Abstract
Introduction
Oral minoxidil is an antihypertensive vasodilator known to stimulate hair growth. The use of low-dose oral minoxidil for the treatment of male androgenetic alopecia (AGA) is receiving increasing attention. The aim of this study was to evaluate the efficacy and safety of oral minoxidil for the treatment of male AGA.
Methods
This was an open-label, prospective, single-arm study. Thirty men aged 24–59 years with AGA types III vertex to V were treated with oral minoxidil 5 mg once daily for 24 weeks. Efficacy was evaluated by hair counts, hair diameter measurements, photographic assessment, and self-administered questionnaire. The safety of the treatment was closely monitored by means of physical examinations and laboratory investigations.
Results
There was a significant increase in total hair counts from baseline at weeks 12 (mean change + 26, range 182.5–208.5 hairs/cm2) and 24 (mean change + 35.1, range 182.5–217.6 hairs/cm2) (both p = 0.007). Photographic assessment of the vertex area by an expert panel revealed 100% improvement (score > + 1), with 43% of patients showing excellent improvement (score + 3, 71–100% increase). The frontal area also showed a significant response but less than that of the vertex area. Common side effects were hypertrichosis (93% of patients) and pedal edema (10%). No serious cardiovascular adverse events and abnormal laboratory findings were observed.
Conclusion
Oral minoxidil 5 mg once daily effectively increased hair growth in our male patients with AGA and had a good safety profile in healthy subjects. However, oral minoxidil should be used carefully with men who have severe hypertension and increased risk for cardiovascular events.
Electronic supplementary material
The online version of this article (10.1007/s13555-020-00448-x) contains supplementary material, which is available to authorized users.
Keywords: Androgenetic alopecia, Asian, Male pattern hair loss, Oral minoxidil, Side effects, Treatment
Digital Features
This article is published with digital features to facilitate understanding of the article. To view the digital features for this article go to 10.6084/m9.figshare.12937661.
Key Summary Points
| The use of oral minoxidil as a treatment for androgenetic alopecia (AGA) is limited mainly because of the side effects of severe and uncontrolled hypertension reported at standard doses of 10–40 mg. |
| The aim of this open-label, prospective, single-arm study was to evaluate the effectiveness and the safety of oral minoxidil at the low dose of 5 mg once daily in men with AGA types III vertex to V. |
| Oral minoxidil significantly increased hair growth, with a mean change in total hair counts + 19.23% from baseline at week 24; global photographic assessment revealed 100% improvement (increase > + 1 score), with 43% of patients showing excellent improvement. |
| Our results show that low-dose oral minoxidil can be considered a safe treatment for male AGA in healthy subjects, with only minor side effects of hypertrichosis (93%) and pedal edema (10%). There was no significant change in blood pressure (BP) and pulse rate (PR) at 24 weeks after treatment, with mean systolic BP decreasing by − 3.8 mmHg, mean diastolic BP decreasing by − 1.1 mmHg, and PR increasing by + 0.6/min. |
Introduction
Topical minoxidil 2–5%, oral finasteride, and low-level laser therapy are current the standard first-line treatments for androgenetic alopecia (AGA). Minoxidil in an oral formulation has been previously used in general medicine for the treatment of severe and uncontrolled hypertension at a dose of 10–40 mg [1]. Unintentionally, the early trials of oral minoxidil as an antihypertensive drug documented side effects such as hypertrichosis and hirsutism with chronic use and reported the drug’s potential for stimulating hair growth [2, 3]. These findings led to the subsequent development of a topical minoxidil formulation for the treatment of AGA. However, the use of oral minoxidil for AGA is limited mainly because of the side effects at standard doses. Nevertheless, increasing attention is focusing on the use of low doses of oral minoxidil (0.25–5 mg once daily) for alopecia [4, 5]. The objectives of this study were to evaluate the effectiveness and safety of oral minoxidil at a dose of 5 mg once daily for the treatment of male AGA.
Methods
Patient Population
Thirty men with mild to moderate AGA (III vertex, IV, V modified Norwood–Hamilton classification) were recruited to the study. All inclusion and exclusion criteria are shown in Electronic Supplementary Material (ESM) Table S1.
Study Design
This was an open-label, prospective, single-arm outpatient study conducted at King Chulalongkorn Memorial Hospital, Bangkok, Thailand. The study involved a screening phase (up to 4 weeks), a treatment phase (24 weeks), and a 2-week follow-up phase. Patients were asked to take minoxidil 5 mg (T.O. Pharma Co. Ltd, Bangkok, Thailand) once daily every morning for 24 weeks, with the exception that the first dose of oral minoxidil was given to patients at nighttime to prevent orthostatic hypotension. The primary study outcome was efficacy of the treatment, evaluated by target area hair counts on the vertex at 12 and 24 weeks. Secondary outcomes were hair thickness, photographic assessment, self-assessment questionnaires, and safety assessments. Patients were asked to return for evaluations at 4, 12, 24, and 26 weeks after treatment initiation. Compliance was evaluated by counting the remaining tablets at each visit. The efficacy and safety assessment schedule is presented in ESM Table S2.
The study protocol and informed consent form were reviewed and approved by the Institutional Review Board of Faculty of Medicine, Chulalongkorn University (IRB number 496/55). This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Informed consent for publication was obtained for each participant.
Efficacy Assessment
Hair Count and Hair Thickness
A 1-cm2 target area of the thinning area at the midscalp or anterior edge of vertex area was marked with a tattoo to facilitate precise determination of hair density and thickness. Macrophotographs of the reference area were taken using a dermoscopic device with a 1-cm2 grid (3Gen DermLite II Pro HR; 3Gen LCC, San Juan Capistrano, CA, USA) connected to a cell phone (iPhone 6; Apple Inc., Cupertino, CA, USA). The hairs in this area were manually counted by the same experienced technician, who was blinded to treatment, and reported as total hair and non-vellus hair counts. Hair thickness of five representative hairs in the reference area was measured using Adobe photoshop analysis (Adobe Inc., Mountain View, CA, USA) and then averaged.
Global Photographic Assessments
Standardized global photographs were taken using a digital camera (model EOS 650D DSLR; Canon Inc., Tokyo, Japan). A stereotactic positioning device with continuous light was used to fix the head position and control the lighting. A panel of three blinded dermatologists independently assessed any improvement in hair growth at the vertex and frontal views using a standardized 7-point rating scale (− 3 to + 3).
Patient Self-Assessment Questionnaires
Each patient was requested to perform self-assessment of hair growth and treatment satisfaction using the Hair Growth Index (HGI) and Hair Growth Satisfaction Scale (HGSS).
Safety Assessments
Safety assessment was performed using the combined results of history taking, physical examinations, laboratory test evaluations, and imaging at every visit. A history of nausea, vomiting, orthostatic hypotension, chest pain, dyspnea, orthopnea, hypertrichosis, and sexual function were recorded and evaluated. The physical examination included vital signs, blood pressure (2 positions: sitting and standing), height, weight, waist circumference, abdominal circumference, pitting edema, and assessment of hypertrichosis or presence of vellus hairs on the face and forearm using the modified Ferriman–Gallwey scoring system for evaluating and quantifying hirsutism. Laboratory evaluation included complete blood count (CBC), blood urea (BUN), creatinine (Cr), electrolytes, liver function tests, chest X-ray, and electrocardiography (EKG).
Statistical Analysis
All analyses were performed with the SPSS version 18 statistical software package (IBM Corp., Armonk, NY, USA) based on the modified intent-to-treat population. P values of < 0.05 were considered to be statistically significant. For hair counts, a general linear analysis model adjusted for treatment, cluster, and baseline value was used. A general linear analysis model that included effects of treatment and cluster was used to compare median panel assessment, and total HGI scores. A Fisher exact test was used to compare all adverse events (AEs) and severe AEs (SAEs).
Results
A total of 30 men aged 24–59 years were enrolled and completed the study. Patient mean age was 38 years with the mean age at which patients began losing hair was 24.8 years and the mean duration of hair loss was 12.9 years. The most common modified Norwood-Hamilton classification was type IV followed by type V and type IIIv. Patient demographic data is summarized in Table 1.
Table 1.
Patient demographic data and characteristics
| Patient characteristics | Valuesa |
|---|---|
| Age (years) | |
| Mean | 38 ± 10 |
| Minimum–maximum | 24–59 |
| Age 20–40.9 years | 19 (63.3%) |
| Age 41–60 years | 11 (36.7%) |
| Age hair loss first noticed (years) | 24.8 ± 8.7 |
| Duration of hair loss (years) | 12.9 ± 11 |
| Family members experiencing AGA | 28 (93.3%) |
| Previous treatment | |
| Finasteride | 2 (20.03%) |
| Topical 5% minoxidil | 5 (16.7%) |
| Topical herbal extracts | 10 (33.3%) |
| Current smokers | 4 (13.3%) |
| Former smokers | 4 (13.3%) |
| Alcohol consumers | 20 (66.7%) |
| Underlying diseases | |
| Diabetes | 3 (10%) |
| Dyslipidemia | 7 (23.3%) |
| Hypertension | 3 (10%) |
| Metabolic syndrome | 4 (13.3%) |
| Baseline Norwood–Hamilton classification | Total patient cohort (n = 30) | Patient cohort aged < 41 years (n = 19) | Patient cohort aged ≥ 41 years (n = 11) |
|---|---|---|---|
| III vertex | 8 (26.7%) | 8 (42.1%) | 0 (0%) |
| IV | 12 (40.0%) | 4 (21.1%) | 8 (72.7%) |
| V | 10 (33.3%) | 7 (36.8%) | 3(27.3%) |
Values in table are presented as the mean ± standard deviation (SD) or as a number with the percentage in parenthesis
AGA Androgenetic alopecia
aTotal number of patients (n) = 30
Efficacy Assessment
Target Area Hair Count (Total and Non-Vellus Hair Counts)
The total hair counts continuously increased during the 12 and 24 weeks following treatment initiation. Compared to baseline, the mean change in total hair counts was significantly higher at 12 weeks (total ± standard deviation [SD] + 26 ± 19.5 hairs/cm2; P = 0.023) and 24 weeks (+ 35.1 ± 18.9 hairs/cm2; P = 0.003) of treatment. Non-vellus hair counts were also significantly higher increase at 12 and 24 weeks compared to baseline (+ 26 ± 18.4 hairs/cm2 [P = 0.006] and + 35.9 ± 15.6 hairs/cm2 [P < 0.001], respectively) (Table 2).
Table 2.
Total, non-vellus hair count, and hair diameter
| Hair count (hairs/cm2) | Total patient cohort (n = 30) | Patient cohort aged < 41 years (n = 19) | Patient cohort aged ≥ 41 years (n = 11) | P valueb |
|---|---|---|---|---|
| Total hair count | ||||
| Baseline | 182.5 ± 43.3 | 180.4 ± 40.6 | 186.3 ± 49.5 | 0.726 |
| 12 weeks | 208.5 ± 42.8 | 208.2 ± 39.3 | 209.1 ± 50.3 | 0.958 |
| 24 weeks | 217.6 ± 44.9 | 221 ± 42.7 | 211.6 ± 49.9 | 0.578 |
| P value | 0.007a | |||
| Difference from baseline | ||||
| 12 weeks | 26.0 ± 19.5 | 27.8 ± 20.3 | 22.8 ± 18.3 | 0.505 |
| 24 weeks | 35.1 ± 18.9 | 40.7 ± 17.5 | 25.4 ± 17.7 | 0.029 |
| Percentage increase = 100 × (([end count − baseline count)]baseline count) | ||||
| 12 weeks | 14.25% | 15.41% | 12.24% | |
| 24 weeks | 19.23% | 22.56% | 13.63% | |
| Non-vellus hair count | ||||
| Baseline | 152.3 ± 33.0 | 152.9 ± 32.7 | 150.7 ± 35.1 | 0.863 |
| 12 weeks | 178.1 ± 38.0 | 180.9 ± 37.0 | 173.3 ± 41.1 | 0.603 |
| 24 weeks | 188.1 ± 37.0 | 192.9 ± 37.6 | 179.7 ± 36.2 | 0.357 |
| P value | 0.001a | |||
| Difference from baseline | ||||
| 12 weeks | 26.0 ± 18.4 | 28.0 ± 20.2 | 22.5 ± 15.1 | 0.444 |
| 24 weeks | 35.9 ± 15.6 | 39.9 ± 15.3 | 29.0 ± 14.2 | 0.063 |
| Percentage increase = 100 × (([end count − baseline count]/baseline count) | ||||
| 12 weeks | 17.07% | 18.31% | 14.93% | |
| 24 weeks | 23.57% | 26.10% | 19.24% | |
| Hair diameter [mean (SD)] | ||||
| Baseline | 58.5 ± 11.8 | 62.9 ± 9.6 | 50.9 ± 12.0 | 0.006 |
| 12 weeks | 64.7 ± 15.2 | 70.2 ± 14.2 | 55.1 ± 12.1 | 0.006 |
| 24 weeks | 67.4 ± 4.5 | 71.5 ± 14.3 | 60.2 ± 12.3 | 0.037 |
| P value (12th week-baseline) | 0.002a | |||
| P value (24th week-baseline) | 0.001a | |||
| Difference from baseline | ||||
| 12 weeks | 6.2 ± 9.8 | 7.3 ± 11.4 | 4.1 ± 6.4 | 0.402 |
| 24 weeks | 8.9 ± 8.6 | 8.6 ± 10.4 | 9.3 ± 4.7 | 0.852 |
| Percentage increase = 100 × ([end count − baseline count]/baseline count) | ||||
| 12 weeks | 10.60% | 11.61% | 8.06% | |
| 24 weeks | 15.21% | 13.67% | 18.27% | |
Values in table are presented as the mean ± SD unless indicated othewrise
aRepeated measures analysis, least significant difference (LSD)
bIndependent t test
Hair Diameter
Hair diameter significantly increased from baseline by 10.6% (from 58.5 ± 11.8 to 64.7 ± 15.2 μm) at 12 weeks (P = 0.002) and by 15.21% (from 58.5 ± 11.8 to 67.4 ± 14.5 μm) at 24 weeks (P < 0.001) (Table 2).
Photographic Assessment
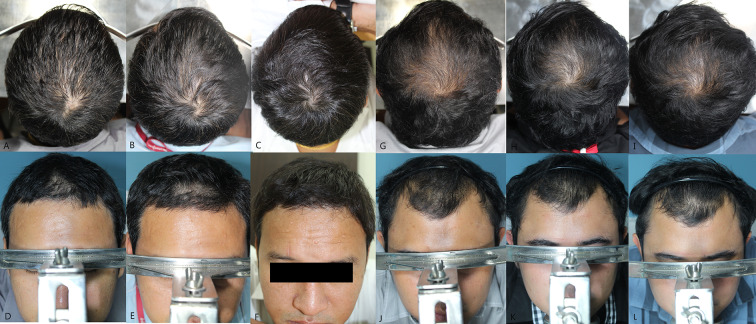
Expert panel global photographic assessment of vertex area, as shown in Table 3, revealed a 100% improvement (score > + 1). Remarkably, 43% of patients had achieved an excellent improvement (score + 3, 71–100% increase) by the end of study. An additional 12 weeks of treatment resulted in greater efficacy outcomes; the median (range) score increased from 2 (0–3) to 3 (1–3) (P < 0.001) and the ratio of excellent improvement increased from 6.7 to 43.3% at weeks 12 and 24, respectively (P < 0.001). On the frontal area, the median (range) score increased from 1 (0–3) to 2 (1–3) at weeks 12 and 24, respectively (P < 0.001). Representative photographs are shown in Fig. 1.
Table 3.
Expert panel global photographic assessment on vertex and frontal area at weeks 12 and 24 of treatment compared to baseline
| 7-Point rating scalea | 12 weeks, n (%) | 24 weeks, n (%) |
|---|---|---|
| Vertex area | ||
| − 3 | 0 (0) | 0 (0) |
| − 2 | 0 (0) | 0 (0) |
| − 1 | 0 (0) | 0 (0) |
| 0 | 1 (3.3) | 0 (0) |
| + 1 | 7 (23.3) | 2 (6.7) |
| + 2 | 20 (66.7) | 15 (50) |
| + 3 | 2 (6.7) | 13 (43.3) |
| Frontal area | ||
| − 3 | 0 (0) | 0 (0) |
| − 2 | 0 (0) | 0 (0) |
| − 1 | 0 (0) | 0 (0) |
| 0 | 51 (16.7) | 0 (0) |
| + 1 | 11 (36.7) | 8 (26.7) |
| + 2 | 12 (40) | 19 (63.3) |
| + 3 | 2 (6.7) | 3 (10) |
aA standardized 7-point rating scale (− 3 to + 3) was used, in which − 3 indicates greatly decreased; − 2, moderately decreased; − 1, minimally decreased; 0, no change; + 1, minimally increased; + 2, moderately increased; + 3, greatly increased
Fig. 1.
Representative global photographs on vertex and frontal area of two patients (a–f patient no. 1, g–l patient no. 2) at baseline (a, b and g, h) and weeks 12 (c, d and i, j) and 24 (e, f and k, l) after initiating treatment with oral minoxidil 5 mg once daily
Subgroup Analysis by Age
Subjects in the older group (> 41–60 years) achieved a higher score than their younger counterparts (20–40 years) on the modified Norwood–Hamilton classification. Comparison of these two age groups revealed that at 24 weeks of treatment younger men achieved a significant increase in number of total hairs (+ 40.7 hairs/cm2 increase) compared to older men (+ 25.4 hairs/cm2 increase) (P = 0.029) (Table 2). In addition, younger men had a larger hair diameter than to older men at baseline (P = 0.006), 12 weeks (P = 0.006), and 24 weeks (P = 0.037). However, there was no significant difference in diameter change after treatment between groups.
Patient Self-Assessment Questionnaires
The clinical improvement could be observed by most patients. The mean (± SD) total HGI score of three aspects (− 9 to + 9) was 3.9 ± 2.8 at 12 weeks and 5.0 ± 2.1 at 24 weeks (P = 0.018). All patients were also satisfied with the results in terms of mean total HGSS score of 5 aspects (− 15 to + 15), with a score of 5.3 ± 4.9 at 12 weeks and 8.0 ± 4.1 at 24 weeks (P = 0.006) (see ESM Tables S3 and S4 for details).
Safety Assessment
The side effects of oral minoxidil are shown in ESM Table S5. After the first dose of oral minoxidil, the mean blood pressure (BP) decreased from 134.2/78 to 131.7/78.5 mmHg and the mean pulse rate (PR) decreased from 75.6 to 71.8/min after 60 min (P > 0.05), without any reflex tachycardia from the vasodilatory effect. Orthostatic hypotension was observed in two patients (6.7%) at 30 min and in one patient (3.3%) at 60 min after taking the first dose of medication without any symptoms. The BP of these three patients had returned to normal at the follow-up BP measurement taken at 30 and 60 min after the dosing. At 24 weeks into treatment, the mean BP showed a non-significant change from 134.2/78 to 130.3/76.9 mmHg, and the mean PR had increased from 75.6 to 76.2/min (P = 0.792).
Regarding cardiac monitoring, abnormal EKG findings were found in six patients (20%), two of whom had occasional premature ventricular contraction and four had with new T wave inversion in one lead (mostly V1) without any symptoms. This T-wave inversion in V1 was classified as a non-ischemic pattern. No serious cardiovascular adverse effects of minoxidil were found.
Pedal edema was present in three patients (10%) at 1–2 months into treatment, which spontaneously resolved after 2–3 months. Focusing on patients with pedal edema, two patients had obesity (body mass index > 30 kg/m2) without abnormal physical or laboratory abnormalities and one patient with hypertension was being treated with calcium channel blocker. The edema decreased after salt intake was rigorously restricted. Hypertrichosis was the most common side effect, present in 27 (90%) and 28 (93.3%) patients at 12 and 24 weeks, respectively. The most common locations were limbs (forearm) and face (forehead, temples and over the malar prominences). However, this condition was well-tolerated by most patients.
Laboratory and Imaging
After 24 weeks of treatment, CBC, BUN, Cr, and electrolytes were within normal limits in all patients. There were slight increases in aspartate aminotransferase (40–60 U/L) in one patient (3.3%) and alanine aminotransferase (40–60 U/L) in nine patients (30%). Chest X-rays were normal in all patients at baseline and one patient (3.3%) showed a borderline heart size at 24 weeks.
Discussion
Minoxidil is known to be an effective treatment for decreasing the rate of hair loss and promoting hair regrowth by increasing both hair diameter and density, but its mechanism of action is not yet fully understood [6, 7]. Minoxidil can also promote premature anagen entry to resting hair follicles and prolong the anagen phase [8, 9]. Thus, shortening of the telogen phase and acceleration of the telogen–exogen phase lead to synchronization of the hair cycle and increased hair shedding after the initiation of minoxidil therapy [10].
Low-dose oral minoxidil has been used in many Asian countries for > 30 years to treat male AGA, albeit there have been very few case reports. In 2018, a pilot study demonstrated the effectiveness of oral minoxidil 0.25–1.25 mg once daily and spironolactone 25 mg for the treatment of Sinclair stage 2–5 female pattern hair loss. One hundred women completed the study, with a mean reduction in hair shedding score (range 1–6) of 2.3 at 6 months and 2.6 at 12 months. [11] In addition, a recent retrospective review showed that low-dose minoxidil (2.5–5 mg once daily), given either as monotherapy or as an additional therapy for 6–12 months, was both effective and safe in 41 male patients with AGA [12, 13]. Therianou et al. also reported the effectiveness of using oral minoxidil in patients allergic to topical minoxidil [14].
The results of our study showed that oral minoxidil improved hair growth in men with AGA according to all of the assessments applied. Compared to baseline, total hair count at the vertex increased by 14.25% at 12 weeks of treatment (from 182.5 to 208.5 hairs/cm2) and by 19.23% at 24 weeks of treatment (from 182.5 to 217.6 hairs/cm2). Non-vellus hair counts were even more improved: compared to baseline there was an increase of + 17.07% (from 152.3 to 178.1 hairs/cm2) at week 12 and an increase of + 23.5% (from 152.3 to 188.1 hairs/cm2) at week 24. As expected, the younger patients (< 41 years) showed better response to treatment than the older ones (≥ 41 years). Compared to baseline, at 24 weeks, total hair counts were significantly different in these two groups in favor of the younger patients, with an increase of + 40.7 hairs/cm2 in the younger patients versus + 25.4 hairs/cm2 in the older ones. This difference is likely due to the shorter duration of hair loss, the lower classification of AGA severity and the larger hair diameter in the younger group at baseline, although the baseline hair counts were not significantly different in the two groups. In addition, the aging process also contributes to the treatment outcomes: hair density and diameter decrease with advancing age, due not only to androgen-mediated processes but also to the systemic senescence process, such as oxidative stress and stem cell apoptosis, found in those aged > 50 years [15]. The authors of a previous study reported that finasteride is also unlikely to stimulate significant hair regrowth in older men, and older men who take finasteride 5 mg for prostate gland hypertrophy show no significant hair growth [16].
Hair growth in patients on oral minoxidil was clearly observed in the global photographic assessment, with a 100% improvement (score > + 1 or + 1–40% improvement) on the vertex area at 24 weeks. An improvement scale score of + 2 (moderate increase, + 41–70% improvement) and 3 (large increase, > + 70–100% improvement) were reported in 93.3% of patients in the vertex and 73.3% of patients in the frontal area. The frontal area also showed a significant response, but less than that of the vertex, which is consistent with many previous reports [17, 18]. In previous studies, the vertex type of AGA showed a more rapid and obvious improvement with oral 1 mg finasteride compared with the other areas [18, 19].
Although we did make a direct comparison of oral minoxidil 5 mg once daily to other standard medications for AGA, our results suggest that oral minoxidil at this dose is superior to a 2–5% minoxidil solution, 5% minoxidil foam, finasteride 1 mg, and dutasteride 0.5 mg daily in all measurements (Table 4). Our global photographic assessment illustrates distinctive results in the oral minoxidil group, with all patients showing positive response. A comparison of total hair counts in patients treated with oral minoxidil in our study with those in other studies (see Table4) showed a 19.2% increase among the patients in our study, followed by those treated with 5% minoxidil foam (13.4% increase), dutasteride (11.67%), and oral finasteride (7.2–9%).
Table 4.
Comparison of oral minoxidil with other standard medical treatments
| Study | Drugs | Duration of treatment | Number of subjects in study | Panel | Total hair count | Non-vellus hair count |
|---|---|---|---|---|---|---|
| This study | Minoxidil oral 5 mg/day | 24 weeks | 30 | 100.0% | 19.2% | 23.5% |
| Olsen et al.a | 5% Minoxidil solution, vs 2% minoxidil vs placebo | 48 weeks | 393 | 57.9% vs 40.8% vs 23.2% | 12.3% vs 8.8 vs 2.6% | |
| Olsen et al.b | 5% Minoxidil foam, vs placebo | 16 weeks | 352 | 38.3% vs 5.2% | 13.4% vs 3.4% | |
| Roberts et al.c | Finasteride 1 mg/day, vs placebo | 12 months | 693 | 54% vs 3% | 9.0% vs − 2.1 | |
| Neste et al.d | Finasteride 1 mg/day, vs placebo | 48 weeks | 212 | 7.2 vs − 10.1 (8.3% better) | ||
| Harcha et al.e | Dutasteride 0.5 mg/day vs finasteride 1 mg/day vs placebo | 24 weeks | 153 | 47% vs 36% vs 7% | 11.67% vs 7.4% vs − 0.64% |
aOlsen EA et al. J Am Acad Dermatol. 2002;47(3):377–85
bOlsen EA et al. J Am Acad Dermatol. 2007;57(5):767–74
cRoberts JL et al. J Am Acad Dermatol. 1999;41(4):555–63
dNeste D et al. Br J Dermatol. 2000;143(4):804–810
eHarcha WG et al. J Am Acad Dermatol. 2014;70(3):489–98
In terms of safety, the results of physical examinations, laboratory evaluations, and imaging were assessed in our study. The common side effects of oral minoxidil were hypertrichosis (93%) and pedal edema (10%). Hypertrichosis is a well-recognized adverse effect of either oral or topical minoxidil, and the extent of this AE has been suggested to be dose dependent [20]. This condition is typically reversible within 3–5 months and does not always require discontinuation of therapy [21, 22]. However, for patients with marked hypertrichosis or psychosocial disturbances, laser hair removal is an option to get rid of unwanted hairs [23]. All patients in this study were able to accept the hypertrichosis, which usually resolves within 2–3 weeks after stopping the medication [24]. We observed pedal edema in patients with obesity and poorly controlled hypertension. This condition is caused by sodium and water retention, with blood flow redistributed from the outer to the inner cortex of the kidney [1, 25]. Therefore, a low salt diet should be recommended to patients who are taking oral minoxidil. However, the prevalence of edema in patients on low-dose oral minoxidil is far less than that in patients taking the standard dose (10–40 mg/day), which ranges from 7 to 100% of patients [1].
Regarding the hemodynamic effects, the first case of orthostatic hypotension was found in two patients, with a drop in systolic BP of + 2.5 mm/Hg. Based on this observation, we recommend that the first dose of oral minoxidil be administrated before bedtime to avoid this side effect [1, 26]. At low dosage, there was no significant change in BP and PR at 24 weeks after treatment, with a mean systolic BP decrease of − 3.8 mmHg, mean diastolic BP decrease of − 1.1 mmHg and a PR increase of + 0.6/min. The most serious side effects of minoxidil reported to date are cardiovascular complications, with ischemic heart disease, pericarditis, pericardial effusion and tamponade, pulmonary hypertension, and high output cardiac failure reported [2]. Angina may worsen or appear for the first time during minoxidil treatment, probably due to the increased oxygen demands associated with increased heart rate and cardiac output [27, 28]. In one study, pericardial effusion caused by minoxidil occurred in approximately 3–5% of patients, but the mechanism remained unknown [28]. An association between pericardial effusion and renal status is assumed. In some studies, pericardial effusion was often associated with renal disease, uremic syndrome, a connective tissue disease, congestive heart failure, or marked fluid retention [2, 3, 29]. When pericardial effusion is suspected, EKG should be performed. In earlier studies, EKG changes, such as ST-segment depression and T-wave flattening and inversion, were reported in up to 90% of patients in the first 2 weeks of minoxidil treatment for hypertension [26, 30]; basic blood chemistry and chest X-rays were largely within normal range. In our study, no SAEs were found and low-dose oral minoxidil was generally well-tolerated. From the safety point of view, we recommend not prescribing oral minoxidil to elderly patients with an increased risk for myocardial infarction, heart failure, chronic renal failure, or severe hypertension; oral minoxidil should be considered only in healthy subjects.
To our knowledge, this is the first prospective clinical trial studying the therapeutic effects of oral minoxidil in male AGA using a standardized hair count method, hair mass index, global photographic assessment, and patient satisfaction. The strength of our study is the comprehensive investigations on safety the profile of oral minoxidil, which is the main concern of most physicians. A limitation of the current study is that it was neither randomized nor controlled but rather an open-label clinical trial. A study population of 30 patients can be considered to be a relatively small sample. In the future, randomized, double-blind, placebo-controlled studies with longer study period are required to accurately identify the efficacy and long-term safety of low-dose oral minoxidil for the treatment of AGA.
Conclusion
Oral minoxidil at a dose of 5 mg once daily significantly increased hair growth in men with AGA after 12 and 24 weeks of treatment. This medication is widely available as 5 mg tablets, is inexpensive, and can be considered safe in healthy subjects, with minor side effects of hypertrichosis and pedal edema. However, the use of oral minoxidil in people who have severe hypertension and risk of cardiovascular events should be carefully planned.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Dr. Pravit Asawanonda, who provided key intellectual support and revised the manuscript. We also thank the study participants, without whom this study would not have been accomplished.
Funding
This study was funded by the Ratchadapisek Sompoch Endowment Fund, Chulalongkorn University, and the Pericardia Program of The Royal College of Physicians of Thailand. The Rapid Service Fee was funded by the Faculty of Medicine, Chulalongkorn University.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
RP developed and designed the manuscript and participated in collecting and interpreting the data. SL collected, analyzed, and interpreted the data and coordinated the project. RP wrote the manuscript and revised it critically. All authors read and approved the final version of manuscript.
Disclosures
Ratchathorn Panchaprateep and Saoraya Lueangarun have nothing to disclose.
Compliance with Ethics Guidelines
The study protocol and informed consent form were reviewed and approved by the Institutional Review Board of Faculty of Medicine, Chulalongkorn University (IRB number 496/55). This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Informed consent for publication was obtained for each participant.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files and also available from the corresponding author on reasonable request.
Footnotes
Digital Features
To view digital features for this article go to 10.6084/m9.figshare.12937661.
References
- 1.Gilmore E, Weil J, Chidsey C. Treatment of essential hypertension with a new vasodilator in combination with beta-adrenergic blockade. N Engl J Med. 1970;282(10):521–527. doi: 10.1056/NEJM197003052821001. [DOI] [PubMed] [Google Scholar]
- 2.Hagstam KE, Lundgren R, Wieslander J. Clinical experience of long-term treatment with minoxidil in severe arterial hypertension. Scand J Urol Nephrol. 1982;16(1):57–63. doi: 10.3109/00365598209179641. [DOI] [PubMed] [Google Scholar]
- 3.Devine BL, Fife R, Trust PM. Minoxidil for severe hypertension after failure of other hypotensive drugs. Br Med J. 1977;2(6088):667–669. doi: 10.1136/bmj.2.6088.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jimenez-Cauhe J, Saceda-Corralo D, Rodrigues-Barata R, et al. Effectiveness and safety of low-dose oral minoxidil in male androgenetic alopecia. J Am Acad Dermatol. 2019;81:648–649. doi: 10.1016/j.jaad.2019.04.054. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka Y, Aso T, Ono J, Hosoi R, Kaneko T. Androgenetic alopecia treatment in Asian men. J Clin Aesthet Dermatol. 2018;11(7):32–35. [PMC free article] [PubMed] [Google Scholar]
- 6.Wester RC, Maibach HI, Guy RH, Novak E. Minoxidil stimulates cutaneous blood flow in human balding scalps: pharmacodynamics measured by laser Doppler velocimetry and photopulse plethysmography. J Invest Dermatol. 1984;82(5):515–517. doi: 10.1111/1523-1747.ep12261084. [DOI] [PubMed] [Google Scholar]
- 7.Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150(2):186–194. doi: 10.1111/j.1365-2133.2004.05785.x. [DOI] [PubMed] [Google Scholar]
- 8.Mori O, Uno H. The effect of topical minoxidil on hair follicular cycles of rats. J Dermatol. 1990;17(5):276–281. doi: 10.1111/j.1346-8138.1990.tb01641.x. [DOI] [PubMed] [Google Scholar]
- 9.Badri T, Nessel TA, Kumar DD. Minoxidil. StatPearls [Internet]. Treasure Island: StatPearls Publishing; 2020.
- 10.Bardelli A, Rebora A. Telogen effluvium and minoxidil. J Am Acad Dermatol. 1989;21(3 Pt 1):572–573. doi: 10.1016/S0190-9622(89)80231-2. [DOI] [PubMed] [Google Scholar]
- 11.Sinclair RD. Female pattern hair loss: a pilot study investigating combination therapy with low-dose oral minoxidil and spironolactone. Int J Dermatol. 2018;57(1):104–109. doi: 10.1111/ijd.13838. [DOI] [PubMed] [Google Scholar]
- 12.Jimenez-Cauhe J, Saceda-Corralo D, Rodrigues-Barata R, et al. Effectiveness and safety of low-dose oral minoxidil in male androgenetic alopecia. J Am Acad Dermatol. 2019;81(2):648–649. doi: 10.1016/j.jaad.2019.04.054. [DOI] [PubMed] [Google Scholar]
- 13.Aldhalimi MA, Hadi NR, Ghafil FA. Promotive effect of topical ketoconazole, minoxidil, and minoxidil with tretinoin on hair growth in male mice. ISRN Pharmacol. 2014;2014:575423. doi: 10.1155/2014/575423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Therianou A, Vincenzi C, Tosti A. How safe is prescribing oral minoxidil in patients allergic to topical minoxidil? J Am Acad Dermatol. 2020 doi: 10.1016/j.jaad.2020.04.027. [DOI] [PubMed] [Google Scholar]
- 15.Karnik P, Shah S, Dvorkin-Wininger Y, Oshtory S, Mirmirani P. Microarray analysis of androgenetic and senescent alopecia: comparison of gene expression shows two distinct profiles. J Dermatol Sci. 2013;72(2):183–186. doi: 10.1016/j.jdermsci.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mirmirani P. Age-related hair changes in men: mechanisms and management of alopecia and graying. Maturitas. 2015;80(1):58–62. doi: 10.1016/j.maturitas.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Leyden J, Dunlap F, Miller B, et al. Finasteride in the treatment of men with frontal male pattern hair loss. J Am Acad Dermatol. 1999;40(6 Pt 1):930–937. doi: 10.1016/S0190-9622(99)70081-2. [DOI] [PubMed] [Google Scholar]
- 18.Shin JW, Chung EH, Kim MB, Kim TO, Kim WI, Huh CH. Evaluation of long-term efficacy of finasteride in Korean men with androgenetic alopecia using the basic and specific classification system. J Dermatol. 2019;46(2):139–143. doi: 10.1111/1346-8138.14719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Z, Song S, Gao Z, Wu J, Ma J, Cui Y. The efficacy and safety of dutasteride compared with finasteride in treating men with androgenetic alopecia: a systematic review and meta-analysis. Clin Interv Aging. 2019;14:399–406. doi: 10.2147/CIA.S192435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baral J. Scalp comedones after topical minoxidil. J Am Acad Dermatol. 1985;13(6):1051–1052. doi: 10.1016/S0190-9622(85)80495-3. [DOI] [PubMed] [Google Scholar]
- 21.Peluso AM, Misciali C, Vincenzi C, Tosti A. Diffuse hypertrichosis during treatment with 5% topical minoxidil. Br J Dermatol. 1997;136(1):118–120. doi: 10.1046/j.1365-2133.1997.d01-1156.x. [DOI] [PubMed] [Google Scholar]
- 22.Dawber RP, Rundegren J. Hypertrichosis in females applying minoxidil topical solution and in normal controls. J Eur Acad Dermatol Venereol. 2003;17(3):271–275. doi: 10.1046/j.1468-3083.2003.00621.x. [DOI] [PubMed] [Google Scholar]
- 23.Benmously Mlika R, Ben Hamida M, Hammami H, Dorbani Ben Thabet I, Rouatbi M, Mokhtar I. Long-pulsed Nd:YAG laser in the treatment of facial hypertrichosis during topical minoxidil therapy. J Cosmet Laser Ther. 2013;15(4):217–218. doi: 10.3109/14764172.2013.764434. [DOI] [PubMed] [Google Scholar]
- 24.Keusch GW, Weidmann P, Campese V, Lee DB, Upham AT, Massry SG. Minoxidil therapy in refractory hypertension analysis of 155 patients. Nephron. 1978;21(1):1–15. doi: 10.1159/000181366. [DOI] [PubMed] [Google Scholar]
- 25.Linas SL, Nies AS. Minoxidil. Ann Intern Med. 1981;94(1):61–65. doi: 10.7326/0003-4819-94-1-61. [DOI] [PubMed] [Google Scholar]
- 26.Franciosa JA, Jordan RA, Wilen MM, Leddy CL. Minoxidil in patients with chronic left heart failure: contrasting hemodynamic and clinical effects in a controlled trial. Circulation. 1984;70(1):63–68. doi: 10.1161/01.CIR.70.1.63. [DOI] [PubMed] [Google Scholar]
- 27.Miller DD, Love DW. Evaluation of minoxidil. Am J Hosp Pharm. 1980;37(6):808–814. [PubMed] [Google Scholar]
- 28.Lowenthal DT, Affrime MB. Pharmacology and pharmacokinetics of minoxidil. J Cardiovasc Pharmacol. 1980;2(Suppl 2):S93–S106. doi: 10.1097/00005344-198000022-00002. [DOI] [PubMed] [Google Scholar]
- 29.Kosman ME. Evaluation of a new antihypertensive agent. Minoxidil. JAMA. 1980;244(1):73–75. doi: 10.1001/jama.1980.03310010059037. [DOI] [PubMed] [Google Scholar]
- 30.Hall D, Charocopos F, Froer KL, Rudolph W. ECG changes during long-term minoxidil therapy for severe hypertension. Arch Intern Med. 1979;139(7):790–794. doi: 10.1001/archinte.1979.03630440052018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article/as supplementary information files and also available from the corresponding author on reasonable request.