Abstract
Objectives
Patients with sickle cell anemia (SCA) are immunocompromised and at an increased risk of developing infections. Our aim was to establish the clinical, laboratory, and radiological manifestations of respiratory viral infections in SCA at Sultan Qaboos University Hospital (SQUH), Oman and assess its impact on disease morbidity and mortality, with special emphasis on H1N1.
Methods
We undertook a retrospective study in SCA patients with respiratory viral infections following up at the hematology department at SQUH. We collected demographic data and clinical, radiological, and laboratory parameters.
Results
In 84 SCA patients with 109 admission episodes for vaso-occlusive crisis (VOC), molecular diagnostic techniques confirmed 125 respiratory viral infections. Rhinovirus was the most prevalent infection (35.8%), whereas H1N1 virus infection was seen only in 10.1%. Laboratory investigations revealed a significant fall in mean hemoglobin levels, mean white blood cell, and platelet counts from baseline, whereas there was a significant rise in the mean lymphocyte and retic count, serum lactate dehydrogenase, and C-reactive protein levels during infective episodes (p < 0.050, Wilcoxon signed rank test). One-third (32.1%) of the VOC episodes progressed to acute chest syndrome (ACS), but in the H1N1 cohort, only two episodes of ACS was seen (18.2%).
Conclusions
Rhinovirus was the commonest respiratory virus infections in SCA patients, whereas parainfluenza 3 was associated with a significant adverse outcome. H1N1 was associated with a mild course. ACS was seen in approximately one-third of this group of patients.
Keywords: Acute Chest Syndrome; Influenza A Virus, H1N1 Subtype; Rhinovirus; Retrospective Studies; Anemia, Sickle Cell
Introduction
Hemoglobinopathies are very common in Oman (partly due to high consanguineous marriage), with an estimate of more than two per 1000 live births affected.1-3 The prevalence of a serious hemoglobinopathies is about 10% with the most significant being sickle cell anemia (SCA) and β-thalassemia major.3
SCA is marked by a point mutation, where glutamic amino acid is replaced by valine leading to abnormal β chain production.4,5 About 40% of hemoglobin in the sickle cell trait (heterozygous state) is HbS, but in the homozygous state it can reach up to 80–95%.6 HbS can polymerize under the low oxygen tension, causing vaso-occlusive events leading to tissue inflammation, infarction, and end organ damage.7 The disease is characterized by the triad of recurrent painful episodes (crises): hemolytic anemia, and a predisposition to repeated infections.7,8 The clinical manifestations of SCA vary from patient to patient, and it is influenced by hereditary and environmental factors, including the level of fetal hemoglobin in postnatal life.9
SCA patients are at an increased risk of infections, due to splenic dysfunction, altered complement regulation, low oxygen tension, medications, and recurrent admissions to hospital with exposure to nosocomial pathogens.6,8 The prevalence of infections has apparently increased owning to the improved diagnostics in recent times.10
Among these infections, respiratory viral infections are very common, and can affect both upper and lower respiratory tract. The Cooperative Study of Sickle Cell Disease in the US demonstrated that viruses contributed to 6.7% of cases progressing to acute chest syndrome (ACS).7 Previous studies showed that three new respiratory viruses had emerged, namely SARS (a novel Corona virus) in 2003, influenza A H5N1 (avian flu) in 2004, and the pandemic caused by the new reasserting influenza A (H1N1) in April 2009, which was first identified in Mexico and California with subsequent spread worldwide.11 In Oman, 2681 persons were reported to be infected with the influenza A (H1N1) virus, of which 24 patients died, with these deaths occurring in the very young, elderly, or those with underlying disease.12
Respiratory infections are a risk factor for triggering ACS in SCA patients and often lead to several complications. Influenza-associated hospitalization and complications in pediatric SCA patients were reported to be 56-times higher than in non-SCA patients.13 Measures to control these infections will immensely benefit these patients, who are highly susceptible to infections; in particular with increasing prevalence of viral infections after the pandemic of H1N1. The Center for Disease Control and Prevention also recommends regular vaccination for the prevention and control of seasonal influenza,14 and this is essential in SCA patients with their inherent immunocompromised state.
However, there has been limited data in the published literature studying the effect of respiratory viral infections and in particular H1N1 infections on SCA patients. A report from the UK estimated H1N1 in 50% of patients with SCA presenting to hospital with 25% developing ACS.15 Another report found that in 48 cases of H1N1 influenza, 21% progressed to ACS.16 A study in children and young adults with SCA identified 29 cases of confirmed H1N1 infection, with 34% developing ACS and 17% required intensive care including mechanical ventilation in 10%.10
Our aim was to establish the clinical, laboratory, and radiological manifestations of respiratory viral infections in SCA patients at Sultan Qaboos University Hospital (SQUH) and assess its impact on the disease morbidity and mortality, with special emphasis on H1N1.
Methods
We conducted a retrospective electronic medical record (EMR) review after approval by the local hospital medical research and ethics committee (MREC #1321). A list of 428 patients with SCA who were tested for viral infections were obtained from the EMR, but only 84 patients with a total of 109 episodes met all the inclusion criteria. These were: aged3 15 years, confirmation of SCA by high-performance liquid chromatography and/or DNA studies, and confirmed to have respiratory viral infection by molecular studies (viral RNA/DNA PCR). All of these events occurred in the study period between June 2013 and July 2018. Demographic information, past medical history, clinical and laboratory findings, chest radiographs, complications, co-infections, interventions, and drugs given related to the episodes of respiratory viral infections in these patients were retrieved for analysis.
We used SPSS (IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp.) to analyze the collected data. Continuous variables were expressed as mean and standard deviation if normally distributed, and median and inter quartile range (IQR) for data not following normal distribution. Nonparametric tests like chi-square was used to determine the statistical significance and the unpaired t-test Wilcoxon signed ranks test were used to compare means of continuous variables between groups. A p-value < 0.050 was considered significant.
Results
Males and females were equally represented (n = 42 each) in our cohort. The mean age was 27.0+7.0 years (range = 15–57 years). Admission episodes were equally divided with 55 and 54 episodes in the male and female patients, respectively. Clinical details and confounding factors showed that 89.0% of patients were on penicillin prophylaxis, whereas 61.5% were on hydroxyurea therapy. On abdominal ultrasound, 38.5% showed asplenia, either owing to surgical splenectomy or autosplenectomy, whereas 58.7% had a previous history of ACS.
Generally, the clinical presentations were similar in all types of respiratory viral infections. The most frequent signs and symptoms seen in these SCA patients were pain (87.2%), fever (55.1%) with a mean duration of 3.0 days, and cough (55.1%) ranging from dry to productive. Other frequent symptoms were throat congestion (23.8%), crepitation (22.9%), chest pain (22.1%), reduced air entry (16.5%), headache (11.9%), and rhinorrhea (14.7%) [Figure 1]. Comparatively, H1N1 patients had a higher incidence of cough (72.7% vs. 53.1%), rhinorrhea (36.4% vs. 14.7%), reduced air entry (18.2% vs. 16.3%), and crepitation (27.3% vs. 22.9%) than other non-H1N1 respiratory viral infected SCA patients in this cohort.
Figure 1.
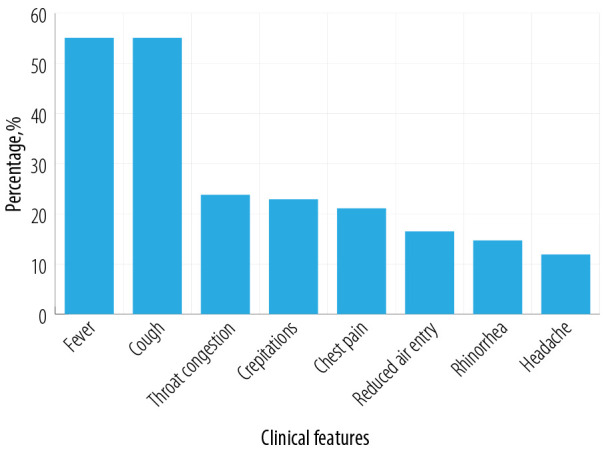
Clinical features in sickle cell anemia patients with respiratory viral infection (n = 84; 109 vaso-occlusive crisis episodes).
Table 1 shows the details of hematological parameters by comparison between the baseline and the data at the time of infection. It was observed that the median hemoglobin at baseline was significantly higher than the median hemoglobin [nadir] during the infection. The mean white blood cell count at baseline was significantly decreased during infection as was the mean platelets count. The median reticulocyte count at baseline was significantly higher than during infection. The median lymphocytes count at baseline was significantly lower than the median lymphocytes during the infection.
Table 1. Hematological laboratory investigations of the study population.
| Characteristics | Median | IQR | Mean | + SD | p-value |
|---|---|---|---|---|---|
| Hemoglobin baseline, g/dL | 11.45 | 1.50 | 9.8 | 1.3 | < 0.001* |
| Hemoglobin nadir, g/dL | 7.25 | 1.20 | 7.2 | 0.9 | |
| WBC baseline, ×109/L | 8.40 | 6.50 | 8.9 | 3.4 | < 0.002* |
| WBC nadir, ×109/L | 7.20 | 4.70 | 6.8 | 2.5 | |
| Lymphocytes count baseline, ×109/L | 2.70 | 0.90 | 2.6 | 0.8 | < 0.001* |
| Lymphocytes count maximum, ×109/L | 3.70 | 1.80 | 4.1 | 2.0 | |
| Platelets count baseline, ×109/L | 313.00 | 233.00 | 350.6 | 252.4 | < 0.001* |
| Platelets count nadir, ×109/L | 238.50 | 232.00 | 285.2 | 207.9 | |
| ANC basaline, ×109/L | 4.20 | 1.60 | 3.9 | 0.9 | 0.273* |
| ANC nadir, ×109/L | 2.60 | 1.60 | 2.5 | 1.2 | |
| Reticulocyte basaline, ×109/L | 214.50 | 230.90 | 223.1 | 129.2 | < 0.005* |
| Reticulocyte nadir, ×109/L | 169.50 | 84.00 | 172.0 | 155.2 | |
| HbS, % | 85.10 | 7.00 | 86.3 | 3.8 | |
| HbF, % | 8.00 | 9.40 | 8.3 | 4.7 |
IQR: inter quartile range; SD: standard deviation; WBC: white blood cell; ANC: absolute neutrophil count; HbS: sickle cell hemoglobin; HbF: fetal hemoglobin. *Statistically significant (Wilcoxon signed ranks test).
Table 2 summarizes the biochemical parameters. We observed that the median C-reactive protein (CRP) level at admission was significantly lower than the median CRP during the infection. Median lactate dehydrogenase (LDH) significantly increased to from its baseline value during the infection.
Table 2. Biochemistry laboratory investigations of the study population.
| Characteristics | Median | IQR | Mean | + SD | p-value |
|---|---|---|---|---|---|
| LDH baseline, U/L | 350.00 | 162.00 | 338.0 | 105.0 | < 0.001* |
| LDH maximum, U/L | 710.50 | 991.00 | 693.0 | 132.0 | |
| CRP at admission, mg/L | 18.50 | 41.00 | 37.7 | 21.8 | < 0.001* |
| CRP maximum, mg/L | 143.50 | 147.00 | 153.0 | 44.4 | |
| BUN, mmol/L | 3.80 | 1.70 | 3.4 | 0.8 | |
| Total bilirubin, µmol/L | 29.00 | 51.00 | 53.8 | 17.6 | |
| Direct bilirubin, µmol/L | 12.50 | 28.00 | 47.9 | 14.1 |
IQR: inter quartile range; SD: standard deviation; LDH: lactate dehydrogenase; CRP: C-reactive protein; BUN: blood urea nitrogen.
*Statistically significant (Wilcoxon signed ranks test).
Abnormal chest radiological findings were present in 25.0%, with segmental (10.0%) or multi-segmental atelectasis (8.0%), and acute respiratory distress syndrome (ARDS) (7.0%). Pleural effusions were seen in 22.0% with 19.0% going through a mild course, but 3.0% had a moderate effusion.
Figure 2 shows the interventions and complications in these patients with respiratory virus infection. Interventions included blood transfusions (44.9%), non-invasive ventilation (12.8%), need for central venous catheter (7.3%), and urinary catheter (3.7%). One-third (32.1%) of these episodes were complicated by ACS and associated with one death. Overall, two patients died in this cohort and both the patients were infected with parainfluenza 3 virus. The first patient had a co-associated Salmonella and developed sepsis and died. The second patient had a co-associated ACS and developed seizures with neurological symptoms and bradycardia that was managed by implantable cardiac defibrillator, but the patient also died.
Figure 2.
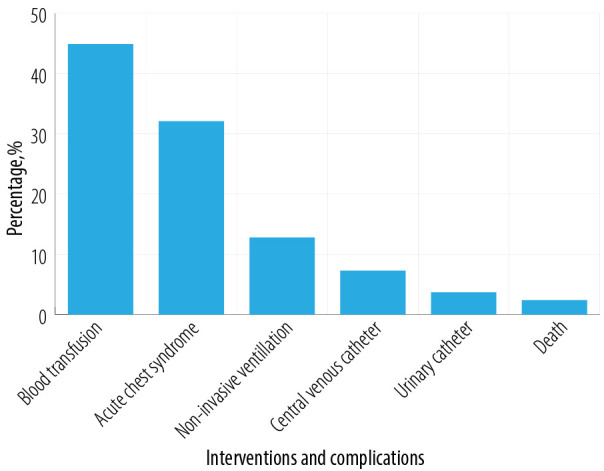
Complications and interventions in sickle cell anemia patients with respiratory viral infections (n = 84; 109 vaso-occlusive crisis episodes).
Figure 3 shows the spectrum of respiratory viruses (n = 125) observed in this study with 10.1% showing H1N1. The most prevalent non-H1N1 respiratory virus infection was rhinovirus (35.8%) followed by adenovirus (16.5%), influenza A (14.7%), respiratory syncytial virus (12.8%), influenza B (11.9%), parainfluenza 3 (6.4%), parainfluenza 4 (2.8%), enterovirus (1.8%), and parainfluenza 2 (1.8%). In all cases, the diagnosis of the specific viral infection was made by molecular studies with reverse-transcriptase polymerase chain reaction to identify the relevant viral RNA or DNA.
Figure 3.
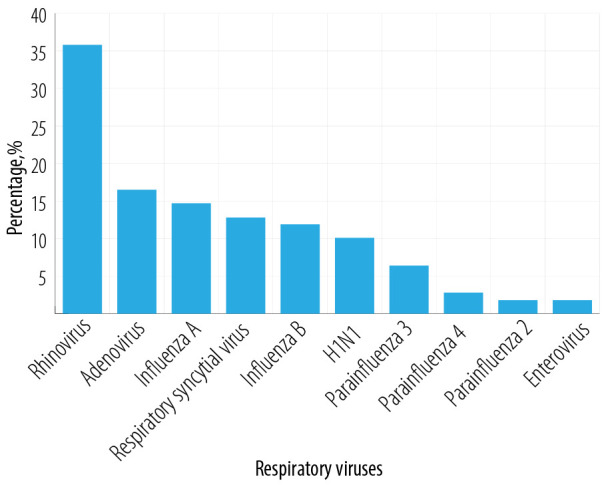
Spectrum of respiratory viruses found in sickle cell anemia patients (n = 84; 109 vaso-occlusive crisis episodes, 125 microorganisms).
In 84.4%, infection was due to a single virus, whereas in the remaining 15.6%, multiple viral organisms were found. Importantly, less than a third of these patients with multiple viruses were associated with ACS (23.5%), but significantly, all H1N1 cases were co-associated with influenza A virus (100%). Additionally, bacterial co-infection in five instances with six different microorganisms, and two instances of a co-infection with both a viral and a fungal microorganism were also observed.
Table 3 shows the comparison of the clinical and radiological features, interventions, and complications in patients with and without H1N1 infections. There were no statistically significant differences seen.
Table 3. Comparison of clinical presentations, radiological features, interventions, and complications between H1N1 infected patients (n = 10) and other respiratory viruses (n = 74).
| Variables | Patients with H1N1 (VOC = 11) n (%) |
Patients without H1N1 (VOC = 98) n (%) |
p-value Chi-square test |
|---|---|---|---|
| Pain | 9 (81.8) | 86 (87.7) | 0.88* |
| Fever | 6 (54.5) | 54 (55.1) | 1.00* |
| Cough | 8 (72.7) | 52 (53.1) | 0.52* |
| Chest pain | 2 (18.2) | 22 (22.4) | 0.38* |
| Reduced air entry | 2 (18.2) | 16 (16.3) | 1.00* |
| Crepitations | 3 (27.3) | 22 (22.4) | 0.77* |
| Rhinorrhea | 4 (36.4) | 12 (12.2) | 0.19* |
| Acute chest syndrome | 2 (18.2) | 33 (33.7) | 0.43* |
| Non-invasive ventilation | 1 (9.1) | 13 (13.3) | 0.72* |
| Blood transfusion | 4 (36.4) | 45(45.9) | 0.70* |
| Death | 0 (0.0) | 2 (2.0) |
*Not significant (p > 0.050); VOC: vaso-occlusive crisis.
Almost all these patients received empiric therapy with antibiotics and antiviral agents, namely oseltamivir (n = 78, 92.9%), azithromycin (n = 72, 85.7%), piperacillin/tazobactam (n = 53, 63.1%), moxifloxacin (n = 52, 6.9%), and cefuroxime (n = 40, 47.6%).
Discussion
The purpose of this study was to establish the clinical, laboratory, and radiological manifestations of respiratory viral infections in SCA patients, and assess its impact on disease morbidity and mortality, with a special emphasis on H1N1. Our data showed that these patients presented with upper respiratory tract infection symptoms with varying degrees of severity, but there were no significant differences in clinical presentations between the different types of the respiratory viruses. We observed more severe symptoms in patients infected with adenovirus and parainfluenza 3 virus. These findings were consistent with the previous reports on the impact of respiratory viruses in patients with SCA.7 Two-thirds of this cohort were on hydroxyurea and had a history of ACS. One-third had splenectomy or asplenia, with ACS seen in 32.1% with a low mortality. Treatment with hydroxyurea may have had a positive impact on this outcome.
The mean age in H1N1 patients was higher than the non-H1N1 patient cohort (28.8 vs. 26.5 years). There were no differences in the common symptoms of infection between the two groups; in H1N1 patients, fever and rhinorrhea were relatively predominant, whereas in the non-H1N1 patient’s painful episodes, blood transfusions, and ACS were more prominent, but these differences were not statistically significant [Table 3]. The clinical course in the H1N1 patients was mild with only two patients needing morphine and one needing chest physiotherapy and incentive spirometry. It was known that H1N1 infections were more severe in the older age and previous pediatric reports showed significant morbidity.
Although the cooperative sickle cell study from US demonstrated that viruses contributed to only 6.7% of cases of ACS,7 influenza-associated hospitalization in SCA is reported to be 56-times higher.12 George et al,16 reported that the impact of H1N1 influenza pandemic on SCA patients was mild, with 21% progressing to ACS. A prior study by Strouse et al,10 had also reported significant morbidity. In their study on H1N1 influenza patients, 34% progressed to ACS, requiring intensive care in 17%, while 10% needed mechanical ventilation. In our H1N1 cohort (n = 11), two (18.2%) patients progressed to ACS, and although none needed intensive care, one (9.1%) required non-invasive ventilation, but there was no death in this subgroup of patients. We believed that the overall improvement in the SCA management may be responsible for these improved outcomes, as well as the role of early institution of oseltamivir needs to be emphasized, as all our patients received it early, when the diagnosis was suspected.
Expectedly, the laboratory parameters worsened after infection, similar to previous studies.7,15,17 We found a decline in oxygen saturation and hemoglobin levels with development of thrombocytopenia, leucopenia, and lymphocytosis. In addition, there was significant increase in CRP and serum LDH indicating intense ongoing inflammation with ischemia and tissue infarction. These findings were similar to previous reports on all respiratory viruses in SCD.15
Monitoring by chest imaging was done in all patients, as they were most likely to trigger the development of ACS. Our findings showed that the most severe radiological changes were seen with adenoviruses. A study from Korea reported that adenovirus pneumonia progressed to ARDS in 63%, with 50% mortality.18 Adenovirus respiratory infections are usually associated with bilateral patchy parenchymal or ground-glass opacities on chest radiographs.19 The predominant radiological pattern seen in H1N1 infection was bilateral ground-glass opacities and alveolar consolidation.20 However, the radiological changes seen in H1N1 cases in this study were mild and unilateral.
Although the number of H1N1 patients in this cohort was small, we feel that the improved management of SCA in general and specifically the early administration of oseltamivir may have played an important role here. Nevertheless, further larger prospective studies will be needed to explain these differences.
It has been found that viral infections can promote bacterial adherence and immune-mediated interactions.21 Our study demonstrated that the co-infections were mostly detected with rhinovirus followed by adenovirus showing the highest prevalence of co-association with bacterial organisms. However, our study cohort showed a lower prevalence of bacterial co-infections (6.0%) than a previous study by Seo et al,22 that reported a prevalence of 26.5%. However, despite the low prevalence, there was prolonged hospital stay, more severe infection course, and higher mortality in these patients with bacterial co-infection.
Conclusion
To the best of our knowledge, this is the first study from Oman that reports the impact of respiratory viruses on clinical, radiological, and laboratory manifestations in SCA patients. Our findings indicated that SCA patients displayed a mild course of H1N1 infection and the most prevalent respiratory virus in this cohort was rhinovirus. Fever and cough were the most frequent presenting symptoms, leading to significant anemia, reticulocytopenia, leucopenia, and thrombocytopenia. Also, LDH and CRP were significantly elevated. Nearly one-third had ACS and almost half required blood transfusions. Further, the most severe course was seen in SCA patients infected with adenovirus or parainfluenza 3 virus, with mortality associated with the latter. However, studies with larger numbers of patients with respiratory viral infections in SCA are necessary to evaluate the predisposing risk factors to its development.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
Acknowledgements
We wish to thank the hospital administration for the use of hospital material in this study.
References
- 1.Rajab A, Patton MA. A study of consanguinity in the Sultanate of Oman. Ann Hum Biol 2000. May-Jun;27(3):321-326. 10.1080/030144600282208 [DOI] [PubMed] [Google Scholar]
- 2.Al-Riyami AA, Suleiman AJ, Afifi M, Al-Lamki ZM, Daar S. A community-based study of common hereditary blood disorders in Oman. East Mediterr Health J 2001. Nov;7(6):1004-1011. [PubMed] [Google Scholar]
- 3.Alkindi S, Al Zadjali S, Al Madhani A, Daar S, Al Haddabi H, Al Abri Q, et al. Forecasting hemoglobinopathy burden through neonatal screening in Omani neonates. Hemoglobin 2010. Jan;34(2):135-144. 10.3109/03630261003677213 [DOI] [PubMed] [Google Scholar]
- 4.Hassan SM, Vossen RH, Chessa R, den Dunnen JT, Bakker E, Giordano PC, et al. Molecular diagnostics of the HBB gene in an Omani cohort using bench-top DNA Ion Torrent PGM technology. Blood Cells Mol Dis 2014. Sep;53(3):133-137. 10.1016/j.bcmd.2014.05.002 [DOI] [PubMed] [Google Scholar]
- 5.Bunn HF. Pathogenesis and treatment of sickle cell disease. N Engl J Med 1997. Sep;337(11):762-769. 10.1056/NEJM199709113371107 [DOI] [PubMed] [Google Scholar]
- 6.Porth CM. Essentials of pathophysiology, concepts of altered health status. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2011. p. 286-288. [Google Scholar]
- 7.Vichinsky EP, Neumayr LD, Earles AN, Williams R, Lennette ET, Dean D, et al. National Acute Chest Syndrome Study Group Causes and outcomes of the acute chest syndrome in sickle cell disease. N Engl J Med 2000. Jun;342(25):1855-1865. 10.1056/NEJM200006223422502 [DOI] [PubMed] [Google Scholar]
- 8.Alkindi S, Matwani S, Al-Maawali A, Al-Maskari B, Pathare A. Complications of PORT-A-CATH® in patients with sickle cell disease. J Infect Public Health 2012. Mar;5(1):57-62. 10.1016/j.jiph.2011.10.004 [DOI] [PubMed] [Google Scholar]
- 9.Hassan SM, Al Muslahi M, Al Riyami M, Bakker E, Harteveld CL, Giordano PC. Sickle cell anemia and α-thalassemia: a modulating factor in homozygous HbS/S patients in Oman. Eur J Med Genet 2014. Nov-Dec;57(11-12):603-606. 10.1016/j.ejmg.2014.09.005 [DOI] [PubMed] [Google Scholar]
- 10.Strouse JJ, Reller ME, Bundy DG, Amoako M, Cancio M, Han RN, et al. Severe pandemic H1N1 and seasonal influenza in children and young adults with sickle cell disease. Blood 2010. Nov;116(18):3431-3434. 10.1182/blood-2010-05-282194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bautista E, Chotpitayasunondh T, Gao Z, Harper SA, Shaw M, Uyeki TM, et al. Writing Committee of the WHO Consultation on Clinical Aspects of Pandemic (H1N1) 2009 Influenza Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med 2010. May;362(18):1708-1719. 10.1056/NEJMra1000449 [DOI] [PubMed] [Google Scholar]
- 12.Al-Mahrezi A, Samir N, Al-Zakwani I, Al-Muharmi Z, Balkhair A, Al-Shafaee M. Clinical characteristics of influenza A H1N1 versus other influenza-like illnesses amongst outpatients attending a university health center in Oman. Int J Infect Dis 2012. Jul;16(7):e504-e507. 10.1016/j.ijid.2012.02.015 [DOI] [PubMed] [Google Scholar]
- 13.Bundy DG, Strouse JJ, Casella JF, Miller MR. Burden of influenza-related hospitalizations among children with sickle cell disease. Pediatrics 2010. Feb;125(2):234-243. 10.1542/peds.2009-1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (CDC) Prevention and control of seasonal influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices–United States, 2013-2014. MMWR Recomm Rep 2013. Sep;62(RR-07):1-43. [PubMed] [Google Scholar]
- 15.Inusa B, Zuckerman M, Gadong N, Afif M, Arnott S, Heath P, et al. Pandemic influenza A (H1N1) virus infections in children with sickle cell disease. Blood 2010. Mar;115(11):2329-2330. 10.1182/blood-2009-12-260836 [DOI] [PubMed] [Google Scholar]
- 16.George A, Benton J, Pratt J, Kim MO, Kalinyak KA, Kalfa TA, et al. The impact of the 2009 H1N1 influenza pandemic on pediatric patients with sickle cell disease. Pediatr Blood Cancer 2011. Oct;57(4):648-653. 10.1002/pbc.23030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vichinsky EP, Styles LA, Colangelo LH, Wright EC, Castro O, Nickerson B, Cooperative Study of Sickle Cell Disease Acute chest syndrome in sickle cell disease: clinical presentation and course. Blood 1997. Mar;89(5):1787-1792. 10.1182/blood.V89.5.1787 [DOI] [PubMed] [Google Scholar]
- 18.Cha MJ, Chung MJ, Lee KS, Kim TJ, Kim TS, Chong S, et al. Clinical features and radiological findings of adenovirus pneumonia associated with progression to acute respiratory distress syndrome: a single center study in 19 adult patients. Korean J Radiol 2016. Nov-Dec;17(6):940-949. 10.3348/kjr.2016.17.6.940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chong S, Lee KS, Kim TS, Chung MJ, Chung MP, Han J. Adenovirus pneumonia in adults: radiographic and high-resolution CT findings in five patients. AJR Am J Roentgenol 2006. May;186(5):1288-1293. 10.2214/AJR.05.0128 [DOI] [PubMed] [Google Scholar]
- 20.Abdelsalam M, Diab HS, Ragab Y. Radiological findings in patients with H1N1 influenza pneumonia. Egypt J Chest Dis Tuberc 2016;65(1):135-142 . 10.1016/j.ejcdt.2015.07.001 [DOI] [Google Scholar]
- 21.McCullers JA, McAuley JL, Browall S, Iverson AR, Boyd KL, Henriques Normark B. Influenza enhances susceptibility to natural acquisition of and disease due to Streptococcus pneumoniae in ferrets. J Infect Dis 2010. Oct;202(8):1287-1295. 10.1086/656333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seo YB, Song JY, Choi MJ, Kim IS, Yang TU, Hong KW, et al. Etiology and clinical outcomes of acute respiratory virus infection in hospitalized adults. Infect Chemother 2014. Jun;46(2):67-76. 10.3947/ic.2014.46.2.67 [DOI] [PMC free article] [PubMed] [Google Scholar]


