Abstract
An organism’s capacity to cope with stressful experiences is dependent on its ability to appropriately engage central and peripheral systems, such as the hypothalamic-pituitary-adrenal (HPA) axis, to adapt to changing environmental demands. The HPA axis is a primary neuroendocrine mediator of neural and behavioral responses to stress, and dysfunction of this system is linked to increased risk for developing mental health disorders such as depression, anxiety, and post-traumatic stress disorder. However, the mechanisms by which dysregulated HPA function results in abnormal behavioral responses to stress are poorly understood. Here, we tested how corticosterone (CORT)-induced HPA axis disruption affects behavioral responses to stress in male C57BL/6N mice, and probed correlates of these behaviors in the brain. We show that chronic HPA disruption blunts acute stress-induced grooming and rearing behaviors in the open field test, effects which were accompanied by decreased FOS immunoreactivity in the paraventricular nucleus of the hypothalamus (PVH) and paraventricular nucleus of the thalamus (PVT). Blockade of CORT secretion with metyrapone injection prior to acute stress did not recapitulate the effects of chronic HPA disruption on open field behavior, and acute CORT replacement did not rescue normal behavioral stress responses following chronic HPA disruption. This suggests that under acute conditions, CORT is not necessary for these responses normally, nor sufficient to rescue the deficits of chronic HPA dysregulation. Together, these findings support the hypothesis that chronic HPA dysregulation causes adaptation in stress-related brain circuits and demonstrate that these changes can influence an organism’s behavioral response to stress exposure.
1. Introduction
Multiple physiological systems are recruited to aid an organism in overcoming or adapting to stress. Proper engagement of these systems is crucial not only for the adequate response to threat, but also for enabling an organism to return to biological equilibrium once the stressor is terminated (or escaped from). Consequently, many pathological conditions are characterized by inadequate or improper responses to stress (de Kloet et al., 2006; McEwen, 1998; Pariante and Lightman, 2008). Exposure to stress rapidly activates the hypothalamic-pituitary-adrenal (HPA) axis, which triggers brain and body-wide physiological changes through the secretion of glucocorticoids. In the HPA axis-mediated stress response, activation of corticotropin-releasing hormone (CRH) neurons of the paraventricular nucleus of the hypothalamus (PVH) stimulates secretion of adrenocorticotropic hormone (ACTH) in the anterior pituitary, in turn promoting glucocorticoid synthesis and release from the adrenal cortex (Herman et al., 2016; Ulrich-Lai and Herman, 2009). Glucocorticoids, such as corticosterone (CORT), exert effects on the brain through rapid engagement of cellular signaling systems as well as through slower-acting effects on cellular adaptation via control of gene expression (Datson et al., 2008; Evanson et al., 2010; Joels et al., 2012). Given these different mechanisms of action, glucocorticoids can have rapid influences on neural circuits at the time of stress exposure, as well as long term effects on neural circuits in the form of functional or structural plasticity. These changes in neural function can significantly impact how an organism reacts to stress at the level of behavior (McEwen, 2017; McEwen et al., 2015). As the HPA axis plays a large role in both short- and long-term responses to stress, it is not surprising that dysfunction of this system has negative implications for health, including links to anxiety disorders and depression (de Kloet et al., 2006; Faravelli et al., 2012; Pariante and Lightman, 2008). While HPA dysregulation is strongly linked to the development and symptomology of these disorders, the HPA phenotype (e.g. hyporesponsive or hyperresponsive glucocorticoid secretion) is highly variable across different disorders, and even within the same disorder it is variable between individuals (Gillespie and Nemeroff, 2005; Juruena et al., 2017; Zaba et al., 2015). Furthermore, despite numerous studies highlighting these links, it is still unclear whether the neural and behavioral effects of HPA dysfunction in these disorders are mediated by the long-term effects of glucocorticoid dysregulation vs. the acute dysregulation of glucocorticoid signaling during exposure to stress.
In the present set of studies, we experimentally disrupted normal HPA axis function in male C57BL/6N mice in order to better understand how this system contributes to behavioral and neural responses to stress. We previously reported that chronic CORT administration in the drinking water of mice suppresses HPA axis function, with reduction of both hypothalamic CRH expression as well as stress-induced ACTH and CORT secretion. Our previous work also showed that this treatment alters stress-induced grooming and rearing behaviors in the open field test (Kinlein et al., 2015). However, the neuroendocrine mechanisms by which HPA axis disruption affects neural function and behavior have not been explored.
To answer this question, we conducted two studies to test both the acute necessity and sufficiency of CORT in the behavioral and neural outcomes of HPA disruption. Our findings demonstrate that chronic HPA axis disruption reduces the effect of stress on normal behavioral coping strategies as well as the activity of two key brain regions associated with emotional responses to stress, the PVH and paraventricular nucleus of the thalamus (PVT). It is noteworthy that these altered responses were not rescued by acute CORT replacement during stress, nor mimicked by acute blockade of CORT synthesis, suggesting that chronic HPA disruption causes changes through long-term adaptation in stress-related brain circuits rather than through the acute actions of stress-induced CORT during stress exposure.
2. Materials and Methods
2.1. Animals and corticosterone treatment:
Adult male mice C57BL/6N mice were obtained from Envigo (Livermore, CA). Upon arrival, mice were group-housed (4 per cage) in standard shoebox cages on a 12-hour light-dark cycle (lights on at 6:00h; Lux = 70-100 at cage level) and given access to standard rodent chow (LabDiet 5001) and drinking water ad libitum. To disrupt normal HPA axis function, after 7 days of acclimation to the facility, drinking water was replaced with a solution containing 25 μg/mL corticosterone (CORT, Sigma Inc., St. Louis, MO; “HPA-X” group) dissolved in 100% ethanol, and then diluted in regular drinking water to a final concentration of 1% ethanol (Vehicle, “Control” group). CORT or Vehicle treatments were administered for 4 weeks in all experiments, with treatment solutions being replaced weekly during cage changes. This dose and duration of CORT treatment is based off previous studies from our lab and others, showing that it results in blunted hypothalamic CRH mRNA as well as blunted stress-induced ACTH and CORT secretion (Karatsoreos et al., 2010; Kinlein et al., 2015; Shahanoor et al., 2017). Our group has conducted extensive characterizations of the physiological impacts of this model of chronic CORT in the drinking water (Cassano et al., 2012; Karatsoreos et al., 2010; Kinlein et al., 2017). To prevent any confounds of oral CORT consumption immediately before our experiments, water bottles were removed from all cages 1 hour before stress exposure. All procedures were approved by the Washington State University Institutional Animal Care and Use Committee.
2.2. Experiment 1
To test how behavior and neural function are affected by CORT-induced HPA axis dysfunction, and to probe the sufficiency of CORT to restore normal neural and behavioral responses to acute stress, following 4 week CORT exposure, mice (N=64 total) were exposed to a single forced swim stress (10 minutes, 21-23°C) in addition to a bolus injection of CORT or Vehicle solution between 11:00-13:00h (ZT5-7). Either Vehicle (5% 2-Hydroxypropyl-β-cyclodextrin in 0.1 M PBS, Sigma) or CORT (2.5mg/kg in 2-Hydroxypropyl-β-cyclodextrin vehicle) were injected i.p. immediately prior to the start of the swim stress. Following swim stress, mice were briefly dried with paper towels and placed back into home cages in the behavioral testing room until behavioral testing in an open field chamber. For the unstressed (“Baseline”) groups, mice were injected and then immediately returned to the home cage. 60 minutes after the start of stress (or injection for baseline groups), mice were anaesthetized with ketamine (15mg/ml) / xylazine (1mg/ml) cocktail and then transcardially perfused with heparinized saline and 4% paraformaldehyde. Brains were then collected and processed for immunohistochemical analysis. On the day of stress and behavioral testing, cages were run such that there was alternation between treatment group (Control vs. HPA-X) and injection type (VEH vs. CORT) to ensure a balanced design.
A group of unstressed mice (n=20) were acutely injected with either Vehicle or CORT solutions to determine the effects of injection on plasma CORT concentrations. Mice were killed via rapid decapitation 25 minutes after the injection (to align with the above experimental timeline) and trunk blood was collected for analysis of plasma CORT.
2.3. Experiment 2
To test the necessity of stress-induced CORT secretion for normal behavioral responses to acute stress, a separate group of mice (n=16 total) received a forced swim stress following a single injection of metyrapone, an inhibitor of CORT synthesis. In this experiment, mice were injected i.p. with either vehicle (5% Tween 20 in 0.1 M PBS, Sigma) or metyrapone (50mg/kg in Tween 20 vehicle, Sigma) 60 minutes prior to the start of the stress. As in Experiment 1, a single forced swim (10 minutes, 21-23°C) was administered between 11:00-13:00h (ZT5-7) to acutely stress mice, and following the forced swim, mice were briefly dried with paper towels and placed back into home cages in the behavioral testing room until open field testing. No unstressed group was tested in this experiment. Mice were killed via rapid decapitation immediately following open field testing, at which time trunk blood was collected for analysis of plasma CORT.
2.4. Corticosterone ELISA:
Blood was centrifuged (1500 rcf for 15 minutes at 4°C) to separate plasma from whole blood, and stored at −20°C until analysis. Plasma CORT concentrations were measured with ELISA kits (Cayman Chemical, Ann Arbor, MI) using manufacturer specifications and protocols. Two ELISA plates were run for the experiments above. For plasma collected in Experiment 1, the intra-assay coefficient of variability and lower limit of detectability were 8.3% and 1.07 ng/mL, respectively. For plasma collected in Experiment 2, the intra-assay coefficient of variability and lower limit of detectability were 6.9% and 4.16 ng/mL, respectively.
2.5. Open field behavioral analysis:
An open field test was used to probe behavioral responses to novel environments after acute stress exposure, as previously described (Kinlein et al., 2015). Ten minutes following the end of the forced swim (or 20 minutes after injection for unstressed mice), mice were placed in an open field chamber (27.5cm x 27.5cm x 20cm, 55-65 lux at floor level) and allowed to roam freely for 5 minutes before being removed. The chamber was cleaned with 70% ethanol before each trial. Locomotor behavior was digitally scored using Noldus Ethovision XT software (Leesburg, VA, USA), with a center area (10cm x 10cm) digitally added to video files. In addition to locomotor behavior, grooming and rearing behaviors were scored as ethologically relevant stress coping responses. Grooming was quantified as the cumulative time that a mouse spent grooming its face or body throughout the duration of the test, while rearing bouts were quantified as the number of times an animal stood up completely on its rear legs, whether touching the sides of the chamber with front paws or without support. Rearing and grooming behaviors were scored manually by an observer blinded to the experimental groups.
2.6. Tissue processing, immunohistochemistry, and confocal microscopy:
Following extraction, brains were post-fixed with 4% paraformaldehyde for 24 hours, and then incubated in one step each of 20% and 30% sucrose in 0.1M PB for 24 hours. Brains were then coronally sectioned at 40μm on a cryostat microtome, and sections containing the rostral to caudal extent of the paraventricular nucleus of the hypothalamus (PVH, bregma −0.58 to −0.94 A-P) and posterior subdivision of the paraventricular nucleus of the thalamus (PVT, bregma −1.58 to −1.94 A-P) (Franklin and Paxinos, 2007) were collected. Slices then underwent immunohistochemical processing. Briefly, tissue sections were washed in 0.1M PB followed by a 1 hour incubation in 2% normal donkey serum (NDS) in 0.1M PB with 0.1% Triton X-100 (PBT, Sigma). Next, tissue sections were incubated for 48 hours in goat anti-FOS (1:4000, Santa Cruz Biotechnology, Dallas TX, sc-52-G). Tissue sections were then washed in 0.1M PBT and incubated for 1 hour in donkey anti-goat Alexa Fluor 594 (1:200, Jackson ImmunoReseach laboratories, Inc., West Grove, PA), followed by a final wash in 0.1M PB. The tissue sections were then mounted on gelatin-coated glass slides, and then incubated in 50%, 70%, 95% (x2), and 100% (x2) ethanol for 5 minutes each, followed by clearing agent (Citrisolv, Fisher Scientific, Waltham, MA) for 5 minutes, and cover slipped with DPX (Sigma).
Images were captured using a Leica SP5 confocal microscope (Leica, Wetzlar, Germany) at 10X magnification for FOS quantification. For PVH imaging, the entire bilateral extent of the region was captured in a single image, with the bottom edge of the images positioned at the base of the ventral third ventricle. For PVT imaging, the entire bilateral extent of the region was captured in a single image, with the top edge of the images positioned within the dorsal portion of the third ventricle. Imaging parameters were based on measurements from a stereotaxic brain atlas (Franklin and Paxinos, 2007), and kept the same for all samples.
2.7. Semi-Automated FOS analysis:
For quantification of FOS-positive cells in the PVH and PVT, a semi-automated workflow was performed in ImageJ software (National Institutes of Health). After opening images, the “Z Project… [Max Intensity]” option was applied, following which the contour of each region was created by the experimenter using the “Polygon” tool. For the PVH, contours were drawn around the left and right extent of the area separately. For the PVT, a single contour was drawn around the bilateral extent of the area. Following the creation of contours, a brightness threshold was applied to the images to reduce background fluorescence. Threshold values were determined for each image by multiplying the measure of the mean gray value by 1.6. This threshold correction was determined by the experimenter prior to data analysis by adjusting the brightness threshold to the point where the only visible cell profiles were fully-labeled FOS-positive cells in the “Control + stress + VEH i.p.” group. After adjusting the brightness threshold in each image, the “Smooth”, “Watershed”, and “Despeckle” options were applied to facilitate accurate quantification of FOS-positive cells. Quantification of FOS-positive cells was accomplished using the “Analyze Particles” tool, with the options as follows: size = 25-150μm, circularity = 0.50-1.00. Total number of cells detected was then divided by the area of the respective contour to determine FOS-positive cell density. Average contour areas for the PVH and PVT were 84,180μm2 and 162,010μm2, respectively. For the PVH, FOS density of the left and right side of the brain was averaged within each brain slice. For both brain regions, 1-2 brain slices were imaged from each animal, and FOS immunofluorescence measurements were averaged between slices for analysis.
2.8. Statistical analysis:
Data are presented as mean + SEM. For all figures from Experiment 1, three-way ANOVAs were performed in R Studio (RStudio, Inc.) to compare the effects of stress, chronic treatment, and injection. As no interactions with injection as a factor were observed, VEH i.p. and CORT i.p. groups were combined, and two-tailed Student’s t tests were used to compare the differences between stress and chronic treatment groups. In all graphs where an interaction was observed, significance is depicted only for the interaction. If no interaction was observed, significance is depicted for any main effects observed. For Experiment 2, two-tailed Student’s t tests were used to compare vehicle- vs. metyrapone-injected groups. Results were considered statistically significant at the p < 0.05 level.
3. Results
3.1. Disruption of normal HPA axis function alters stress-induced behaviors through a mechanism independent of acute glucocorticoid signaling.
Our previous findings show that chronic corticosterone (CORT) administration prevents the stress-induced rise of endogenous plasma CORT and alters immediate behavioral stress responses in the open field (Kinlein et al., 2015). Here, we tested if replacing CORT is sufficient to restore these responses by injecting CORT i.p. at the start of an acute forced swim stress, to mimic the normal stress induced increase of endogenous CORT, and subsequently measuring open field behaviors (Fig. 1). First, in a cohort of unstressed mice (as described in methods) we verified that injection of CORT was sufficient to raise plasma CORT concentrations in HPA-X mice to within the physiological stress-induced range (Control + CORT i.p. = 327.16 ± 57.93ng/ml, HPA-X + CORT i.p. = 555.72 ± 88.67ng/ml). CORT concentrations achieved by injection were similar to plasma CORT concentrations typically observed after swim stress in Control mice as reported previously (Kinlein et al., 2015). Further, there was no difference between body weights in these groups (Control = 25.28 ± 0.28g, HPA-X = 25.58 ± 0.37g) at the end of the experiment, also in line with our previous findings.
Figure 1.
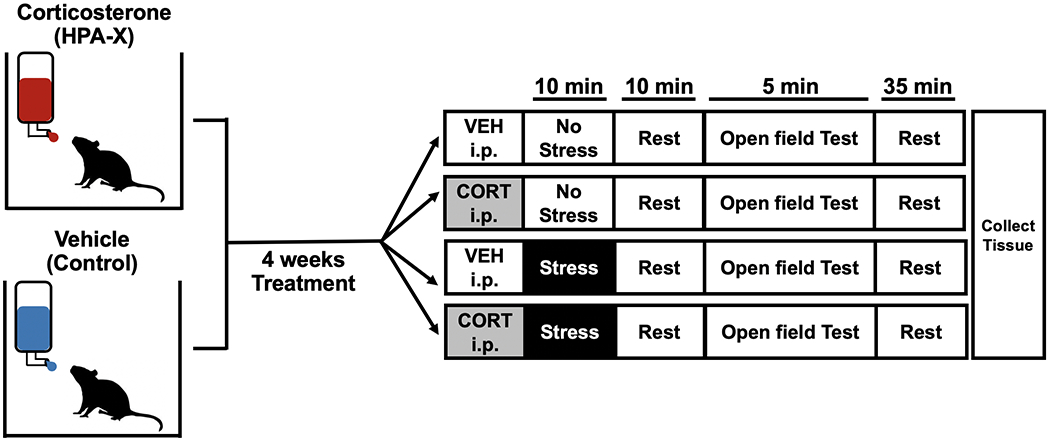
Experimental design. Mice were treated with corticosterone (CORT, 25μg/ml) or Vehicle (1% EtOH) administered orally in drinking water for 4 weeks, following which half of the mice were exposed to stress with an injection of either CORT (2.5mg/kg) or Vehicle i.p. Behavior was then assayed in an open field test.
Locomotor behaviors were first scored as a general measure of anxiety and stress-responsivity. We found that exposure to stress reduced open field center time, center entries, and distance traveled similarly in both Control and HPA-X mice (Fig. 2B–D). This suggests that chronic HPA axis disruption does not alter stress-induced locomotor behavior. As augmentation of CORT had no effect on these behavioral measures, this suggests that plasma CORT levels, in the acute sense, do not drive the stress-induced changes in these open field measures.
Figure 2.
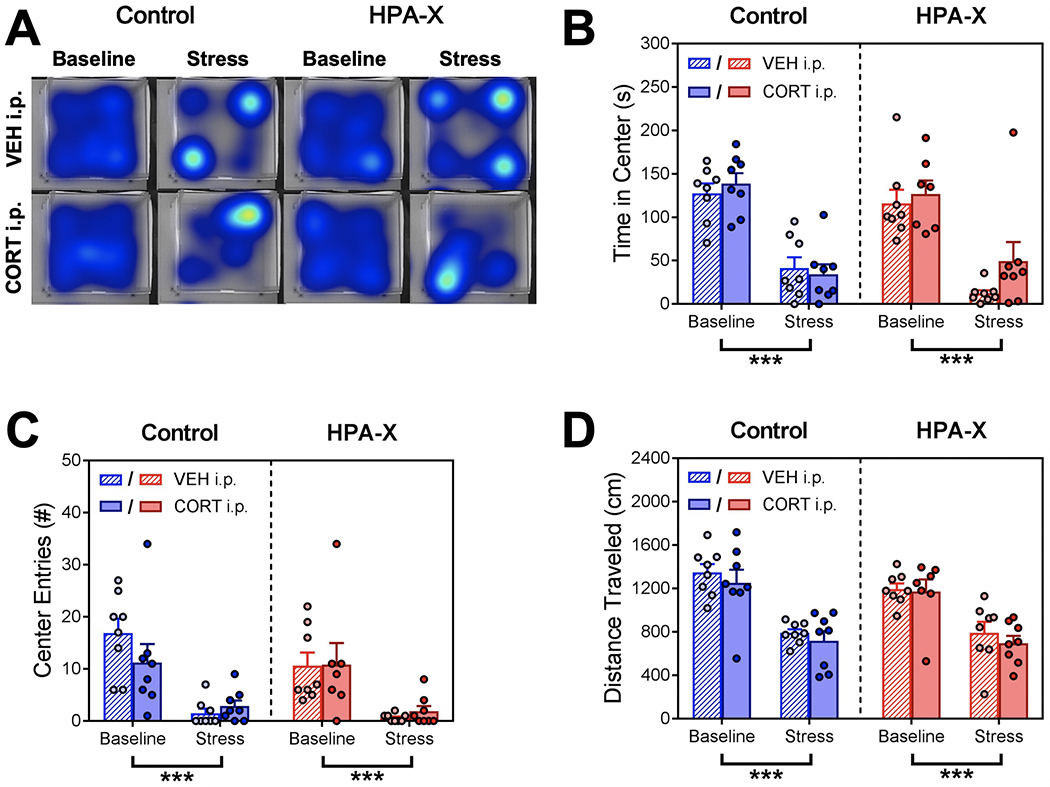
Disruption of the HPA axis with chronic corticosterone has no effect on stress-induced open field locomotor behavior. (A) Representative heatmap plots for each group showing the patterns of movement in the open field test, with warmer colors representing more time spent in an area. (B) Cumulative time spent in the center of the open field. We observed a main effect of stress (F1, 55 = 90.40, p < 0.001), but no effect of chronic treatment or injection. Stress decreased center time in both Control (p < 0.001) and HPA-X mice (p < 0.001). (C) Number of entries into the center of the open field. We observed a main effect of stress (F1, 55 = 41.53, p < 0.001), but no effect of chronic treatment or injection. Stress decreased center entries in both Control (p < 0.001) and HPA-X mice (p < 0.001). (D) Total distance traveled in the open field. Similar to center time and center entry results, we observed a main effect of stress (F1, 55 = 66.40, p < 0.001), but no effect of chronic treatment or injection. Stress decreased distance traveled in both Control (p < 0.001) and HPA-X mice (p < 0.001). Bars represent means + SEM; ***p < 0.001, n = 7-8 mice/group. Three way ANOVAs were used to analyze data (stress x chronic treatment x injection), and two-tailed Student’s t tests were used to compare differences between stress and chronic treatment groups.
In addition to open field locomotor measures, we also quantified ethologically relevant behaviors related to stress-coping responses. Self-grooming behavior is evoked by stress in rodents and is thought to play a role in de-arousal (Kalueff et al., 2016; van Erp et al., 1994). We found that stress exposure increased open field grooming behavior in both Control and HPA-X mice, however this increase in grooming behavior was significantly blunted in HPA-X mice (Fig. 3A–B). As an additional behavioral measure, we quantified rearing in the open field. Rearing is an exploratory behavior thought to represent an organism’s natural drive to survey its environment (Sturman et al., 2018; Thiel et al., 1999). Importantly, rearing bouts increase when rodents are placed into a novel environment, and the amount of rearing is sensitive to stress and other anxiogenic manipulations (Nosek et al., 2008; Sturman et al., 2018; Zimprich et al., 2014). We found that stress exposure decreased open field rearing behavior in both Control and HPA-X mice, however the decrease was significantly blunted in HPA-X mice (Fig. 2C–D). Notably, replacement of CORT in HPA-X mice did not restore normal open field grooming or rearing behaviors, suggesting that the changes observed in HPA-X mice are driven through a mechanism independent of stress-induced CORT secretion.
Figure 3.
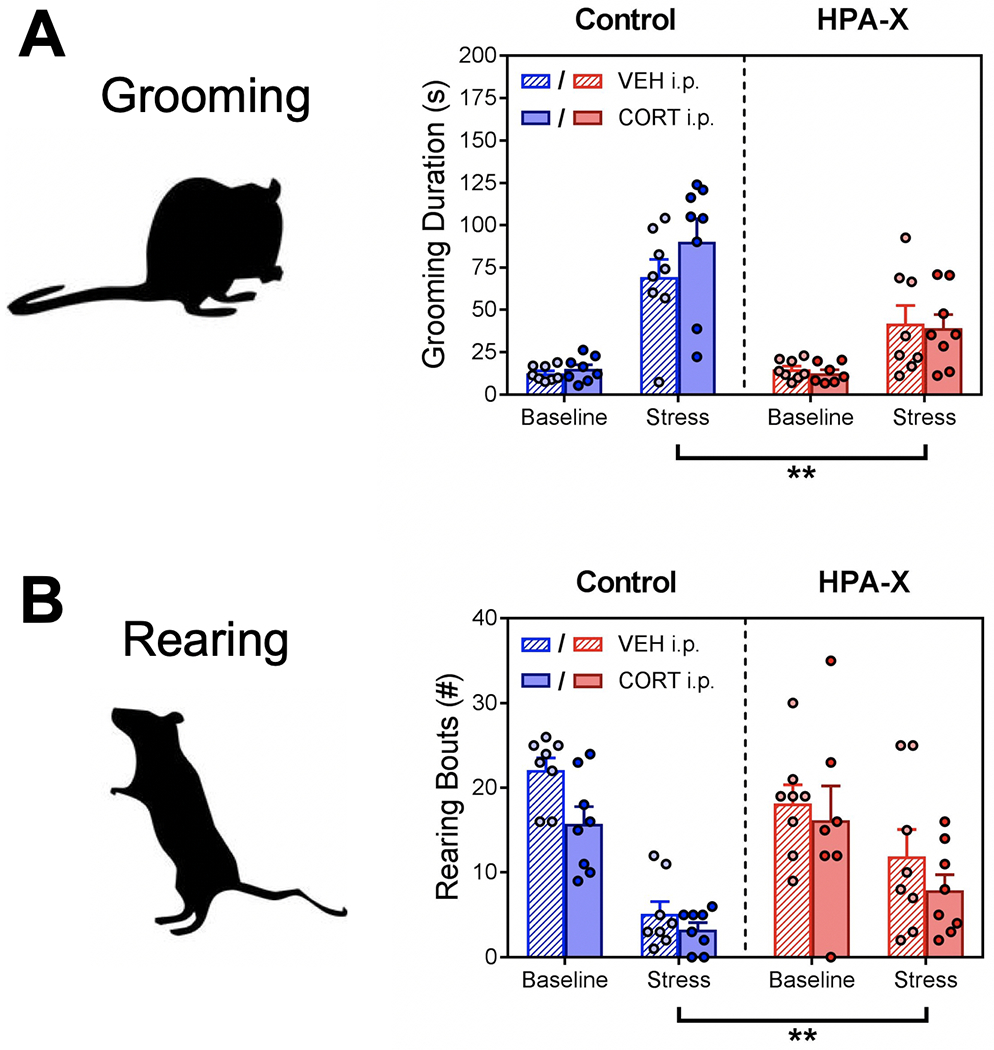
Disruption of the HPA axis with chronic corticosterone blunts normal coping responses to stress. (A) Grooming behavior in the open field. We observe a main effect of stress (F1, 55 = 68.08, p < 0.001), chronic treatment (F1, 55 = 11.53, p = 0.001), and a significant interaction (stress x chronic treatment, F1, 55 = 11.91, p = 0.001). No effect of injection was observed. Stress increased grooming in both Control (p < 0.001) and HPA-X mice (p < 0.001), however this increase was significantly blunted in HPA-X mice (p = 0.001). (B) Rearing behavior in the open field. We observe a main effect of stress (F1, 55 = 47.10, p < 0.001), injection (F1, 55 = 5.25, p = 0.025), and a significant interaction (stress x chronic treatment, F1, 55 = 5.47, p = 0.023). Stress decreased rearing in both Control (p < 0.001) and HPA-X mice (p = 0.016), however this decrease was significantly blunted in HPA-X mice (p = 0.009). Bars represent means + SEM; **p < 0.01, n = 7-8 mice/group. Three way ANOVAs were used to analyze data (stress x chronic treatment x injection), and two-tailed Student’s t tests were used to compare differences between stress and chronic treatment groups.
To test the necessity of the stress-induced rise in CORT for grooming and rearing behavior, we also measured open field behavior after pharmacological inhibition of CORT synthesis in treatment-naïve mice with metyrapone. Similar to the findings above, inhibition of CORT synthesis prior to the forced swim stress did not alter stress-induced grooming or rearing behaviors (Fig. 4). This further suggests that the rise in stress-induced CORT secretion is not necessary for the expression of these behaviors.
Figure 4.
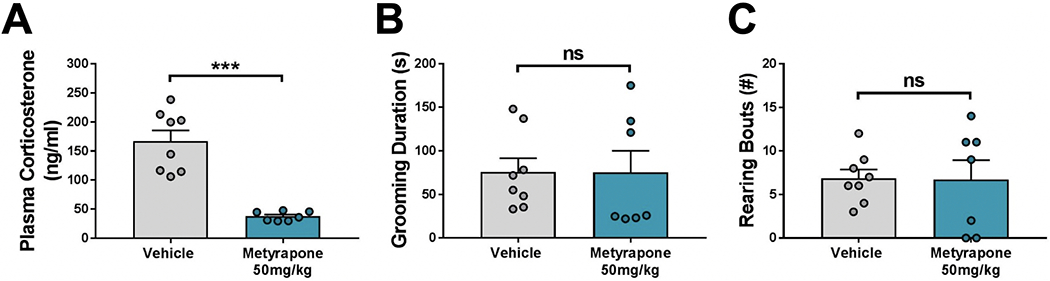
Acute corticosterone (CORT) blockade with metyrapone does not alter normal coping responses to stress. (A) Plasma CORT concentrations after a forced swim test. Pretreatment with metyrapone significantly blunted stress-induced plasma CORT (p < 0.001). (B) Grooming and (C) rearing behaviors in the open field. Pretreatment with metyrapone had no effect on stress-induced grooming (p = 0.982) or rearing (p = 0.946). Bars represent means + SEM; ***p < 0.001, n = 7-8 mice/group. Two-tailed Student’s t tests were used to compare differences between vehicle- and metyrapone-injected groups.
3.2. Chronic HPA axis disruption alters stress-induced activation of the PVH and PVT through a mechanism independent of acute glucocorticoid signaling.
To further understand the effects of chronic HPA axis disruption on brain circuits known to modulate both rearing and grooming, we quantified FOS protein expression in the paraventricular nucleus of the hypothalamus (PVH) and the paraventricular nucleus of the thalamus (PVT). These brain regions are regulators of the neuroendocrine stress response and have been implicated in the control of stress-induced emotional behaviors including grooming and rearing (Fuzesi et al., 2016; Hsu et al., 2014; Kirouac, 2015; Li et al., 2010).
We found that acute exposure to stress increased PVH FOS expression in both Control and HPA-X mice, however PVH FOS expression was significantly lower in HPA-X mice under both baseline conditions and after stress exposure (Fig. 5A–5B). Likewise, we observed that exposure to stress increased PVT FOS in both Control and HPA-X mice, however this increase in PVT FOS expression was significantly blunted in HPA-X mice (Fig. 5C–5D). In parallel with our behavioral measures, CORT replacement in HPA-X mice did not normalize PVH nor PVT FOS expression.
Figure 5.
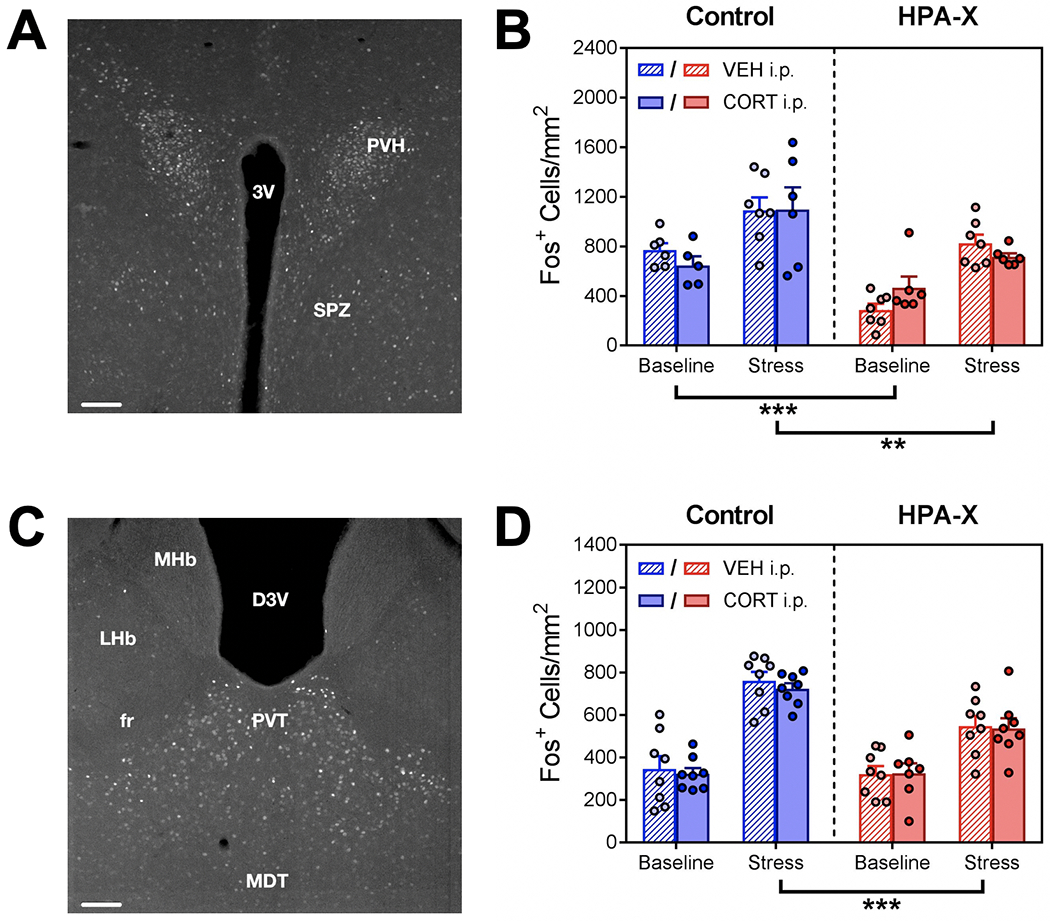
Disruption of the HPA axis with chronic corticosterone alters hypothalamic and thalamic response to stress. (A) Photomicrograph of FOS expression (bright cells) in the paraventricular nucleus of the hypothalamus (PVH). (B) PVH FOS expression, as measured by the number of FOS-positive nuclei per counting area. We observe a main effect of stress (F1, 42 = 36.83, p < 0.001) and chronic treatment (F1, 42 = 28.93, p < 0.001), but no effect of injection. Stress increased PVH FOS expression in both Control (p = 0.003) and HPA-X mice (p < 0.001), however this increase was significantly lower in HPA-X mice under both baseline (p < 0.001) and stress conditions (p = 0.005). (C) Photomicrograph of FOS expression in the paraventricular nucleus of the thalamus (PVT). (D) PVT FOS expression, as measured by the number of FOS-positive nuclei per counting area. We observe a main effect of stress (F1, 55 = 106.12, p < 0.001), chronic treatment (F1, 55 = 11.14, p = 0.002), and a significant interaction (stress x chronic treatment, F1, 55 = 9.50, p = 0.003). No effect of injection was observed. Stress increased FOS expression in both Control (p < 0.001) and HPA-X mice (p < 0.001), however this increase was significantly blunted in HPA-X mice (p < 0.001). Bars represent means + SEM; **p < 0.01; ***p < 0.001, n = 5-8 mice/group. Three way ANOVAs were used to analyze data (stress x chronic treatment x injection), and two-tailed Student’s t tests were used to compare differences between stress and chronic treatment groups. Abbreviations: 3V = Third ventricle; PVH = Paraventricular hypothalamic nucleus; SPZ = Subparaventricular zone; D3V = Dorsal third ventricle; fr = fasciculus retroflexus; LHb = Lateral habenular nucleus; MDT = Medial dorsal thalamic nuclei; MHb = Medial habenular nucleus; PVT = Paraventricular thalamic nucleus. Scale bars for photomicrographs = 100μm.
4. Discussion
Understanding the mechanisms by which HPA axis dysregulation leads to changes in neurobehavioral function could provide insights as to the basic concepts that underlie long-term adaptation of the stress-response system. This contributes to our fundamental understanding of these processes and could contribute to development of potential treatment strategies for individuals that have altered HPA responses to stress that interfere with normal emotional function.
Our experiments used a model of HPA disruption that we have shown clearly impacts normal function of both the neuroendocrine stress axis, as well as the behavioral and neural responses to stress (Karatsoreos et al., 2010; Kinlein et al., 2015). While there may be some caveats of this model, including some variability in dosing, our approach to HPA disruption has several advantages in comparison to other traditionally used models such as adrenalectomy and hormone replacement. Specifically, this method is non-invasive and requires no surgical manipulations or injections to achieve downregulation of stress-induced plasma CORT secretion. Importantly, this method decreases the size of the adrenal zona fasciculata (site of glucocorticoid production) while not affecting the adrenal zona glomerulosa (site of aldosterone production) (Cassano et al., 2012). Thus, the structures supporting other physiological functions of the adrenal gland are maintained while HPA downregulation is achieved, though whether the production or secretion of aldosterone and/or other adrenal factors (e.g. catecholamines) is altered in this model has not been explored. Furthermore, since the adrenal is not removed, this model allows one to investigate recovery, as many of the physiological changes are reversible (Cassano et al., 2012; Kinlein et al., 2017), allowing for the study of long-term consequences of HPA disruption after recovery of normal HPA function.
A limitation of our previous work was understanding the potential neuroendocrine mechanisms by which changes in brain and behavior take place following HPA axis disruption, and the neural circuitry underlying the behavioral outputs. In the current study, HPA-X mice had significantly reduced behavioral grooming and rearing responses to acute stress exposure, which was mirrored by reduced FOS expression in the paraventricular nucleus of the hypothalamus (PVH) and thalamus (PVT). Since a major neuroendocrine consequence of HPA-X is an inability for mice to release CORT endogenously (Kinlein et al., 2015), we posited that if this acute loss of a stress-induced CORT signal was the proximate cause of the neural and behavioral outcomes, then replacement of CORT at the time of stress in HPA-X mice should restore normal behavioral or neural responses to stress exposure (as in Figs. 2, 3, 5). Similarly, acutely blocking endogenous CORT release in mice with an intact HPA should cause a similar effect as HPA-X treatment (as in Fig. 4). However, our results demonstrate that replacement of plasma CORT in stressed HPA-X mice does not rescue the effects of the HPA-X treatment, nor does acute blockade of endogenous CORT secretion with metyrapone in stressed HPA-intact mice mimic the effects of HPA-X treatment. Furthermore, augmentation of plasma CORT in Control mice had no effect on the behavioral or neural outcomes measured, supporting the interpretation that the immediate actions of CORT are not sufficient to cause these effects alone. This suggests that the observed effects in HPA-X mice are likely driven by longer-term effects of CORT on neural adaptation, rather than the acute CORT deficiency during the time of stress exposure.
Immediately following exposure to stress, organisms begin showing behaviors that are hypothesized to combat, or cope, with the immediate physiological challenges presented. These initial responses to stress have been implicated in long-term resilience and vulnerability to stress-related disorders (Franklin et al., 2012; Koolhaas, 2008; Taylor and Stanton, 2007). We observed no differences in general open field locomotor activity between Control and HPA-X mice, nor an effect of CORT augmentation in either group on these measures. These results are bolstered by previous studies showing that adrenalectomy, acute metyrapone injection, or acute glucocorticoid augmentation alone does not affect open field locomotor activity in rats (Joffe et al., 1972; Mikics et al., 2005; Moyer, 1966). Furthermore, acute metyrapone injection alone has been shown to have no effect on open field or elevated plus maze locomotor behaviors in mice (Aliczki et al., 2013). We did find acute swim stress caused a similar decrease in open field locomotor measures in both Control and HPA-X mice independent of CORT manipulation (Fig. 2), suggesting that this effect of stress is not affected by our manipulation.
Our results further showed that the stress-induced rise in glucocorticoids was neither necessary nor sufficient to alter stress-induced grooming and rearing behaviors following swim stress. In line with these findings, Fuzesi et al. (2016) previously reported similar results in grooming and rearing following foot shock stress after pharmacological blockade of CORT synthesis with metyrapone. Together these findings suggest that even under different stress modalities, stress-induced CORT secretion does not affect these immediate behavioral stress responses. However, the neural and behavioral effects of the HPA-X model have not been directly compared between multiple different stressor types, or in different environmental conditions. Stressor type (e.g. physical vs psychological) can differentially impact the HPA axis and autonomic responses to stress, and can have different effects on behavioral outcomes of stress exposure (Finnell et al., 2017; Pacak, 2000; Pijlman et al., 2003). Exploring these differences in the HPA-X model could reveal important mechanistic distinctions by which glucocorticoids or other physiological responses to stress exert their effects on neural circuits and behavior.
We chose to measure activity in the PVH and PVT as neural correlates of stress-induced behavior, as grooming and rearing behavior have been shown to be influenced by stimulation of the PVH and PVT independently (Fuzesi et al., 2016; Li et al., 2010). Fuzesi et al. (2016) showed that grooming and rearing behaviors were influenced by activity of CRH neurons in the PVH. Further, photoinhibition of PVH CRH neurons decreased stress-induced grooming, and increased stress-induced rearing behaviors, while photostimulation of these neurons had the opposite effect (Fuzesi et al., 2016). These findings are in line with our PVH FOS expression and behavioral results, as HPA-X mice displayed blunted stress-induced PVH FOS expression, decreased grooming, and increased rearing compared to Control mice. In addition, we previously showed that chronic CORT administration decreases PVH CRH mRNA expression, indicating that changes in efferent PVH CRH signaling may be an important factor mediating the observed behavioral effects in HPA-X mice (Kinlein et al., 2015). Not only does this strengthen the idea that the PVH is a part of the circuitry driving these behaviors, it further suggests that chronic HPA axis dysfunction alters behavior through suppression of PVH function.
The posterior subdivision of the PVT is known to regulate habituation to repeated stress exposure by inhibiting HPA axis output following stress (Bhatnagar et al., 2002; Hsu et al., 2014; Jaferi and Bhatnagar, 2006). Importantly, this inhibition of HPA output by the PVT appears to driven by negative feedback actions of CORT through the glucocorticoid and mineralocorticoid receptors, as repeated stress-induced ACTH secretion is reduced by intra-PVT corticosterone implants, and acute stress-induced ACTH secretion is potentiated by combined glucocorticoid and mineralocorticoid receptor antagonism (Jaferi and Bhatnagar, 2006). Additionally, Li et al. (2010) showed that grooming and rearing behaviors in the open field were influenced by acute pharmacological PVT stimulation with orexin. In their study, stimulation of the posterior PVT increased grooming and decreased rearing (Li et al., 2010). These findings are consistent with our results, as HPA-X mice display blunted stress-induced posterior PVT FOS expression, decreased grooming, and increased rearing compared to Control mice, suggesting that chronic HPA axis dysfunction alters behavior through suppression of PVT function.
The patterns of PVH and PVT FOS expression are consistent in how they are affected by HPA-X treatment and with how they are thought to affect behavior. This indicates these brain regions are likely part of a neural circuit which drives immediate behavioral coping responses to stress. The PVH and PVT are connected by both direct and indirect pathways, with direct projections from the PVH to the posterior PVT, and indirect projections from the PVT to the PVH through a relay in the bed nucleus of the stria terminalis (Chen and Su, 1990; Hsu et al., 2014; Kirouac, 2015; Li and Kirouac, 2008, 2012; Otake et al., 1995). Thus, the blunted responses of both brain regions following stress exposure in HPA-X mice suggests that chronic HPA axis dysfunction downregulates the activity of these pathways. These brain regions also share connections with corticolimbic structures including as the prefrontal cortex, possibly representing a neural circuit through which chronic HPA axis disruption can shape long term changes in emotionality or cognitive function associated with stress (Jurik et al., 2015; Vertes, 2004). Exploration of these pathways is a crucial next step for determining the impact of HPA axis dysfunction on an organism’s vulnerability to developing stress-related neuropathology.
Of note, the behavioral differences we observed indicate that HPA-X mice seem “resistant” to the effects of stress compared to Control mice, in that stress does not cause as large a change in grooming or rearing in HPA-X mice after stress exposure. This is in line with our previous findings (Kinlein et al., 2015), and while seemingly advantageous, we suggest the blunting of these responses indicates that the appropriate neural circuits, and the behaviors they underlie, are not being recruited adequately. In the face of an ethologically-relevant threat (e.g. predation, social challenge, resource scarcity), an inadequate behavioral response hinders the chances of an organism’s survival. Restated, reduced “normal” behavioral responses to stress are maladaptive in nature, and rather than a boon to the organism may instead represent a negative outcome.
As we did not find that behavioral and neural measures were mediated by the stress-induced rise in CORT, it is important to consider the mechanisms that could be driving the differences between Control and HPA-X mice. Through the glucocorticoid and mineralocorticoid receptors, CORT can have rapid effects on neural function via direct engagement of intracellular signaling systems, as well as protracted effects via genomic actions (Datson et al., 2008; Evanson et al., 2010; Joels et al., 2012). As our acute administration of CORT occurred very shortly before our measures were taken (20 minutes prior to behavior and 60 minutes prior to brain collection), it is likely that any observed effects of this administration method would be due to the rapid effects of CORT. However, since we observed minimal effects of acute CORT administration, this suggests that the differences in behavior and brain function in HPA-X mice were driven by the protracted effects of CORT, likely via genomic mechanisms acting throughout the course of chronic treatment. Given that we observed decreased stress-induced FOS expression in the PVH and PVT of HPA-X mice, it is possible that HPA-X treatment decreases sensitivity of neurons in these brain regions to incoming stress-related signals from elsewhere in the brain. A general mechanism by which this could occur is through the well described actions of glucocorticoids on synaptic function via their effects on presynaptic neurotransmitter release, postsynaptic sensitivity, and dendritic structural morphology (McEwen et al., 2015). More specifically, as orexin actions in the PVT can impact behavior and PVH function (Heydendael et al., 2011; Li et al., 2010), it is possible that chronic CORT treatment alters stress-induced grooming and rearing behaviors through glucocorticoid effects on orexin signaling. Both the PVH and PVT contain glucocorticoid receptors which makes this a likely mechanism (Jaferi and Bhatnagar, 2006; Tasker and Herman, 2011). In order to determine the mechanisms driving the behavioral and neural differences in HPA-X mice, it will be necessary to analyze neural function at each of these levels. For example, decreased neural activity in the PVH and PVT after HPA-X could be caused by decreased excitatory synaptic tone, increased inhibitory synaptic tone, or through changes in synaptic contacts with afferent neurons.
5. Conclusions
Our study shows that chronic dysfunction of the HPA axis leads to neural adaptation in stress-related brain circuits, which has consequences for an organism’s behavioral responses to stress exposure. While behavioral and neural responses to stress were blunted in our model of chronic HPA axis dysregulation, these responses were not rescued or recapitulated by acute manipulation of glucocorticoids at the time of stress, suggesting a long-term, genomic effect of glucocorticoids as the likely mediating factor.
While HPA dysregulation is strongly linked to the development of stress-related psychiatric disorders, there are many biological factors beyond glucocorticoid secretion that contribute to their development and symptomology. Further studies will be needed to determine how HPA hormonal signals interact with other biological systems involved in emotional regulation in the progression of these disorders. Examining how these complex interactions affect disease progression is a necessary step in the detection of risk factors and the development of treatments for these illnesses.
Acknowledgements
We would like to thank the WSU Center for Reproductive Biology for the use of their imaging equipment in this study, Dr. David Dewitt for his advice and expertise in imaging and analysis, and the WSU vivarium staff for their role in supporting our research. This work was supported by the National Institutes for Health [AG050054] and National Science Foundation [NSF CAREER 1553067].
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to declare.
6. References
- Aliczki M, Zelena D, Mikics E, Varga ZK, Pinter O, Bakos NV, Varga J, Haller J, 2013. Monoacylglycerol lipase inhibition-induced changes in plasma corticosterone levels, anxiety and locomotor activity in male CD1 mice. Hormones and behavior 63, 752–758. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Huber R, Nowak N, Trotter P, 2002. Lesions of the posterior paraventricular thalamus block habituation of hypothalamic-pituitary-adrenal responses to repeated restraint. Journal of neuroendocrinology 14, 403–410. [DOI] [PubMed] [Google Scholar]
- Cassano AE, White JR, Penraat KA, Wilson CD, Rasmussen S, Karatsoreos IN, 2012. Anatomic, hematologic, and biochemical features of C57BL/6NCrl mice maintained on chronic oral corticosterone. Comparative medicine 62, 348–360. [PMC free article] [PubMed] [Google Scholar]
- Chen S, Su HS, 1990. Afferent connections of the thalamic paraventricular and parataenial nuclei in the rat--a retrograde tracing study with iontophoretic application of Fluoro-Gold. Brain research 522, 1–6. [DOI] [PubMed] [Google Scholar]
- Datson NA, Morsink MC, Meijer OC, de Kloet ER, 2008. Central corticosteroid actions: Search for gene targets. European journal of pharmacology 583, 272–289. [DOI] [PubMed] [Google Scholar]
- de Kloet CS, Vermetten E, Geuze E, Kavelaars A, Heijnen CJ, Westenberg HG, 2006. Assessment of HPA-axis function in posttraumatic stress disorder: pharmacological and non-pharmacological challenge tests, a review. Journal of psychiatric research 40, 550–567. [DOI] [PubMed] [Google Scholar]
- Evanson NK, Herman JP, Sakai RR, Krause EG, 2010. Nongenomic actions of adrenal steroids in the central nervous system. Journal of neuroendocrinology 22, 846–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faravelli C, Lo Sauro C, Lelli L, Pietrini F, Lazzeretti L, Godini L, Benni L, Fioravanti G, Talamba GA, Castellini G, Ricca V, 2012. The role of life events and HPA axis in anxiety disorders: a review. Current pharmaceutical design 18, 5663–5674. [DOI] [PubMed] [Google Scholar]
- Finnell JE, Lombard CM, Padi AR, Moffitt CM, Wilson LB, Wood CS, Wood SK, 2017. Physical versus psychological social stress in male rats reveals distinct cardiovascular, inflammatory and behavioral consequences. PloS one 12, e0172868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KB, Paxinos G, 2007. The mouse brain in stereotaxic coordinates, Third ed. Academic Press, New York. [Google Scholar]
- Franklin TB, Saab BJ, Mansuy IM, 2012. Neural mechanisms of stress resilience and vulnerability. Neuron 75, 747–761. [DOI] [PubMed] [Google Scholar]
- Fuzesi T, Daviu N, Wamsteeker Cusulin JI, Bonin RP, Bains JS, 2016. Hypothalamic CRH neurons orchestrate complex behaviours after stress. Nature communications 7, 11937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie CF, Nemeroff CB, 2005. Hypercortisolemia and depression. Psychosomatic medicine 67 Suppl 1, S26–28. [DOI] [PubMed] [Google Scholar]
- Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, Scheimann J, Myers B, 2016. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Comprehensive Physiology 6, 603–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydendael W, Sharma K, Iyer V, Luz S, Piel D, Beck S, Bhatnagar S, 2011. Orexins/hypocretins act in the posterior paraventricular thalamic nucleus during repeated stress to regulate facilitation to novel stress. Endocrinology 152, 4738–4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu DT, Kirouac GJ, Zubieta JK, Bhatnagar S, 2014. Contributions of the paraventricular thalamic nucleus in the regulation of stress, motivation, and mood. Frontiers in behavioral neuroscience 8, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaferi A, Bhatnagar S, 2006. Corticosterone can act at the posterior paraventricular thalamus to inhibit hypothalamic-pituitary-adrenal activity in animals that habituate to repeated stress. Endocrinology 147, 4917–4930. [DOI] [PubMed] [Google Scholar]
- Joels M, Sarabdjitsingh RA, Karst H, 2012. Unraveling the time domains of corticosteroid hormone influences on brain activity: rapid, slow, and chronic modes. Pharmacological reviews 64, 901–938. [DOI] [PubMed] [Google Scholar]
- Joffe JM, Mulick JA, Rawson RA, 1972. Effects of adrenalectomy on open-field behavior in rats. Hormones and behavior 3, 87–96. [DOI] [PubMed] [Google Scholar]
- Jurik A, Auffenberg E, Klein S, Deussing JM, Schmid RM, Wotjak CT, Thoeringer CK, 2015. Roles of prefrontal cortex and paraventricular thalamus in affective and mechanical components of visceral nociception. Pain 156, 2479–2491. [DOI] [PubMed] [Google Scholar]
- Juruena MF, Bocharova M, Agustini B, Young AH, 2017. Atypical depression and non-atypical depression: Is HPA axis function a biomarker? A systematic review. Journal of affective disorders. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Stewart AM, Song C, Berridge KC, Graybiel AM, Fentress JC, 2016. Neurobiology of rodent self-grooming and its value for translational neuroscience. Nature reviews. Neuroscience 17, 45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatsoreos IN, Bhagat SM, Bowles NP, Weil ZM, Pfaff DW, McEwen BS, 2010. Endocrine and physiological changes in response to chronic corticosterone: a potential model of the metabolic syndrome in mouse. Endocrinology 151, 2117–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinlein SA, Shahanoor Z, Romeo RD, Karatsoreos IN, 2017. Chronic Corticosterone Treatment During Adolescence Has Significant Effects on Metabolism and Skeletal Development in Male C57BL6/N Mice. Endocrinology 158, 2239–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinlein SA, Wilson CD, Karatsoreos IN, 2015. Dysregulated hypothalamic-pituitary-adrenal axis function contributes to altered endocrine and neurobehavioral responses to acute stress. Frontiers in psychiatry 6, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirouac GJ, 2015. Placing the paraventricular nucleus of the thalamus within the brain circuits that control behavior. Neuroscience and biobehavioral reviews 56, 315–329. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, 2008. Coping style and immunity in animals: making sense of individual variation. Brain, behavior, and immunity 22, 662–667. [DOI] [PubMed] [Google Scholar]
- Li S, Kirouac GJ, 2008. Projections from the paraventricular nucleus of the thalamus to the forebrain, with special emphasis on the extended amygdala. The Journal of comparative neurology 506, 263–287. [DOI] [PubMed] [Google Scholar]
- Li S, Kirouac GJ, 2012. Sources of inputs to the anterior and posterior aspects of the paraventricular nucleus of the thalamus. Brain structure & function 217, 257–273. [DOI] [PubMed] [Google Scholar]
- Li Y, Li S, Wei C, Wang H, Sui N, Kirouac GJ, 2010. Changes in emotional behavior produced by orexin microinjections in the paraventricular nucleus of the thalamus. Pharmacology, biochemistry, and behavior 95, 121–128. [DOI] [PubMed] [Google Scholar]
- McEwen BS, 1998. Stress, adaptation, and disease. Allostasis and allostatic load. Annals of the New York Academy of Sciences 840, 33–44. [DOI] [PubMed] [Google Scholar]
- McEwen BS, 2017. Neurobiological and Systemic Effects of Chronic Stress. Chronic stress 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Bowles NP, Gray JD, Hill MN, Hunter RG, Karatsoreos IN, Nasca C, 2015. Mechanisms of stress in the brain. Nature neuroscience 18, 1353–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikics E, Barsy B, Barsvari B, Haller J, 2005. Behavioral specificity of non-genomic glucocorticoid effects in rats: effects on risk assessment in the elevated plus-maze and the open-field. Hormones and behavior 48, 152–162. [DOI] [PubMed] [Google Scholar]
- Moyer KE, 1966. Effect of ACTH on open-field behavior, avoidance, startle, and food and water consumption. The Journal of genetic psychology 108, 297–302. [DOI] [PubMed] [Google Scholar]
- Nosek K, Dennis K, Andrus BM, Ahmadiyeh N, Baum AE, Solberg Woods LC, Redei EE, 2008. Context and strain-dependent behavioral response to stress. Behavioral and brain functions : BBF 4, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otake K, Ruggiero DA, Nakamura Y, 1995. Adrenergic innervation of forebrain neurons that project to the paraventricular thalamic nucleus in the rat. Brain research 697, 17–26. [DOI] [PubMed] [Google Scholar]
- Pacak K, 2000. Stressor-specific activation of the hypothalamic-pituitary-adrenocortical axis. Physiological research 49 Suppl 1, S11–17. [PubMed] [Google Scholar]
- Pariante CM, Lightman SL, 2008. The HPA axis in major depression: classical theories and new developments. Trends in neurosciences 31, 464–468. [DOI] [PubMed] [Google Scholar]
- Pijlman FT, Wolterink G, Van Ree JM, 2003. Physical and emotional stress have differential effects on preference for saccharine and open field behaviour in rats. Behavioural brain research 139, 131–138. [DOI] [PubMed] [Google Scholar]
- Shahanoor Z, Sultana R, Baker MR, Romeo RD, 2017. Neuroendocrine stress reactivity of male C57BL/6N mice following chronic oral corticosterone exposure during adulthood or adolescence. Psychoneuroendocrinology 86, 218–224. [DOI] [PubMed] [Google Scholar]
- Sturman O, Germain PL, Bohacek J, 2018. Exploratory rearing: a context- and stress-sensitive behavior recorded in the open-field test. Stress, 1–10. [DOI] [PubMed] [Google Scholar]
- Tasker JG, Herman JP, 2011. Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic-pituitary-adrenal axis. Stress 14, 398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Stanton AL, 2007. Coping resources, coping processes, and mental health. Annual review of clinical psychology 3, 377–401. [DOI] [PubMed] [Google Scholar]
- Thiel CM, Muller CP, Huston JP, Schwarting RK, 1999. High versus low reactivity to a novel environment: behavioural, pharmacological and neurochemical assessments. Neuroscience 93, 243–251. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP, 2009. Neural regulation of endocrine and autonomic stress responses. Nature reviews. Neuroscience 10, 397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Erp AM, Kruk MR, Meelis W, Willekens-Bramer DC, 1994. Effect of environmental stressors on time course, variability and form of self-grooming in the rat: handling, social contact, defeat, novelty, restraint and fur moistening. Behavioural brain research 65, 47–55. [DOI] [PubMed] [Google Scholar]
- Vertes RP, 2004. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 51, 32–58. [DOI] [PubMed] [Google Scholar]
- Zaba M, Kirmeier T, Ionescu IA, Wollweber B, Buell DR, Gall-Kleebach DJ, Schubert CF, Novak B, Huber C, Kohler K, Holsboer F, Putz B, Muller-Myhsok B, Hohne N, Uhr M, Ising M, Herrmann L, Schmidt U, 2015. Identification and characterization of HPA-axis reactivity endophenotypes in a cohort of female PTSD patients. Psychoneuroendocrinology 55, 102–115. [DOI] [PubMed] [Google Scholar]
- Zimprich A, Garrett L, Deussing JM, Wotjak CT, Fuchs H, Gailus-Durner V, de Angelis MH, Wurst W, Holter SM, 2014. A robust and reliable non-invasive test for stress responsivity in mice. Frontiers in behavioral neuroscience 8, 125. [DOI] [PMC free article] [PubMed] [Google Scholar]


