Abstract
Background Blood procalcitonin (PCT) levels usually increase during infectious diseases and might be helpful to differentiate bacterial from non-bacterial origin. COVID-19 patients could present co-infections at initial presentation in the Emergency Department and nosocomial infections during stay in the ICU. However, the published literature has not established whether PCT changes could aid in the diagnosis of infectious complication during the COVID-19 pandemic. Methods Retrospective, single-center, cohort study, including COVID-19 patients admitted between March and May 2020. The data were prospectively collected for department purposes; laboratory results were collected automatically at admission and during the whole patient admission. Results 56 patients were analyzed (female 32%, male 68%), 35 were admitted to ICU, and 21 received general ward care. 21 ICU patients underwent mechanical ventilation (88%), and 9 died during admission (26%). Non-survivors had higher initial blood PCT levels than survivors at ICU admission (p.
1. Introduction
Patients admitted to Intensive Care Units (ICU) are at risk of nosocomial infections. Sepsis is a life-threatening organ dysfunction due to a dysregulated host response to infection [1] that requires early diagnosis, appropriate antibiotic treatment and other prompt measures such as intravenous fluids, vasopressor therapy and oxygen supply, as any delay may portend poor outcomes.
It is established that unnecessary use of antibiotics increases antibiotic resistance, adverse effects and healthcare costs [2,3].
Sometimes, sepsis appears with heterogeneous, or even markedly ambiguous manifestations, Procalcitonin (PCT) is a biomarker that has been proposed as a helpful tool in these situations [4]. PCT is produced by C thyroid cells with no hormonal activity, and by neuroendocrine pulmonary cells [5] in response to endotoxin bacterial stimuli and the release of interleukin-1 (IL-1) and tumor necrosis factor-alpha (TNF-α).
A range of studies endorse the use of PCT in ICU patients as an effective diagnostic tool in sepsis and a prognosis marker [6,7], as plasma levels increase rapidly in 3 to 6 h and reach their peak in 12–48 h in response to bacterial infection [8,9].
Viral infections usually result to low blood PCT level, due to inhibition of TNF-α production and increase of interferon-gamma (IFN-γ) that inhibits PCT synthesis [5,8]. In addition, PCT has a low sensitivity for the diagnosis of bacterial infection in patients with renal failure [10].
The coronavirus disease 2019 (COVID-19) pandemic, caused by a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV2), has revealed new systemic disturbances that could cause severe pneumonia and develop acute respiratory distress syndrome (ARDS) with occasional progression to multi-organ dysfunction. Moreover, the incidence of renal failure in COVID-19 is high [11,12] and many critically ill patients need dialysis (usually continuous renal replacement therapy) [12,13]. At this stage, various studies have reported that PCT levels may have prognostic value [[14], [15], [16], [17]] but their value as a diagnostic marker of nosocomial bacterial infection in critically ill COVID-19 patients remains unknown.
Our objective has been to investigate whether blood PCT level is a useful marker of bacterial coinfection at Emergency Department (ED) and nosocomial bacterial infection in ICU patients with pneumonia due to SARS-CoV2.
2. Method
We performed a retrospective, observational, cohort study in patients admitted to our hospital during March–May 2020 with COVID-19. All patients met the diagnosis criteria of pneumonia due to SARS-CoV2 defined as a positive result on real-time reverse transcription-polymerase chain reaction (RT-PCR) test of nasopharyngeal swab specimens and compatible positive chest imaging test (X-ray or computerized tomography).
Patients under the age of 18, pregnant women, or with do-not-resuscitate (DNR) or do-not-intubate (DNI) orders were excluded from this study. Demographic data and prognostic indices were obtained on admission to the hospital. These included the Simplified Acute Physiology Score (SAPS II) [18] and the Sequential Organ Failure Assessment scores (SOFA) [19], both scores being calculated within the first 24 h of admission.
Blood PCT levels were measured at admission and then prospectively whether there was a suspicion of a bacterial infection. All the laboratory test results were collected automatically by our critical care information system (IntelliSpace Critical Care and Anesthesia, Philips, Germany) during the whole admission. The glomerular filtration rate (GFR) was calculated by the Modification of Diet in Renal Disease formula (MDRD-7) [21].
We also collected data regarding the need for mechanical ventilation (invasive and non-invasive), renal replacement therapy (did you mean renal replacement therapy here or the actual technique = hemodialysis vs hemofiltration etc. here?), and patient survival.
Respiratory bacterial co-infection was defined as an infection diagnosed with one or more positive cultures obtained from blood, valid sputum, tracheal aspirate or pleural effusion at hospital admission of patients with COVID-19. Antigen test for detection of S. pneumoniae and L. pneumophila in urine and serum samples for serological tests for M. pneumoniae, C. pneumoniae and L. pneumoniae were also used.
Microbiologically documented nosocomial infection was defined as an illness caused by organ invading pathogens with compatible clinical symptoms, imaging or laboratory findings and a positive culture, which justified the use of antibiotics.
The study was approved by the Ethics Committee of the hospital (Institut d'Investigació Sanitària Pere Virgili Ref. CEIM: 168/2020). Patient's identification remained anonymous and informed consent was waived due to the retrospective nature of the study.
3. Statistical analysis
Results are shown as means and medians with their respective standard deviations and interquartile ranges. The normal distribution of the variables was determined by the Shapiro-Wilk test. Comparisons between means were performed with the Student t-test or the Mann-Whitney test, when appropriate. Pearson or Spearman coefficients were used to evaluate the correlations between different variables. The interactions between variables were analyzed by multiple linear and binary logistic regression, incorporating confounding factors. Differences in p values less than 0.05 were considered statistically significant. The statistical analyses were performed with the SPSS 24.0 program (Chicago, USA).
4. Results
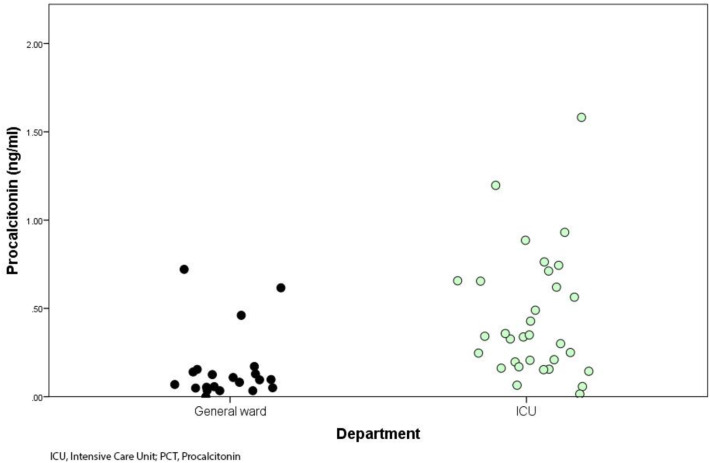
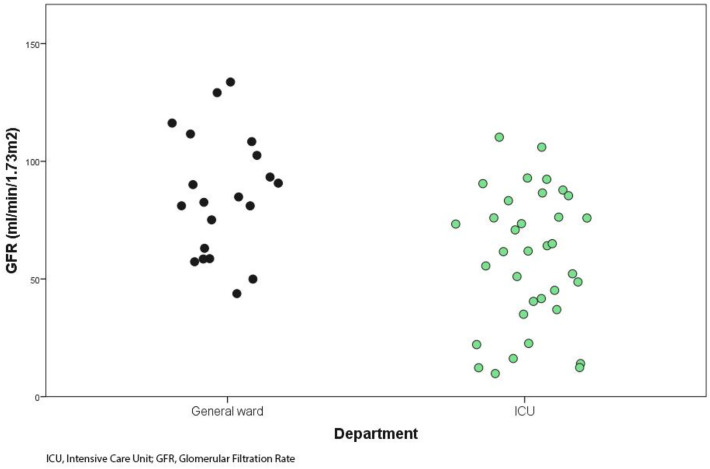
During the study period 56 patients with acute pneumonia due to SARS-CoV2 were included (67.9% males, 32.1% females), 35 required admission to ICU and 29 (82.9%) of them underwent invasive ventilation. The remaining 21 patients were treated in the general ward. Median (IQR 25–75) age was 68 years (60.0–73.8), median (IQR 25–75) blood PCT level was 0.22 ng/ml (0.06–0.63) and mean (SD) estimated GFR was 69 ml/min/1.73 m2 (48–89). The general characteristics of the study population with and without ICU admission are shown in Table 1 . Patients admitted to ICU differed significantly blood PCT levels (0.30 ng/ml vs 0.06 ng/ml) (Fig. 1 ) and estimated GFR (88 ml/min/1.73 m2 vs 57 ml/min/1.73 m2) (Fig. 2 ) from those who entered general ward.
Table 1.
Characteristics of the study population.
| General ward (n = 21) | ICU (n = 35) | Statistical tests |
||
|---|---|---|---|---|
| t-test⁎ Mann-Whitney⁎ | ||||
| Categorical Variable, percentage (n) | ||||
| Sex F/M | 32.1 (18) / 67.9 (38) | |||
| Continuous variables, mean (SD) o median (IQR25–75) | ||||
| Age (years) | 68 (60.0–73.8) | 72 (59.6–83.0) | 67(60–72) | 0.098 |
| PCT (ng/mL) | 0.22 (0.60–0.63) | 0.06 (0.04–0.18) | 0.30 (0.17–0.67) | <0.05⁎ |
| GFR (ml/min/1.73m2) | 69.0 (32.5) | 88.1 (31.5) | 57.2 (27.3) | <0.05⁎ |
| Urea (mg/dL) | 51.8 (31.8–71.3) | 34.0 (23.0–55.5) | 64.8 (40.3–90.0) | <0.05⁎ |
| Creatinine (mg/dL) | 0.80 (0.65–1.09) | 0.77 (0.62–0.86) | 0.91 (0.67–1.48) | <0.05⁎ |
| Albumin (gr/dL) | 3.1 (2.6–3.7) | 3.7 (3.5–4.1) | 2.8 (2.3–3.1) | <0.05⁎ |
Abbreviations: ICU, Intensive Care Unit, PCT, Procalcitonin; GFR, Glomerular Filtration Rate.
Differences with values of p < .05 were considered statistically significant.
Fig. 1.
Distribution of initial blood PCT levels.
Fig. 2.
Glomerular filtration rate at hospital admission.
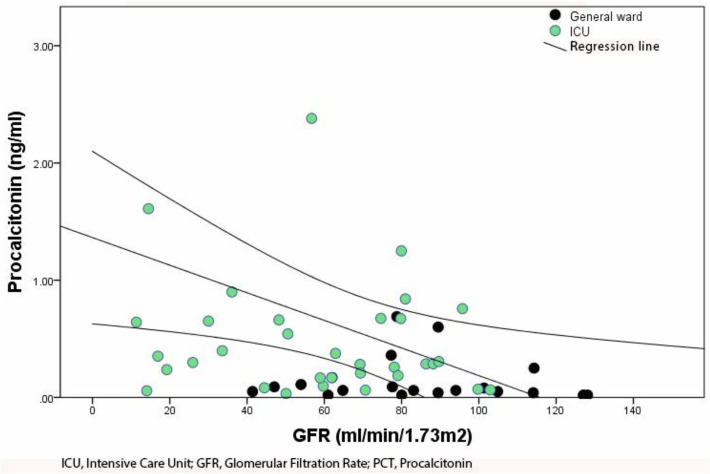
We found an inverse correlation between blood PCT levels and estimated GFR at hospital admission (p < .05) (Fig. 3 ). The multiple linear regression analysis did not show any significant confounding factor in this linear association.
Fig. 3.
Correlation between initial blood PCT levels and GFR.
Nine of the patients admitted to the ICU died during admission. Medians (IQR 25–75) for age, initial blood PCT level and estimated GFR were 66 years (59–71), 0.31 ng/ml (0.17–0.67), and 61.9 ml/min/1.73 m2 (30–79) respectively. The characteristics of ICU patients at admission are shown in Table 2 .
Table 2.
Characteristics of ICU patients.
| Global (n = 35) | Non-survivors (n = 9) | Survivors (n = 26) | Statistical tests |
|
|---|---|---|---|---|
| χ2 Logistic regression⁎ Spearman⁎ | ||||
| Categorical Variable, percentage (n) | ||||
| Sex F/M | 28.6 (10) / 71.4 (25) | 22.2 (2) / 77.8 (7) | 30.8 (8) / 69.2 (18) | 0.625 |
| Mortality | 25.7 (9) | |||
| Nosocomial Infection | 42.9 (15) | 55.6 (5) | 38.5 (10) | 0.372 |
| Antibiotics | 60.0 (21) | 66.7 (6) | 57.7 (15) | 0.636 |
| HFOT | 65.7 (23) | 55.6 (5) | 69.2 (18) | 0.456 |
| IMV | 82.9 (29) | 88.9 (8) | 80.8 (21) | 0.577 |
| CRRT | 20.0 (7) | 33.3 (3) | 15.4 (4) | 0.246 |
| Continuous variables, median (IQR25–75) | ||||
| Age (years) | 66.6 (59.6–71.9) | 71.7 (67.7–72.2) | 61.6(56.6–71.6) | 0.075 |
| SAPS II | 30 (24–37) | 38 (30–42) | 28 (20–36) | <0.05⁎ |
| SOFA | 4 (2–5) | 5 (4.5–7.5) | 3 (2–5) | <0.05⁎ |
| PCT (ng/mL) | 0.31 (0.17–0.67) | 0.67 (0.42–5.96) | 0.28 (0.09–0.64) | <0.05⁎ |
| GFR (ml/min/1.73m2) | 61.9 (30–79.8) | 48.2 (26–50.6) | 69.9 (32.7–86.8) | 0.110 |
Abbreviations: PCT, Procalcitonin; GFR, Glomerular Filtration Rate; HFOT, High-Flow Oxygen Therapy; IMV, Invasive Mechanical Ventilation; CRRT, Continuous Renal Replace Therapy, IQR, Interquartile Range.
Differences with values of p < .05 were considered statistically significant.
Non-survivors had a higher initial blood PCT level (median, IQR 25–75: 0.67 ng/ml, 0.42–5.96) than survivors (median, IQR 25.75: 0.28 ng/ml, 0.09–0.64) with a significant positive non-parametric correlation (p < .006). 21 ICU patients (60%) received antibiotic therapy on suspicion of 31 bacterial nosocomial infections, some patients were suspected of more than one nosocomial infection, and in 15 (42.9%) at least one infection was microbiologically confirmed.
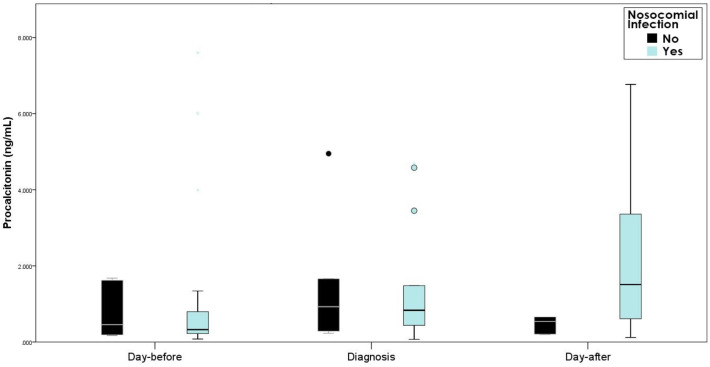
31 empirical antibiotic treatments were initiated because of suspected nosocomial infections and 25 of them were microbiologically confirmed. The empirical antibiotic therapy was adequate in 22 of the confirmed infections (88%). The blood PCT levels at the moment of diagnosis or blood PCT level variations from the day-before and the day-after the diagnosis did not significantly differ between those with confirmed infection and the non-confirmed cases, as shown in Table 3 , Fig. 4 . We also did not find difference in the GFR between the two groups.
Table 3.
Characteristics of nosocomial bacterial infection suspected cases⁎.
| Total cases (n = 31) | Confirmed infections (n = 25) | Non-confirmed infections (n = 6) | Statistical tests |
|
|---|---|---|---|---|
| Man-Whitney | ||||
| Continuous variables, median (IQR25–75) | ||||
| PCT (day of infection) | 0.9 (0.37–3) | 0.9 (0.42–3.73) | 0.93 (0.27–2.48) | 0.682 |
| PCT (day before) | 0.32 (0.2–0.82) | 0.32 (0.21–0.81) | 0.46 (0.19–1.64) | 0.903 |
| PCT variation (with respect to day before) | 0.15 (0.003–0.98) | 0.25 (0.041–0.85) | 0.05 (−0.07–1.75) | 0.494 |
| PCT (day after) | 0.83 (0.49–3.38) | 1.74 (0.59–3.7) | 0.54 (0.2–3.43) | 0.175 |
| PCT variation (with respect to day after) | −0.08 (−0.86–2.06) | −0.08 (−1.36–2.29) | −0.06 (−0.83–1.7) | 0.977 |
| GFR (ml/min/1.73m2) | 47 (21.1–91.7) | 52.6 (20.1–102.2) | 44.5 (25–61.3) | 0.542 |
Abbreviations: PCT, Procalcitonin ng/ml; GFR, Glomerular Filtration Rate; IQR, Interquartile Range.
Differences with values of p < .05 were considered statistically significant.
Fig. 4.
Procalcitonin variations.
5. Discussion
The normal blood PCT level in a non-infected individual is 0.033 ng/ml (±0.003 ng/ml) [21] and is undetectable by the commonly used methods in clinical laboratories. Blood PCT levels below the detection limit of the assay offer excellent negative predictive value for bacterial community-acquired respiratory infection [22] and higher values are a useful guide to start and maintain antibiotic therapy in patients with acute respiratory failure [17].
In critically ill patients, PCT is recommended as a diagnostic biomarker of infection in febrile population and is associated with mortality [6]. It has recently been used for early diagnosis of sepsis in these patients [25,26]. In patients with ARDS, it has been proposed that blood PCT values above the usually accepted cut-off rule out a non-septic cause [23]. Hence, PCT emerges as a useful tool to establish antibiotic therapeutic algorithms [24].
As in our experience, the vast majority of studies show low initial blood PCT levels [16,17,27,28] in non-severely ill COVID-19 patients admitted to hospital. Our ICU patients had higher blood PCT levels than those admitted to the general ward, which suggest the possibility of PCT being an early marker of disease severity or associated comorbidities. Furthermore, we observed higher blood PCT levels in those patients who died (0.67 ng/ml) in concordance with other authors [[15], [16], [17],27,29] and confirming the utility of initial blood PCT level as a marker of mortality.
Low blood PCT levels are associated with a negative predictive value of 94% for bacterial infection in critically ill patients infected by Influenza A [30]. Furthermore, a low incidence of co-infection has been documented in COVID-19 patients and low PCT levels [28].
Even though initial PCT high levels were found at ICU admission in comparison to non-ICU patients, we did not detect episodes of bacterial co-infection. Nevertheless, at ICU admission 91.4% of our patients were empirically treated with ceftriaxone plus azithromycin for 5 to 7 days due to the possibility of community-acquired bacterial pneumonia.
After the first week of ICU admission, 60% (21/35) of patients received empirical antibiotic treatment (usually meropenem plus linezolid) on suspicion of nosocomial bacterial infection. We recorded 25 episodes of microbiologically-diagnosed nosocomial infections in 15 out of 35 patients. Comparisons of sequential results of PCT in patients with bacterial infection did not show a significant difference among the day-before, the day-after and the day of the nosocomial infection diagnosis. In a context of patients on mechanical ventilation due to severe hypoxemia, continuous fever, multiple inserted devices and diffuse pulmonary opacities in chest X-ray, PCT did not help to identify cases of nosocomial infection.
SARS-CoV and SARS-CoV-2 invade human cells by using angiotensin-converting enzyme 2 (ACE2) as the entry receptor. ACE2 is amply found in the alveolar epithelium [31,32] and the renal tubule. Detection of antibodies against nucleoproteins of virus [33] and anatomopathological kidney changes [34] demonstrate cell invasion in kidney structures. Hirsch et al. [12] confirm that 36.6% of the hospitalized patients present some degree of renal failure, 31.1% of which are acute kidney injury (AKI) III and 14.3% need dialysis (96.8% of them require mechanical ventilation). Renal failure is associated with a high risk of mortality [35] and acts as a marker of worse prognosis [36]. Our ICU patients had worse oxygenation (82% underwent mechanical ventilation during the first 24 h of admission) and renal function in comparison to patients admitted at the general ward (57 vs 88 ml/min/1.73m2) (Fig. 2). In our series, 7 patients (20%) required dialysis. High blood PCT levels are described in patients undergoing chronic hemodialysis in relation to a chronic systemic inflammatory state (monocytes activation and subsequent inflammatory cytokines release) [37]. We did not find any differences in renal function parameters between patients with and without nosocomial infections. However, we found a significant correlation between high blood PCT levels and worse renal function. This disclosure was independent from the presence of sepsis, invalidating it as a reliable marker in patients with renal failure when conventional cut-off concentrations are used [38].
A recent study reveals that blood PCT levels appeared to be disease severity-dependent in the COVID-19 pandemic. Notably, co-infection rates were only 20% and 50% in severe and critically ill patients, whereas high blood PCT levels' rates were 50% and 80% respectively [39]. It seems contradictory that a viral infection may occur with high blood PCT level without concomitant bacterial infection, however severely ill COVID-19 patients are known to have high blood PCT levels [[14], [15], [16],24,29,39]. In this scenario, they frequently suffer organ failures, e.g. kidney failure, which is another target of this infection [11,35,36]. PCT has a low sensitivity for the diagnosis of bacterial infection in patients with renal failure [10], hence we believe that there may be a link between the kidney failure and high blood PCT levels found in these patients, that hinders the identification of a possible bacterial infection.
Our study has some limitations. First, it is a single-center retrospective study limited by the sample size. Second, we do not have a procalcitonin-guided antibiotic stewardship protocol, therefore, PCT assays are not routinely used and were measured at the clinician's discretion. Third, we do not know whether blood PCT levels were influenced by the massive use of empirical antibiotic therapy.
6. Conclusions
Our study provide evidence that initial blood PCT level at Emergency Department in COVID-19 patients is a useful marker of severity and poor prognosis. We also found that higher blood PCT levels at ICU admission of these patients were associate with higher mortality. These findings suggest that blood PCT levels could be used (in addition to other measures) for triaging critically ill patients in the ED.
During stay in the ICU, blood PCT levels were high in COVID-19 patients and variations in serial blood PCT levels did not help to detect bacterial nosocomial infections. We found an inverse correlation between PCT concentrations and GFR estimation. The impaired renal function may have played a role in the high serum PCT concentrations detected.
Further studies are needed to establish the impact of these findings.
Contributions
All authors: Final approval of manuscript.
Funding
The authors received no specific funding for this work.
Declaration of Competing Interest
The authors have no conflicts of interest to declare.
Acknowledgements
We acknowledge all health-care workers involved in the treatment of our patients, and we thank Jordi Camps (Institut d'Investigació Sanitària Pere Virgili, Universitat Rovira i Virgili, Reus, Spain) and Gabor Zilahi (St George's Hospital, London, UK) for review of the manuscript.
Appendix A. Appendix
Serum concentrations of PCT, urea, creatinine and albumin were measured at admission and then prospectively at the clinician's discretion when there was suspicion of bacterial infection. It's a retrospective study and there was no specific designed protocol for the extraction of blood samples, but in fact analyses were requested at least at 24 h intervals.
All the laboratory test results were collected automatically by our critical care information system (IntelliSpace Critical Care and Anesthesia, Philips, Germany) during the whole admission. Laboratory tests were analyzed by standard test in Roche/Hitachi Modular Analytics P800 System (Roche Diagnostics, Basel, Switzerland).
For the real-time RT-PCR RNA was extracted from clinical samples (140 μL) on a Qiacube using the Rneasy Mini kit (Qiagen)) following a viral inactivation step using Lysis Buffer according to the manufacturer's instructions. RNA elution occurred in 50 μL RNAse free water and 5 μL were used for the RT-PCR. Reverse transcription and RT-PCR were performed on an LC480 thermocycler (Roche) based on Corman et al. [40] protocol for the detection of RdRP and E genes using the Taqman Fast Virus 1-StepMaster Mix (Thermo Fisher). Primers and probes (Eurogentec, Belgium) were used as described by the authors [40].
The glomerular filtration rate (GFR) was calculated by the Modification of Diet in Renal Disease formula (MDRD) [20].
References
- 1.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., et al. The third international consensus definitions for Sepsis and septic shock (Sepsis-3) JAMA. 2016 Feb 23;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zilahi G., McMahon M.A., Povoa P., Martin-Loeches I. Duration of antibiotic therapy in the intensive care unit. J Thorac Dis. 2016 Dec;8(12):3774–3780. doi: 10.21037/jtd.2016.12.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jee Y., Carlson J., Rafai E., Musonda K., Huong T.T.G., Daza P., et al. Antimicrobial resistance: a threat to global health. Lancet Infect Dis. 2018 Sep 1;18(9):939–940. doi: 10.1016/S1473-3099(18)30471-7. [DOI] [PubMed] [Google Scholar]
- 4.Lee H. Procalcitonin as a biomarker of infectious diseases. Korean J Intern Med. 2013 May;28(3):285–291. doi: 10.3904/kjim.2013.28.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maruna P., Nedelníková K., Gürlich R. Physiology and genetics of procalcitonin. Physiol Res. 2000;49(Suppl. 1):S57–S61. [PubMed] [Google Scholar]
- 6.Tsangaris I., Plachouras D., Kavatha D., Gourgoulis G.M., Tsantes A., Kopterides P., et al. Diagnostic and prognostic value of procalcitonin among febrile critically ill patients with prolonged ICU stay. BMC Infect Dis. 2009 Dec 22;9:213. doi: 10.1186/1471-2334-9-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuetz P., Wirz Y., Sager R., Christ-Crain M., Stolz D., Tamm M., et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017;10 doi: 10.1002/14651858.CD007498.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilbert D.N. Use of plasma procalcitonin levels as an adjunct to clinical microbiology. J Clin Microbiol. 2010 Jul;48(7):2325–2329. doi: 10.1128/JCM.00655-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linscheid P., Seboek D., Schaer D.J., Zulewski H., Keller U., Müller B. Expression and secretion of procalcitonin and calcitonin gene-related peptide by adherent monocytes and by macrophage-activated adipocytes. Crit Care Med. 2004 Aug;32(8):1715–1721. doi: 10.1097/01.ccm.0000134404.63292.71. [DOI] [PubMed] [Google Scholar]
- 10.Lu X.-L., Xiao Z.-H., Yang M.-Y., Zhu Y.-M. Diagnostic value of serum procalcitonin in patients with chronic renal insufficiency: a systematic review and meta-analysis. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc Eur Ren Assoc. 2013 Jan;28(1):122–129. doi: 10.1093/ndt/gfs339. [DOI] [PubMed] [Google Scholar]
- 11.Zhu J., Ji P., Pang J., Zhong Z., Li H., He C., et al. Clinical characteristics of 3,062 COVID-19 patients: a meta-analysis. J Med Virol. 2020 Oct;92(10):1902–1914. doi: 10.1002/jmv.25884. https://onlinelibrary.wiley.com/doi/10.1002/jmv.25884 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirsch J.S., Ng J.H., Ross D.W., Sharma P., Shah H.H., Barnett R.L., et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020 Jul;98(1):209–218. doi: 10.1016/j.kint.2020.05.006. https://www.kidney-international.org/article/S0085-2538(20)30532-9/abstract Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu Y., Xu D., Fu S., Zhang J., Yang X., Xu L., et al. Patients with COVID-19 in 19 ICUs in Wuhan, China: a cross-sectional study. Crit Care. 2020 May 14;24(1):219. doi: 10.1186/s13054-020-02939-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lippi G., Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin Chem Lab Med CCLM. 2020 Mar;58(7):1131–1134. doi: 10.1515/cclm-2020-0198. https://www.degruyter.com/view/journals/cclm/ahead-of-print/article-10.1515-cclm-2020-0198/article-10.1515-cclm-2020-0198.xml Available from: [DOI] [PubMed] [Google Scholar]
- 15.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 Apr 30;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb 15;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Gall J.R., Lemeshow S., Saulnier F. A new simplified acute physiology score (SAPS II) based on a European/north American multicenter study. JAMA. 1993 Dec 22;270(24):2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 19.Vincent J.-L., Moreno R., Takala J., Willatts S., De Mendonça A., Bruining H., et al. The SOFA (Sepsis-related organ failure assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996 Jul 1;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 20.Levey A.S. A more accurate method to estimate glomerular filtration rate from serum Creatinine: a new prediction equation. Ann Intern Med. 1999 Mar 16;130(6):461. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 21.Becker K.L., Snider R., Nylen E.S. Procalcitonin assay in systemic inflammation, infection, and sepsis. Clin Utility Limitat Crit Care Med. 2008 Mar;36(3):941–952. doi: 10.1097/CCM.0B013E318165BABB. [DOI] [PubMed] [Google Scholar]
- 22.Gilbert D.N. Role of procalcitonin in the management of infected patients in the intensive care unit. Infect Dis Clin North Am. 2017;31(3):435–453. doi: 10.1016/j.idc.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Tsantes A., Tsangaris I., Kopterides P., Kapsimali V., Antonakos G., Zerva A., et al. The role of procalcitonin and IL-6 in discriminating between septic and non-septic causes of ALI/ARDS: a prospective observational study. Clin Chem Lab Med. 2013 Jul;51(7):1535–1542. doi: 10.1515/cclm-2012-0562. [DOI] [PubMed] [Google Scholar]
- 24.Schuetz P., Raad I., Amin D.N. Using procalcitonin-guided algorithms to improve antimicrobial therapy in ICU patients with respiratory infections and sepsis. Curr Opin Crit Care. 2013 Oct;19(5):453–460. doi: 10.1097/MCC.0b013e328363bd38. [DOI] [PubMed] [Google Scholar]
- 25.Wacker C., Prkno A., Brunkhorst F.M., Schlattmann P. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect Dis. 2013 May 1;13(5):426–435. doi: 10.1016/S1473-3099(12)70323-7. [DOI] [PubMed] [Google Scholar]
- 26.Kondo Y., Umemura Y., Hayashida K., Hara Y., Aihara M., Yamakawa K. Diagnostic value of procalcitonin and presepsin for sepsis in critically ill adult patients: a systematic review and meta-analysis. J Intensive Care. 2019 Apr;7:22. doi: 10.1186/s40560-019-0374-4. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6466719/ Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J.-J., Dong X., Cao Y.-Y., Yuan Y.-D., Yang Y.-B., Yan Y.-Q., et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75(7):1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 28.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020 Feb 15;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J., et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63(3):364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodríguez A.H., Avilés-Jurado F.X., Díaz E., Schuetz P., Trefler S.I., Solé-Violán J., et al. Procalcitonin (PCT) levels for ruling-out bacterial coinfection in ICU patients with influenza: a CHAID decision-tree analysis. J Infect. 2016 Feb;72(2):143–151. doi: 10.1016/j.jinf.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003 Nov 27;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. (e8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su H., Yang M., Wan C., Yi L.-X., Tang F., Zhu H.-Y., et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020 Jul;98(1):219–227. doi: 10.1016/j.kint.2020.04.003. https://www.kidney-international.org/article/S0085-2538(20)30369-0/abstract Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang F., Shi S., Zhu J., Shi J., Dai K., Chen X. Analysis of 92 deceased patients with COVID-19. J Med Virol. 2020;92(11):2511–2515. doi: 10.1002/jmv.25891. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pei G., Zhang Z., Peng J., Liu L., Zhang C., Yu C., et al. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol. 2020 Jun 1;31(6):1157–1165. doi: 10.1681/ASN.2020030276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ali H., Daoud A., Mohamed M.M., Salim S.A., Yessayan L., Baharani J., et al. Survival rate in acute kidney injury superimposed COVID-19 patients: a systematic review and meta-analysis. Ren Fail. 2020;42(1):393–397. doi: 10.1080/0886022X.2020.1756323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt M., Burchardi C., Sitter T., Held E., Schiffl H. Procalcitonin in patients undergoing chronic hemodialysis. Nephron. 2000 Feb;84(2):187–188. doi: 10.1159/000045570. [DOI] [PubMed] [Google Scholar]
- 38.El-Sayed D., Grotts J., Golgert W.A., Sugar A.M. Sensitivity and specificity of procalcitonin in predicting bacterial infections in patients with renal impairment. Open Forum Infect Dis. 2014 Sep;1(2) doi: 10.1093/ofid/ofu068. (ofu068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu R., Han C., Pei S., Yin M., Chen X. Procalcitonin levels in COVID-19 patients. Int J Antimicrob Agents. 2020 Aug;56(2):106051. doi: 10.1016/j.ijantimicag.2020.106051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020 Jan;25(3) doi: 10.2807/1560-7917.ES.2020.25.3.2000045. https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2020.25.3.2000045 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]