Summary
This report describes the care provided to a 64‐year‐old woman presenting with airway obstruction following recovery from COVID‐19 pneumonitis, prolonged tracheal intubation and tracheostomy weaning. Her initial admission was with COVID‐19 pneumonitis during the first surge of cases in early 2020, and was complicated by multiple bilateral segmental pulmonary emboli, a 28‐day stay in intensive care, 16 days of mechanical ventilation and finally, a tracheostomy with subsequent weaning of respiratory support and rehabilitation. On presentation, her symptoms of airway obstruction were because of significant granuloma of the posterior glottis and subglottis, as well as a mild lambdoid deformity at the site of her previous tracheostomy. The key learning points described relate to the use of apnoeic oxygenation during the COVID‐19 pandemic, managing the shared airway, as well as the management of post‐intubation laryngotracheal complications.
Keywords: airway obstruction, apnoeic oxygenation, COVID‐19, jet ventilation
Introduction
Anaesthesia during airway surgery is challenging because of the need to simultaneously oxygenate the patient and operate through the same anatomic channel. Transnasal humidified rapid‐Insufflation ventilatory exchange (THRIVE), now a well‐established method of apnoeic ventilation, employs high‐flow oxygen through wide‐bore nasal cannula to establish a tubeless operative field. The main benefit of THRIVE is the extended apnoea time permitted to establish access to the airway.
The coronavirus disease 2019 (COVID‐19) pandemic continues to have a significant impact on healthcare, with up to 10% of those admitted to hospital requiring tracheal intubation and mechanical ventilation. Furthermore, patients with post‐intubation complications presenting to hospital may have airway pathology precluding tracheal intubation. As such, full access to the airway through a closed circuit is at times difficult to attain. As more is understood about COVID‐19 and the aerosolisation properties of THRIVE and high‐frequency jet ventilation (HFJV), the ability to reinstate tubeless methods for airway surgery has improved. Although supraglottic jet ventilation is suitable, the extended apnoea time of THRIVE allows the operator to navigate the airway and obtain adequate exposure. We report a case of surgical management of obstructing laryngeal granulomata following tracheal intubation in a patient with COVID‐19‐affected lungs, supported by THRIVE.
Report
A 64‐year‐old woman of African descent presented to hospital with stridor, cough and difficulty clearing secretions 2 months after admission with COVID‐19. Her comorbidities included non‐insulin‐dependent diabetes mellitus, hypertension and an elevated body mass index of 33.1 kg.m‐2. Her prior admission with COVID‐19 was severe, and she was found to have multisegment pulmonary emboli. The patient was in intensive care for 28 days and her trachea was intubated for a total of 16 days. She failed tracheal extubation twice, resulting in a tracheostomy which was in‐situ for 9 days.
A computed tomography (CT) scan on her second admission demonstrated scarring at both lung apices, the lingula and the right middle lobe, some residual ground‐glass change in the right lung, and a mass below the vocal folds. She was referred to the ear, nose and throat service and bedside nasendoscopy demonstrated granulomata of the subglottis with posterior glottic obstruction. Anticipated operative challenges related to her underlying lung disease, obesity and relative micrognathia. The anaesthetic plan was to employ THRIVE to allow the surgical team adequate time to gain an acceptable view of the glottis/subglottis, and then proceed with HFJV in the event of hypoxia. Risk of viral transmission to staff members was minimised by confirming a negative COVID‐19 reverse‐transcriptase polymerase chain‐reaction swab test on admission, limiting the number of staff in theatre and the wearing of appropriate personal protective equipment (FFP3 masks; visors; gloves; gowns; and hats).
The patient was pre‐oxygenated with 30 l.min‐1 high‐flow nasal oxygen increased to 70 l.min‐1 before induction of anaesthesia. Anaesthesia was induced using total intravenous anaesthesia with target‐controlled infusions of propofol and remifentanil. Once unconscious, 1 mg.kg‐1 rocuronium was given intravenously. Ability to bag‐mask ventilate was confirmed before laryngoscopy as a fail‐safe technique should the case be abandoned due to inadequate access. With 30º elevation of the head to minimise atelectasis, THRIVE was used as the primary method to maintain intra‐operative oxygenation, and an adequate view of the glottis was attained by 6 mins with a suspension laryngoscope (Fig. 1A). Cricoarytenoid joint mobility was confirmed, as was the presence of granuloma arising from two points in the posterior subglottis, consistent with tracheal injury. There was also a mild, non‐obstructing lambdoid deformity at the site of her previous tracheostomy. Methyprednisolone was injected into the base of the granuloma, followed by cold steel subtotal excision (Fig. 1B). A slow desaturation of approximately 1% per minute occurred after a total apnoea time of 11 min. At this point, supraglottic HFJV was commenced and THRIVE stopped, with oxygen saturations maintained above 94% for the rest of the procedure. At the end of the procedure, neuromuscular blockade was reversed with sugammadex 2 mg.kg‐1 and she was recovered from anaesthesia. She had an uncomplicated recovery, and postoperative respiratory function tests demonstrated a peak expiratory flow 73% of predicted at 247 l.min‐1. Despite symptomatic improvement, there was some vocal indrawing on forced inspiration and she was referred for respiratory muscle retraining. At follow‐up microlaryngoscopy 6 weeks later there was no granuloma, subglottic stenosis or posterior glottic scar (Fig. 1C).
Figure 1.
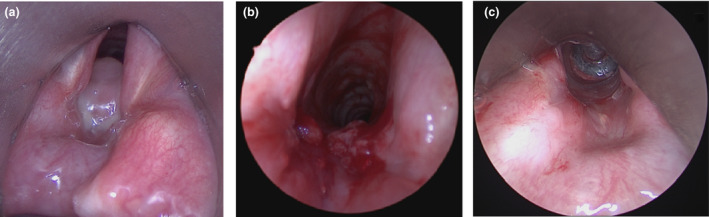
(a) Endoscopic image of subglottic granulomata before excision, then (b) after excision. (c) Endoscopic appearance of posterior commissure at follow‐up microlaryngoscopy, 6 weeks later.
Discussion
This case demonstrates two key learning points. The first relates to the successful use of THRIVE in a recovered patient with significant COVID‐19, most notably multiple pulmonary emboli. The second is a typical example of post‐intubation airway complications which will become more common because of the pandemic. To our knowledge, none of the staff involved in this case tested positive for COVID‐19 in the days or weeks afterwards, further supporting the effectiveness of maintaining high standards of personal protective equipment for airway surgery. We nevertheless recognise that it would be difficult to definitively rule out causal links due to many confounding factors.
Surgery of the laryngotracheal junction typically requires a tubeless field to enable access to the surgical site. Passing a tracheal tube may be impossible or detrimental to the airway. As such, HFJV using a supraglottic cannula or subglottic catheter has been the mainstay of anaesthesia for many years. Difficulties may arise in the obese patient or in those with pre‐existing lung disease, where poor access to the larynx increases the apnoea time until HFJV can be established. The technique of THRIVE was developed as a method of pre‐oxygenation in a flow‐dependant manner, until adequate access for alternative ventilation is achieved, if required. As mentioned, this typically relates to supraglottic HFJV [1]. The technique of THRIVE has previously been demonstrated as a feasible method of apnoeic ventilation during airway surgery [2] and is now in some instances the preferred method. In this patient, THRIVE successfully achieved 11 min of apnoea time allowing airway patency to be re‐established through granuloma excision. This was only 3 months after ventilator‐dependent COVID‐19‐related respiratory failure complicated by thromboembolic phenomena. Granuloma excision was performed with cold steel techniques, however, should laser have been required it could have been employed, as all members of staff in theatre had been appropriately fit‐tested for their masks and had full personal protective equipment. Although it is likely cold steel techniques have a reduced risk of aerosolisation, if a better surgical outcome can be achieved with other means, such as laser, this will minimise the number of aerosol‐generating procedures over time and should be used.
Post‐intubation injuries can be at every level of the laryngotracheal complex. In the hours to weeks following extubation, oedema, erythema and ulceration are common. With time, other findings include tracheal or subglottic stenosis as well as posterior glottic scar or granulation. Vocal fold paresis may occur and there are reports of cricoarytenoid joint injury. Our patient had obstructing granuloma and a mild lambdoid deformity of the trachea.
A recent systematic review of laryngeal injury following tracheal intubation and mechanical ventilation suggested an injury prevalence of 83% [3]. Moderate to severe injuries were present in up to 31% of patients. Granuloma such as in our case had a prevalence of 27%. Women may be at higher risk of post‐intubation laryngeal injury [4], so too for those with diabetes mellitus [5]. A recent audit suggested 7355 (71.9%) of COVID‐19 positive patients in critical care required advanced respiratory support, such as tracheal intubation [6]. Many of these patients will have comorbidities and those presenting with post‐intubation laryngeal injuries will be a major peri‐operative challenge.
Unfortunately, our patient’s story is not uncommon and it is likely there will be many more in a similar position over the coming months requiring acute airway intervention. This case report provides an example of the viability of apnoeic oxygenation in the recovered COVID‐19 patient. Although there is some experimental evidence that high‐flow nasal oxygen may be no worse than facemask oxygenation when it comes to bioaerosol dispersal [7], it is still important to proceed carefully and ensure appropriate safety measures are taken. It is important for centres that restart tubeless airway surgery to have an agreed pathway to use THRIVE and HFJV, incorporating pre‐COVID‐19 techniques and virus transmission reduction methods. Irrespective of the COVID‐19 swab result, our centre practices airway surgery in the same fashion and we recommend: a full team briefing where the risks are discussed; not allowing high‐risk staff to include themselves; ensuring those remaining use appropriate personal protective equipment; minimising operating theatre staff numbers; and minimising movement in and out of the operating theatre during the case until there is sufficient global evidence to prove otherwise. This will ensure that the risk of transmission is minimised, even in cases that, by necessity, need to be undertaken in patients that are still infectious.
Acknowledgements
Published with the written consent of the patient. SARN has received research, travel and consultancy support from Fisher and Paykel Healthcare Ltd. No other external funding or competing interests declared.
References
- 1. Patel A, Nouraei SAR. Transnasal Humidified Rapid‐Insufflation Ventilatory Exchange (THRIVE): a physiological method of increasing apnoea time in patients with difficult airways. Anaesthesia 2015; 70: 323–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. To K, Harding F, Scott M, Milligan P, Nixon IJ, Adamson R, et al. The use of transnasal humidified rapid‐Insufflation ventilatory exchange in 17 cases of subglottic stenosis. Clinical Otolaryngolology 2017; 42: 1407–10. [DOI] [PubMed] [Google Scholar]
- 3. Brodsky MB, Levy MJ, Jedlanek E, et al. Laryngeal injury and upper airway symptoms after oral endotracheal intubation with mechanical ventilation during critical care: a systematic review. Critical Care Medicine 2018; 46: 2010–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pacheco‐Lopez PC, Berkow LC, Hillel AT, Akst LM. Complications of airway management. Respiratory Care 2014; 59: 1006–21. [DOI] [PubMed] [Google Scholar]
- 5. Zias N, Chroneou A, Tabba MK, et al. Post tracheostomy and post intubation tracheal stenosis: report of 31 cases and review of the literature. BMC Pulmonary Medicine 2008; 8: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Intensive Care National Audit and Research Centre . ICNARC Report on COVID‐19 in Critical Care. London, UK: ICNARC, 2020. https://www.icnarc.org/DataServices/Attachments/Download/baa7de02‐3f00‐eb11‐912b‐00505601089b (Accessed 01/10/2020). [Google Scholar]
- 7. Li J, Fink JB, Ehrmann S. High‐flow nasal cannula for COVID‐19 patients: low risk of bio‐aerosol dispersion. European Respiratory Journal 2020; 55(5): 2000892. [DOI] [PMC free article] [PubMed] [Google Scholar]


