Summary
Acquired tracheomegaly is a rare condition associated with pulmonary fibrosis, connective tissue disease and the use of cuffed tracheal tubes. We describe the urgent tracheal re‐intubation and subsequent tracheal repair of a previously well 58‐year‐old man who developed tracheostomy‐related tracheomegaly during prolonged mechanical ventilation for coronavirus disease 2019 pneumonitis. Urgent tracheal re‐intubation was required due to a persistent cuff leak, pneumomediastinum and malposition of the tracheostomy tube. We describe the additional challenges and risks associated with airway management in patients with tracheomegaly, and discuss how even in urgent cases these can be mitigated through planning and teamwork. We present a stepwise approach to tracheal re‐intubation past a large tracheal dilatation, including the use of an Aintree catheter inserted via the existing tracheal stoma for oxygenation or tracheal re‐intubation if required. Computed tomography imaging was valuable in characterising the defect and developing a safe airway management strategy before starting the procedure. This report emphasises the role of planning, teamwork and the development of an appropriate airway strategy in the safe management of complex cases.
Keywords: airway management, tracheomalacia, tracheomegaly, tracheostomy
Introduction
Tracheomegaly is a rare but underdiagnosed condition. It is defined radiologically as a tracheal diameter exceeding the upper limits of normal (25 or 21 mm coronal, 27 or 23 mm sagittal; in men and women, respectively) [1]. Although tracheomegaly is usually congenital, it can be acquired in association with connective tissue diseases, the use of cuffed tracheal tubes, inflammatory conditions such as chronic bronchitis, and pulmonary or cystic fibrosis [2, 3, 4]. Prior case reports describe tracheostomy‐related tracheomegaly in patients undergoing long‐term ventilation for chronic respiratory failure [3, 5]. The management described in these reports is based on tracheal re‐intubation with the cuff placed outside the dilatation using devices such as double cuffed and adjustable flange tracheostomy tubes. The challenges presented by airway management in tracheomegaly include that it can be challenging to advance the tracheal tube beyond the dilatation, and cuff leaks are likely if the cuff is not optimally placed. Failure to ventilate secondary to massive cuff leak has been reported during tracheostomy insertion in a patient with undiagnosed tracheomegaly [6]. In this report, we describe the emergent and subsequent management of acquired tracheomegaly in a previously well patient receiving prolonged ventilatory support for coronavirus disease 2019 (COVID‐19) pneumonitis.
Report
After 42 days of invasive ventilation for COVID‐19 pneumonitis and subsequent pulmonary fibrosis, a 58‐year‐old man underwent surgical tracheostomy and insertion of an 8 mm cuffed tube (Portex Blue Line Ultra Suctionaid, Smiths Medical, Minneapolis, MN, USA). A cuff leak, noted following return from theatre, was managed by adding air; the cuff pressure increased from 40 to 85 cmH2O over the course of the day due to the repeated addition of air. The leak persisted and the tracheostomy tube was upsized to a 9mm Portex on day 45, then a 10mm TRACOE twist (TRACOE Medical GmbH, Nieder‐Olm, Germany) on day 50. Despite the addition of more air (peak cuff pressure 120 cmH2O) the leak continued. Computed tomography (CT) imaging on day 55 demonstrated new tracheomegaly with associated severe pneumomediastinum (Figure 1a). Following multidisciplinary discussion, oral tracheal re‐intubation with the cuff placed distal to the dilatation was planned.
Figure 1.
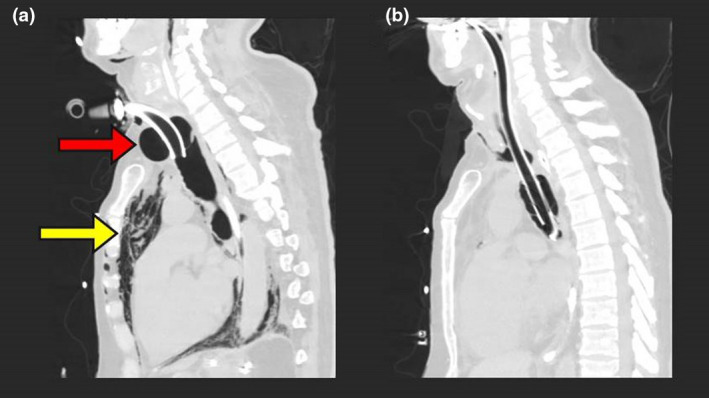
(a) Sagittal computed tomography scan demonstrating tracheomegaly with tracheostomy cuff morphology and an anteroposterior diameter of 52.2 mm (red arrow), with associated severe pneumomediastinum (yellow arrow). (b) Sagittal computed tomography scan following oral tracheal re‐intubation below the defect, demonstrating the tracheal tube cuff in the lower trachea and a reduction in tracheal diameter.
Tracheal re‐intubation was performed in the operating theatre with the ear, nose and throat (ENT) and scrub teams present. The patient's FiO2 was increased to 1.0, existing remifentanil and propofol infusions were titrated to a bispectral index of 30–45 and vecuronium 10 mg i.v. was administered. Airborne precautions personal protective equipment was worn, and ventilation of the patient's lungs was ceased during circuit disconnections [7]. Our airway management strategy was written on the operating theatre whiteboard during the team briefing.
Step 1: Assessment of the upper airway
A McGrath videolaryngoscope with a size 4 MAC blade (Medtronic, Watford, UK) was used to inspect the upper airway. Secretions were cleared using a Yankauer suction catheter.
Step 2: Rescue front‐of‐neck airway
An Aintree airway exchange catheter (Cook Medical, Bloomington, IN, USA) was loaded onto a flexible bronchoscope (aScope 4 regular, Ambu, Ballerup, Denmark) and advanced through the tracheostomy tube via a bronchoscopy angle‐piece. The Aintree catheter was secured with the tip positioned 1 cm above the carina.
Step 3: Tracheal intubation beyond the defect
The flexible bronchoscope, loaded with a 9mm tracheal tube (PRO‐Breathe SuctionPlus, PROACT Medical Ltd, Corby, UK) was advanced through the glottis via the mouth. It was noted that the cuff had herniated above the outer bend of the tracheostomy tube. Following the aspiration of approximately 50 ml of air from the cuff, the tracheostomy tube was removed. The bronchoscope was advanced, and the tracheal tube railroaded so that its tip was 1 cm above the carina. The Aintree catheter was removed, and the tracheal tube cuff inflated. The tracheostomy wound was closed with sutures.
Because ventilation of the patient's lungs was resumed between steps 2 and 3, to minimise the risk of exposure to aerosolised respiratory secretions the Aintree catheter was occluded using a Luer lock Rapi‐fit adapter (Cook Medical, Bloomington, IN, USA) and obturator.
Subsequent management
Having mitigated the immediate risks of tube migration and pneumomediastinum and obtained repeat CT imaging (Figure 1b), another tracheostomy was inserted on day 66. The FiO2 was increased to 1.0, sevoflurane (minimum alveolar concentration of 0.7) was given alongside remifentanil and propofol infusions and rocuronium 100 mg i.v. was administered for neuromuscular blockade. Apnoeic circuit disconnections and airborne precautions were observed [7, 8].
Having confirmed that the tracheal mucosa appeared viable, the antero‐superior part of the defect was repaired with perichondrial sutures and the inferior part was refashioned to form a tracheal window. The tracheal tube was removed and a 9mm adjustable flange tracheostomy tube (UniPerc, Portex, Smiths Medical, Minneapolis, MN, USA) was inserted and the tip was positioned 1.5 cm above the carina under bronchoscopic guidance (FNL‐10RP3; Pentax Medical, Slough, UK).
At the time of writing, the patient continues to wean slowly from invasive ventilation.
Discussion
Tracheomegaly is a recognised complication of prolonged tracheal intubation, particularly when cuff pressures exceed 30 cmH2O [3, 9]. High cuff pressures can reduce local perfusion and damage tracheal mucosa and cartilage; a cuff:trachea diameter ratio >1.5 is associated with cartilaginous destruction [9]. Conversely, low cuff pressures are associated with cuff leaks, aspiration and pneumonia. In this case, higher cuff pressures may have been required to enable the ventilation of poorly compliant lungs, and concerns regarding aerosolised secretions and the risk of severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) transmission may have led to staff feeling unable to tolerate even minor cuff leaks [8]. The requirement for high cuff pressures soon after the initial tracheostomy implies that trauma on insertion may have been a contributory factor. However, additional potential contributory factors include pulmonary fibrosis, hypothesised to cause tracheomegaly through traction on the trachea [2], and airway inflammation secondary to COVID‐19 [10]. Furthermore, the patient in this report received steroid therapy to treat pulmonary fibrosis, which may have impaired wound healing. It is likely that the cause of tracheomegaly was multifactorial in this case, and it cannot be determined whether elevated cuff pressures or abnormal tracheal morphology arose first.
The CT imaging (Figure 1a) provides clear demonstration of why airway management in tracheomegaly may be challenging. Complications could have included tracheal collapse on cuff deflation, tracheal tube tip passing into the sac of the dilatation, creation of a false passage within an already compromised tracheal mucosa and cuff placement within the defect. The imaging also demonstrates why tracheal re‐intubation was urgently required in this case: the tracheostomy tube tip was close to the anterior edge of the tracheal defect and severe pneumomediastinum indicated loss of tracheo‐bronchial integrity. Dislodgement of the tube or worsening of the pneumomediastinum could have resulted in catastrophic loss of ventilation. Imaging was not only useful in diagnosing and characterising the tracheomegaly, but also in planning airway management, anticipating problems and determining the appropriate cuff location.
As with all airway management, defining an overall strategy and considering contingencies in a ‘plan A‐B‐C’ approach was vital. In this already complex case, with the additional considerations of managing a patient recovering from COVID‐19 [7, 8], team briefing and multidisciplinary co‐ordination was of particular importance. Rescue strategies were defined for each stage and written out explicitly during the team briefing: if a patent glottis could not be viewed in step 1, we planned to re‐intubate the patient's trachea via the tracheal stoma with an uncut tracheal tube using an exchange catheter technique. I oral tracheal re‐intubation was not possible in step 3, we planned to use the Aintree catheter for emergency oxygenation and as a means to railroad a backup airway device. I if bonchoscopy was difficult due to abnormal tracheal morphology we planned to use the Aintree catheter as a guide alongside which to advance the flexible bronchoscope. The use of video‐assisted devices allowed findings at each stage to be viewed by all members of the multidisciplinary team so that consensus on the next steps could be reached.
Management of cuff‐associated tracheomegaly requires repositioning of the cuff to allow the trachea to heal. This can be achieved with oral tracheal intubation or the use of adjustable flange or double cuffed tracheostomy tubes [3]. More invasive management such as tracheal resection may be indicated but should be carefully balanced against each patient's comorbidities. Improvement in the extent of the tracheomegaly in this case was seen following repositioning of the cuff (Figure 1b). However, following decannulation and cessation of positive pressure ventilation patients with tracheomegaly can experience tracheomalacia [3]. Therefore, the potential for variable airway obstruction should be anticipated if and when decannulation is attempted.
This case demonstrates the value of teamwork and planning in the management of complex airways. Despite the urgency of intervention, the time taken to assemble an appropriate team and develop an airway strategy was well spent. Though the airway management plan was executed uneventfully, this may not have been the case. By establishing a rescue plan and explicitly communicating contingencies we were able to offer the safest possible airway management in a difficult clinical setting.
Acknowledgements
Published with the written consent of the patient's next of kin. The authors wish to thank Dr S Knight for his review of a draft version of the manuscript. CLS is an assistant editor of Anaesthesia Reports. No external funding or competing interests declared.
Contributor Information
S. Harper, Email: stephharper@nhs.net.
J. Hobson, @JonathanHobson.
C. L. Shelton, @DrCliffShelton.
References
- 1. Breatnach E, Abbott G, Fraser R. Dimensions of the normal human trachea. American Journal of Roentgenology 1984; 142: 903–6. [DOI] [PubMed] [Google Scholar]
- 2. Woodring J, Barrett P, Rehm NP. Acquired tracheomegaly in adults as a complication of diffuse pulmonary fibrosis. American Journal of Roentgenology 1989; 152: 743–7. [DOI] [PubMed] [Google Scholar]
- 3. Yang JH, Kim TW, Lee BJ, Yoon JA, Shin MJ, Shin YB. Successful management of acquired tracheomalacia of patients with amyotrophic lateral sclerosis: a report of three cases. Annals of Rehabilitation Medicine 2018; 42: 368–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schmitt P, Dalar L, Jouneau S, et al. Respiratory conditions associated with tracheobronchomegaly (Mounier‐Kuhn syndrome): a study of seventeen cases. Respiration 2016; 91: 281–7. [DOI] [PubMed] [Google Scholar]
- 5. Lee DH, Yoon TM, Lee JK, Lim SC. Tracheomegaly secondary to tracheotomy tube cuff in amyotrophic lateral sclerosis: a case report. Medicine 2015; 94: e1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim MY, Kim EJ, Min BW, Ban JS, Lee SK, Lee JH. Anesthetic experience of a patient with tracheomegaly – a case report‐. Korean Journal of Anesthesiology 2010; 58: 197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McGrath BA, Brenner MJ, Warrilow SJ, et al. Tracheostomy in the COVID‐19 era: global and multidisciplinary guidance. Lancet Respiratory Medicine 2020; 8: 717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cook TM, El‐Boghdadly K, McGuire B, McNarry AF, Patel A, Higgs A. Consensus guidelines for managing the airway in patients with COVID‐19. Anaesthesia 2020; 75: 785–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khan F, Reddy NC. Enlarging intratracheal tube cuff diameter: a quantitative roentgenographic study of its value in the early prediction of serious tracheal damage. Annals of Thoracic Surgery 1977; 24: 49–53. [DOI] [PubMed] [Google Scholar]
- 10. McGrath BA, Wallace S, Goswamy J. Laryngeal oedema associated with COVID‐19 complicating airway management. Anaesthesia 2020; 75: 972. [DOI] [PubMed] [Google Scholar]


