Dear Editor,
We read with interest the recent paper by Yang et al., concluding that corticosteroids overall have a negative impact on COVID-19 outcomes from a meta-analysis 1. Critical COVID-19, characterized by refractory hypoxemia caused by acute respiratory distress syndrome (ARDS), is a life-threatening multi-organ dysfunction syndrome resulted from host response to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Glucocorticoid (GC) was one of the anti-inflammatory medications used in critical patients 2. Efficacy of glucocorticoids has been reported in numerous clinical studies in the treatment of coronavirus pneumonia 3. Yang and colleagues demonstrated that patients treated with GC had a higher mortality 1, suggesting that not all patients could benefit from GC treatment. Present study aimed to evaluate the effect of GC on different patient population. Since critical patients were more likely to receive GC therapy, only severe type and critical type patients, according to clinical classification of the Chinese Recommendations for Diagnosis and Treatment of Novel Coronavirus (SARSCoV2) infection (Trial 7th version) 4, were enrolled in present study. We retrospective collected the clinical and outcome data of critical COVID-19 patients, and taking methylprednisolone (MP) treatment, the most used GC during clinical treatment, as an exposure factor analyzed the outcome. This study provides information on MP clinical application in treatment of SARS-CoV-2 infection, including patient selection and administration time and dosage.
Present multicenter retrospective cohort study was performed in 4 government designated treatment centers for COVID-19 patients in 3 cities in China, Wuhan, Guangzhou, and Shenzhen. The data collection period was from December 2019 to March 2020. This study was approved by the Research Ethics Commission of General Hospital of Southern Theater Command of PLA (HE-2020–08) and the requirement for informed consent was waived by the Ethics Commission. Inclusion Criteria for all patients: (1) Adult aged >= 18 years old; (2) Laboratory (RT-PCR) confirmed SARS-COV-2 infection in throat swab and/or sputum and/or lower respiratory tract samples; or conformed plasma positive of specific antibody (IgM or/and IgG) against SARS-COV-2; (3) In-hospital treatment ≥72 h. The patients in who meet any one of the following criteria were enrolled in severe type group 4: (a) Respiratory rate >= 30/min; or (b) Rest SPO2 <= 90%; or (c) PaO2/FiO2 <= 300 mmHg. The patients in who meet any one of the following criteria were enrolled in critical type group 4: (a) Respiratory failure and needs mechanical ventilation; or (b) Shock occurs; or (c) Multiple organ failure and needs ICU monitoring. Exclusion Criteria: (1) Exist of other evidences that can explain pneumonia including but not limited to influenza A virus, influenza B virus, bacterial pneumonia, fungal pneumonia, noninfectious causes, etc.; (2) Women who are pregnant or breast-feeding.
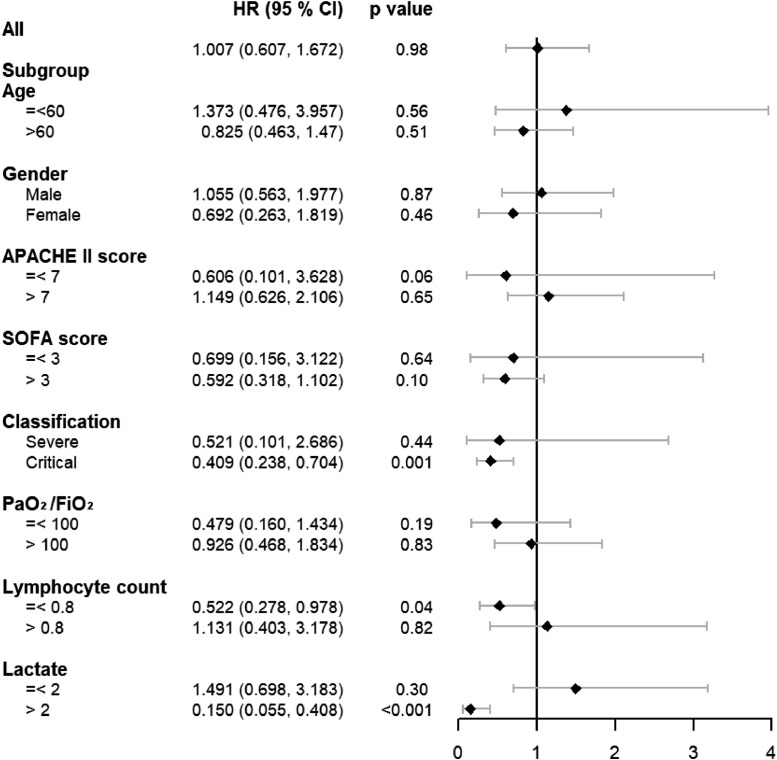
Data from 338 patients diagnosed as critical COVID-19 were collected in present study. According to whether methylprednisolone was employed during treatment, 164 in Non-MP group and 174 in MP group. For all cases, MP treatment did not show benefit in prognosis (Fig. 1 ). The effects of MP differed between different clinical classification and baseline lactate concentration. MP treatment on the critical type patients could decrease the 60-day fatality (HR: 0.409, 95% CI: 0.238–0.704, p-value: 0.001), while it has no influence on the fatality of severe type patients. In addition, patients with higher lactate concentration on baseline could get more benefit from MP treatment. For the patients with lactic acid concentration over 2 mmol/L, MP treatment could significantly decrease the 60-day fatality (HR: 0.150, 95% CI: 0.055–0.408, p-value: < 0.001). These results showed that MP treatment have more efficiency on the patients with serious condition. Glucocorticoid could suppress lung inflammation and but also inhibit immune responses. Therefore, balancing the risk and benefit is crucial during the treatment, that is, not all patients could benefit from GC therapy. Critical type COVID-19 patients showed excessive inflammatory responses and MP treatment could alleviate the cytokine storm by inhibiting the inflammatory cells activation 5. In addition, critical illness-related corticosteroid insufficiency occurs across a broad spectrum of critical illness due to the impairment of the hypothalamic pituitary axis 6. Inadequate endogenous glucocorticoid resulted in insufficient anti-inflammatory activity, and MP treatment could adverse this issue. Moreover, MP could improve the microcirculation in critical patients 7. That explained that our subgroup analysis results, which showed that MP treatment reduced the fatality of the patients with increased lactate.
Fig. 1.
Effect of methylprednisolone therapy on the 60-day fatality in different subgroup (n = 338).
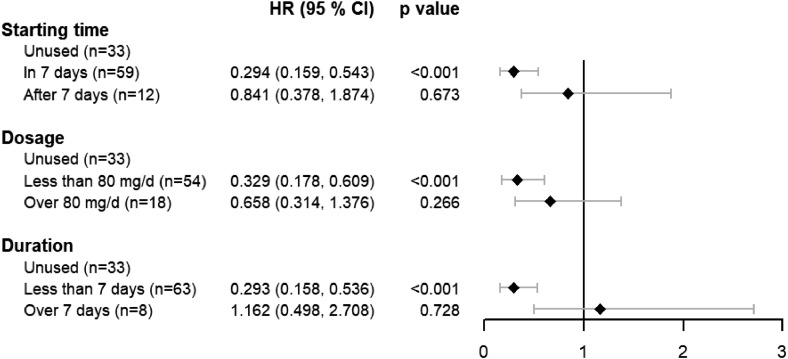
Few studies have discussed the application time, dosage and duration of MP, which were mostly based on the physician experience. To further clarify when and how to employ MP application on the critical type patients, the hazards ratios were analyzed in each group according to the starting time, dosage, and treatment duration (Fig. 2 ). In all 107 critical type patients, 33 of them were not received MP treatment, 59 of them received MP treatment in 7 days after admission to hospital and 12 of them were received after 7 days. 3 patients received MP treatment, but the starting time were missed, and they were not enrolled in analysis. Our results showed MP treatment in 7 days after admission could decrease the 60-day fatality (HR: 0.294, 95% CI: 0.159–0.543, p-value < 0.001), while MP treatment after 7 days has no effect on the fatality. Subgroup with different doses of MP (=< 80 mg/d or > 80 mg/d) were also analyzed. We found that small dose MP showed significant effect on the fatality (HR: 0.329, 95% CI: 0.178–0.605, pvalue < 0.001). In addition, most patients benefited from MP were received treatment no more than 7 days. MP long-term treatment might increase the death risk.
Fig. 2.
Effect of different starting time, dosage, and duration of methylprednisolone on the on the 60-day fatality of critical type patients (n = 107).
Present multicenter retrospective cohort study showed that methylprednisolone therapy could decrease the 60-fatality for the COVID-19 patients diagnosed as critical type, that is, those occurred respiratory failure and needs mechanical ventilation, or shock, or multiple organ failure and needs ICU monitoring. Early (starting in 7 days after admission), low-dose (no more than 80 mg/d), and short-term (no more than 7 days) methylprednisolone therapy could significant decrease the 60-day fatality.
Declarations
Funding
This work was supported by grants from the National Natural Science Foundation of China (NO. 82,072,143), PLA Logistics Research Project of China (18CXZ030, 17CXZ008), Sanming Project of Medicine in Shenzhen (SZSM20162011) and Clinical Research Project of Shenzhen municipal health commission (SZLY2017007).
Role of funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Ethics approval
The study was approved by the Research Ethics Commission of General Hospital of Southern Theater Command of PLA.
Consent to participate
The requirement for informed consent was waived by the Ethics Commission.
Consent for publication
All authors reviewed the manuscript and approved the publication.
Availability of data and material
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Authors' contributions
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Zhifeng Liu was responsible for study concept and design. Ziyun Shao, Ming Wu, Qifeng Xie, Zheying Liu, Zhifeng Liu, Li Zhong, and Conglin Wang were responsible for collecting the data. Jingjing Ji, Ming Wu, Zhifeng Liu and Li Zhong were responsible for statistical analysis. Zhifeng Liu, Jingjing Ji and Ming Wu were responsible for drafting the manuscript. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Reference
- 1.Zhenwei Yang, Jialong Liu, Yunjiao Zhou, Xixian Zhao, Qiu Zhao, Jing Liu. The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis. J Infect. 2020;81(1):e13–e20. doi: 10.1016/j.jinf.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo-Wei Tu, Yi Shi, Yi-Jun Zheng, Min-Jie Ju, Hong-Yu He, Guo-Guang Ma. Glucocorticoid attenuates acute lung injury through induction of type 2 macrophage. J Transl Med. 2017;15(1):181. doi: 10.1186/s12967-017-1284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wen Zhang, Yan Zhao, Fengchun Zhang, Qian Wang, Taisheng Li, Zhengyin Liu. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the Perspectives of clinical immunologists from China. Clin Immunol. 2020;214 doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Health Commission of the People`s Republic of China. Chinese recommendations for diagnosis and treatment of novel coronavirus (SARSCoV2) infection (Trial 7th version) 2020.
- 5.Gregory Julia L., Pam Hall, Michelle Leech, Morand Eric F., Hickey Michael J. Independent roles of macrophage migration inhibitory factor and endogenous, but not exogenous glucocorticoids in regulating leukocyte trafficking. Microcirculation. 2009;16(8):735–748. doi: 10.3109/10739680903210421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Annane Djillali, Stephen M. Pastores, Bram Rochwerg, Wiebke Arlt, Robert A. Balk, Albertus Beishuizen. Guidelines for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in critically ill patients (Part I): society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Intensive Care Med. 2017;43(12):1751–1763. doi: 10.1007/s00134-017-4919-5. [DOI] [PubMed] [Google Scholar]
- 7.Nina Kumowski, Tobias Hegelmaier, Jonas Kolbenschlag, Tina Mainka, Beate Michel-Lauter, Christoph Maier. Short-term glucocorticoid treatment normalizes the microcirculatory response to remote ischemic conditioning in early complex regional pain syndrome. Pain Pract. 2019;19(2):168–175. doi: 10.1111/papr.12730. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.