Abstract
Solid dispersion is the preferred technology to prepare efficacious forms of BCS class-II/IV APIs. To prepare solid dispersions, there exist a wide variety of polymeric carriers with interesting physicochemical and thermochemical characteristics available at the disposal of a formulation scientist. Since the advent of the solid dispersion technology in the early 1960s, there have been more than 5000 scientific papers published in the subject area. This review discusses the polymeric carrier properties of most extensively used polymers PVP, Copovidone, PEG, HPMC, HPMCAS, and Soluplus® in the solid dispersion technology. The literature trends about preparation techniques, dissolution, and stability improvement are analyzed from the Scopus® database to enable a formulator to make an informed choice of polymeric carrier. The stability and extent of dissolution improvement are largely dependent upon the type of polymeric carrier employed to formulate solid dispersions. With the increasing acceptance of transfer dissolution setup in the research community, it is required to evaluate the crystallization/precipitation inhibition potential of polymers under dynamic pH shift conditions. Further, there is a need to develop a regulatory framework which provides definition and complete classification along with necessarily recommended studies to characterize and evaluate solid dispersions.
Supplementary Information
The online version contains supplementary material available at 10.1208/s12249-020-01849-z.
Keywords: solid dispersion, polymers, dissolution, solubility, stability
INTRODUCTION
In the quest to access the complex targets, most of the New Chemical Entities (NCEs) in the development pipeline are becoming increasingly lipophilic, thereby restricting their aqueous solubility. Therefore, a drug candidate with good permeability but limited aqueous solubility is not drug-like due to consequent lower bioavailability(1,2). For such candidates, which belong to Biopharmaceutical Classification class-II & IV (BCS-II & IV), improving the aqueous solubility is the most prudent option. To that end, various techniques have been employed to improve the solubility like micro/nanoparticle drug delivery (3), co-crystal formation (4,5), complexation with cyclodextrins (6,7), and salt formation (8). Nevertheless, success is usually marginal due to the inability of these techniques to generate and sustain the supersaturated state (9,10). In this regard, solid dispersion (SD) is considered as a promising technique, owing to its ability to generate and sustain supersaturation, leading to improved bioavailability (11,12).
The first reported solid dispersion attempt was from Sekiguchi and Obi in 1961(13). However, the term solid dispersion was defined by Chiou and Riegelman in 1971 as “a dispersion of one or more active ingredients in an inert carrier at the solid-state prepared by the melting (fusion), solvent, or melting-solvent method” (14). In order to prepare efficacious and stable solid dispersion, the choice of carrier is of great significance. In a solid dispersion, an ideal carrier is required to perform multiple functions (Fig. 1-supplementary material). First, the carrier is expected to improve the solubility and dissolution of the principal drug. This can be achieved by various mechanisms like particle size reduction, improved wettability, and amorphous state of the drug or combination thereof. Second, the carrier is required to impart the physical stability to the prepared dispersion. This chiefly holds well when the physical state of the dispersion is amorphous. The stability to dispersion or amorphous solid dispersion (ASD) is imparted through high glass transition temperature (Tg) of a carrier, intimate mixing, and molecularly dispersed drug in a carrier, intermolecular adhesive interactions or combination thereof (15–21).
Early attempts (1960’s decade) of formulating solid dispersions were focused mainly on using small molecular weight water-soluble crystalline carriers like urea and sugars. Many of these reported solid dispersions belonged to the category of Eutectics. They exhibited excellent thermodynamic stability due to their crystalline nature; nevertheless, the drug release from such formulations was found much slower than amorphous forms (15,22). These limitations of crystalline carriers created an impetus to formulate solid dispersions by employing carriers that have the ability to stabilize drugs in their amorphous state. The space of such carriers has arguably been assumed by polymers, especially amorphous polymers. A solid dispersion prepared by using polymeric carrier falls in the subcategory of solid solutions, glass solutions, or solid suspensions (Table I) (12,14,23). Polymeric carriers have been proven to be the most successful and extensively investigated subgroup. Beginning from mid-2000, there has been a shift in the strategy to formulate solid dispersion, which is driven by two important trends. First, instead of the composite function of solubility and stability improvement in a single carrier, there is new wisdom of using a mixture of polymer and surfactant as a carrier. Wherein, the polymer with high Tg is expected to impart stability, while the solubility and dissolution can be improved by surfactant through increased wettability or micellar formation. Second, the polymers with high glass transition temperature are made pliable to formulate by the fusion method by adding a plasticizer (24,25). In the recent past, however, there is an upward trend in using polymeric surfactants like block copolymers, i.e., Soluplus® and various grades of poloxamers in solid dispersion technology (16,22).
Table I.
Various Types of Solid Dispersions and Their Relative Potential for Stability and Solubility Improvement
| SN. | API state | Carrier state | Phase solubility | SD subtype | Stability | Solubility |
|---|---|---|---|---|---|---|
| 1 | Crystal | Crystal | No | Solid suspension | +++ | ++ |
| 2 | Crystal | Crystal | Yes | Eutectics | +++ | + |
| 3 | Crystal | Amorphous | No | Solid suspension | +++ | ++ |
| 4 | Amorphous | Crystal | No | Solid suspension | + | +++ |
| 5 | Amorphous | Crystal | Yes | Solid solution | +++ | +++ |
| 6 | Amorphous | Amorphous | No | Glass suspension | + | +++ |
| 7 | Amorphous | Amorphous | Yes | Glass solution | +++ | +++ |
Part of the classification has been reproduced from the reference (23)
Since 1961, there is prolific literature available on the topic of solid dispersion technology. This is evident by almost 5457 records in the Scopus® database on the topic “Solid Dispersion” OR “Solid Dispersions” as of March 2020. The vast number of polymeric carriers like poly vinyl pyrrolidone (PVP), poly ethylene glycol (PEG), and natural polymers like cellulose derivatives (hydroxypropyl methyl cellulose-HPMC, hydroxy propyl methyl cellulose acetate succinate-HPMCAS, ethyl cellulose-EC) have been actively researched. These polymeric carriers possess interesting physicochemical and thermodynamic properties, which enables their usage in formulating solid dispersions. Aplenty dissolution and stability improvement mechanisms were deduced in the literature for each polymer. Seldom, there exists a single mechanism responsible for dissolution improvement. The same is true for the stability of prepared dispersions. Such numerous and overlapping mechanisms may overwhelm the early carrier researcher and limit his/her comprehension to select an appropriate polymer. In this context, the review article attempts to compile all the existing common knowledge and literature trends of dissolution and stability improvement mechanisms of commonly used polymers in solid dispersion technology to help make an informed decision, in particular for early-career researchers in the field. This was achieved by searching through the Scopus® database. The review will cover polymeric carriers employed in binary solid dispersions; however, the discussion about small molecular weight carriers is out of the scope of this manuscript, as this space is assumed by so-called co-amorphous technology. The interested readers are advised to read many interesting reviews about co-amorphous technology (26,27).
CLASSIFICATION OF POLYMERIC CARRIERS
There are multiple bases to classify the polymeric carriers employed in the solid dispersion technology, like the physical state, relative hydrophilicity, ionization potential, and source of the carrier (15,16,22,23). Of which, the physical state of polymeric carrier is a very important criterion for classification, as it has direct functional relevance to the dispersion prepared. The polymeric carriers can either be disordered amorphous state or ordered crystalline state. It is widely documented that the crystalline polymeric carriers have limited drug solubility (12,28). Thus, a limited amount of drugs can get molecularly dissolved in the crystalline polymer. If the resultant system is a single-phase, then it is usually classified into a sub-category of solid solutions (12,14,23) (Table I) wherein a limited part of a drug is dissolved in the crystalline carrier. For such systems, oftentimes, the difference between the molecular size of the drug and polymeric carrier is very large. Hence, it is hypothesized that a drug gets dispersed in the interstitial spaces of the polymeric carrier. In particular, such systems are called interstitial solid solutions (12,29). PEG is the representative example of this class (15,30,31). The dispersion consisting of amorphous polymeric carriers has a drug molecularly dissolved or form an amorphous precipitate within the carrier. Amorphous carriers tend to form a single-phase amorphous systems with drugs. The systems can be classified as the single-phase glass solutions (Table I) or colloquially called as amorphous solid dispersion (ASD) (12,14,16,32). Solid dispersions with amorphous carriers usually exhibit higher solubility and dissolution rates due to the high energy amorphous phase of the drug. However, these systems demonstrated to have limited physical stability at room temperature, as the drug can possibly recrystallize within polymeric matrix upon storage. Polymers like PVP, polyvinylpyrrolidone vinyl acetate (PVP VA), and HPMC, which have been extensively used in solid dispersion technology, belong to the class of amorphous polymeric carriers.
Furthermore, the polymeric carriers can be classified based on their relative hydrophilicity into the categories of hydrophilic, hydrophobic, and amphiphilic polymer-carriers (Fig. 2-supplementary material). The utility of hydrophobic polymeric carriers in solid dispersion technology, which attempts to improve aqueous solubility, seems counterintuitive. However, hydrophobic polymers tend to form excellent single-phase systems with less soluble drugs, which are inherently more lipophilic (BCS-II & IV). The intimately mixed or molecularly dissolved drug within the polymeric carrier is usually inclined to stabilize in the amorphous state and rendering them comparatively more physically stable than hydrophilic counterparts (33–35). The physical stability can be attributed to the excellent phase solubility with drug candidates. If the physical state of the polymer is amorphous, then the phase-soluble systems can be categorized as glass solutions, and phase-separated systems are called glass suspension (Table I). Though the carriers are hydrophobic, the solubility and dissolution improvement is seen in such dispersions due to the amorphous state of the drug (36,37), as the amorphous state does not require the excess energy, compared to the crystalline state, to break down the drug’s crystal lattice in order to solubilize it. Further, hydrophobic carriers are believed to inhibit drug aggregation in solution phase, thus sustaining supersaturation of the drug with respect to its crystalline solubility (37–39). Water-insoluble polymers like cellulose acetate (CA), cellulose acetate phthalate (CAP), ethyl cellulose (EC), methacrylates, and Eudragits fall under hydrophobic polymeric carrier category. Hydrophilic polymeric carriers are by far the most extensively researched topic in solid dispersion technology. If a drug is molecularly dissolved in the hydrophilic polymer carrier, then such type of dispersions are sub-categorized as glass solutions, while phase separated systems are sub-categorized as solid suspensions (Table I) (12,16,40). Though hydrophilic polymeric carriers appear an ideal choice for solid dispersion, being hydrophilic, they exhibit very limited solubility with usually hydrophobic drugs (BCS-II & IV). So, to molecularly dissolve a drug, large quantities of polymers are required (12,41,42). Further, hydrophilic polymers are deliquescent. The limited drug loading in a high proportion of polymer and deliquescent nature of hydrophilic polymers compounds the problem of phase separation, rendering the solid dispersion physically unstable (16,41,43,44). However, hydrophilic polymeric carriers have been demonstrated to be very efficient in solubility and dissolution improvement. Mechanistically, the drug molecule gets released as the hydrophilic carrier dissolves, subsequently forming a supersaturated solution of the drug (45–47). Polymers like PVP, PEG belong to this category. The polymeric surfactants like di-block and tri-block copolymers demonstrate amphiphilic properties. The amphiphilic polymers tend to strike a balance between solubility and stability improvement. The solubility improvement is achieved due to surfactant properties through micellar solubilization and increase in wettability, while the polymeric nature helps to stabilize a drug in a disordered amorphous state. Block copolymers like Soluplus® and various grades of poloxamers are categorized as amphiphilic polymers (48–51). Furthermore, the polymeric carriers are also classified based on their ionization tendencies. Usually, these polymers have pH-dependent solubility and dissolution properties. The interested readers can be advised to follow the literature (88).
There exist other miscellaneous grounds to classify polymeric carriers like ionization tendency and source of polymers. Unlike afore discussed classification, these categories are not functionally relevant and hence not discussed in detail in this review.
SELECTION CRITERIA FOR POLYMERIC CARRIERS
The desirability of polymeric carriers for preparation of solid dispersion can broadly be influenced by three factors, i.e., safety, kinetic, and thermodynamic aspects (Fig. 3-supplementary material). It is conventional wisdom that any excipient used in pharmaceutical formulations should be inert both chemically and pharmacologically. The same criterion holds good for polymeric carriers employed in the preparation of solid dispersions. The carrier must preferably be Generally Recognized As Safe (GRAS) status (12,22,40,52). From the kinetic perspective, the polymer should be able to inhibit the precipitation of drugs in gastro-intestinal (GI) milieu into their respective crystalline form. Particularly, weakly basic drugs are amenable for precipitation in the intestine due to the predominance of unionized form therein (53,54). Further, if the polymer possesses surfactant properties, then partitioning of the drug in the micelles may help in improving the solubility. Such solubility advantage may be over, and above the solubility, the advantage gained through high energy amorphous state (55–57). The hydrophilic and amorphous polymers are deliquescent in nature. For low load solid dispersions, which usually is a case, the polymer is present in significantly higher proportion, and de-vitrification of amorphous state and subsequent phase-separation pose a significant challenge for physical stability (58,59). Further, thermodynamic factors are important from the stability point of view for dispersions whose physical state is amorphous. The polymer must have a high glass transition temperature (Tg), so prepared dispersion remains stable at room temperature, lowering the probability of Johri-Goldestain (JG) relaxations (20,60–62). If the objective of the formulation scientist is to prepare an amorphous solid dispersion, then the polymer employed must have a good glass-forming ability with a variety of compounds. The high phase-solubility of a polymer with the drug is a pre-requisite to form a single-phase amorphous system (11,52,63). Also, a high probability of adhesive intermolecular interactions through hydrogen bond acceptor and donor properties is desirable to form stable and single-phase amorphous dispersion. From the manufacturing point of view, the solubility of a polymer in organic solvents and phase-solubility aid the preparation of solid dispersion through the solvent and melt method, respectively.
VINYL PYRROLIDONE DERIVATIVES
The polymerization of N-vinylpyrrolidone yielded a water-soluble product named as polyvinylpyrrolidone and patented in 1939. N-vinyl pyrrolidone derivatives are classified based on crosslinking (Fig. 4-Supplementary material). Since then, PVP has become a widely used excipient in the pharmaceutical industry (64). Further, it was learned that the N-Vinylpyrrolidone undergoes crosslinking at a temperature over 100°C in the presence of alkali hydroxide by the process so-called as popcorn polymerization. The crosslinked product came to be known as Crospovidone demonstrates different physicochemical properties than PVP. The Crospovidone is water-insoluble due to its cross-linkages and hence not widely used in solid dispersions (65,66). Furthermore, to strike a balance between water-soluble PVP and insoluble Crospovidone, the co-polymer of N-vinyl pyrrolidone with vinyl acetate was synthesized which exhibited intermediate polarity between PVP and Crospovidone. The product named as Copovidone is vinyl acetate co-polymer of vinylpyrrolidone in a ratio of 6:4, where 6 parts are vinylpyrrolidone and 4 parts are vinyl acetate. The Copovidone exhibits certain interesting physicochemical properties which enable it to use in formulating solid dispersions (67). The subsequent sections discuss the utility of PVP and Copovidone in the solid dispersion technology.
POLY VINYL PYRROLIDONE (PVP)
PVP is one of the most commonly used polymeric carriers to formulate solid dispersion. The search through the Scopus database yielded around 939 records of PVP-based solid dispersions from 1978 to date. PVP is commercially available in different grades based on average (mean) molecular weight ranging from 10,000 (10K) to 120,000 (120 K) (Table II) (68,69). The K values assigned to various grades of PVP polymer represent a function of the average molecular weight, the degree of polymerization, and the intrinsic viscosity. The K values are derived from viscosity measurements and are calculated according to Fikentscher’s formula (Supplementary material). It is a hydrophilic polymer that has an amorphous physical state. It is soluble in water, ethanol, iso-propyl alcohol, and chloroform (16,47).
Table II.
Physicochemical Properties of Polymers Commonly Used in the Preparation of Solid Dispersions
| SN. | Polymer | Physical form | Molecular weight (g/Mol) | Water solubility | Hygroscopicity | Glass transition/Melting temperature (°C) | Degradation temperature (°C) | Solubility parameter (Mpa1/2) |
|---|---|---|---|---|---|---|---|---|
| 1 | Poly-Vinyl-Pyrrolidone (PVP) | |||||||
| 1.1 | PVP K-12 | Amorphous | 5000 | Water soluble | High | 120a | 196 | 21.74 |
| 1.2 | PVP K-15 | Amorphous | 9700 | Water soluble | High | 130a | 215 | 21.63 |
| 1.3 | PVP K-30 | Amorphous | 66,800 | Water soluble | High | 163a | 171 | 21.69 |
| 1.4 | PVP K-60 | Amorphous | 396,000 | Water soluble | High | 170a | NA | 21.70 |
| 1.5 | PVP K-90 | Amorphous | 1,570,000 | Water soluble | High | 174a | 194 | 21.73 |
| 1.6 | PVP K-120 | Amorphous | 3,470,000 | Water soluble | High | 174a | 194 | 21.70 |
| 2 | Copovidone | Amorphous | 45,000–70,000 | Amphiphilic | Moderate | 100–105 | 230 | 21.20 |
| 3 | Poly ethylene glycol (PEG) | |||||||
| 3.1 | PEG 400 | Crystalline | 400 | Intermediate | High | NA | NA | 18.9 |
| 3.2 | PEG 600 | Crystalline | 600 | Intermediate | High | NA | 160 | 23.70 |
| 3.3 | PEG 800 | Crystalline | 800 | Intermediate | High | NA | 160 | 23.70 |
| 3.4 | PEG 1000 | Crystalline | 1000 | Intermediate | Moderate | 37–40 | 160 | 23.70 |
| 3.5 | PEG 1500 | Crystalline | 1500 | Intermediate | Moderate | 44–48 | 160 | 23.70 |
| 3.6 | PEG 2000 | Crystalline | 2000 | Intermediate | Moderate | 45–50 | 160 | 17.6 |
| 3.7 | PEG 3000 | Crystalline | 3000 | Intermediate | Moderate | 48–54 | NA | NA |
| 3.8 | PEG 4000 | Crystalline | 4000 | Intermediate | Moderate | 50–58 | NA | NA |
| 3.9 | PEG 6000 | Crystalline | 6000 | Intermediate | Moderate | 55–63 | NA | NA |
| 3.10 | PEG 8000 | Crystalline | 8000 | Intermediate | Moderate | 60–63 | NA | 19.8 |
| 3.11 | PEG 10000 | Crystalline | 10,000 | Intermediate | Moderate | 62–65 | NA | 16.6 |
| 3.12 | PEG 20000 | Crystalline | 20,000 | Intermediate | Moderate | 60–63 | NA | NA |
| 4 | Hydroxypropyl methylcellulose (HPMC) | |||||||
| 4.1 | HPMC-E | Amorphous | 85,000–150,000 | Water soluble | High | 141c | NA | 29.95b |
| 4.2 | HPMC-F | Amorphous | 85,000–150,000 | Water soluble | High | 160c | 240 | 29.05b |
| 4.3 | HPMC-K | Amorphous | 85,000–150,000 | Water soluble | High | 172c | 260 | 30.57b |
| 5 | Hydroxypropyl methylcellulose acetate succinate (HPMCAS) | |||||||
| 5.1 | HPMCAS L | Amorphous | 50,000 | Water soluble above pH 5.0 | High | 119 | 204 | 29.10 |
| 5.2 | HPMCAS M | Amorphous | 50,000 | Water soluble above pH 5.0 | High | 120 | 190 | 29.10 |
| 5.3 | HPMCAS H | Amorphous | 50,000 | Water soluble above pH 5.0 | High | 122 | NA | 29.10 |
| 6 | Soluplus® | Amorphous | 118,000 | Amphiphilic | Moderate | 70 | 250 | 19.40 |
NA, not available
aReference (68). bHansen solubility parameter reported from (124). cDSC data for samples heated from 0 to 230°C at a heating rate of 10°C/min (43), part of the table has been reproduced from reference (88, 172, 173), the molecular weights used in the table are average molecular weights
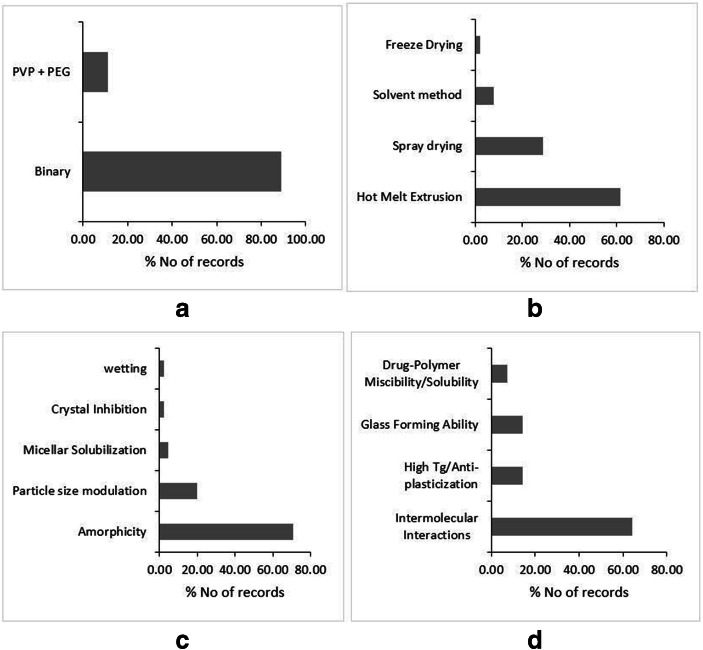
Owing to the solubility in volatile solvents like alcohol, PVP is a suitable candidate for the preparation of solid dispersion through the solvent method. The solvent method can be scaled-up by using spray drying. Therefore, a large number of studies attempted to formulate PVP-based dispersions through solvent and spray drying methods (Fig. 1a, b). The Tg of PVP is proportional to its molecular weight (Table II). Most of the high molecular weight PVP has usually high glass transition temperature (Tg), one of the highest within the category, which makes it unsuitable for the melt quench method, particularly for low melting point and thermo-labile drugs. Nevertheless, many reports claim the use of the hot melt extrusion (HME) method for preparation (Fig. 1b). Wherein, the majority of these dispersions belong to the ternary category with additional plasticizer employed to formulate the dispersion (Fig. 1a). Furthermore, the rate of dissolution of PVP-based solid dispersion is found highly dependent on the molecular weight of PVP employed to prepare the dispersions. An increase in the molecular weight correlated negatively with the rate of dissolution, as an increase in the molecular weight results in an increase in the viscosity and swelling of PVP within the solution phase (Table-1-supplementary material). This consequently decreases the diffusion of drug molecules from the surface boundaries of the viscous material into the bulk of the solution, leading to retarded dissolution (70). The optimum balance between dissolution rate and polymer grade has led to the prolific utility of PVP 30 K grade, among others, to prepare PVP-based dispersions. The drugs Sulfisoxazole (71), Sulfathiazole (72), Phenytoin (73), Chloramphenicol (74), and furosemide (75) are the examples where the decrease in molecular weight of PVP resulted in the improvement of dissolution rates. Also, the dissolution rate and maintenance of supersaturation are attributed to crystal growth-inhibiting properties of PVP(76). Baghel et al. (2018) attributed the extended supersaturation to the reduced crystal growth rates of Dypyridamol and Cinnarazine in the presence of PVP K-30 (77). However, for hydrophilic carriers like PVP, the important dissolution improvement mechanism remains to be the release of molecularly dispersed high energy amorphous drug, which releases as hydrophilic carrier dissolves (Fig. 1c) (78). The reduced particle size is also reported as one of the mechanisms of solubility and dissolution improvement (Fig. 1c). The particle size reduction is achieved by techniques like spray drying, electrospinning, and supercritical aerosol solvent extraction techniques (79–81). However, seldom the reduction in the particle size is alone responsible for dissolution improvement, as it is the interplay between amorphicity and particle size reduction, which achieves the goal of a higher dissolution rate.
Fig. 1.
Literature trends of PVP-based dispersions by type of dispersion (a), preparation methods (b), solubility and dissolution mechanism (c), and stability mechanism (d). Source: Scopus® database
As the physical state of PVP is amorphous, it demonstrated to form single-phase glass solutions. For the physical stability of glass solutions, the primary goal is to prevent the phase separation and subsequent recrystallization of a drug within the polymeric matrix. In this regard, the phase solubility/miscibility of a drug in the polymeric carrier is considered as the vital factor in the physical stability of these systems, and evidently, the PVP-based dispersions are no exception for this (Fig. 1d). In addition, the intermolecular interactions are also responsible for physical stability, particularly carbonyl functionality of PVP usually forms hydrogen bonds with weakly basic drugs containing –NH2 and –OH functionalities. The physical stability of Telaprevir-PVP solid dispersion is attributed to the intermolecular hydrogen bonding N-H----O and O-H---O between PVP and Telaprevir (82). Furthermore, the abovementioned stability mechanisms are always supported by higher Tg of PVP (Fig. 1d). Even the compounds, which are fragile glasses with low Tg, also demonstrated to form stable dispersions with PVP due to its high Tg (83,84). Nevertheless, the relative hydrophilicity of PVP makes it a deliquescent compound. Therefore, PVP is amenable for moisture uptake, which may lead to amorphous-amorphous or crystal-amorphous phase separation. Kapourani et al., (2019) found that Rivaroxaban solid dispersion with PVP underwent amorphous-amorphous de-mixing and subsequent API recrystallization after significant moisture uptake by PVP (85). Interestingly, Chen et al., (2018) reported that though there is a significant risk of moisture-induced amorphous phase separation in PVP-based dispersions, such phase separation does not have a profound impact on drug dissolution of slow-crystallizer compounds (86). Completely withstanding with the report, Milne et al., (2015) also showed that high relative humidity and an increase in the temperature did not result in the recrystallization of Zopiclone from PVP-based amorphous dispersion(87). The strengths, weaknesses, opportunities, and threats (SWOTs) analysis of PVP with regard to its implementation in solid dispersions are outlined in Fig. 5 in supplementary material.
COPOVIDONE
Copovidone is a co-polymer of vinyl-pyrrolidone and vinyl-acetate in a ratio of 6:4, which is also popularly known by trade names Kollidon VA64 (BASF, Germany) and Plasdone S-630 (Ashland, USA). This is amorphous, water-soluble polymer, which has traditionally been used as a binder and film-forming agent in the pharmaceutical industry (67). However, Abbott laboratories successfully repurposed the polymer to formulate the solid dispersion of Lopinavir-Ritonavir combination under the brand name Kaletra (88). Only after the success of Kaletra (after 2010) did the scientific community put the focus on the polymer as a candidate for excipient in solid dispersion technology (Fig. 2a). Copovidone has a significant edge over PVP from the processability perspective. First, owing to the relatively less hydrophilic vinyl acetate substituent, Copovidone is able to exhibit phase solubility with a wide range of APIs having varied polarity values. Second, the Copovidone has a glass transition temperature around 100°C, while degrading at a much higher temperature, i.e., 230°C (67,88,89). This wide temperature window presents an opportunity to employ the polymer to formulate solid dispersions of low as well as high melting temperature APIs alike using hot melt extrusion (HME) technology. Therefore, HME is the most widely used method followed by spray drying for preparing Copovidone-based solid dispersions (Fig. 2b).
Fig. 2.
Literature trends of Copovidone-based dispersions by type of dispersion (a), preparation methods (b), solubility and dissolution mechanism (c), and stability mechanism (d). Source: Scopus® database
Copovidone usually forms a single-phase glassy solutions with APIs. The amorphicity of API within solid dispersion is thought of as a major dissolution improvement mechanism, where the molecularly dispersed amorphous API dissolves along with the water-soluble polymer (Fig. 2c). Taylor et al., (2019) concluded that Copovidone accounts for the ideal release of Ledipasvir from the solid dispersion making the process polymer controlled. This generated the supersaturated state of Ledipasvir, even exceeding the amorphous solubility of the drug leading to the formation of a colloidal drug rich phase. Such drug rich phase acts as a reservoir which replenishes the absorbed drug fraction leading to the sustained supersaturation (90). Furthermore, Copovidone-based dispersions are also found to generate nanoparticles during dissolution, contributing to improved solubility and permeability. Harmon et al., (2016) demonstrated that Anacetrapib forms nanoparticles during the rapid dissolution of amorphous domains of solid dispersions driven by Copovidone. However, the study employed TPGS as a surfactant to prevent rapid, local drug domain aggregation events (91). Recently, Moseson et al., (2020) reported that Copovidone has strong crystal nucleation and growth inhibition properties through polymer adsorption onto the drug crystals. Such mechanism demonstrated to prevent the de-supersaturation of the drug and translated into the sustained parachute in the dissolution profile(92).
As revealed by the literature, intermolecular interactions are reported as a major stabilization mechanism for Copovidone-based solid dispersion (Fig. 2d). Among all types of intermolecular interactions, hydrogen bonding is deduced as a type of supramolecular bond responsible for the physical stability of Copovidone. The carbonyl functionality of both pyrrolidone and vinyl acetate blocks was found promiscuous to form hydrogen bonding. Nevertheless, the recent report claimed that Copovidone tends to form intermolecular halogen bonding. The halogen-containing APIs, namely Clotrimazole, Loratidine, Brotrimazole, and Me-DIBF, were found to form halogen bonds with the amide carbonyl of the vinyl pyrrolidone moiety in Copovidone-based solid dispersions (93). Furthermore, Copovidone has a reasonably high glass transition temperature (Table II). Thus, if it forms a single-phase glass solution with APIs, which is usually the case, then the overall Tg-50°C temperature is expected to have a value higher than the room temperature. The APIs exhibiting low glass transition temperatures like Clotrimazole (Tg 30°C) (94) and Nifedipine (Tg 45°C) (95) are reported to be stable in Copovidone-based solid dispersions. Copovidone is relatively less hygroscopic than PVP. This aids in the storage stability of Copovidone-based dispersions. Sotthivirat et al., (2013) analyzed the stability of MK-0364 in Copovidone- and PVP-based solid dispersions under varied temperature and humidity conditions. Unlike PVP-based solid dispersions, no degradation was observed in Copovidone-based solid dispersion. Furthermore, under stress conditions, relatively less crystallinity was observed in MK-0364-Copovidone dispersions (96). However, Copovidone has more moisture uptake potential than Soluplus® and HPMCAS. Kapourani et al., (2019) investigated polymeric carrier-based solid dispersions of Rivaroxaban under elevated relative humidity conditions, and found that Copovidone-based dispersion resulted in less physically stable dispersion than its HPMCAS and Soluplus®-based counterparts (85). The SWOT analysis of Copovidone with regard to its implementation in solid dispersions is outlined in Fig. 6-supplementary material.
POLY ETHYLENE GLYCOL (PEG)
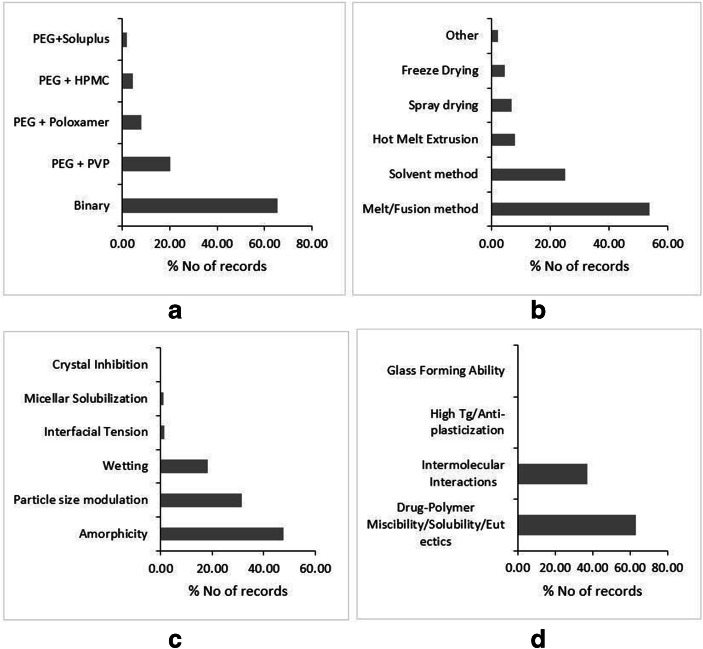
PEG is the polymer of ethylene oxide having a molecular weight in the range from 200 to 300,000 g/mol. The physical state of PEG is dependent on its molecular weight. PEG having a molecular weight below 600 are viscous liquids at room temperature, while the polymer with molecular weights up to 8000 and 20,000 are waxy and dry solids, respectively (16,40,47). PEG is a semi-crystalline polymer with both crystalline and amorphous domains within its structure (97). It has a low melting range from 55 to 68°C and good solubility in water, as well as many volatile organic solvents like methanol, ethanol, and chloroform. The excellent solubility of PEG in volatile solvents makes it a suitable candidate to fabricate solid dispersions by the solvent method (Fig. 3b). The low melting range is advantageous in preparing the solid dispersions of low melting drugs by melt (fusion method). Furthermore, such a low melting characteristic of PEG is uniquely leveraged by certain studies to formulate solid dispersions through combination of solvent-melt method, wherein the drug solution in volatile solvents like methanol/chloroform is added into the molten mass of PEG under constant stirring. The method claimed to stabilize drugs in the microcrystalline state within the PEG matrix (98–100). The cursory look of the published reports on PEG-based polymers reveals that comparatively very few studies have followed the scaled-up approaches for the Solvent method and melt method (Fig. 3b). This may be attributed to the trend in the recent past that the utility of semi-crystalline polymers like PEG to prepare commercial binary solid dispersions is outweighed by the amorphous carriers like PVP, Soluplus®, and advantages thereof. Therefore, crystalline polymer like PEG is largely used in small-experimental-scale mechanistic studies or re-purposed as a plasticizer in conjugation with other polymers like PVP in ternary dispersions or to prepare polymeric nanoparticles (Fig. 3a). Historically, it was hypothesized that the large molecular weight crystalline lamellar polymer like PEG traps small molecular weight drugs within crystalline interstitial spaces of the polymer. Thus, these solid dispersions were considered as interstitial solid solutions (14). However, later it was demonstrated that the molecularly dispersed drug resides in the amorphous domains of PEG(30). Further, PEG also demonstrated to form eutectic mixtures with variety of drugs (Fig. 3d).
Fig. 3.
Literature trends of PEG-based dispersions by type of dispersion (a), preparation methods (b), solubility and dissolution mechanism (c), and stability mechanism (d). Source: Scopus® database
Unlike PVP-based dispersions, PEG-based dispersions do not exhibit any consistent correlation between dissolution rate and molecular weight of PEG (47,101). There exists a negative correlation for Tolbutamide (102), Indomethacin (103), Phenylbutazone (103), and Griseofulvin (98), while Furosemide (104) and Papaverine (105) dissolution rates correlate positively with the molecular weight of PEG. However, as the formation of PEG-based solid solutions and eutectics are dictated by drug-polymer composition, dissolution and stability are highly dependent on the drug-polymer ratio. In these cases, the rate of dissolution of polymer dictates the dissolution rate of the drug. Therefore, the dissolution rate increases as a function of weight fraction of polymer within the dispersion. Multitude of drugs like Felodipine (106), Lorazepam (107), and Prednisone (108) demonstrated the increasing dissolution rate with increase in the polymer weight fraction. However, this is not a thumb rule, and the drugs with high glass-forming ability show exactly the opposite trend, where increase in the drug weight fraction leads to the improvement in the dissolution rate. Duong et al., (2015) contended that higher drug loading of Indomethacin, a good glass-forming agent, increases the amorphicity of the polymer and inhibiting the crystallization of PEG, leading to the higher dissolution rates (109). Furthermore, the molecularly dispersed drug in microcrystalline or nano-crystalline form within the polymeric matrix also reported as a chief dissolution improvement mechanism for PEG-based dispersions (Fig. 3c).
As the crystalline polymer like PEG is believed to form solid solutions and eutectics, understandably, the phase-solubility/miscibility is a pre-requisite to form thermodynamically stable systems (Fig. 3d). Due to the large difference in the molecular weight of a drug and the polymer, usually, drugs have limited solubility within the polymer, and hence, such systems can best be categorized as monotectics (110). Furthermore, the physical stability of PEG-based dispersions is dictated by the physical state of the drug and polymer in the dispersion, i.e., either PEG/drug is present in crystalline or amorphous form. If a drug and polymer are both present in the crystalline phase, as the case in eutectics or monotectics, then such systems have high physical stability at room temperature (Table I). However, if either one or both the components of binary dispersion are in the amorphous form, then thermodynamic factors such as phase-solubility/separation and subsequent recrystallization of the amorphous phase drive the physical stability of these solid dispersions. The first scenario, where a drug is molecularly dispersed in the amorphous domains of PEG, wherein the recrystallization of a drug is usually observed after storing the dispersion at room temperature. The scenario is described for weak glasses like paracetamol (111), Telmisartan (112), and Celecoxib (113). The second scenario, where the polymer is in amorphous form, herein, it is established that PEG has the potential to vitrify after fusion and subsequent cooling. Dordunoo et al., (1997) demonstrated that a significant fraction of PEG stabilized in the amorphous state post-fusion and cooling, albeit the amorphous form reverted to recrystallized state gradually upon storage(114). The same phenomenon was observed for Haloperidol-PEG and Fenofibrate-PEG dispersions, where PEG recrystallized rapidly upon storage (115). Nevertheless, if a drug has a good glass-forming ability, then in such cases, the recrystallization of PEG was found arrested by drugs rendering the dispersions physically stable (Fig. 3d). PEG is relatively less hygroscopic than PVP. However, it is comparable or even more hygroscopic than Soluplus®. The presence of ethoxy and hydroxyl groups in the structure of PEG contributes to the absorption of moisture and forms hydrogen bonds with water molecules (116). Thijs et al., (2007) reported that albeit the crystalline nature of PEG may restrict the water uptake, nevertheless, once the hydration shell is formed during the prolonged exposure to higher relative humidity, then the moisture uptake enhances exponentially (117). Baird et al., (2010) demonstrated that the deliquescent nature of PEG depends on the molecular weight of the polymer, temperature, and humidity. The moisture sorption ability of the PEG negatively correlates with its molecular weight due to the hydrophobicity of the polymer by an increase in the chain length. It positively correlates with the temperature (116). The behavior of PEG at higher humidity levels depends on its molecular weight, which might lead to recrystallization, cake formation, or formation of viscous liquid (116). Bley et al., (2010) observed the dissolution performance of Nifedipine/PEG1500 solid dispersion system was decreased and phase-separated after 6 months of storage. It can be inferred that due to the hygroscopic nature of PEG, there is a high chance of phase separation, and it may affect the dissolution performance as well (118). PEG is being frequently used as hydrogels in preparation of pharmaceutical formulations over a decade. Albeit the popularity, PEG cannot form hydrogels by just adding water. Inevitably, PEGs require crosslinking agents for the formation of networks chemically such as acrylate, methacrylate, vinyl sulfone, maleimide, vinyl ether, and allyl ether. Other techniques involve subjecting PEG to UV radiation or photoinitiators (119,120). PEG hydrogels can be modified according to the need of the formulator. Thus, when crosslinked, PEG can withhold the drug release leading to the controlled release of the formulation. On the contrary, non-linked PEG could burst release suggesting its use in immediate release dosage forms. On the other hand, other polymers mentioned in this review apart from Copovidone are reported to absorb the water and form crosslinking networks resulting in hydrogel formation. This ability of the lot can result in limiting the drug release (41,75,121,122). The possible reason for less physical stability can be attributed to the extremely low glass transition temperature (Tg) of PEG and plasticizer potential thereof. Due to the plasticizing nature of PEG, the final dispersion usually tends to be waxy. To put it in a more technical term, this waxy form is oftentimes found to be supercooled liquid, which has α-relaxations (global mobility) responsible for recrystallization. The SWOT analysis of PEG with regard to its implementation in solid dispersions is outlined in Fig. 7-supplementary material.
Notwithstanding the continued active research interest since 1961, only one commercial product of PEG-based solid dispersions has been marketed so far viz. Gris-PEG. The commercial utility of PEG-based solid dispersion is limited by several issues, which include the poor glass-forming ability of the polymer, large variability in physicochemical properties as a function of change in the process parameters, stability issues of polymer and drug, and scale-up and manufacturing challenges (40). The physical state of PEG is crystalline, and it is a poor glass former, which leads to the crystallization of the polymer in addition to the recrystallization of the drug during storage. Furthermore, for fusion methods, the crystallinity of the prepared dispersions is found dependent upon the rate of cooling during processing. Save et al. found that the rapid cooling of PEG-4000/6000-Nifedipine melts resulted in the formation of metastable amorphous form, while slow cooling of melts yielded crystalline drug within the dispersion (123). In another study, the same pattern was observed, where slow-cooled Tolbutamide-PEG 6000 solid dispersion exhibited a greater degree of crystallinity as compared to flash-cooled samples. Change in the fusion temperature during the processing of PEG-based solid dispersion was also found to manifest in a variable dissolution rate (124). Such variable physicochemical properties, along with stability issues, were demonstrated to pose a significant challenge during the scale-up of the laboratory processing methods. Furthermore, the plasticizing potential compounded with the poor glass-forming ability of PEG also proves troublesome during formulation development. In particular, the development of tablet dosage form employing a wet granulation step could induce the recrystallization of PEG or drug (125). The low glass transition temperature of PEG leads to the soft and waxy dispersion at room temperature. This translates into the slow dissolution of the dosage form, also complicating the manufacturing of solid dispersion, in particular, compression operations(40).
CELLULOSE DERIVATIVES
Cellulose derivatives are commonly used polymers in the stabilization of amorphous solid dispersions. Its popularity is because of high molecular weight, inability to get absorbed from the GI tract, strong interaction with the drug molecule, and high Tg. Cellulose is a polysaccharide that consists of linear chains of 1-4-linked β-D-anhydroglucopyranose units in variable length. Since the native form of cellulose is weakly water-soluble because of 40–60% of crystallinity and strong inter- and intramolecular hydrogen bonding between the individual chains in cellulose, chemical modification is carried out via etherification or esterification of hydroxyl groups. Ether and ester derivative are water-soluble forms of cellulose. In ether derivatives of cellulose, alkyl/mixed alkyl groups are added in place of hydrogen atoms of hydroxyl groups of repetitive anhydroglucose units. Methylcellulose (MC), ethylcellulose (EC), hydroxypropylcellulose (HPC), hydroxyethyl cellulose (HEC), and hydroxypropyl methylcellulose (HPMC) are commonly used ether derivatives of cellulose. Esterification of cellulose alkyl ethers involves the reaction of ω-carboxyalkanoyl groups with monobenzyl adipoyl, suberoyl, and sebacoyl chlorides, and subsequent benzyl ester hydrogenolysis, to avoid cross-linking. Cellulose derivatives are also used as bonding, coating, stabilizing and film-forming agents, plastic sheets, and emulsion stabilizers in formulations. Cellulose derivatives can be classified based on their water solubility, chemical substituents, and pH responsiveness (Fig. 8-supplementary material). Owing to the availability of multiple cellulosic polymers and extensive scientific records thereof, the discussion under this section will be restricted to the prominently used cellulosic polymer in solid dispersion technology, i.e., HPMC & HPMCAS. The interested readers are advised to follow the rigorous review of the cellulosic polymers (41).
HYDROXY PROPYL METHYL CELLULOSE (HPMC)
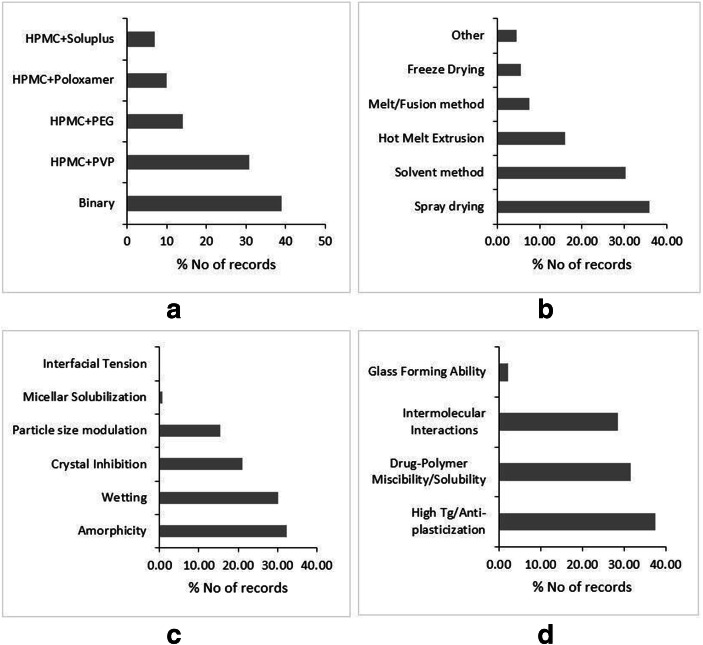
HPMC belongs to the broad category of cellulose-based polymers. Cellulose is ubiquitous in structural components of plants and has extensively been used for various applications as a raw material for over 150 years. The important physicochemical and thermodynamic properties of different grades of HPMC are compiled in Table 2-supplementary material (43,126,127). HPMC is a non-ionic, hydrophilic polymer, which is soluble in water, and most of the organic solvents, including methanol, ethanol, propanol, and dichloromethane. The solubility in volatile organic solvents makes it amenable for formulating solid dispersions through solvent evaporation and its automated scaled-up iteration, i.e., spray drying. In contrast, the aqueous solubility helps to utilize the freeze-drying technique (Fig. 4a, b). Though pure cellulose is semi-crystalline (50–60% crystallinity), HPMC is present in an amorphous state exhibiting a reasonably high glass transition temperature (Tg) of 180°C (Table II) (43). Therefore, for HPMC-based dispersions, fusion methods, including Hot Melt Extrusion, are not methods of choice, certainly not for the low melting APIs. Nonetheless, the fusion methods have been utilized extensively in ternary dispersion, wherein HPMC is being used for its role as a crystal nucleation inhibitor and anti-plasticizer (Fig. 4a).
Fig. 4.
Literature trends of HPMC-based dispersions by type of dispersion (a), preparation methods (b), solubility and dissolution mechanism (c), and stability mechanism (d). Source: Scopus® database
For HPMC-based dispersions, the predominant mechanism for improved dissolution rate and sustained supersaturation is believed to be the crystallization inhibition potential of HPMC (Fig. 4c). In this regard, HPMC is a more potent precipitation inhibitor than PVP (128). HPMC has proven useful to maintain the supersaturation of weakly basic drugs like Felodipine (129), Itraconazole (130), and Celecoxib(131), which are amenable for precipitation in the intestinal part of the GI tract. Before the disordered API undergoes crystallization in the solution phase, the small nuclei of the crystal build-up in the supersaturated solution, the thermodynamic phenomenon called crystal nucleation. Consequently, it is followed by the kinetic occurrence of the growth of crystals around the crystal nuclei (132). It has been demonstrated by multiple studies that HPMC has a pronounced effect on inhibiting the thermodynamics of nucleation by restricting the mobility of the disordered phase. Xie et al., (2016) investigated and found that the inhibitory potential of HPMC on precipitation and solution nucleation of Celecoxib. Nevertheless, the study cautioned that in the presence of seed crystals emanating from the undissolved crystalline drug, it is difficult to prevent crystal growth, particularly at high supersaturation levels (131). It is believed that the intermolecular interactions between API and polymer aid to retard the conversion of the disordered phase into a more ordered crystal nucleus, thus reducing the nucleation rate (Fig. 4c). The hydrogen-bonding interactions between Felodipine and HPMC reduced the nucleation rate by increasing the kinetic barrier to nucleation (129). Chavan et al., (2018) also found the additional contribution of hydrogen bonding between API and HPMC towards preventing the crystal nucleation and growth in supersaturated solutions (76). However, the role of high energy amorphous form stabilized within the HPMC matrix in generating the supersaturated solutions cannot be overemphasized. Okada et al., (2020) observed that upon dissolution of Nifedipine/HPMC solid dispersion, the Nifedipine undergoes phase separation and dissolves independent of HPMC from amorphous Nifedipine rich phase (133). Furthermore, the unique property of glassy HPMC to swell in contact with water is exploited to extend the release of certain APIs through solid dispersion technology. Puncochova et al., (2015) demonstrated that dry glassy HPMC transforms into the wet rubbery state after influx of water. This creates a gel layer, and diffusion of drugs through the gel layer is considered as the rate-limiting step during the dissolution of solid dispersion(121).
The numerous enabling properties regarding the physical stability make HPMC a polymer of choice for commercial manufacturing of solid dispersions, as evident by the fact that more than 50% of the marketed solid dispersion products are HPMC based (41). The vast majority of published literature reported that APIs are generally dispersed molecularly in the amorphous state within the HPMC matrix leading to the formation of glass solutions (Fig. 4c). The HPMC-based glass solutions are rendered physically stable at room temperature due to the high glass transition temperature of HPMC (Fig. 4c). As per the Tg-50 K rule, HPMC prevents the β relaxations from undercooled melts preventing the slow de-vitrification process at room temperature (134), even for fragile glasses with low Tg like Nimodipine (135). Boghra et al., (2011) showed that HPMC slowed down the de-vitrification of Irbesartan due to its anti-plasticization potential (136). However, to make use of the anti-plasticization property of HPMC, the drug-polymer solubility/miscibility is the pre-requisite, which forms a single-phase amorphous system (12,137). As HPMC has both hydrophilic (hydroxy) and lipophilic (ether) groups within the structure, it exhibits excellent drug-polymer miscibility/solubility with a wide variety of APIs (41). Tian et al., (2016) implemented the fluorescent-based method to investigate the indomethacin-HPMC miscibility. The study reported that the miscibility is highly dependent on relative fraction of a drug within the dispersion. They observed that the dispersion samples with less than 50% drug loading were maintained in amorphous form, while the samples with drug loading higher than 50% crystallized within 15 days (138). The drug-polymer miscibility is also found to be dependent on the preparation technique employed to fabricate solid dispersion. The spray-dried solid dispersions of ABT-102 (model drug)/HPMC exhibited strong drug-polymer miscibility with negative Gibb’s free energy manifesting into the greater extent of melting point depression than solvent evaporated dispersions (139). Furthermore, the intermolecular interactions between drug and polymer also contribute towards the stability of the amorphous phase (Fig. 4d), albeit the contribution of such interactions is relatively lesser than PVP. Felodipine formed intermolecular hydrogen bonds with HPMC, thereby increasing the kinetic barrier to the nucleation rate throughout the storage period, thus preventing the phase separation (129). The physical stability of Resveratrol in HPMC-based solid dispersion was found dependent upon the type and strength of intermolecular interactions between drug and polymer (140). Interestingly, a sizable number of studies contends that the presence of HPMC disturbs the cohesive intramolecular interactions within drug molecules, consequently preventing the crystallization of API. Hormann et al., (2018) attributed the stability of Nimodipine/HPMC dispersion to disruption of intramolecular hydrogen bonding within the drug molecules (135). Conversely, the strong intramolecular hydrogen bonding within Curcumin molecules impeded the crystallization inhibition potential of HPMC, preventing the molecular level interaction between the polymer and API (141). Therefore, the contribution of intramolecular hydrogen bonding in prevention of recrystallization within HPMC-based solid dispersion is contended in the literature and it warrants further research on the topic. The SWOTs analysis of HPMC with regard to its implementation in solid dispersions is outlined in Fig. 9-supplementary material.
HYDROXY PROPYL METHYL CELLULOSE ACETATE SUCCINATE (HPMCAS)
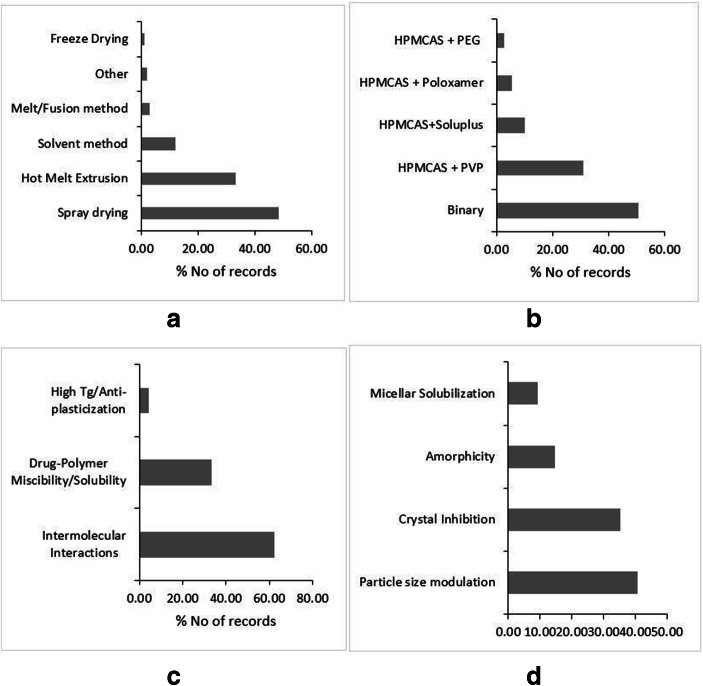
HPMCAS is a cellulose succinate mixed ester, which is an amorphous amphiphilic derivative of cellulose. Depending upon the acetyl and succinoyl content, HPMCAS is available in three grades, namely L, M, and H (Table 3-supplementary material) (88). Owing to the presence of a succinate group within the structure, HPMCAS exhibits ionization potential. The succinate group within HPMCAS structure has a pKa value 5.0; therefore, the polymer is predominantly unionized below pH 4.0 and ionized above pH 6.0. This ionization behavior manifests in the pH-dependent solubility of the polymer (41,142). HPMCAS is amphiphilic, stable at high temperature, and soluble in organic solvents. These properties put together make spray drying a preferred choice to manufacture HMPCAS-based solid dispersions (Fig. 5a). Further, the large temperature window between the glass transition (120°C) and degradation temperature (270°C) (270–120 = 150°C) is also plentifully explored to prepare solid dispersions by hot melt extrusion (HME) technology (Table II, Fig. 5a) for both low as well as high melting APIs alike (88).
Fig. 5.
Literature trends of HPMCAS-based dispersions by preparation methods (a), type of dispersion (b), stability mechanism (c), and solubility and dissolution mechanism (d). Source: Scopus® database
As mentioned earlier, HPMCAS predominantly remains in the ionized form in the intestinal pH conditions (5.0 to 7.4). However, the presence of lipophilic methoxy and acetate substituents limits the solubility of the polymer at pH values above 5.0. Hence, once the solubility limit is exceeded, HPMCAS forms the colloidal phase of Nano-dimension in the intestinal pH conditions, and the negatively charged succinate group keeps the in situ nanostructure stable (143). Such nanostructures are found to dissolve rapidly, leading to a faster dissolution rate (Fig. 5d). Much like HPMC, HPMCAS also exhibits recrystallization inhibition properties. Such recrystallization inhibition is believed to propagate through drug-polymer intermolecular interactions and surface adsorption of the polymer on an API crystal seed (Fig. 5c). The drug-polymer interactions in the solution phase are found responsible for recrystallization inhibition for Nimodipine (144), Carbamazepine, and Phenytoin (145), thereby enhancing the solubility and dissolution rate. Albeit, Ueda et al., (2014) put forth an alternate narrative about crystallization inhibition potential of HPMCAS. The group demonstrated that HPMCAS suppresses crystallization of Carbamazepine, Nifedipine, Mefenamic acid, and Dexamethasone through molecular level hydrophobic interactions between the drug and polymer(146). Kapourani et al., (2019) demonstrated that during crystal growth of Rivaroxaban, small API crystals adhere to the polymer surface due to hydrogen bonding interactions, consequently inhibiting the crystal growth and translating into the improved dissolution (85).
HPMCAS has a higher propensity to form drug-polymer interactions than HPMC. Such intermolecular interactions are responsible for preventing the phase separation in the solid-state, which renders the solid dispersion physically stable (Fig. 5c). Ueda et al., (2020) evaluated the intermolecular interactions between Carbamazepine and HPMCAS by employing 1D -1H NMR spectroscopy and found that the mobility of Carbamazepine was strongly suppressed in the presence of HPMCAS due to intermolecular interactions (39). Huang et al., (2017) applied coarse grain simulation approach to study the interactions between Phenytoin and HPMCAS and demonstrated that the drug and polymer form a complex due to strong intermolecular interactions. Interestingly though, the protonated polymer chains were found more effective than deprotonated ones in inhibiting the recrystallization of the drug (147). Like HMPC, HPMCAS also has a reasonably high Tg (Table II), which can aid in physical stability. Nevertheless, there are very few reports claiming high Tg of HPMCAS as the sole reason for stability (148). Part of the reason can be attributed to the fact that unlike HPMC, the promiscuous ability of HPMCAS to form intermolecular interactions always confound in many HPMCAS-based solid dispersions as a stability mechanism. Furthermore, the presence of lipophilic substituents over a hydrophilic cellulose backbone gives the wider solubility window to HPMCAS. Therefore, HPMCAS has an ability to be soluble in APIs with varied logP values, and such drug-polymer solubility/miscibility is reported as a physical stability mechanism for HPMCAS-based solid dispersions. The SWOT analysis of HPMCAS with regard to its implementation in solid dispersions is outlined in Fig. 10-supplementary material.
SOLUPLUS®
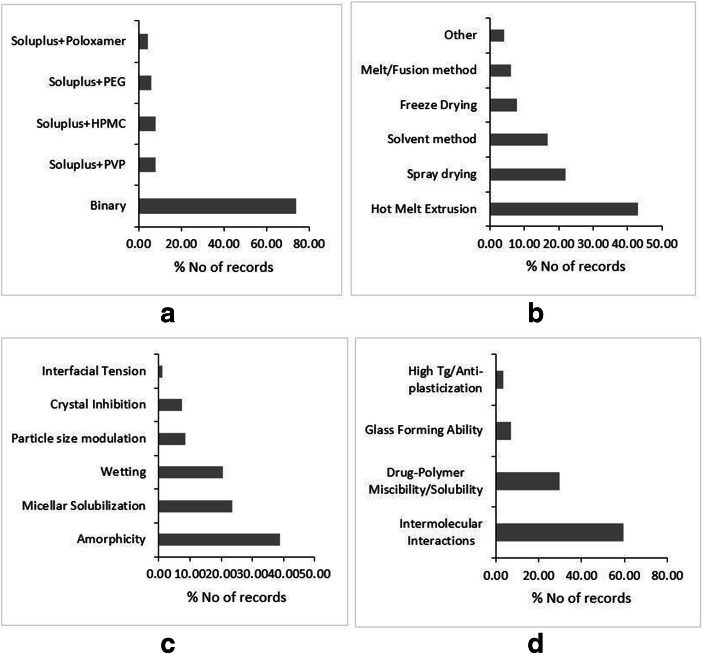
Soluplus® is a triblock graft copolymer consisting of polyethylene glycol (13% PEG 6000), polyvinyl caprolactam (57%), and polyvinyl acetate (30%). It is an amphiphilic polymer, wherein PEG provides hydrophilicity, while vinyl caprolactam and vinyl acetate domains are lipophilic within the polymer matrix. The molecular weight of Soluplus® usually ranges from 90,000 to 1,40,000 g/mol. It is an amorphous polymer with a relatively low glass transition temperature (Tg) 70°C (149,150). Soluplus® was conceptualized and purpose-developed as an excipient amenable for processing through the hot-melt extrusion process (151). Notwithstanding its plasticization potential, Soluplus® is not widely used in ternary solid dispersions in combination with high Tg polymers like PVP and HPMC (Fig. 6a). Due to the potential of Soluplus® to impart the stability to the glass solutions through mechanisms other than high Tg, it is employed in ASDs without any additional polymers. However, limited studies combined Soluplus® with PVP to make the dispersion pliant for hot-melt extrusion process at low temperature, especially for thermolabile APIs (Fig. 6b). On account of amphiphilic property, Soluplus® is soluble in aqueous as well as organic solvents alike. Solubility in volatile organic solvents makes it suitable candidate to formulate dispersions by solvent evaporation and spray drying. Freeze drying is less frequently employed to formulate Soluplus®-based dispersions even though Soluplus® exhibits good aqueous solubility (Fig. 6b).
Fig. 6.
Literature trends of Soluplus®-based dispersions by type of dispersion (a), preparation methods (b), solubility and dissolution mechanism (c), and stability mechanism (d). Source: Scopus® database
Soluplus® is a good glass former, and it forms stable glass solutions with a variety of APIs. For Soluplus®-based dispersions, API is generally dispersed molecularly within the polymer matrix (152). Therefore, upon exposure to the solvent or dissolution media, the API forms a supersaturated solution as it dissolves along with the polymer (Fig. 6c) (149). Owing to the high energy of amorphous API in a supersaturated solution, there exists a thermodynamic driving force working upon API molecules, making them undergo nucleation and crystal growth (132). Therefore, the objective of Soluplus®-based solid dispersions is to maintain the supersaturation. This can be achieved by various mechanisms. Prominent among them is the formation of intermolecular interactions between a drug and polymer (Fig. 6c). Song et al., (2019) demonstrated that hydrogen bonding interactions between Andrographolide and Soluplus® lead to enhanced interface wetting, consequently leading to improved dissolution rate (153). Griseofulvin also showed remarkable supersaturation from Soluplus®-based dispersion due to inhibition of API recrystallization through stronger intermolecular interactions (154). There are very few reports outlining the crystallization or precipitation inhibition properties of Soluplus® (Fig. 6c). Guan et al., (2019) reported that Soluplus® synergistically inhibited crystal nucleation and growth of Lacidipine, leading to the prolonged supersaturation (155). Soluplus® also exhibits swelling property, which may offset the dissolution rate due to limited diffusion through the swelled polymer. Slamova et al., (2020) reported that Tadalafil release from Soluplus® dispersion retarded due to swelling of the polymer during dissolution (122). Nevertheless, the swelling property of Soluplus® can be leveraged to formulate delayed release solid dispersions. Furthermore, owing to its amphiphilic property, Soluplus® forms micelles having a hydrophobic core. Such micelles are capable of a solubilizing variety of solutes, thereby contributing to the improved extent and rate of dissolution (Fig. 6c). More often, the micelles formed with Soluplus® are of nano dimensions, where the average particle size could be sub 100 nm. Micellar solubilization synergistically coupled with reduced particle size contributes to the improved dissolution rate of Soluplus®-based dispersions (150). Zeng et al., (2017) demonstrated that Scopoletin/Soluplus® dispersion formed drug entrapped Soluplus® micelles with an average size of 59.4 nm after dissolution (156). However, the solubility and dissolution advantage gained through micellar solubilization can offset in the presence of biorelevant media. It is found that the Soluplus® micelles interact with the bile and lecithin micelles in biorelevant media. This interaction leads to the formation of mixed micelles with increased particle size and altered polarity of the microenvironment of micelles. Pinto et al., (2020) found that the solubilization capacity of Candesartan Cilexetil from Soluplus®-based dispersion reduced in biorelevant media (57). Furthermore, if a drug has significant food effect through entrapment into the bile and lecithin micelles of biorelevant media, then micellar solubilization advantage gained by Soluplus®-based dispersion usually diminish. Lakshman et al., (2020) found that the dissolution advantage of API from Dolutegravir/Soluplus® dispersion in USP phosphate buffer pH 6.8 was found bridged in biorelevant media. The bridging in the dissolution extent was due to the improvement of dissolution of pure Dolutegravir in biorelevant media which is attributed to micellar solubilization of Dolutegravir in the bile and lecithin micelles, and much less due to the retardation of dissolution from solid dispersion (56).
As Soluplus® is a purpose developed to have good extrudability, it shows relatively lower glass transition temperature (Tg). Therefore, seldom, high Tg is attributed to the physical stability of Soluplus®-based dispersions (Fig. 6d). One of the prominent stability mechanisms for Soluplus®-based dispersions is intermolecular interactions (Fig. 6d). The carbonyl functionality of vinyl caprolactam and vinyl acetate is mainly involved in intermolecular interactions, especially hydrogen bond interactions (139). However, structured intermolecular bonding between API and polymer can be proven complicated for the stabilization of the disordered phase as the disruption of these bonding patterns may manifest into the phase separation. Jog et al., (2016) demonstrated the physical stability of ABT-102/Soluplus® dispersion as a consequence of strong hydrogen bonding between –C=O function of Soluplus® and –N-H moiety of the drug(139). Singh et al., (2016) prepared Itraconazole/Soluplus® solid dispersion through hot-melt extrusion. The dispersion was found stabilized due to hydrogen bonding interactions between API and polymer. However, while attempting to formulate the tablets from prepared dispersion, Soluplus®-rich regions were formed during compression. It was indicated that the disruption of hydrogen bonding leads to phase separation (157). Oftentimes, the hydrogen bonding interactions are complemented by API-polymer solubility/miscibility with regard to the solid state. In fact, higher API-Soluplus® miscibility may be the result of stronger hydrogen bonding there between. The SWOTs analysis of Soluplus® with regard to its implementation in solid dispersions is outlined in Fig. 11-supplementary material.
MANUFACTURING ISSUES OF POLYMERS IN SOLID DISPERSION TECHNOLOGY
Solid dispersions are usually prepared by solvent, fusion, or solvent-fusion methods (14). At the manufacturing scale, spray drying constitutes a principal scaled-up version of solvent methods, while the space of fusion methods is unarguably assumed by hot melt extrusion (HME) technology. These techniques have been extensively employed to commercially manufacture various solid dispersion products (Table-4-supplementary material), owing, in part, to their scalability and continuous capabilities (158,159). However, these technologies also have their own set of challenges. Oftentimes, such challenges are dependent on the physicochemical properties of specific polymers, as much they are encountered due to the inherent complexity of the technology. This section discusses the polymer dependent challenges/issues of manufacturing technologies, i.e., spray drying and HME.
Spray drying technology offers many advantages with respect to the manufacturing process-ability of solid dispersion, which includes non-requirement of separate drying and deep-cooling steps, process-ability of thermo-labile compounds, control on the particle morphology, to mention a few (159,160). However, hygroscopic polymer like PVP may lead to amorphous-amorphous phase separation during or after processing. Paudel et al. reported the highest residual solvent content in Naproxen-PVP solid dispersion at lower inlet temperature and airflow, while phase separation was observed in response to increased inlet temperature and airflow rate (161). If not instantaneously during the processing, the relatively high residual solvent post-processing can consequently affect the storage stability of the dispersion. Felodipine-PVP solid dispersion was not stable upon exposure to 40°C/75%RH conditions for 8 weeks (162). The issue of residual solvents can be addressed by adding secondary drying unit operation, which employs vacuum dryer or convection tray dryers. Nevertheless, such an additional processing step can incur significant production costs and an increase in production time. It must be borne in mind while preparing PVP-based solid dispersions that certain drugs can get degraded in the presence of PVP solutions. Temazepam was found degrading in the presence of a PVP-K-30 solution in a concentration-dependent manner (163). The problems of high residual solvent content and subsequent instability are also observed in cellulose derivative-based solid dispersions prepared through spray drying. In particular, the inlet temperature has emerged as a critical factor for processing polymers, which have a high probability of hydrogen bond formation like HPMC, HPMCAS (159,160). The glass transition temperatures of HPMC-based solid dispersions were found to correlate directly with inlet temperature. Therefore, a small variation in inlet temperature can translate into the inconsistent Tg and subsequent stability of prepared dispersions through spray drying (161). Furthermore, the surface coverage of drug particles of HPMC-based solid dispersion prepared through spry drying was also found significantly higher than the rota-evaporated product. Such preferential accumulation of drug molecules on the particle surface can be detrimental for dissolution (164).
Though HPMCAS-based spray-dried solid dispersions have been projected as a platform technology by many pharmaceutical industries, it faces several challenges. The major challenge for the preparation of HPMCAS-based solid dispersion in the requirement of large volumes of organic solvents. It is difficult to find a common solvent for water-insoluble drugs and water-soluble HPMCAS (143,159). Curatolo et al. used 10 g of acetone to dissolve 133 mg of API and 67 mg of HPMCAS (165). This case in point is further elaborated by Solanki et al. that it would require 9480 l of acetone to manufacture a batch of solid dispersion containing 100 kg of the API (166). Multiple studies employed organic solvents like acetone, methanol, ethanol, or combination thereof in the drug-organic solvent ratio in the window of 1:40 to 1:75%w/v (162,167–169). There are two-fold implications of employing higher volumes of organic solvents. First, there is a possibility of a higher amount of residual organic solvent in the product, which could be toxic and thus not accepted by regulatory authorities. Second, employing such high volumes of organic solvents may pose an environmental hazard. Furthermore, it has been established in the literature that some HPMCAS-based solid dispersions prepared by spray drying were not truly single-phase molecularly dissolved dispersions. Connely et al. analyzed Incinek™, which is marketed HPMCAS-telaprevir solid dispersion prepared by spray drying and found that the DSC thermogram of the product showed multiple glass transition temperatures and a melting endotherm of the drug. This indicates that the product is a solid suspension containing multiple amorphous phases and a crystalline phase (170).
HME is a widely adopted technology by the pharmaceutical industry to manufacture solid dispersions (Table-4-supplementary material). Touted as a disruptive technology, it has the potential to make a paradigm shift in pharmaceutical research and manufacturing. The technology has various strengths like the ability to continuously manufacture, scalability, high throughput, customizable, and solvent-free nature (158). Regardless of the popularity, HME faces few issues with regard to chemical degradation and viscoelastic properties of the polymers employed for preparing solid dispersion (171). PVP is the choice of polymer to prepare solid dispersions through HME technology, in particular, due to relatively high glass transition temperature and availability of a wider window between glass transition and degradation temperature. Such anti-plasticization property of PVP requires higher processing temperatures to enable the manufacturing of solid dispersion through HME technology. This often proves deleterious for thermo-labile APIs, limiting the utility of HME technology. Furthermore, the extrusion operation is only possible with complex viscosity of neat polymer between 10,000 to 1000 Pas. Beyond 10,000 Pas complex viscosity limits the extrusion processing capabilities, which pushes the torque limitations of extruders. Lower molecular grades of PVP have a narrow processing window, while higher grades of PVP are too viscous to process without exceeding the torque limitations of the extruder. Polymers, in particular, PVP, HPMC, and HPMCAS exhibit relatively high glass transition temperature and a wider temperature window between glass transition and degradation temperature. However, these polymers cannot be extruded below the degradation temperature due to complex viscosity exceeding 10,000 Pas (171–173). The issue can be and have been remedied by using additional plasticizer, which provides a sufficiently wide processing window below the degradation temperature of the polymers.
CONCLUSION
In this manuscript, extensively used polymers in solid dispersions such as PVP, Copovidone, PEG, HPMC, HPMCAS, and Soluplus® are discussed thoroughly along with the literature trends. Furthermore, to help with the choice of polymer based on API properties such as melting point, intermolecular interactions, glass forming ability, hygroscopicity, and logP, we have put together a discernment table for the above discussed polymers (Table III). The solid dispersion technology has established itself as a novel way to achieve bioavailability improvement, which is evident by a great deal of publications in the subject area. While it has potential to become a platform technology, it is imperative to give the solid dispersion technology a meaning within the ambit of regulations. Especially in the light of the fact that recently USFDA has come up with a draft guideline for co-crystals, it is necessary to develop a regulatory framework which provides definition and complete classification along with necessarily recommended studies to characterize and evaluate solid dispersions.
Table III.
Discernment Table for Choosing Polymer Based on API Properties
| Drug property | Poly-vinyl pyrrolidone | Poly-ethylene glycol | Hydroxy propyl methyl cellulose | Soluplus® | Copovidone | HPMCAS |
|---|---|---|---|---|---|---|
| Melting point | ||||||
| High | Yes | No | Yes | Yes | Yes | No |
| Low | No | Yes | No | Yes | Yes | Yes |
| Intermolecular interactions | ||||||
| Molecular bonding | Yes | No | Yes | Yes | Yes | Yes |
| Glass formability | ||||||
| Rich | Yes | Yes | Yes | Yes | Yes | Yes |
| Poor | Yes | No | Yes | Yes | Yes | Yes |
| Hygroscopicity | ||||||
| High | No | No | No | Yes | Yes | Yes |
| Low | Yes | Yes | No | Yes | Yes | Yes |
| Log P (phase solubility/miscibility) | ||||||
| High | No | No | No | Yes | No | Yes |
| Low | Yes | Yes | Yes | Yes | Yes | Yes |
Electronic Supplementary Material
(PDF 1461 kb)
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal. Mr. VSK Anand, Mr. Y Dani Lakshman, and Ms. KS Navya Sree received Dr. TMA Pai Ph.D. Scholarship from Manipal Academy of Higher Education.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Di L, Fish PV, Mano T. Bridging solubility between drug discovery and development. Drug Discov Today. 2012;17(9–10):486–495. doi: 10.1016/j.drudis.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Di L, Kerns EH, Carter GT. Drug-like property concepts in pharmaceutical design. Curr Pharm Des. 2009;15(19):2184–2194. doi: 10.2174/138161209788682479. [DOI] [PubMed] [Google Scholar]
- 3.Wilczewska AZ, Niemirowicz K, Markiewicz KH, Car H. Nanoparticles as drug delivery systems. Pharmacol reports. 2012;64(5):1020–1037. doi: 10.1016/s1734-1140(12)70901-5. [DOI] [PubMed] [Google Scholar]
- 4.Almarsson Orn, Zaworotko MJ. Crystal engineering of the composition of pharmaceutical phases. Do pharmaceutical co-crystals represent a new path to improved medicines? Chem Commun. 2004;(17):1889–96. [DOI] [PubMed]
- 5.Vishweshwar P, McMahon JA, Bis JA, Zaworotko MJ. Pharmaceutical co-crystals. J Pharm Sci. 2006;95(3):499–516. doi: 10.1002/jps.20578. [DOI] [PubMed] [Google Scholar]
- 6.Loftsson T, Brewster ME. Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J Pharm Sci. 1996;85(10):1017–1025. doi: 10.1021/js950534b. [DOI] [PubMed] [Google Scholar]
- 7.Challa R, Ahuja A, Ali J, Khar RK. Cyclodextrins in drug delivery: an updated review. AAPS PharmSciTech. 2005;6(2):E329–E357. doi: 10.1208/pt060243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serajuddin ATM. Salt formation to improve drug solubility. Adv Drug Deliv Rev. 2007;59(7):603–616. doi: 10.1016/j.addr.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Augustijns P, Brewster ME. Supersaturating drug delivery systems: fast is not necessarily good enough. J Pharm Sci. 2012;101(1):7–9. doi: 10.1002/jps.22750. [DOI] [PubMed] [Google Scholar]
- 10.Brouwers J, Brewster ME, Augustijns P. Supersaturating drug delivery systems: the answer to solubility limited oral bioavailability? J Pharm Sci. 2009;98(8):2549–2572. doi: 10.1002/jps.21650. [DOI] [PubMed] [Google Scholar]
- 11.Baghel S, Cathcart H, O’Reilly NJ. Polymeric amorphous solid dispersions: a review of amorphization, crystallization, stabilization, solid-state characterization, and aqueous solubilization of biopharmaceutical classification system class II drugs. J Pharm Sci. 2016;105(9):2527–2544. doi: 10.1016/j.xphs.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Janssens S, Van den Mooter G. Physical chemistry of solid dispersions. J Pharm Pharmacol. 2009;61(12):1571–1586. doi: 10.1211/jpp/61.12.0001. [DOI] [PubMed] [Google Scholar]
- 13.Sekiguchi K, Obi N. Studies on absorption of eutectic mixture. I. A comparison of the behavior of eutectic mixture of sulfathiazole and that of ordinary sulfathiazole in man. Chem Pharm Bull. 1961;9(11):866–872. [Google Scholar]
- 14.Chiou WL, Riegelman S. Pharmaceutical applications of solid dispersion systems. J Pharm Sci. 1971;60(9):1281–1302. doi: 10.1002/jps.2600600902. [DOI] [PubMed] [Google Scholar]
- 15.Van Duong T, Van den Mooter G. The role of the carrier in the formulation of pharmaceutical solid dispersions. Part I: crystalline and semi-crystalline carriers. Expert Opin Drug Deliv. 2016;13(11):1583–1594. doi: 10.1080/17425247.2016.1198768. [DOI] [PubMed] [Google Scholar]
- 16.Van Duong T, Van den Mooter G. The role of the carrier in the formulation of pharmaceutical solid dispersions. Part II: amorphous carriers. Expert Opin Drug Deliv. 2016;13(12):1681–1694. doi: 10.1080/17425247.2016.1198769. [DOI] [PubMed] [Google Scholar]
- 17.Jadhav KR, Pacharane SS, Pednekar PP, Koshy PV, Kadam VJ. Approaches to stabilize amorphous form-a review. Curr Drug ther. 2012;7(4):255–262. [Google Scholar]
- 18.Guo Y, Shalaev E, Smith S. Physical stability of pharmaceutical formulations: solid-state characterization of amorphous dispersions. TrAC Trends Anal Chem. 2013;49:137–144. [Google Scholar]
- 19.Grohganz H, Priemel PA, L¶bmann K, Nielsen LH, Laitinen R, Mullertz A, et al. Refining stability and dissolution rate of amorphous drug formulations. Expert Opin Drug Deliv. 2014;11(6):977–989. doi: 10.1517/17425247.2014.911728. [DOI] [PubMed] [Google Scholar]
- 20.Dengale SJ, Grohganz H, Rades T, Löbmann K. Recent advances in co-amorphous drug formulations. Adv Drug Deliv Rev. 2016;100:116–25. [DOI] [PubMed]
- 21.Lin X, Hu Y, Liu L, Su L, Li N, Yu J, Tang B, Yang Z. Physical stability of amorphous solid dispersions: a physicochemical perspective with thermodynamic, kinetic and environmental aspects. Pharm Res. 2018;35(6):125. doi: 10.1007/s11095-018-2408-3. [DOI] [PubMed] [Google Scholar]
- 22.Vasconcelos T, Sarmento B, Costa P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov Today. 2007;12(23–24):1068–1075. doi: 10.1016/j.drudis.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Meng F, Gala U, Chauhan H. Classification of solid dispersions: correlation to (i) stability and solubility (ii) preparation and characterization techniques. Drug Dev Ind Pharm. 2015;41(9):1401–1415. doi: 10.3109/03639045.2015.1018274. [DOI] [PubMed] [Google Scholar]
- 24.Alam MA, Ali R, Al-Jenoobi FI, Al-Mohizea AM. Solid dispersions: a strategy for poorly aqueous soluble drugs and technology updates. Expert Opin Drug Deliv. 2012;9(11):1419–1440. doi: 10.1517/17425247.2012.732064. [DOI] [PubMed] [Google Scholar]
- 25.Tran PHL, Duan W, Lee B-J, Tran TTD. Current designs of polymer blends in solid dispersions for improving drug bioavailability. Curr Drug Metab. 2018;19(13):1111–1118. doi: 10.2174/1389200219666180628171100. [DOI] [PubMed] [Google Scholar]
- 26.Chavan RB, Thipparaboina R, Kumar D, Shastri NR. Co amorphous systems: a product development perspective. Int J Pharm. 2016;515(1–2):403–415. doi: 10.1016/j.ijpharm.2016.10.043. [DOI] [PubMed] [Google Scholar]
- 27.Karagianni A, Kachrimanis K, Nikolakakis I. Co-amorphous solid dispersions for solubility and absorption improvement of drugs: composition, preparation, characterization and formulations for oral delivery. Pharmaceutics. 2018;10(3):98. doi: 10.3390/pharmaceutics10030098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tejwani RW, Joshi HN, Varia SA, Serajuddin ATM. Study of phase behavior of poly (ethylene glycol)-polysorbate 80 and poly (ethylene glycol)-polysorbate 80-water mixtures. J Pharm Sci. 2000;89(7):946–950. doi: 10.1002/1520-6017(200007)89:7<946::AID-JPS12>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 29.Findlay A. The phase rule and its applications. Green: Longmans; 1904. [Google Scholar]
- 30.Schachter DM, Xiong J, Tirol GC. Solid state NMR perspective of drug-polymer solid solutions: a model system based on poly (ethylene oxide) Int J Pharm. 2004;281(1–2):89–101. doi: 10.1016/j.ijpharm.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 31.Dreezen G, Ivanov DA, Nysten B, Groeninckx G. Nano-structured polymer blends: phase structure, crystallisation behaviour and semi-crystalline morphology of phase separated binary blends of poly (ethylene oxide) and poly (ether sulphone) Polymer (Guildf) 2000;41(4):1395–1407. [Google Scholar]
- 32.Qian F, Huang J, Hussain MA. Drug-polymer solubility and miscibility: stability consideration and practical challenges in amorphous solid dispersion development. J Pharm Sci. 2010;99(7):2941–2947. doi: 10.1002/jps.22074. [DOI] [PubMed] [Google Scholar]
- 33.HASEGAWA A, KAWAMURA RIE, NAKAGAWA H, SUGIMOTO I. Physical properties of solid dispersions of poorly water-soluble drugs with enteric coating agents. Chem Pharm Bull. 1985;33(8):3429–3435. doi: 10.1248/cpb.33.3429. [DOI] [PubMed] [Google Scholar]
- 34.Arca HC, Mosquera-Giraldo LI, Bi V, Xu D, Taylor LS, Edgar KJ. Pharmaceutical applications of cellulose ethers and cellulose ether esters. Biomacromolecules. 2018;19(7):2351–2376. doi: 10.1021/acs.biomac.8b00517. [DOI] [PubMed] [Google Scholar]
- 35.Li N, Taylor LS. Tailoring supersaturation from amorphous solid dispersions. J Control Release. 2018;279:114–125. doi: 10.1016/j.jconrel.2018.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schver GCRM, Nadvorny D, Lee PI. Evolution of supersaturation from amorphous solid dispersions in water-insoluble polymer carriers: effects of swelling capacity and interplay between partition and diffusion. Int J Pharm. 2020;119292. [DOI] [PubMed]
- 37.Frank DS, Matzger AJ. Effect of polymer hydrophobicity on the stability of amorphous solid dispersions and supersaturated solutions of a hydrophobic pharmaceutical. Mol Pharm. 2019;16(2):682–688. doi: 10.1021/acs.molpharmaceut.8b00972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raina SA, Van Eerdenbrugh B, Alonzo DE, Mo H, Zhang GGZ, Gao Y, et al. Trends in the precipitation and crystallization behavior of supersaturated aqueous solutions of poorly water-soluble drugs assessed using synchrotron radiation. J Pharm Sci. 2015;104(6):1981–1992. doi: 10.1002/jps.24423. [DOI] [PubMed] [Google Scholar]
- 39.Ueda K, Okada H, Zhao Z, Higashi K, Moribe K. Application of solid-state 13C relaxation time to prediction of the recrystallization inhibition strength of polymers on amorphous felodipine at low polymer loading. Int J Pharm. 2020;119300. [DOI] [PubMed]
- 40.Serajuddin A. Solid dispersion of poorly water soluble drugs: early promises, subsequent problems, and recent breakthroughs. J Pharm Sci. 1999;88(10):1058–1066. doi: 10.1021/js980403l. [DOI] [PubMed] [Google Scholar]
- 41.Chavan RB, Rathi S, Jyothi VGSS, Shastri NR. Cellulose based polymers in development of amorphous solid dispersions. Asian J Pharm Sci. 2019;14(3):248–264. doi: 10.1016/j.ajps.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bochmann ES, Neumann D, Gryczke A, Wagner KG. Micro-scale solubility assessments and prediction models for active pharmaceutical ingredients in polymeric matrices. Eur J Pharm Biopharm. 2019;141:111–120. doi: 10.1016/j.ejpb.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 43.Ford JL. Thermal analysis of hydroxypropylmethylcellulose and methylcellulose: powders, gels and matrix tablets. Int J Pharm. 1999;179(2):209–228. doi: 10.1016/s0378-5173(98)00339-1. [DOI] [PubMed] [Google Scholar]
- 44.Schenz TW. Glass transitions and product stability: an overview. Food Hydrocoll. 1995;9(4):307–315. [Google Scholar]
- 45.Knopp MM, Chourak N, Khan F, Wendelboe J, Langguth P, Rades T, Holm R. Effect of polymer type and drug dose on the in vitro and in vivo behavior of amorphous solid dispersions. Eur J Pharm Biopharm. 2016;105:106–114. doi: 10.1016/j.ejpb.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 46.Punčochova K, Vukosavljevic B, Hanus J, Beranek J, Windbergs M, Stepanek F. Non-invasive insight into the release mechanisms of a poorly soluble drug from amorphous solid dispersions by confocal Raman microscopy. Eur J Pharm Biopharm. 2016;101:119–125. doi: 10.1016/j.ejpb.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Owusu-Ababio G, Habib MJ. Polymers applied in solid dispersion technology. Clin Res Regul Aff. 1998;15(1):25–45. [Google Scholar]
- 48.Bhasin N. Current trends in solid dispersion: a review. J Drug Deliv Ther. 2014;4(3):80–86. [Google Scholar]
- 49.Linn M, Collnot E-M, Djuric D, Hempel K, Fabian E, Kolter K, Lehr CM. Soluplus® as an effective absorption enhancer of poorly soluble drugs in vitro and in vivo. Eur J Pharm Sci. 2012;45(3):336–343. doi: 10.1016/j.ejps.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 50.Dumortier G, Grossiord JL, Agnely F, Chaumeil JC. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm Res. 2006;23(12):2709–2728. doi: 10.1007/s11095-006-9104-4. [DOI] [PubMed] [Google Scholar]
- 51.Vo CL-N, Park C, Lee B-J. Current trends and future perspectives of solid dispersions containing poorly water-soluble drugs. Eur J Pharm Biopharm. 2013;85(3):799–813. doi: 10.1016/j.ejpb.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 52.Dengale SJ, Grohganz H, Rades T, Löbmann K. Recent advances in co-amorphous drug formulations. Adv Drug Deliv Rev. 2016;100:116–125. doi: 10.1016/j.addr.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 53.Ruff A, Fiolka T, Kostewicz ES. Prediction of ketoconazole absorption using an updated in vitro transfer model coupled to physiologically based pharmacokinetic modelling. Eur J Pharm Sci. 2017;100:42–55. doi: 10.1016/j.ejps.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 54.Chegireddy M, Hanegave GK, Lakshman D, Urazov A, Sree KN, Lewis SA, et al. The significance of utilizing in vitro transfer model and media selection to study the dissolution performance of weak ionizable bases: investigation using saquinavir as a model drug. AAPS PharmSciTech. 2020;21(2):1–14. doi: 10.1208/s12249-019-1563-0. [DOI] [PubMed] [Google Scholar]
- 55.Zhong Y, Jing G, Tian B, Huang H, Zhang Y, Gou J, et al. Supersaturation induced by Itraconazole/Soluplus® micelles provided high GI absorption in vivo. Asian J Pharm Sci. 2016;11(2):255–264. [Google Scholar]
- 56.Lakshman D, Chegireddy M, Hanegave GK, Sree KN, Kumar N, Lewis SA, Dengale SJ. Investigation of drug-polymer miscibility, biorelevant dissolution, and bioavailability improvement of Dolutegravir-polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol graft copolymer solid dispersions. Eur J Pharm Sci. 2020;142:105137. doi: 10.1016/j.ejps.2019.105137. [DOI] [PubMed] [Google Scholar]
- 57.Pinto JMO, Rengifo AFC, Mendes C, Leao AF, Parize AL, Stulzer HK. Understanding the interaction between Soluplus® and biorelevant media components. Colloids Surfaces B Biointerfaces. 2019;110673. [DOI] [PubMed]
- 58.Hancock BC, Zografi G. The relationship between the glass transition temperature and the water content of amorphous pharmaceutical solids. Pharm Res. 1994;11(4):471–477. doi: 10.1023/a:1018941810744. [DOI] [PubMed] [Google Scholar]
- 59.Nokhodchi ALI, Ford JL, Rowe PH, Rubinstein MH. The influence of moisture content on the consolidation properties of hydroxypropylmethylcellulose K4M (HPMC 2208) J Pharm Pharmacol. 1996;48(11):1116–1121. doi: 10.1111/j.2042-7158.1996.tb03905.x. [DOI] [PubMed] [Google Scholar]
- 60.Yu L. Amorphous pharmaceutical solids: preparation, characterization and stabilization. Adv Drug Deliv Rev. 2001;48(1):27–42. doi: 10.1016/s0169-409x(01)00098-9. [DOI] [PubMed] [Google Scholar]
- 61.Sheri L. Shamblin †, Xiaolin Tang †, Liuquan Chang †, Bruno C. Hancock ‡ and, Michael J. Pikal* †. Characterization of the time scales of molecular motion in pharmaceutically important glasses. 1999;103(20):4113–21.
- 62.Craig DQ, Royall PG, Kett VL, Hopton ML. The relevance of the amorphous state to pharmaceutical dosage forms: glassy drugs and freeze dried systems. Int J Pharm. 1999;179(2):179–207. doi: 10.1016/s0378-5173(98)00338-x. [DOI] [PubMed] [Google Scholar]
- 63.Marsac PJ, Li T, Taylor LS. Estimation of drug–polymer miscibility and solubility in amorphous solid dispersions using experimentally determined interaction parameters. Pharm Res. 2009;26(1):139–151. doi: 10.1007/s11095-008-9721-1. [DOI] [PubMed] [Google Scholar]
- 64.Vieweg R, Reiher M, Scheurlen H. Kunststoff-Handbuch XI. Carl-Hanser-Verlag Munchen. 1971:558–69.
- 65.Barabas ES, Adeyeye CM. Crospovidone. In: Analytical profiles of drug substances and excipients. Elsevier; 1996. p. 87–163.
- 66.Breitenbach JW. IUPAC Symposium Makromol. Ch Budapest. 1967:529–44.
- 67.Buhler V. Polyvinylpyrrolidone excipients for pharmaceuticals: povidone, crospovidone and copovidone. 1st edition. Berlin: Springer-Verlag Berlin Heidelberg; 2005.
- 68.Turner DT, Schwartz A. The glass transition temperature of poly (N-vinyl pyrrolidone) by differential scanning calorimetry. Polymer (Guildf). 1985;26(5):757–762. [Google Scholar]
- 69.Knopp MM, Olesen NE, Holm P, Langguth P, Holm R, Rades T. Influence of polymer molecular weight on drug-polymer solubility: a comparison between experimentally determined solubility in PVP and prediction derived from solubility in monomer. J Pharm Sci. 2015;104(9):2905–2912. doi: 10.1002/jps.24410. [DOI] [PubMed] [Google Scholar]
- 70.NOGAMI H, NAGAI T, KONDO A. Dissolution kinetics of polyvinylpyrrolidone of various molecular weights. Chem Pharm Bull. 1970;18(11):2290–2296. [Google Scholar]
- 71.SEKIKAWA H, NAKANO M, ARITA T. Inhibitory effect of polyvinylpyrrolidone on the crystallization of drugs. Chem Pharm Bull. 1978;26(1):118–126. [Google Scholar]
- 72.Simonelli AP, Mehta SC, Higuchi WI. Dissolution rates of high energy polyvinylpyrrolidone (PVP) sulfathiazole coprecipitates. J Pharm Sci. 1969;58(5):538–549. doi: 10.1002/jps.2600580503. [DOI] [PubMed] [Google Scholar]
- 73.Sekikawa H, Fujiwara, Naganurna T, Nakano M, Arita T. Dissolution behaviors and gastrointestinal absorption of phenytoin in phenytoin-polyvinylpyrrolidone coprecipitate. Chem Pharm Bull. 1978;26:3033–3039. doi: 10.1248/cpb.26.3033. [DOI] [PubMed] [Google Scholar]
- 74.Kassem AA, Zaki SA, Mursi NM, Tayel SA. Antimicrobial activity of chloramphenicol in solid dispersion systems. Die Pharmazie. 1979;34(1):43–4. [PubMed]
- 75.Nogami H, Nagai T, Kondo A. Dissolution kinetics of polyvinylpyrrolidone. Chem Pharm Bull. 1970;18(6):1185–1190. [Google Scholar]
- 76.Chavan RB, Lodagekar A, Shastri NR. Determination of precipitation inhibitory potential of polymers from amorphous solid dispersions. Drug Dev Ind Pharm. 2018;44(12):1933–1941. doi: 10.1080/03639045.2018.1503295. [DOI] [PubMed] [Google Scholar]
- 77.Baghel S, Cathcart H, O’Reilly NJ. Understanding the generation and maintenance of supersaturation during the dissolution of amorphous solid dispersions using modulated DSC and 1 H NMR. Int J Pharm. 2018;536(1):414–425. doi: 10.1016/j.ijpharm.2017.11.056. [DOI] [PubMed] [Google Scholar]
- 78.Doherty C, York P. Mechanisms of dissolution of frusemide/PVP solid dispersions. Int J Pharm. 1987;34(3):197–205. [Google Scholar]
- 79.Browne E, Charifou R, Worku ZA, Babu RP, Healy AM. Amorphous solid dispersions of ketoprofen and poly-vinyl polymers prepared via electrospraying and spray drying: A comparison of particle characteristics and performance. Int J Pharm. 2019;566:173–184. doi: 10.1016/j.ijpharm.2019.05.062. [DOI] [PubMed] [Google Scholar]
- 80.Yan T, Zhang Y, Ji M, Wang Z, Yan T. Preparation of irbesartan composite microparticles by supercritical aerosol solvent extraction system for dissolution enhancement. J Supercrit Fluids. 2019;153:104594. [Google Scholar]
- 81.Smeets A, Koekoekx R, Clasen C, Van den Mooter G. Amorphous solid dispersions of darunavir: comparison between spray drying and electrospraying. Eur J Pharm Biopharm. 2018;130:96–107. doi: 10.1016/j.ejpb.2018.06.021. [DOI] [PubMed] [Google Scholar]
- 82.Xiong X, Zhang M, Hou Q, Tang P, Suo Z, Zhu Y, Li H. Solid dispersions of telaprevir with improved solubility prepared by co-milling: formulation, physicochemical characterization, and cytotoxicity evaluation. Mater Sci Eng C. 2019;105:110012. doi: 10.1016/j.msec.2019.110012. [DOI] [PubMed] [Google Scholar]
- 83.Chokshi RJ, Shah NH, Sandhu HK, Malick AW, Zia H. Stabilization of low glass transition temperature indomethacin formulations: impact of polymer type and its concentration. J Pharm Sci. 2008;97(6):2286–2298. doi: 10.1002/jps.21174. [DOI] [PubMed] [Google Scholar]
- 84.Sakurai A, Sakai T, Sako K, Maitani Y. Polymer combination increased both physical stability and oral absorption of solid dispersions containing a low glass transition temperature drug: physicochemical characterization and in vivo study. Chem Pharm Bull. 2012;60(4):459–464. doi: 10.1248/cpb.60.459. [DOI] [PubMed] [Google Scholar]
- 85.Kapourani A, Vardaka E, Katopodis K, Kachrimanis K, Barmpalexis P. Rivaroxaban polymeric amorphous solid dispersions: moisture-induced thermodynamic phase behavior and intermolecular interactions. Eur J Pharm Biopharm. 2019;145:98–112. doi: 10.1016/j.ejpb.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 86.Chen H, Pui Y, Liu C, Chen Z, Su C-C, Hageman M, Hussain M, Haskell R, Stefanski K, Foster K, Gudmundsson O, Qian F. Moisture-induced amorphous phase separation of amorphous solid dispersions: molecular mechanism, microstructure, and its impact on dissolution performance. J Pharm Sci. 2018;107(1):317–326. doi: 10.1016/j.xphs.2017.10.028. [DOI] [PubMed] [Google Scholar]
- 87.Milne M, Liebenberg W, Aucamp M. The stabilization of amorphous zopiclone in an amorphous solid dispersion. AAPS PharmSciTech. 2015;16(5):1190–1202. doi: 10.1208/s12249-015-0302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shah N, Sandhu H, Choi DS, Chokshi H, Malick AW. Amorphous solid dispersions. Berlin, Ger: Theory Pract Springer; 2014. [Google Scholar]
- 89.Rahman M, Ozkan S, Lester J, Farzana I, Bi V, Durig T. Plasticizer compatibility and thermal and rheological properties of Plasdone™ povidone and copovidone polymers for hot-melt extrusion applications. In: Annual Meeting of the American Association of Pharmaceutical Scientists. 2012.
- 90.Que C, Lou X, Zemlyanov DY, Mo H, Indulkar AS, Gao Y, Zhang GGZ, Taylor LS. Insights into the dissolution behavior of Ledipasvir-Copovidone amorphous solid dispersions: role of drug loading and intermolecular interactions. Mol Pharm. 2019;16(12):5054–5067. doi: 10.1021/acs.molpharmaceut.9b01025. [DOI] [PubMed] [Google Scholar]
- 91.Harmon P, Galipeau K, Xu W, Brown C, Wuelfing WP. Mechanism of dissolution-induced nanoparticle formation from a copovidone-based amorphous solid dispersion. Mol Pharm. 2016;13(5). [DOI] [PubMed]
- 92.Moseson DE, Parker AS, Beaudoin SP, Taylor LS. Amorphous solid dispersions containing residual crystallinity: influence of seed properties and polymer adsorption on dissolution performance. Eur J Pharm Sci. 2020;105276. [DOI] [PubMed]
- 93.Que C, Qi Q, Zemlyanov DY, Mo H, Deac A, Zeller M, et al. Evidence for halogen bonding in amorphous solid dispersions. Cryst Growth Des. 2020;
- 94.Lamm MS, Simpson A, McNevin M, Frankenfeld C, Nay R, Variankaval N. Probing the effect of drug loading and humidity on the mechanical properties of solid dispersions with nanoindentation: antiplasticization of a polymer by a drug molecule. Mol Pharm. 2012;9(11):3396–3402. doi: 10.1021/mp3003013. [DOI] [PubMed] [Google Scholar]
- 95.Yang F, Su Y, Zhang J, DiNunzio J, Leone A, Huang C, Brown CD. Rheology guided rational selection of processing temperature to prepare copovidone-nifedipine amorphous solid dispersions via hot melt extrusion (HME) Mol Pharm. 2016;13(10):3494–3505. doi: 10.1021/acs.molpharmaceut.6b00516. [DOI] [PubMed] [Google Scholar]
- 96.Sotthivirat S, McKelvey C, Moser J, Rege B, Xu W, Zhang D. Development of amorphous solid dispersion formulations of a poorly water-soluble drug, MK-0364. Int J Pharm. 2013;452(1–2):73–81. doi: 10.1016/j.ijpharm.2013.04.037. [DOI] [PubMed] [Google Scholar]
- 97.Tadokoro H, Chatani Y, Yoshihara T, Tahara S, Murahashi S. Structural studies on polyethers,[ (CH2) m O ] n. II. Molecular structure of polyethylene oxide. Die Makromol Chemie Macromol Chem Phys. 1964;73(1):109–127. [Google Scholar]
- 98.Chiou WL, Riegelman S. Preparation and dissolution characteristics of several fast release solid dispersions of griseofulvin. J Pharm Sci. 1969;58(12):1505–1510. doi: 10.1002/jps.2600581218. [DOI] [PubMed] [Google Scholar]
- 99.Usui F, Maeda K, Kusai A, Ikeda M, Nishimura K, Yamamoto K. Dissolution improvement of RS-8359 by the solid dispersion prepared by the solvent method. Int J Pharm. 1998;170(2):247–256. [Google Scholar]
- 100.Vera N, Veiga MD, Cadorniga R. Solid dispersions of oxodipine/PEG 6000: characterization and dissolution study. STP Pharma Sci. 1991;1(2):125–129. [Google Scholar]
- 101.Corrigan OI, Murphy CA, Timoney RP. Dissolution properties of polyethylene glycols and polyethylene glycol-drug systems. Int J Pharm. 1979;4(1):67–74. [Google Scholar]
- 102.Miralles MJ, McGinty JW, Martin A. Combined water soluble carriers for coprecipitates of tolbutamide. J Pharm Sci. 1982;71(3):302–304. doi: 10.1002/jps.2600710309. [DOI] [PubMed] [Google Scholar]
- 103.Ford JL, Stewart AF, Dubois J-L. The properties of solid dispersions of indomethacin or phenylbutazone in polyethylene glycol. Int J Pharm. 1986;28(1):11–22. [Google Scholar]
- 104.Shin SC. Dissolution characteristics of furosemide-polymer coprecipitates. Arch Pharm Res. 1979;2:35–48. [Google Scholar]
- 105.El-Gindy NA, Karara AH, El-Khalek MM. Enhanced dissolution rate of papaverine via the solid dispersion technique. Sci Pharm. 1976;44(4).
- 106.Patil AK, Dinesh BM, Hiremath S. Preparation and evaluation of solid dispersion of felodipine.
- 107.Al-Angary AA, Al-Mahrouk GM, Al-Meshal MA. Interaction of polyethylene glycols with lorazepam. Pharm Ind. 1996;58(3):260–263. [Google Scholar]
- 108.Leonardi D, Barrera MG, Lamas MC, Salomon CJ. Development of prednisone: polyethylene glycol 6000 fast-release tablets from solid dispersions: solid-state characterization, dissolution behavior, and formulation parameters. AAPS PharmSciTech. 2007;8(4):221. doi: 10.1208/pt0804108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Van Duong T, Van Humbeeck J, Van den Mooter G. Crystallization kinetics of indomethacin/polyethylene glycol dispersions containing high drug loadings. Mol Pharm. 2015;12(7):2493–2504. doi: 10.1021/acs.molpharmaceut.5b00299. [DOI] [PubMed] [Google Scholar]
- 110.Vippagunta SR, Wang Z, Hornung S, Krill SL. Factors affecting the formation of eutectic solid dispersions and their dissolution behavior. J Pharm Sci. 2007;96(2):294–304. doi: 10.1002/jps.20754. [DOI] [PubMed] [Google Scholar]
- 111.Katona G, Sipos P, Frohberg P, Ulrich J, Szabo-Revesz P, Jojart-Laczkovich O. Study of paracetamol-containing pastilles produced by melt technology. J Therm Anal Calorim. 2016;123(3):2549–2559. [Google Scholar]
- 112.Tran PH-L, Tran TT-D, Park J-B, Min DH, Choi H-G, Han H-K, Rhee YS, Lee BJ. Investigation of physicochemical factors affecting the stability of a pH-modulated solid dispersion and a tablet during storage. Int J Pharm. 2011;414(1–2):48–55. doi: 10.1016/j.ijpharm.2011.04.065. [DOI] [PubMed] [Google Scholar]
- 113.Obaidat RM, AlTaani B, Ailabouni A. Effect of different polymeric dispersions on in-vitro dissolution rate and stability of celecoxib class II drug. J Polym Res. 2017;24(4):58. [Google Scholar]
- 114.Dordunoo SK, Ford JL, Rubinstein MH. Physical stability of solid dispersions containing triamterene or temazepam in polyethylene glycols. J Pharm Pharmacol. 1997;49(4):390–396. doi: 10.1111/j.2042-7158.1997.tb06812.x. [DOI] [PubMed] [Google Scholar]
- 115.Zhu Q, Harris MT, Taylor LS. Modification of crystallization behavior in drug/polyethylene glycol solid dispersions. Mol Pharm. 2012;9(3):546–553. doi: 10.1021/mp200546p. [DOI] [PubMed] [Google Scholar]
- 116.Baird JA, Olayo-Valles R, Rinaldi C, Taylor LS. Effect of molecular weight, temperature, and additives on the moisture sorption properties of polyethylene glycol. J Pharm Sci. 2010;99(1):154–168. doi: 10.1002/jps.21808. [DOI] [PubMed] [Google Scholar]
- 117.Thijs HML, Becer CR, Guerrero-Sanchez C, Fournier D, Hoogenboom R, Schubert US. Water uptake of hydrophilic polymers determined by a thermal gravimetric analyzer with a controlled humidity chamber. J Mater Chem. 2007;17(46):4864–4871. [Google Scholar]
- 118.Bley H, Fussnegger B, Bodmeier R. Characterization and stability of solid dispersions based on PEG/polymer blends. Int J Pharm [Internet] 2010;390(2):165–173. Available from: 10.1016/j.ijpharm.2010.01.039 [DOI] [PubMed]
- 119.Degradable poly (ethylene glycol) hydrogels for 2D and 3D cell culture | Sigma-Aldrich [Internet]. Available from: https://www.sigmaaldrich.com/technical-documents/articles/material-matters/degradable-polyethylene-glycol-hydrogels.html. Accessed 24 June 2020.
- 120.Milen D, Nikolai L, Stoicho S, Veneta D, Baranovski Vladimir Y. Hydrogels based on the chemically crosslinked polyacrylic acid: Biopharmaceutical characterization. Acta Pharm [Internet]. 2003;53(1):25–31. Available from: http://acta.pharmaceutica.farmaceut.org/2503.html. Accessed 25 June 2020. [PubMed]
- 121.Punčochova K, Ewing AV, Gajdosova M, Sarvasova N, Kazarian SG, Beranek J, et al. Identifying the mechanisms of drug release from amorphous solid dispersions using MRI and ATR-FTIR spectroscopic imaging. Int J Pharm. 2015;483(1–2):256–267. doi: 10.1016/j.ijpharm.2015.02.035. [DOI] [PubMed] [Google Scholar]
- 122.Slamova M, Skolakova T, Skolakova A, Patera J, Zamostny P. Preparation of solid dispersions with respect to the dissolution rate of active substance. J Drug Deliv Sci Technol. 2020;101518.
- 123.Save T, Venkitachalam P. Studies on solid dispersions of nifedipine. Drug Dev Ind Pharm. 1992;18(15):1663–1679. [Google Scholar]
- 124.McGinity JW, Maincent P, Steinfink H. Crystallinity and dissolution rate of tolbutamide solid dispersions prepared by the melt method. J Pharm Sci. 1984;73(10):1441–1444. doi: 10.1002/jps.2600731030. [DOI] [PubMed] [Google Scholar]
- 125.Ford JL, Rubinstein MH. Formulation and ageing of tablets prepared from indomethacin-polyethylene glycol 6000 solid dispersions. Pharmaceutica acta Helvetiae. 1980;55(1):1–7. [PubMed]
- 126.McCrystal CB. Characterisation of the fundamental interactions between water and cellulose ether polymers. 1998. http://researchonline.ljmu.ac.uk/id/eprint/4912/1/DX212995.pdf.
- 127.Archer WL. Determination of Hansen solubility parameters for selected cellulose ether derivatives. Ind Eng Chem Res. 1991;30(10):2292–2298. [Google Scholar]
- 128.Xie T, Taylor LS. Dissolution performance of high drug loading celecoxib amorphous solid dispersions formulated with polymer combinations. Pharm Res. 2016;33(3):739–750. doi: 10.1007/s11095-015-1823-y. [DOI] [PubMed] [Google Scholar]
- 129.Konno H, Taylor LS. Influence of different polymers on the crystallization tendency of molecularly dispersed amorphous felodipine. J Pharm Sci. 2006;95(12):2692–2705. doi: 10.1002/jps.20697. [DOI] [PubMed] [Google Scholar]
- 130.Jung J-Y, Yoo SD, Lee S-H, Kim K-H, Yoon D-S, Lee K-H. Enhanced solubility and dissolution rate of itraconazole by a solid dispersion technique. Int J Pharm. 1999;187(2):209–218. doi: 10.1016/s0378-5173(99)00191-x. [DOI] [PubMed] [Google Scholar]
- 131.Xie T, Taylor LS. Improved release of celecoxib from high drug loading amorphous solid dispersions formulated with polyacrylic acid and cellulose derivatives. Mol Pharm. 2016;13(3):873–884. doi: 10.1021/acs.molpharmaceut.5b00798. [DOI] [PubMed] [Google Scholar]
- 132.De Yoreo JJ, Vekilov PG. Principles of crystal nucleation and growth. Rev Mineral geochemistry. 2003;54(1):57–93. [Google Scholar]
- 133.Okada H, Ueda K, Yasuda Y, Higashi K, Inoue M, Ito M, Noguchi S, Kawakami K, Moribe K. Correlation between drug dissolution and resistance to water-induced phase separation in solid dispersion formulations revealed by solid-state NMR spectroscopy. Int J Pharm. 2020;577:119086. doi: 10.1016/j.ijpharm.2020.119086. [DOI] [PubMed] [Google Scholar]
- 134.Bhattacharya S, Suryanarayanan R. Local mobility in amorphous pharmaceuticals—characterization and implications on stability. J Pharm Sci [Internet]. 2009 Sep [cited 2016 Nov 14];98(9):2935–53. Available from: http://linkinghub.elsevier.com/retrieve/pii/S002235491633060X. Accessed 14 Nov 2016. [DOI] [PubMed]
- 135.Hormann TR, Jager N, Funke A, Murb R-K, Khinast JG, Paudel A. Formulation performance and processability window for manufacturing a dual-polymer amorphous solid dispersion via hot-melt extrusion and strand pelletization. Int J Pharm. 2018;553(1–2):408–421. doi: 10.1016/j.ijpharm.2018.10.035. [DOI] [PubMed] [Google Scholar]
- 136.Boghra RJ, Kothawade PC, Belgamwar VS, Nerkar PP, Tekade AR, Surana SJ. Solubility, dissolution rate and bioavailability enhancement of irbesartan by solid dispersion technique. Chem Pharm Bull. 2011;59(4):438–441. doi: 10.1248/cpb.59.438. [DOI] [PubMed] [Google Scholar]
- 137.Meng F, Trivino A, Prasad D, Chauhan H. Investigation and correlation of drug polymer miscibility and molecular interactions by various approaches for the preparation of amorphous solid dispersions. Eur J Pharm Sci. 2015;71:12–24. doi: 10.1016/j.ejps.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 138.Tian B, Tang X, Taylor LS. Investigating the correlation between miscibility and physical stability of amorphous solid dispersions using fluorescence-based techniques. Mol Pharm. 2016;13(11):3988–4000. doi: 10.1021/acs.molpharmaceut.6b00803. [DOI] [PubMed] [Google Scholar]
- 139.Jog R, Gokhale R, Burgess DJ. Solid state drug-polymer miscibility studies using the model drug ABT-102. Int J Pharm. 2016;509(1–2):285–295. doi: 10.1016/j.ijpharm.2016.05.068. [DOI] [PubMed] [Google Scholar]
- 140.Wegiel LA, Mauer LJ, Edgar KJ, Taylor LS. Crystallization of amorphous solid dispersions of resveratrol during preparation and storage-impact of different polymers. J Pharm Sci. 2013;102(1):171–184. doi: 10.1002/jps.23358. [DOI] [PubMed] [Google Scholar]
- 141.Wegiel LA, Zhao Y, Mauer LJ, Edgar KJ, Taylor LS. Curcumin amorphous solid dispersions: the influence of intra and intermolecular bonding on physical stability. Pharm Dev Technol. 2014;19(8):976–986. doi: 10.3109/10837450.2013.846374. [DOI] [PubMed] [Google Scholar]
- 142.Riedel A, Leopold CS. Degradation of omeprazole induced by enteric polymer solutions and aqueous dispersions: HPLC investigations. Drug Dev Ind Pharm. 2005;31(2):151–160. doi: 10.1081/ddc-200047787. [DOI] [PubMed] [Google Scholar]
- 143.Friesen DT, Shanker R, Crew M, Smithey DT, Curatolo WJ, Nightingale JAS. Hydroxypropyl methylcellulose acetate succinate-based spray-dried dispersions: an overview. Mol Pharm. 2008;5(6):1003–1019. doi: 10.1021/mp8000793. [DOI] [PubMed] [Google Scholar]
- 144.Zhang Q, Zhao Y, Zhao Y, Ding Z, Fan Z, Zhang H, Liu M, Wang Z, Han J. Effect of HPMCAS on recrystallization inhibition of nimodipine solid dispersions prepared by hot-melt extrusion and dissolution enhancement of nimodipine tablets. Colloids Surfaces B Biointerfaces. 2018;172:118–126. doi: 10.1016/j.colsurfb.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 145.Chavan RB, Thipparaboina R, Kumar D, Shastri NR. Evaluation of the inhibitory potential of HPMC, PVP and HPC polymers on nucleation and crystal growth. RSC Adv. 2016;6(81):77569–77576. [Google Scholar]
- 146.Ueda K, Higashi K, Yamamoto K, Moribe K. The effect of HPMCAS functional groups on drug crystallization from the supersaturated state and dissolution improvement. Int J Pharm. 2014;464(1–2):205–213. doi: 10.1016/j.ijpharm.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 147.Huang W, Mandal T, Larson RG. Computational modeling of hydroxypropyl-methylcellulose acetate succinate (HPMCAS) and phenytoin interactions: A systematic coarse-graining approach. Mol Pharm. 2017;14(3):733–745. doi: 10.1021/acs.molpharmaceut.6b01013. [DOI] [PubMed] [Google Scholar]
- 148.Kallakunta VR, Sarabu S, Bandari S, Batra A, Bi V, Durig T, et al. Stable amorphous solid dispersions of fenofibrate using hot melt extrusion technology: effect of formulation and process parameters for a low glass transition temperature drug. J Drug Deliv Sci Technol. 2019;101395. [DOI] [PMC free article] [PubMed]
- 149.Tsinman O, Tsinman K, Ali S. Excipient update-Soluplus®: an understanding of supersaturation from amorphous solid dispersions. Drug Deliv Technol. 2015;15(1).
- 150.Alopaeus JF, Hagesæther E, Tho I. Micellisation mechanism and behaviour of Soluplus®-furosemide micelles: preformulation studies of an oral nanocarrier-based system. Pharmaceuticals. 2019;12(1):15. doi: 10.3390/ph12010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shi N-Q, Lai H-W, Zhang Y, Feng B, Xiao X, Zhang H-M, Li ZQ, Qi XR. On the inherent properties of Soluplus and its application in ibuprofen solid dispersions generated by microwave-quench cooling technology. Pharm Dev Technol. 2018;23(6):573–586. doi: 10.1080/10837450.2016.1256409. [DOI] [PubMed] [Google Scholar]
- 152.Bochmann ES, Neumann D, Gryczke A, Wagner KG. Micro-scale prediction method for API-solubility in polymeric matrices and process model for forming amorphous solid dispersion by hot-melt extrusion. Eur J Pharm Biopharm. 2016;107:40–48. doi: 10.1016/j.ejpb.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 153.Song B, Wang J, Lu SJ, Shan LN. Andrographolide solid dispersions formulated by Soluplus to enhance interface wetting, dissolution, and absorption. J Appl Polym Sci. 2020;137(6):48354. [Google Scholar]
- 154.Rahman M, Ahmad S, Tarabokija J, Bilgili E. Roles of surfactant and polymer in drug release from spray-dried hybrid nanocrystal-amorphous solid dispersions (HyNASDs) Powder Technol. 2020;361:663–678. [Google Scholar]
- 155.Guan J, Huan X, Liu Q, Jin L, Wu H, Zhang X, Mao S. Synergetic effect of nucleation and crystal growth inhibitor on in vitro-in vivo performance of supersaturable lacidipine solid dispersion. Int J Pharm. 2019;566:594–603. doi: 10.1016/j.ijpharm.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 156.Zeng Y, Li S, Liu C, Gong T, Sun X, Fu Y, Zhang ZR. Soluplus micelles for improving the oral bioavailability of scopoletin and their hypouricemic effect in vivo. Acta Pharmacol Sin. 2017;38(3):424–433. doi: 10.1038/aps.2016.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Singh A, Bharati A, Frederiks P, Verkinderen O, Goderis B, Cardinaels R, Moldenaers P, van Humbeeck J, van den Mooter G. Effect of compression on the molecular arrangement of itraconazole-soluplus solid dispersions: induction of liquid crystals or exacerbation of phase separation? Mol Pharm. 2016;13(6):1879–1893. doi: 10.1021/acs.molpharmaceut.6b00046. [DOI] [PubMed] [Google Scholar]
- 158.Repka MA, Bandari S, Kallakunta VR, Vo AQ, McFall H, Pimparade MB, Bhagurkar AM. Melt extrusion with poorly soluble drugs-an integrated review. Int J Pharm. 2018;535(1–2):68–85. doi: 10.1016/j.ijpharm.2017.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Singh A, Van den Mooter G. Spray drying formulation of amorphous solid dispersions. Adv Drug Deliv Rev. 2016;100:27–50. doi: 10.1016/j.addr.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 160.Paudel A, Worku ZA, Meeus J, Guns S, Van den Mooter G. Manufacturing of solid dispersions of poorly water soluble drugs by spray drying: formulation and process considerations. Int J Pharm. 2013;453(1):253–284. doi: 10.1016/j.ijpharm.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 161.Paudel A, Loyson Y, Van den Mooter G. An investigation into the effect of spray drying temperature and atomizing conditions on miscibility, physical stability, and performance of naproxen-PVP K 25 solid dispersions. J Pharm Sci. 2013;102(4):1249–1267. doi: 10.1002/jps.23459. [DOI] [PubMed] [Google Scholar]
- 162.Mahmah O, Tabbakh R, Kelly A, Paradkar A. A comparative study of the effect of spray drying and hot-melt extrusion on the properties of amorphous solid dispersions containing felodipine. J Pharm Pharmacol [Internet]. 2014 Feb [cited 2016 Nov 14];66(2):275–84. Available from: http://doi.wiley.com/10.1111/jphp.12099 [DOI] [PubMed]
- 163.Van den Mooter G, Augustijns P, Blaton N, Kinget R. Physico-chemical characterization of solid dispersions of temazepam with polyethylene glycol 6000 and PVP K30. Int J Pharm. 1998;164(1–2):67–80. [Google Scholar]
- 164.Dahlberg C, Millqvist-Fureby A, Schuleit M. Surface composition and contact angle relationships for differently prepared solid dispersions. Eur J Pharm Biopharm. 2008;70(2):478–485. doi: 10.1016/j.ejpb.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 165.Curatolo W, Nightingale JA, Herbig SM. Utility of hydroxypropylmethylcellulose acetate succinate (HPMCAS) for initiation and maintenance of drug supersaturation in the GI milieu. Pharm Res. 2009;26(6):1419–1431. doi: 10.1007/s11095-009-9852-z. [DOI] [PubMed] [Google Scholar]
- 166.Solanki NG, Lam K, Tahsin M, Gumaste SG, Shah AV, Serajuddin ATM. Effects of surfactants on itraconazole-HPMCAS solid dispersion prepared by hot-melt extrusion I: miscibility and drug release. J Pharm Sci. 2019;108(4):1453–1465. doi: 10.1016/j.xphs.2018.10.058. [DOI] [PubMed] [Google Scholar]
- 167.Li B, Konecke S, Wegiel LA, Taylor LS, Edgar KJ. Both solubility and chemical stability of curcumin are enhanced by solid dispersion in cellulose derivative matrices. Carbohydr Polym. 2013;98(1):1108–1116. doi: 10.1016/j.carbpol.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 168.Li B, Konecke S, Harich K, Wegiel L, Taylor LS, Edgar KJ. Solid dispersion of quercetin in cellulose derivative matrices influences both solubility and stability. Carbohydr Polym. 2013;92(2):2033–2040. doi: 10.1016/j.carbpol.2012.11.073. [DOI] [PubMed] [Google Scholar]
- 169.Al-Obaidi H, Buckton G. Evaluation of griseofulvin binary and ternary solid dispersions with HPMCAS. AAPS PharmSciTech. 2009;10(4):1172–1177. doi: 10.1208/s12249-009-9319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Connelly PR, Quinn BP, Johnston S, Bransford P, Mudunuri P, Peresypkin A, et al. Translational development of amorphous dispersions. Pharm Sci Encycl Drug Discov Dev Manuf. 2010:1–29.
- 171.LaFountaine JS, McGinity JW, Williams RO. Challenges and strategies in thermal processing of amorphous solid dispersions: a review. AAPS PharmSciTech. 2016;17(1):43–55. doi: 10.1208/s12249-015-0393-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gupta SS, Meena A, Parikh T, Serajuddin ATM. Investigation of thermal and viscoelastic properties of polymers relevant to hot melt extrusion-I: Polyvinylpyrrolidone and related polymers. J Excipients Food Chem. 2016;5(1):1001. [Google Scholar]
- 173.Gupta SS, Solanki N, Serajuddin ATM. Investigation of thermal and viscoelastic properties of polymers relevant to hot melt extrusion, IV: Affinisol™ HPMC HME polymers. AAPS PharmSciTech. 2016;17(1):148–157. doi: 10.1208/s12249-015-0426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1461 kb)