeh and Namazi, 2017). The PEIMnF and PEINF nanomaterials were succesfully synthesized as revealed by ATR-FTIR and SEM studies. Also the two major regions of weight loss in the TGA curves exhibited by the samples confirms PEI coating. The polyethyleneimine through its amine groups can stabilize the nanoparticles through coordination and this aspect is of great importance in the polyethyleneimine-coated nanoparticles for biomedical applications. It has been shown that, after the absorption of nanoparticles unto polyethyleneimine, steric effects would make them very stable and dispersed (Dong et al. 2016). Many antimicrobial agents show high activity but often face some drawbacks like low stability and complicated preparation processes. These limit their practical application and therefore synthesis of stable antibacterial agents through simple routes is an interesting field (Dong et al. 2016). The amino groups of polyethyleneimine are chemically reactive and therefore are susceptible to chemical modifications and also able to go into coordinate bonding with nanoparticles thereby improving their properties and suitability for diverse applications. Polyethyleneimine therefore has appropriate physicochemical properties and its positive charge and hydrophobicity makes it a possible antimicrobial agent (Yudovin-Farber et al. 2010).
A synthetic strategy involving polyethyeleneimine-coated nanoparticles is very important as it confers a number of physicochemical properties and biological activities making them to find applications in various domains such as in medicine and material science. Coating imparts functionality to nanoparticles, improving their penetration and drug delivery properties and can bind them to drugs, proteins, enzymes, antibodies and nucleotides (Solodov et al. 2018).
It should be noted that, there is no significant difference in the antimicrobial activity of the PEIMnF and PEINF. It could be possible that the antimicrobial activity observed is conferred to these nanoparticles by the polyethyleneimine coating since in previous studies, polyethylenenimine has been proven to possess molecular weight-dependent antimicrobial activity on the same microorganisms Staphylococcus aureus, Escherichia coli and C. albicans (Barros et al. 2015). In previous studies, Fe3O4 showed antibacterial effect against Gram-positive and Gram-negative bacteria which clearly indicates that these nanoparticles are effective antibacterial agents. It was suggested that the bactericidal activity is due to the presence of reactive oxygen species (ROS) generated by different nanoparticles such as hydrogen peroxide which enters the bacterial cells and eventually kill them (Tran et al. 2010; Prabhu et al. 2015). The synergistic effect of the antimicrobial potential of polyethyleneimine and the nanoparticles could better account for the observed antimicrobial properties. It is observed that both samples had very good antibiofilm activities of above 80% inhibition on all tested microorganisms at concentrations of 10 mg/mL of polyethyleneimine-coated nanoparticles. Nanomaterials possess intrinsic antimicrobial activities and can overcome or disrupt bacterial resistance such as biofilm formation and quorum sensing. Nanomaterials are able to interfer with quorum sensing within the biofilm consortium as well as inhibit efflux pumps which are suitable strategies to combat multidrug-resistant biofilms (Manaf et al. 2018). The antibiofilm activity of the samples were close for the same microorganism at the same concentration. As explained for MIC values, polyethyleneimine has been shown to possess antibiofilm activities (Barros et al. 2015). Equally the iron oxide nanoparticles have been shown to possess antibiofilm activities which increases with an increase in the concentration of test samples (Al-Shabib et al. 2018; Mehran and Naser, 2019). Studies have shown some unique properties of magnetic nanoparticle including large surface to volume ratio augment reactivity with surrounding materials, as such, the generation of reactive oxygen species resulting from them and the shape of the magnetic nanoparticles can disrupt the biofilm formation by bacteria (Duran et al. 2016; Mu et al. 2016). Magnetic nanoparticles can also penetrate and damage biofilm by release of ions (Qayyum and Khan 2016). Therefore, the profound antibiofilm activity could result from the synergy of antibiofilm potential of both nanoparticles and polyethyleneimine polymer coat. The disruption of bacterial biofilms is very important in combatting microbial resistance since it provides protective and suitable survival conditions for the bacteria in harsh milieu and in the presence of antibiotics posing a serious health threat. The ability of synthesized nanomaterials to inhibit biofilms is very advantageous. Most conventional antibiotics remedy the symptoms arising from the activities of planktonic bacteria which are outside the biofilms while bacteria within established biofilms can still grow and multiply and consequently re-establish communicative networks known as quorum sensing. Microbial biomass reduction and cell death by nanoparticles also results from prevention of capsule-forming exopolysaccharides and proteins (Joo and Aggarwal 2018).
PEINF showed antiquorum sensing activity while PEIMnF was not able to inhibit quorum sensing at tested concentrations. PEINF being a bimetallic nanoparticle has higher advantage such as enhanced surface plasmon resonance (SPR) of their core and shell parts which has made the to find uses in used specifically as antimicrobial, drug delivery, bio-imaging and biosensors agents (Mehran and Naser 2018). It could be possible that in the PEINF (Nickel ferrite) nanoparticle, the inhibition of violacein formation and the anti-quorum inhibition observed results from the presence of Nickel. This is so because the PEIMnF in which nickel is lacking didn’t show violacein inhibition and anti-quorum sensing activity. In a previous study, nickel and cadmium were shown to inhibit biofilm formation by the bacterium Burkholderia multivorans through the inhibition of acyl-homoserine lactone quorum sensing (Leticia et al. 2014). Furthemore, at the same concentration, swimming and swarming motility inhibitions were higher for PEINF than PEIMnF and these activities were dose-dependent. The higher anti-motility activity of PEINF than PEIMnF can also be attributed to the prensence of nickel in PEINF. Hence nickel could be important component in nanoparticles which will find applications in disruption of bacteria cell-to-cell communication via quorum-sensing mediated processes. The antiadhesive properties of nanomaterials also play an important role in the prevention of biofilms since decrease in adherence of the bacterium to the cellular and inert surfaces via swarming and swimming inhibition is an early stage of biofilm formation especially for flagellated bacteria such as PA01 bacterium and these specialized form of flagellum-driven motility is usually triggered by chemical signals (Cinzia et al. 2005; Soheili et al. 2019). Bacterial motility by flagellated P. aeroginosa PA01 plays a pivotal role in its microbial surface colonization and contribute to the formation of structured surface-associated communities of bacteria called biofilms (O’May and Tufenkji, 2011). Thus inhibiting these motilities implies inhibition of biofilm by P. aeroginosa PA01.
Conclusion
The alarming emergence of multidrug resistant microorganisms resulting from poor usage of conventional antibiotics is a serious health problem. For this reason, interest in new antimicrobial substances capable of overcoming this phenomenon is an field of research with growing interest. Magnetic nanomaterials are mainly composed of oxides of iron and other suitable metal oxides and they find applications in the fields of analytical chemistry, biosensing, and nanomedicine. They have also been found to possess antibacterial potential and thus the necessity to evaluate their effect on quorum sensing mediated bacterial processes which are the root causes of microbial resistance. Two magnetic nanoparticles, magnetite and ternary nickel ferrite nanoparticles were synthesized and stabilized by coating with polyethyleneimine polymer. These polyethyleneimine-coated nanoparticles PEIMnF and PEINF showed excellent antibiofilm activities on pathogenic bacteria S. aureus, E. coli and C. albicans. The antimicrobial and antibiofilm potentials of these particles could result from a synergistic action of polyethyleneimine and the magnetic nanoparticles which have both been proven to possess antimicrobial and antibiofilm activities. The nickel ferrite (PEINF) nanomaterial showed antiquorum sensing activity and this could be attributed to the presence of Nickel, an antiquorum sensing metal. Both nanomaterials inhibited P. aeruginosa swarming and swimming motilities. This study shows that polyethyleneimine-coated magnetite and nickel ferrite nanomaterials could be a potential solution in combatting microbial resistance.
Compliance with ethical standards
Conflict of interest
The authors have no conflict of interest to declare.
Contributor Information
Ozgur Ceylan, Email: ozgurceylan@mu.edu.tr.
Alfred Ngenge Tamfu, Email: macntamfu@yahoo.co.uk.
References
- Al-Shabib NA, Husain FM, Ahmed F, Khan RA, Khan MS, Ansari FA, Alam MZ, Ahmed MA, Khan MS, Baig MH, Khan JM, Shahzad SA, Arshad M, Alyousef A, Ahmad I. Low Temperature Synthesis of Superparamagnetic Iron Oxide (Fe3O4) Nanoparticles and Their ROS Mediated Inhibition of Biofilm Formed by Food-Associated Bacteria. Front Microbiol. 2018;9:2567. doi: 10.3389/fmicb.2018.02567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros J, Ana D, Miguel AR, Cidália P-V, Maria AL, Irene P-V. Antibiofilm and antimicrobial activity of polyethylenimine: an interesting compound for endodontic treatment. The J Contemp Dental Prac. 2015;16(6):427–432. doi: 10.5005/jp-journals-10024-1701. [DOI] [PubMed] [Google Scholar]
- Blanco-Esqueda IG, Ortega-Zarzosa G, Martínez JR, Guerrero AL. Preparation and characterization of nickel ferrite-SiO2/Ag core/shell nanocomposites. Adv Mat Sci Eng. 2015;1(7):678739. doi: 10.1155/2015/678739. [DOI] [Google Scholar]
- Cakmak I, Ulukanli Z, Tuzcu M, Karabuga S, Genctav K. Synthesis and characterization of novel antimicrobial cationic polyelectrolytes. European Pol J. 2004;40(10):2373–2379. doi: 10.1016/j.eurpolymj.2004.06.004. [DOI] [Google Scholar]
- Chen-yu W, Pooyan M, Ehsan NZ, Franklin RT, Li-Na N. Advances in antimicrobial organic and inorganic nanocompounds in biomedicine. Adv Therap. 2020;3:2000024. doi: 10.1002/adtp.202000024. [DOI] [Google Scholar]
- Chhabra V, Ayyub P, Chattopadhyay S, Maitra AN. Preparation of acicular γ–Fe2O3 particles from a microemulsion-mediated reaction. Mater Lett. 1996;26:21–26. doi: 10.1016/0167-577X(95)00200-6. [DOI] [Google Scholar]
- Cinzia C, Francesco C, Emilia G, Giuseppe A, Sara S, Fabrizio C, Alessandro G, Sonia S. Swarming differentiation and swimming motility in Bacillus subtilis are controlled by swra, a newly identified dicistronic operon. J Bacteriol. 2005;187(5):5356–5366. doi: 10.1128/JB.187.15.5356-5366.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doğaç YI, Teke M. Synthesis and characterisation of biocompatible polymer-conjugated magnetic beads for enhancement stability of urease. Appl Biochem Biotechnol. 2016;179(1):94–110. doi: 10.1007/s12010-016-1981-3. [DOI] [PubMed] [Google Scholar]
- Dong X, Qingyun W, Tao Y, Jianzhong C, Qinlu L, Zhiqin Y, Le L. Polyethyleneimine capped silver nanoclusters as efficient antibacterial agents. Int J Environ Res Public Health. 2016;13:334. doi: 10.3390/ijerph13030334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran N, Duran M, de Jesus MB. Silver nanoparticles: a new view on mechanistic aspects on antimicrobial activity. Nanomed Nanotechnol Biol Med. 2016;12:789–799. doi: 10.1016/j.nano.2015.11.016. [DOI] [PubMed] [Google Scholar]
- Enomoto N, Akagi JI, Nakagawa Z. Sonochemical powder processing of iron hydroxides. Ultrason Sonochem. 1996;3:97–103. doi: 10.1016/1350-1477(96)00009-3. [DOI] [Google Scholar]
- Ezhilarasi AA, Vijaya JJ, Kaviyarasu K, Maaza M, Ayeshamariam A, Kennedy LJ. Green synthesis of NiO nanoparticles using Moringa oleifera extract and their biomedical applications: Cytotoxicity effect of nanoparticles against HT-29 cancer cells. J Photochem Photobiol. 2016;164:352–263. doi: 10.1016/j.jphotobiol.2016.10.003. [DOI] [PubMed] [Google Scholar]
- Gong P, Li H, He X. Preparation and antibacterial activity of Fe3O4-Ag nanoparticles. Nanotechnology. 2007;18(28):285604. doi: 10.1088/0957-4484/18/28/285604. [DOI] [Google Scholar]
- Grodzinski P, Silver M, Molnar LK. Nanotechnology for cancer diagnostics: promises and challenges. Expert Rev Mol Diagn. 2006;6:307–318. doi: 10.1586/14737159.6.3.307. [DOI] [PubMed] [Google Scholar]
- Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26:3995–4021. doi: 10.1016/j.biomaterials.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Heegn H, Trinkler M, Langbein H. Phase formation and solid state structure on calcination of a nickel ferrite acetate precursor. Cryst Res Technol. 2000;35(3):255–264. doi: 10.1002/1521-4079(200003). [DOI] [Google Scholar]
- Ho KM, Li P. Design and synthesis of novel magnetic core−shell polymeric particles. Langmuir. 2008;24:1801–1807. doi: 10.1021/la702887m. [DOI] [PubMed] [Google Scholar]
- Jeong JH, Byoun YS, Lee YS. Poly(styrene-altmaleic anhydride)-4-aminophenol conjugate: synthesis and antibacterial activity. Reactive Func Pol. 2002;50(3):257–263. doi: 10.1016/S1381-5148(01)00120-1. [DOI] [Google Scholar]
- Joo SH, Aggarwal S. Factors impacting the interactions of engineered nanoparticles with bacterial cells and biofilms: Mechanistic insights and state of knowledge. J Environ Manag. 2018;225:62–74. doi: 10.1016/j.jenvman.2018.07.084. [DOI] [PubMed] [Google Scholar]
- Kale A, Gubbala S, Misra RDK. Magnetic behavior of nanocrystalline nickel ferrite synthesized by the reverse micelle technique. J Magn Magn Mater. 2004;277:350–358. doi: 10.1016/j.jmmm.2003.11.015. [DOI] [Google Scholar]
- Kenawy E-R. Biologically active polymers. IV. Synthesis and antimicrobial activity of polymers containing 8-hydroxyquinoline moiety. J Applied Pol Sci. 2001;82(6):1364–1374. doi: 10.1002/app.1973. [DOI] [Google Scholar]
- Kim YH, Lee DK, Cha HG, Kim CW, Kang YS. Synthesis and characterization of antibacterial Ag-SiO2 advances in materials science and engineering 7 nanocomposite. J Phy Chem C. 2007;111(9):3629–3635. doi: 10.1021/jp068302w. [DOI] [Google Scholar]
- Kothari V, Sharma S, Padia D. Recent research advances on Chromobacterium violaceum. Asian Pac J Trop Med. 2017;10(8):744–752. doi: 10.1016/j.apjtm.2017.07.022. [DOI] [PubMed] [Google Scholar]
- Leticia MV, Jacques M, Yu Y, Barry HP, Robert JCM, Pedro JJA. Nickel and cadmium ions inhibit quorum sensing and biofilm formation without affecting viability in Burkholderia multivorans. Int Biodeter Biodegrad. 2014;91:82–87. doi: 10.1016/j.ibiod.2014.03.013. [DOI] [Google Scholar]
- Li Wu Xiao HHZGX. Effects of synthetic conditions on particle size and magnetic properties of NiFe2O4. Powder Technol. 2010;198:157–166. doi: 10.1016/j.powtec.2009.11.005. [DOI] [Google Scholar]
- Manaf A, Essam AM, Isil V, Fatih K. The role of nanoparticles in the ınhibition of multidrug-resistant bacteria and biofilms. Curr Drug Deliv. 2018;15(4):470–484. doi: 10.2174/1567201815666171207163504. [DOI] [PubMed] [Google Scholar]
- Mehran A, Naser K. Antiplanktonic, antibiofilm, antiswarming motility and antiquorum sensing activities of green synthesized Ag–TiO2, TiO2–Ag, Ag–Cu and Cu–Ag nanocomposites against multi-drug-resistant bacteria. Art Cells Nanomed Biotechnol. 2018;46(3):S399–S413. doi: 10.1080/21691401.2018.1496923. [DOI] [PubMed] [Google Scholar]
- Mehran A, Naser K. Ultrasound assisted-phytofabricated Fe3O4 NPs with antioxidant properties and antibacterial effects on growth, biofilm formation, and spreading ability of multidrug resistant bacteria. Art Cells Nanomed Biotechnol. 2019;47(1):2405–2423. doi: 10.1080/21691401.2019.1624560. [DOI] [PubMed] [Google Scholar]
- Mu H, Tang J, Liu Q. Potent antibacterial nanoparticles against biofilm and intracellular bacteria. Sci Rep. 2016;6:73–80. doi: 10.1038/srep18877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’May C, Tufenkji N. The swarming motility of Pseudomonas aeruginosa is blocked by cranberry proanthocyanidins and other tannin-containing materials. Appl Envtal Microbiol. 2011;77(9):3061–3067. doi: 10.1128/AEM.02677-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osaka T, Matsunaga T, Nakanishi T, Arakaki A, Niwa D, Iida H. Synthesis of magnetic nanoparticles and their application to bioassays. Anal Bioanal Chem. 2006;384:593–600. doi: 10.1007/s00216-005-0255-7. [DOI] [PubMed] [Google Scholar]
- Pooyan M, Chen-yu W, Ehsan NZ, Assunta B, Li-na N, Franklin RT. Metal-based nanomaterials in biomedical applications: antimicrobial activity and cytotoxicity aspects. Adv Funct Mater. 2020;30:1910021. doi: 10.1002/adfm.201910021. [DOI] [Google Scholar]
- Prabhu YT, Venkateswara RK, Siva KB, Vemula SSK, Tambur P. Synthesis of Fe3O4 nanoparticles and its antibacterial application. Int Nano Lett. 2015;5:85–92. doi: 10.1007/s40089-015-0141-z. [DOI] [Google Scholar]
- Prodan AM, Iconaru SL, Chifiriuc CM, Bleotu C, Ciobanu CS, Motelica-heino M, Sizaret S, Predoi D. Magnetic properties and biological activity evaluation of ıron oxide nanoparticles. J Nanomat. 2013 doi: 10.1155/2013/893970. [DOI] [Google Scholar]
- Qayyum S, Khan AU. Nanoparticles vs. biofilms: a battle against another paradigm of antibiotic resistance. Med Chem Commun. 2016;7:1479–1498. doi: 10.1039/C6MD00124F. [DOI] [Google Scholar]
- Rasoulzadeh M, Namazi H. Carboxymethyl cellulose/graphene oxide bio-nanocomposite hydrogel beads as anticancer drug carrier agent. Carbohydr Polym. 2017;168:320–326. doi: 10.1016/j.carbpol.2017.03.014. [DOI] [PubMed] [Google Scholar]
- Rezvan J, Cynthia KY, Yiu ENZ, Li-Na N, Raffaele V, Guojun C, Zhen G, Franklin RT, Pooyan M. Advances in antimicrobial microneedle patches for combating ınfections. Adv Mater. 2020;32:2002129. doi: 10.1002/adma.202002129. [DOI] [PubMed] [Google Scholar]
- Sanpo N, Berndt CC, Wang J. Microstructural and antibacterial properties of zincsubstituted cobalt ferrite nanopowders synthesized by sol-gel methods. J Appl Phys. 2012;112:1–7. doi: 10.1063/1.4761987.60. [DOI] [Google Scholar]
- Sepelak V, Bergmann I, Feldhoff A, Heitjans P, Krumeich F, Menzel D, Litterst FJ, Campbell SJ, Becker KD. Nanocrystalline nickel ferrite, NiFe2O4: mechanosynthesis, nonequilibrium cation distribution, canted spin arrangement and magnetic behavior. J Phys Chem C. 2007;111:5026–5033. doi: 10.1021/jp067620s. [DOI] [Google Scholar]
- Sibhghatulla S, Nazia N, Syed MDR, Khurshid A, Mohammad HB, Eun JL, Inho C. Mechanistic insights into the antimicrobial actions of metallic nanoparticles and their implications for multidrug resistance. Int J Mol Sci. 2019;20:2468. doi: 10.3390/ijms20102468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalickova S, Milosavljevic V, Cihalova K, Horky P, Richtera L, Adam V. Selenium nanoparticles as a nutritional supplement. Nutrition. 2017;33:83–90. doi: 10.1016/j.nut.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Soheili V, Tajani AS, Ghodsi R, Bazzaz BSF. Anti-PqsR compounds as next-generation antibacterial agents against Pseudomonas aeruginosa: A review. Eur J Med Chem. 2019;172:26–35. doi: 10.1016/j.ejmech.2019.03.049. [DOI] [PubMed] [Google Scholar]
- Solodov AN, Julia RS, Evgenia AB, Rustem RA. Polyethyleneimine-modified iron oxide nanoparticles: their synthesis and state in water and in solutions of ligands. Colloid Polym Sci. 2018;296:1983–1993. doi: 10.1007/s00396-018-4425-5. [DOI] [Google Scholar]
- Stoimenov PK, Klinger RL, Marchin GL, Klabunde KJ. Metal oxide nanoparticles as bactericidal agents. Langmuir. 2002;18(17):6679–6686. doi: 10.1021/la0202374. [DOI] [Google Scholar]
- Sun J, Su Y, Rao S, Yang Y. Separation of lysozyme using superparamagnetic carboxymethyl chitosan nanoparticles. J Chromatogr B. 2011;879(23):2194–2200. doi: 10.1016/j.jchromb.2011.05.052. [DOI] [PubMed] [Google Scholar]
- Tank C, Raman S, Karan S, Gosavi S, Lalla NP, Sathe V, Berndt R, Gade WN, Bhoraskar SV, Mathe VL. Antimicrobial activity of silica coated silicon nano-tubes (SCSNT) and silica coated silicon nano-particles (SCSNP) synthesized by gas phase condensation. J Mater Sci Mater Med. 2013;24:1483–1490. doi: 10.1007/s10856-013-4896-3. [DOI] [PubMed] [Google Scholar]
- Tran N, Mir A, Mallik D, Sinha A, Nayar S, Webster TJ. Bactericidal effect of iron oxide nanoparticles on Staphylococcus aureus. Int J Nanomed. 2010;5(1):277–283. doi: 10.2147/ijn.s9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivekanandhan S, Venkateswarlu M, Satyanarayana N. Glycerol-assisted gel combustion synthesis of nano-crystalline LiNiVO4 powders for secondary lithium batteries. Mater Lett. 2004;58:1218–1222. doi: 10.1016/j.matlet.2003.09.011. [DOI] [Google Scholar]
- Wang X, Zhou L, Ma Y, Li X, Gu H. Control of aggregate size of polyethyleneimine-coated magnetic nanoparticles for magnetofection. Nano Res. 2009;2(5):365–372. doi: 10.1007/s12274-009-9035-6. [DOI] [Google Scholar]
- Wu JH, Ko SP, Liu HL, Kim S, Ju JS, Kim YK. Sub 5 nm magnetite nanoparticles: synthesis, microstructure, and magnetic properties. Mater Lett. 2007;61:3124–3129. doi: 10.1016/j.matlet.2006.11.032. [DOI] [Google Scholar]
- Yadollahi M, Farhoudian S, Namazi H. One-pot synthesis of antibacterial chitosan/silver bio-nanocomposite hydrogel beads as drug delivery systems. Int J Biol Macromol. 2015;79:37–43. doi: 10.1016/j.ijbiomac.2015.04.032. [DOI] [PubMed] [Google Scholar]
- Yudovin-Farber I, Jacob G, Nurit B, Ervin IW, Abraham JD. Quaternary ammonium polyethyleneimine: antibacterial activity. J Nanomaterials. 2010 doi: 10.1155/2010/826343. [DOI] [Google Scholar]
- Zintchenko A, Ogris M, Wagner E. Temperature dependent gene expression induced by PNIPAM-based copolymers: potential of hyperthermia in gene transfer. Bioconjug Chem. 2006;17:766–772. doi: 10.1021/bc050292z. [DOI] [PubMed] [Google Scholar]