Abstract
Most reproductive biologists who study female gametes will agree with the 16th century anatomist William Harvey’s doctrine: ‘Ex Ovo Omnia’. This phrase, which literally translates to ‘everything from the egg’, recognizes the centrality of the egg in animal development. Eggs are most impressive cells, capable of supporting development of an entirely new organism following fertilization or parthenogenetic activation. Not so uniformly embraced in the field of reproductive biology is the nomenclature used to refer to the female germ cell. What is an oocyte? What is an egg? Are these terms the same, different, interchangeable? Here we provide functional definitions of the oocyte and egg, and how they can be used in the context of mammalian gamete biology and beyond.
Keywords: oocyte, oocyte maturation, egg, gamete / mammalian
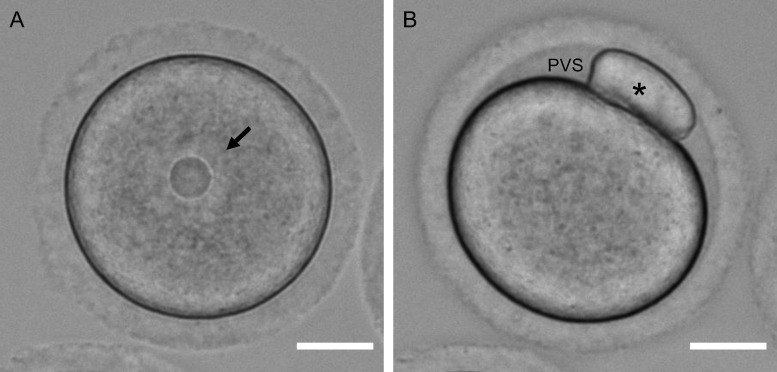
The mammalian oocyte is the ‘founder cell’ and refers to a female germ cell arrested in prophase of meiosis I. In the ovary, follicle-enclosed oocytes are found in follicles at all stages of development, including primordial, primary, secondary, early antral and antral follicles. Although these oocytes may vary in size due to differences in their growth phase, they are all arrested in prophase of meiosis I. Oocytes are characterized by a nucleus, or germinal vesicle, with an intact nuclear envelope that is readily visible by transmitted light microscopy (Fig. 1A). Oocytes also have a minimal perivitelline space or gap between the plasma membrane and zona pellucida (Ueno and Niimura, 2008; Yoshida and Niimura, 2011).
Figure 1.
Morphological differences between an oocyte and an egg. Transmitted light images showing key differences between a mammalian (mouse) (A) oocyte and (B) egg. The egg was obtained following in-vitro maturation. The nucleus or germinal vesicle is highlighted by the arrow and the polar body is highlighted by the asterisk. Note the increased perivitelline space (PVS) in the egg relative to the oocyte. Scale bar = 20 µm.
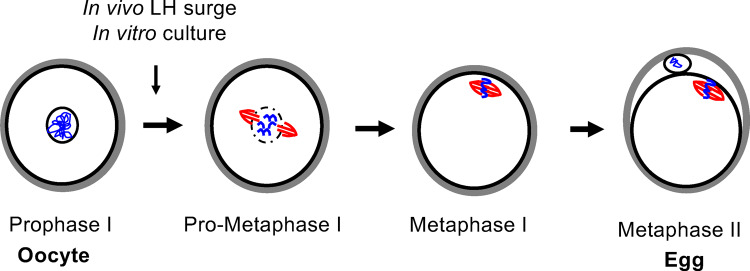
The mammalian oocyte becomes an egg through the process of meiotic maturation (Figs 1, 2, and 3). In response to the luteinizing hormone surge that triggers ovulation, the oocyte resumes meiosis in vivo. Meiotic competence—the ability to undergo meiotic maturation—is achieved once oocytes have reached a critical size threshold, and in mice, meiotic maturation can be induced spontaneously in vitro by removing oocytes from the follicle (Durinzi et al., 1995; Kanatsu-Shinohara et al., 2000). In the first steps of meiotic maturation, chromatin condenses, the nuclear envelope surrounding the oocyte breaks down (often referred to as germinal vesicle break down), and a meiotic spindle forms. The cells complete meiosis I with separation of homologous chromosomes and extrusion of the first polar body. Meiosis I is followed immediately by meiosis II, which occurs without an intervening round of DNA replication. The cells then enter a second meiotic arrest at metaphase of meiosis II (MII) with a characteristic meiotic spindle. We refer to a gamete arrested at MII as an egg because functionally at this stage, the mammalian egg can undergo normal fertilization if sperm are present and support normal embryo development. Upon fertilization, the egg completes meiosis with separation of sister chromatids and extrusion of the second polar body.
Figure 2.
Mammalian meiotic maturation transforms an oocyte into an egg. Schematic of the key steps of meiotic maturation in which an oocyte arrested at prophase of meiosis I resumes meiosis, completes meiosis I and arrests at metaphase of meiosis II. This process is initiated in vivo by the hormonal trigger for ovulation (LH surge) and can occur spontaneously in vitro when the oocyte is removed from the follicle.
Figure 3.
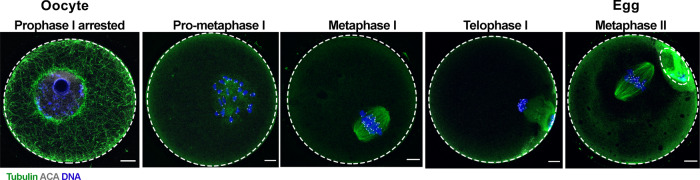
Cell cycle, chromosome and cytoskeleton changes during mammalian meiotic maturation. High-resolution imaging reveals clear differences in cell cycle stage as well as chromosome and microtubule configurations as an oocyte matures into an egg during mammalian meiotic maturation (green = tubulin, blue = DNA, gray = centromeres; anti-centromere antibody [ACA]). The gametes shown here are mouse in origin. Scale bar = 10 µm.
Our nomenclature of an egg differs from others who refer to an egg or mature oocyte as a cell that has completed meiosis. Logically the defining characteristic of a mature egg would be its DNA content with 1N 1C signifying a haploid cell, where N refers to the number of chromosomes and C refers to the number of chromatids or copies of DNA. However, careful consideration of the process of female mammalian meiosis reveals a counterintuitive reality; if an egg is defined as being 1N 1C, then mammals do not have eggs. This reality is because in female mammals, meiosis is not completed until fertilization occurs, and by this time, the sperm genome is already present. Thus, unlike men, women never have purely haploid cells in their body. From an evolutionary perspective, it may be advantageous for mammalian eggs to lack a truly haploid phase. The tight link between the completion of meiosis and fertilization ensures that the haploid female and male genomic content will be present synchronously within the same cytoplasm to enable syngamy of the female and male pronuclei, which is a prerequisite for successful zygote development. Moreover, the interconnected nature of the completion of meiosis and fertilization may be a mechanism to reduce parthenogenesis.
Given the ethereal haploid phase of the egg in mammals, our nomenclature instead relies on a functional definition in which an egg is a cell that can be fertilized to produce a normal embryo. In mammals, this occurs at MII when the cell has a DNA content of 1N 2C (homologous chromosomes have segregated but sister chromatids have not). The meiotic stage of eggs appears to be consistent among all Eutherian mammals although much of our data are based on the mouse model. There is a paucity of information to define at what stage fertilization occurs in the human gamete upon natural ovulation in unstimulated cycles because of the restrictions (practical, legal and ethical) of collecting human eggs from the ampullae. Of note, because ART-conceived children will likely account for 1.4% of the global population by 2100, ART has and will continue to fundamentally change the way we reproduce (Faddy et al., 2018). Conception by ART has demonstrated that it is technically possible to fertilize human gametes prior to MII and even obtain live offspring from these approaches. However, the efficacy is low, and these examples are the exception rather than the rule (Vanhoutte et al., 2005). If fertilizing cells prior to MII resulted in robust outcomes, this methodology would be widely adopted in ART clinics, which is not the case. In the mouse, IVF prior to MII is possible. However, the outcomes improve significantly with more advanced stages of meiotic progression, and the true developmental potential of gametes that are fertilized before MII has not been systematically examined (Iwamatsu and Chang, 1972). In canids, bitches ovulate oocytes at metaphase of meiosis I, but fertilization occurs at 90 h post-ovulation when the cells are at the MII stage (Reynaud et al., 2005; Nagashima et al., 2015). In fact, sperm penetration of immature oocytes is a rare exception, and only occurred 3 out of 112 times in one study (Reynaud et al., 2005).
In mammals, oocytes and eggs are distinct in several ways (Table I, Figs 1 and 3) (Ducibella et al., 1988; Combelles and Albertini, 2001; Sanfins et al., 2003; Duncan et al., 2005, 2016; Ueno and Niimura, 2008; Tartia et al., 2009; Yoshida and Niimura, 2011; Duan and Sun, 2019). Oocytes are found in the ovary, are arrested in prophase of meiosis I, are characterized by an intact nucleus, have an interphase microtubule network, have cortical symmetry (actin, cortical granules, zinc vesicles), have minimal perivitelline space and cannot regulate their volume independently. Oocytes are not capable of being fertilized normally when exposed to sperm because they fail to elicit normal egg activation, including changes in intracellular calcium and extracellular zinc (Mehlmann and Kline, 1994; Jones et al., 1995; Miao and Williams, 2012; Duncan et al., 2016). On the other hand, eggs are found in the oviduct in vivo and are arrested at MII. Eggs are morphologically distinct from oocytes because they lack a nucleus, have a visible first polar body and have an enlarged perivitelline space in the vicinity of the first polar body. Eggs have a spindle and are characterized by cortical asymmetry (actin, cortical granules, zinc vesicles, and polarity markers). Eggs possess the machinery to regulate cell volume independently, and, if fertilized, can support normal embryonic development.
Table I.
Parameters that distinguish a mammalian oocyte from an egg. *
| Oocyte | Egg | |
|---|---|---|
| In vivo tissue environment | Ovary | Oviduct |
| Meiotic stage | Prophase of meiosis I | Metaphase of meiosis II |
| Defining features by transmitted light | Intact nucleus |
Lack of a nucleus; presence of polar body |
| Perivitelline space | Minimal | Enlarged |
| Independent cell volume regulation | Not possible | Possible |
| Microtubule cytoskeleton | Interphase | Meiotic spindle |
| Cortical organization | Symmetric | Asymmetric |
| Normal fertilization | Not possible | Possible |
Here we distinguish between an oocyte and egg, but when the mammalian gamete is neither at prophase of meiosis I nor metaphase of meiosis II, the precise cell cycle stage should be specified (e.g. cell at pro-metaphase I, metaphase I).
Ideally, our terminology should not be unique to mammals but instead would be broadly relevant to most animals. We would do the field a great disservice if the terms we used were only applicable to one type of animal, but the same cell in a different animal had different terminology (Wessel, 2009). Our field is already sufficiently complicated without imposing many different languages. Thus, after initiation of meiotic maturation, ‘eggs’ are fertilized at all stages of meiosis, depending on the species of the animal. For example, female gametes in marine invertebrates are typically fertilized at metaphase I, or, less commonly, at prophase I. Similarly, most insects are fertilized at metaphase I, whereas sea urchins are fertilized after having completed meiosis. The key characteristic of the terminology is at what point the female gamete becomes fertilizable and able to support normal embryo development. When it does, the term for this cell is ‘egg’, and the term for the cell leading up to that point is an ‘oocyte’.
In summary, the terminology of ‘oocyte’ and ‘egg’ is ultimately functional—mammalian oocytes mature to become eggs that are fertilizable and capable of supporting normal subsequent embryo development, whereas oocytes are generally not. We encourage the field of reproductive science and medicine to consider these distinctions and be deliberate when using the terms oocyte and egg to communicate effectively.
Acknowledgements
We acknowledge Dr Nucharin Songsasen for providing us with useful references on canid fertilization. We also thank the reviewer of this editorial for raising important and provocative points which we have integrated into our thinking.
Authors’ roles
F.E.D. conceived of the editorial topic, and all authors contributed to the research, writing and editing of the manuscript. All authors approved the final version of the manuscript.
Funding
This work was supported funding from the National Institutes of Health (NIH): R01HD093726 (F.E.D.), R21HD098498 (F.E.D.), R01 HD091331 (K.S.), R01 GM112801 (K.S.), R01 GM125071 (G.M.W.), R01 GM132222 (G.M.W.) and the Intramural Research Program of the NIH, National Institutes of Environmental Health Sciences, 1ZIAES10298 (C.J.W.).
Conflict of interest
There are no conflicts of interest to declare.
References
- Combelles CM, Albertini DF. Microtubule patterning during meiotic maturation in mouse oocytes is determined by cell cycle-specific sorting and redistribution of gamma-tubulin. Dev Biol 2001;239:281–294. [DOI] [PubMed] [Google Scholar]
- Duan X, Sun SC. Actin cytoskeleton dynamics in mammalian oocyte meiosis. Biol Reprod 2019;100:15–24. [DOI] [PubMed] [Google Scholar]
- Ducibella T, Anderson E, Albertini DF, Aalberg J, Rangarajan S. Quantitative studies of changes in cortical granule number and distribution in the mouse oocyte during meiotic maturation. Dev Biol 1988;130:184–197. [DOI] [PubMed] [Google Scholar]
- Duncan FE, Moss SB, Schultz RM, Williams CJ. PAR-3 defines a central subdomain of the cortical actin cap in mouse eggs. Dev Biol 2005;280:38–47. [DOI] [PubMed] [Google Scholar]
- Duncan FE, Que EL, Zhang N, Feinberg EC, O’Halloran TV, Woodruff TK. The zinc spark is an inorganic signature of human egg activation. Sci Rep 2016;6:24737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durinzi KL, Saniga EM, Lanzendorf SE. The relationship between size and maturation in vitro in the unstimulated human oocyte. Fertil Steril 1995;63:404–406. [DOI] [PubMed] [Google Scholar]
- Faddy MJ, Gosden MD, Gosden RG. A demographic projection of the contribution of assisted reproductive technologies to world population growth. Reprod Biomed Online 2018;36:455–458. [DOI] [PubMed] [Google Scholar]
- Iwamatsu T, Chang MC. Sperm penetration in vitro of mouse oocytes at various times during maturation. J Reprod Fertil 1972;31:237–247. [DOI] [PubMed] [Google Scholar]
- Jones KT, Carroll J, Whittingham DG. Ionomycin, thapsigargin, ryanodine, and sperm induced Ca2+ release increase during meiotic maturation of mouse oocytes. J Biol Chem 1995;270:6671–6677. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Schultz RM, Kopf GS. Acquisition of meiotic competence in mouse oocytes: absolute amounts of p34(cdc2), cyclin B1, cdc25C, and wee1 in meiotically incompetent and competent oocytes. Biol Reprod 2000;63:1610–1616. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Kline D. Regulation of intracellular calcium in the mouse egg: calcium release in response to sperm or inositol trisphosphate is enhanced after meiotic maturation. Biol Reprod 1994;51:1088–1098. [DOI] [PubMed] [Google Scholar]
- Miao YL, Williams CJ. Calcium signaling in mammalian egg activation and embryo development: the influence of subcellular localization. Mol Reprod Dev 2012;79:742–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima JB, Sylvester SR, Nelson JL, Cheong SH, Mukai C, Lambo C, Flanders JA, Meyers-Wallen VN, Songsasen N, Travis AJ. Live births from domestic dog (Canis familiaris) embryos produced by in vitro fertilization. PLoS One 2015;10:e0143930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaud K, Fontbonne A, Marseloo N, Thoumire S, Chebrout M, de Lesegno CV, Chastant-Maillard S. In vivo meiotic resumption, fertilization and early embryonic development in the bitch. Reproduction 2005;130:193–201. [DOI] [PubMed] [Google Scholar]
- Sanfins A, Lee GY, Plancha CE, Overstrom EW, Albertini DF. Distinctions in meiotic spindle structure and assembly during in vitro and in vivo maturation of mouse oocytes. Biol Reprod 2003;69:2059–2067. [DOI] [PubMed] [Google Scholar]
- Tartia AP, Rudraraju N, Richards T, Hammer MA, Talbot P, Baltz JM. Cell volume regulation is initiated in mouse oocytes after ovulation. Development 2009;136:2247–2254. [DOI] [PubMed] [Google Scholar]
- Ueno S, Niimura S. Size of Perivitelline space and incidence of polyspermy in mouse oocytes matured in vivo and in vitro. J Mamm Ova Res 2008;25:44–49. [Google Scholar]
- Vanhoutte L, De Sutter P, Van der Elst J, Dhont M. Clinical benefit of metaphase I oocytes. Reprod Biol Endocrinol 2005;3:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel GM. Eggs over easier…please. Mol Reprod Dev 2009;76:i. [Google Scholar]
- Yoshida N, Niimura S. Size of the perivitelline space and incidence of polyspermy in rabbit and hamster oocytes. Reprod Med Biol 2011;10:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]