Abstract
Introduction
Atopic dermatitis (AD) is a chronic, relapsing, inflammatory skin disease characterized by eczema and pruritus, and frequently impairs sleep quality. Although cyclosporine improves symptoms of AD, objective evaluation of sleep in patients with AD treated with cyclosporine has not been reported. This study was conducted to elucidate the effects of cyclosporine on sleep quality for patients with AD.
Methods
Twelve patients with moderate-to-severe AD were recruited. Nocturnal sleep quality was evaluated for 7 days using a sleep analyzer, which patients wore at the waist before and after cyclosporine was administered at 2.0–4.0 mg/kg per day. Seven parameters of sleep quality were measured before and after cyclosporine administration for a period of 7 days for each patient.
Results
The administration of cyclosporine significantly improved total sleep time in four cases, sleep latency in two cases, wake after sleep onset in six cases, number of awakenings in two cases, sleep efficiency in seven cases, number of awakenings for more than 8 min in three cases, and number of position changes recorded every 2 min in three cases. The mean values of sleep latency significantly decreased after cyclosporine administration (P = 0.023). The mean value of sleep efficiency significantly increased after the administration (P = 0.002).
Conclusion
Cyclosporine improves sleep quality in patients with moderate-to-severe AD.
Electronic supplementary material
The online version of this article (10.1007/s13555-020-00451-2) contains supplementary material, which is available to authorized users.
Keywords: Atopic dermatitis, Cyclosporine, Pruritus, Quality of life, Sleep
Key Summary Points
| Why carry out the study? |
| Effects of cyclosporine on sleep in patients suffering from atopic dermatitis have not been objectively evaluated yet. |
| What was learned from the study? |
| Sleep quality of 12 patients with atopic dermatitis treated with cyclosporine was analyzed by actigraphy, which is an objective analyzer for sleep quality, in this study. |
| Cyclosporine improves sleep quality including sleep latency and sleep efficiency in patients with atopic dermatitis. |
Digital Features
This article is published with digital features to facilitate understanding of the article. You can access the digital features on the article’s associated Figshare page. To view digital features for this article go to 10.6084/m9.figshare.12958025.
Introduction
Atopic dermatitis (AD) is a chronic, relapsing, inflammatory skin disease characterized by eczema and pruritus [1, 2]. Although most patients with AD can achieve adequate disease control with topical agents and/or ultraviolet light, a subpopulation of patients with moderate-to-severe AD does not show adequate control by these treatments [3]. In such uncontrolled patients, the most burdensome symptoms are pruritus followed by excessive dryness/scaling and inflamed skin [4].
Several studies have evaluated sleep quality in patients with AD using a visual analog scale (VAS) as a subjective evaluation tool, included in SCORing Atopic Dermatitis, a scoring system for AD severity [5–9]. Recently, various objective tools have been applied to the evaluation of sleep quality for patients with AD. Indeed, the objective tools such as actigraphy, polysomnography, and infrared video have been reported to potentially detect prolonged sleep onset latency, more wake time after sleep onset, lower sleep efficiency, less non-rapid eye movement (NREM) sleep, more sleep fragmentation, and more scratching and movement in sleep of patients with AD [10].
These objective tools usually require specialists for analysis and handling, resulting in their low utilization. In contrast, the Sleep-Sign-Act system (Kissei Comtec, Matsumoto, Japan), a type of actigraphy, is an alternative choice to evaluate nocturnal sleep quality of patients with AD, owing to (i) ease of device handling; (ii) ease of the results analysis procedure; (iii) small size of the device, which does not prevent the patient's sleep; (iv) high reliability of the device/procedure that has been proven by a high concordance rate of 85–87% with polysomnography [11]; and (v) adequate performance demonstrated by multiple previous studies [12, 13].
Bender et al. examined sleep efficiency and scratching using the objective tools including polysomnography and actigraphy, and subjective tools including self-report in 20 adult patients with AD, and the authors revealed that objective tools, but not self-report, accurately reflected the severity of AD associated with pruritus and sleep disturbance [14]. Therefore, the effects of therapeutic options for AD on sleep quality should be objectively evaluated. This study was conducted to elucidate the effects of cyclosporine on nocturnal sleep quality in patients with AD by using Sleep-Sign-Act system as an alternative objective tool for sleep quality evaluation.
Methods
Patients
The ethics committee of the Sleep Clinic Chofu approved the study protocol (approval number, SC-IRB20200403a), and this study was approved by all institutions. All patients provided written informed consent before enrollment. This study was performed in accordance to the Helsinki Declaration of 1964 and its later amendments.
This study recruited 12 patients with AD (eight male, four female) who met the following inclusion criteria: (i) referral to Sleep Clinic Chofu for subjective sleep symptoms including difficulty in falling asleep, wake after sleep onset, early-morning awakening, and lack of deep sleep; (ii) fulfillment of the diagnostic criteria for AD described by Hanifin and Rajka [15]; (iii) moderate-to-severe AD according to three severity criteria: score ≥ 3 in investigator global assessment, score ≥ 16 in eczema area and severity index (EASI), and score ≥ 10% in body surface area; (iv) treatment with cyclosporine; and (v) agreement of the assessments by actigraphy for nocturnal sleep quality. We excluded patients with AD who met the following exclusion criteria: (i) complication of psychiatric disorder such as schizophrenia and depression; (ii) complication of organic brain disorders such as brain tumor and cerebrovascular disorder; (iii) administration of psychotropic agent, except for hypnotic drugs, of which dose was not changed during the analysis; (iv) administration of systemic immunosuppressants and systemic corticosteroids; (v) treatment with phototherapy; (vi) self-administration of any drugs during the analysis; and (vii) pregnancy and breastfeeding.
The mean age of recruited patients was 36.6 years (range 25–51 years). Cyclosporine was administered at 2.0–4.0 mg/kg per day individually. The patients were allowed to use emollients, topical corticosteroids, topical calcineurin inhibitors, and oral antihistamines; the doses of these drugs were not changed during the analysis, except for case 3, in which class II topical corticosteroid treatment was started at the initial administration of cyclosporine.
Sleep Assessments
Nocturnal sleep quality was examined before initiation of cyclosporine and at 14–28 days after initiation. Sleep quality was evaluated over a period of 7 days using the Sleep-Sign-Act version 2.0 sleep analyzer (Kissei Comtec), which patients wore at their waist. The analyzer recorded the following parameters: (i) total sleep time, defined as the time calculated by subtracting wake time from the duration of sleep onset to final awakening (minutes); (ii) sleep latency, defined as the time from start of recording to onset of sleep (minutes); (iii) wake after sleep onset, defined as the time awake during the period from sleep onset to final awakening (minutes); (iv) number of awakenings, defined as the number of awakenings in the duration from sleep onset to final awakening (times); (v) sleep efficiency, defined as the percentage calculated by total sleep time divided by time in bed (%); (vi) number of awakenings for more than 8 min (times); and (vii) number of position changes recorded every 2 min (times). According to the literature, increasing total sleep time and sleep efficiency along with decreasing sleep latency, wake after sleep onset, number of awakenings, number of awakenings for more than 8 min, and number of position changes recorded every 2 min were regarded as reflecting improvements in sleep quality.
Statistical Analysis
Statistical analysis was performed using SPSS version 22 software (SPSS Japan, Tokyo, Japan). Statistical significance was analyzed by Wilcoxon signed-rank test for the comparison of mean values of median value in each patient before and after cyclosporine administration and two-way factorial analysis of variance followed by Bonferroni’s multiple comparison analysis for the comparison of the values in each individual patient before and after the administration. Values of P < 0.05 were considered as statistically significant.
Results
Changes in Sleep Parameters Before and After Cyclosporine Administration
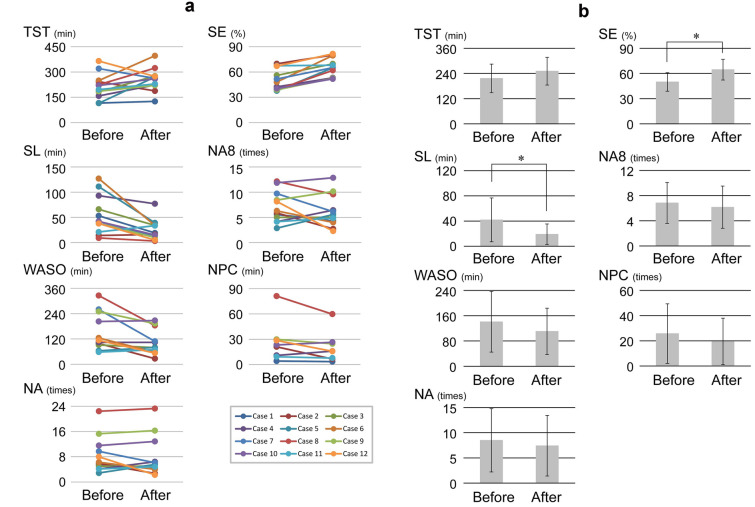
Each parameter of nocturnal sleep quality was measured before and after cyclosporine administration for a period of 7 days for each patient (Fig. 1a). Total sleep time increased in nine patients and decreased in three patients. Sleep latency increased in three patients and decreased in nine patients. Wake after sleep onset increased in eight patients, decreased in three patients, and was unchanged in one patient. Number of awakenings increased in six patients, decreased in five patients, and was unchanged in one patient. Sleep efficiency increased in all 12 patients. Number of awakenings for more than 8 min increased in five patients, decreased in six patients, and was unchanged in one patient. Number of position changes recorded every 2 min increased in two patients and decreased in six patients. None of the 12 patients showed any severe adverse effects during the study period. The mean values of sleep latency was significantly decreased after cyclosporine administration (P = 0.023) (Fig. 1b). The mean value of sleep efficiency was significantly increased after cyclosporine administration (P = 0.002). The mean values of other parameters did not change significantly.
Fig. 1.
Changes in sleep parameters from before to after cyclosporine. a Changes in each sleep parameter of individual patients. “Before” and “After” indicate time points before and after cyclosporine administration, respectively. b Changes in mean values of each sleep parameter. Asterisks indicate significant difference. Error bars represent standard deviation. TST total sleep time (minutes), SL sleep latency (minutes), WASO wake after sleep onset (minutes), NA number of awakenings (times), SE sleep efficiency (%), NA8 number of awakenings for more than 8 min (times), NPC number of position changes recorded every 2 min (times)
Changes in Each Sleep Parameter of Individual Patients
Changes in each sleep parameter of individual patients with AD before and after cyclosporine administration were statistically analyzed (Tables 1, 2). Significant improvements were seen for total sleep time in four patients, sleep latency in two patients, wake after sleep onset in six patients, number of awakenings in two patients, sleep efficiency in seven patients, number of awakenings for more than 8 min in three patients, and number of position changes recorded every 2 min in three patients (Fig. 2). Moreover, significant exacerbation of total sleep time was seen in one patient. These data also indicated that (i) cyclosporine improved at least one of the seven sleep parameters in eight patients, although it did not improve any parameter in four patients; and (ii) sensitivity to cyclosporine was different among parameters.
Table 1.
Median values in each sleep parameter before and after cyclosporine administration in individual patients
| TST (min) | SL (min) | WASO (min) | NA (times) | SE (%) | NA8 (times) | NPC (times) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | Before | After | Before | After | Before | After | |
| Case 1 | 114 | 118 | 20 | 8 | 90 | 70 | 6 | 4 | 44.1 | 50.6 | 6 | 4 | 4 | 3 |
| Case 2 | 232 | 198 | 6 | 14 | 70 | 30 | 6 | 3 | 69.1 | 76.9 | 6 | 3 | 14 | 5 |
| Case 3 | 194 | 282 | 66 | 28 | 64 | 80 | 4 | 6 | 50.9 | 70.5 | 4 | 6 | ND | ND |
| Case 4 | 168 | 190 | 9856 | 60 | 102 | 4 | 6 | 42.9 | 48.9 | 4 | 6 | 10 | 12 | |
| Case 5 | 114 | 242 | 86 | 14 | 68 | 84 | 3 | 4 | 38.3 | 62.7 | 3 | 4 | ND | ND |
| Case 6 | 238 | 382 | 96 | 34 | 130 | 52 | 4 | 4 | 47.4 | 78.9 | 4 | 4 | ND | ND |
| Case 7 | 330 | 282 | 16 | 18 | 268 | 140 | 11 | 6 | 53.6 | 63.9 | 11 | 6 | ND | ND |
| Case 8 | 240 | 288 | 8 | 2 | 314 | 204 | 24 | 23 | 40.8 | 65.0 | 12 | 10 | 78 | 59 |
| Case 9 | 208 | 230 | 36 | 6 | 262 | 240 | 16 | 13 | 38.3 | 48.4 | 8 | 11 | 31 | 27 |
| Case 10 | 204 | 268 | 28 | 10 | 216 | 222 | 12 | 13 | 43.3 | 51.6 | 12 | 13 | 26 | 27 |
| Case 11 | 230 | 234 | 16 | 36 | 60 | 42 | 5 | 4 | 68.6 | 75.2 | 5 | 4 | 10 | 6 |
| Case 12 | 328 | 296 | 28 | 4 | 88 | 60 | 7 | 3 | 61.5 | 81.7 | 7 | 3 | 33 | 17 |
TST total sleep time, SL sleep latency, WASO wake after sleep onset, NA number of awakenings, SE sleep efficiency, NA8 number of awakenings for more than 8 min, NPC number of position changes recorded every 2 min, ND no data
Table 2.
P values in comparisons of sleep parameters before and after cyclosporine administration
| TST | SL | WASO | NA | SE | NA8 | NPC | |
|---|---|---|---|---|---|---|---|
| Case 1 | 0.802 | 0.156 | 0.649 | 0.442 | 0.008 | 0.360 | 0.889 |
| Case 2 | 0.182 | 0.913 | 0.012 | 0.061 | 0.101 | 0.026 | 0.006 |
| Case 3 | 0.016 | 0.175 | 0.628 | 1.000 | 0.036 | 1.000 | ND |
| Case 4 | 0.108 | 0.520 | 0.976 | 0.201 | 0.095 | 0.105 | 0.331 |
| Case 5 | < 0.001 | 0.002 | 0.579 | 0.106 | < 0.001 | 0.055 | ND |
| Case 6 | < 0.001 | < 0.001 | 0.018 | 0.172 | < 0.001 | 0.105 | ND |
| Case 7 | 0.148 | 0.314 | < 0.001 | 0.034 | 0.035 | 0.012 | ND |
| Case 8 | 0.015 | 0.798 | < 0.001 | 0.608 | < 0.001 | 0.068 | < 0.001 |
| Case 9 | 0.534 | 0.230 | 0.045 | 0.549 | 0.033 | 0.223 | 0.304 |
| Case 10 | 0.324 | 0.249 | 0.872 | 0.442 | 0.073 | 0.476 | 0.540 |
| Case 11 | 0.395 | 0.593 | 0.694 | 0.669 | 0.921 | 0.611 | 0.759 |
| Case 12 | 0.024 | 0.163 | 0.034 | 0.001 | 0.024 | < 0.001 | 0.014 |
TST total sleep time, SL sleep latency, WASO wake after sleep onset, NA number of awakenings, SE sleep efficiency, NA8 number of awakenings for more than 8 min, NPC number of position changes recorded every 2 min, ND no data
Fig. 2.
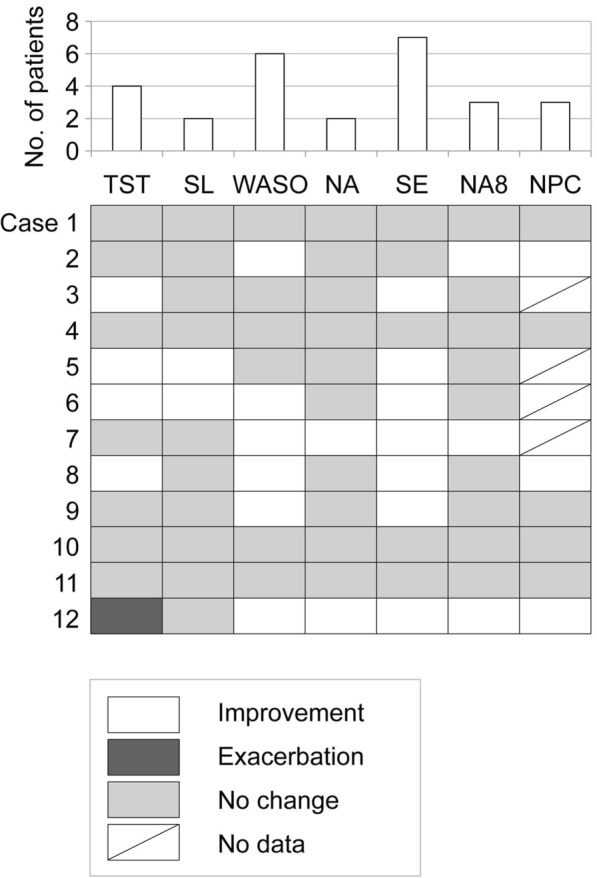
Statistical evaluations for sleep parameter changes in individual patients. Tile plot indicates statistical outcomes of changes in sleep parameters before and after cyclosporine administration of individual patients with AD. White, dark gray, and light gray squares indicate significant improvement, significant exacerbation, and no significant change, respectively. Diagonal marks in white squares indicate lack of data. Upper bar graph indicates number of patients showing significant improvements in each sleep parameter. Tile plot and bar graph share an x-axis, indicating each sleep parameter. TST total sleep time (minutes), SL sleep latency (minutes), WASO wake after sleep onset (minutes), NA number of awakenings (times), SE sleep efficiency (%), NA8 number of awakenings for more than 8 min (times), NPC number of position changes recorded every 2 min (times)
Discussion
This study offers the preliminary objective evidence that cyclosporine improves nocturnal sleep quality in patients with moderate-to-severe AD analyzed by objective procedures. Cyclosporine is potentially associated with improved quality of life in patients with moderate-to-severe AD through the favorable effects on sleep.
From the perspective of each patient, cyclosporine significantly improved at least one of the seven parameters in eight of the 12 examined patients. Case 3 did not show a remarkable improvement compared to other cases, although class II topical corticosteroid treatment was started at the initiation of cyclosporine administration. From the perspective of each parameter, cyclosporine significantly improved sleep efficiency in seven patients and wake after sleep onset in six patients. On the other hand, cyclosporine significantly improved mean values of sleep efficiency and sleep latency, although it did not significantly improve mean values of the other parameters. These observations suggested that (i) cyclosporine can shorten the time until onset of sleep in bed, resulting in better sleep efficiency; and (ii) cyclosporine may not always contribute to relieve the difficulty falling asleep in terms of onset of sleep discontinuation. Overall, cyclosporine is strongly suggested to improve sleep quality in patients with moderate-to-severe AD, which might be explained by two possible mechanisms: (i) cyclosporine reduces pruritus, which impairs sleep quality; and (ii) cyclosporine regulates the cytokines/chemokines associated with sleep.
Sleep quality in patients with AD has been evaluated by various subjective tools. Silverberg et al. examined sleep disturbance in patients with AD by a cross-sectional questionnaire of 34,613 adults and reported that eczema was associated with fatigue [odds ratio (OD), 2.97], regular daytime sleepiness (OD, 2.66), and regular insomnia (OD, 2.36) [16], roughly in line with a study reported by Li et al. in which sleep disturbance was observed in most adult patients with AD [17]. Ramirez et al. studied 13,988 children, including 4938 children with AD, by using questionnaires, and reported that nighttime awakenings, regular early morning awakenings, difficulty falling asleep, and nightmares were detected in 15–65% of children with AD, although total sleep duration was similar between children with active AD and without AD [18]. Thus, the previous studies demonstrated that AD definitely impairs subjective sleep quality. On the other hand, our data objectively showed that cyclosporine administration significantly improved sleep latency but not total sleep time. Cyclosporine might be ineffective on the improvement of total sleep time in this study, because the total sleep time of our patients was not originally impaired by AD only.
As it is known that pruritus impairs sleep quality in patients with AD [10], Kaaz et al. analyzed the impact of pruritus on sleep quality among 100 patients with AD and indicated a substantial association with insomnia [19]. Several recent studies have reported that patients with inadequately controlled AD exhibit a higher burden of pruritus, as well as more frequent sleep disturbance, including longer sleep latency and increasing needs of over-the-counter sleep medications compared to patients with controlled AD [20, 21]. On the other hand, there is limited direct evidence to show the effects of cyclosporine on both sleep and pruritus concurrently in patients with AD; moreover, those effects were evaluated with subjective tools. Sowden et al. reported that cyclosporine administration significantly improved concurrently both loss of sleep and pruritus; (i) each VAS score of loss of sleep was 13.8 and 41.9 in cyclosporine-treated patients and placebo-treated patients, respectively, and (ii) each VAS score of pruritus was 16.6 and 51.2 in cyclosporine-treated patients and placebo-treated patients, respectively [22]. This report provides high-quality but vague evidence of sleep quality improvement by cyclosporine in patients with AD. In addition to that, our study potentially provides the specific information that cyclosporine administration improves sleep efficiency and sleep latency in patients with moderate-to-severe AD.
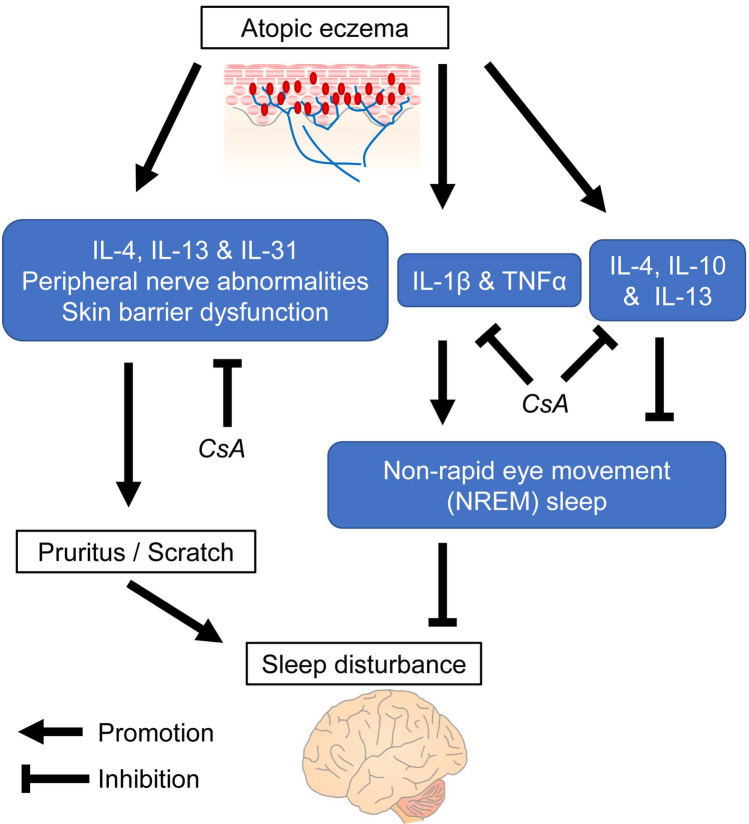
Cyclosporine alleviates pruritus, potentially by inhibiting pruritus-inducing cytokines such as interleukin (IL)-4, IL-13, and IL-31, and by ameliorating peripheral nerve abnormalities and skin barrier dysfunction [23, 24]. These facts strongly support the hypothesis that the favorable effects of cyclosporine on sleep quality in patients with AD are responsible for improvement of pruritus. On the other hand, some cytokines associated with AD directly impair sleep quality. IL-4, IL-10, and IL-13 have been reported to decrease NREM sleep amount [25]. Khattri et al. suggest that cyclosporine reduces expression of type 2 T helper cell (Th2)-related molecules, including IL-4 in patients with AD [23]. Such mechanisms for cyclosporine may contribute to the improvement of sleep quality (Fig. 3). However, cytokines that regulate NREM sleep including tumor necrosis factor-α, interferon-α, IL-1β, IL-2, and IL-6 can also be negatively modulated by cyclosporine in patients with AD [25, 26]. Moreover, the mechanisms of sleep quality improvement by cyclosporine through sleep-associated cytokines/chemokines still cannot be easily understood, as suggested by Xerfan et al. [27]. Therefore, more studies are required to evaluate the effects of cyclosporine on sleep-associated cytokines/chemokines.
Fig. 3.
Hypothetical points where cyclosporine acts on sleep disturbance-related cytokines in atopic dermatitis. Hypothetical signaling pathway in sleep disturbance due to atopic eczema, and points where cyclosporine acts on the pathway are shown. CsA cyclosporine, TNFα tumor necrosis factor-α
For patients with moderate-to-severe AD, systemic immune-modulating treatments including cyclosporine and dupilumab are indicated. Cyclosporine is sometimes preferentially selected as the systemic therapy for patients with AD rather than dupilumab, because (i) the efficacy of cyclosporine for clinical signs of AD has been repeatedly and sufficiently confirmed in multiple clinical trials over the past 20 years [28]; (ii) cyclosporine is usually cheaper than dupilumab; and (iii) the duration of cyclosporine administration is shorter than dupilumab. In addition to aforementioned reasons, this study potentially shows a merit of cyclosporine which improves sleep quality in patients with moderate-to-severe AD.
Some limitations of this study must be considered. First, the therapeutic options other than cyclosporine were not completely controlled before or after cyclosporine administration; in particular, topical corticosteroid treatment was added for case 3 at the initiation of cyclosporine administration. Second, the data on the severity of eruptions and pruritus were lacking after the initiation of cyclosporine administration; such data are important to obtain a clearer understanding about changes in sleep quality. Third, the number of examined patients was relatively small, which may impair the reliability of the data. Fourth, the sleep analyses were performed within 1 month after the initiation of cyclosporine. However, Haw et al. reported that during longer-term cyclosporine administration, effects appeared during the 6-month follow-up period [29]. Therefore, if our analyses were performed at later time points after the initiation of cyclosporine administration, better results would have been demonstrated. Fifth, the selection bias in this study should be considered due to referred patients. Ideally, the patients with AD who are referred to the dermatology clinic should be recruited.
Conclusions
Cyclosporine is strongly suggested to improve sleep quality in patients with moderate-to-severe AD.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Ken-ichi Yasuda, Yozo Ishiuji, Takuro Endo, Katsumi Tanito, Ryoichi Kamide, Yoshimasa Nobeyama and Akihiko Asahina have nothing to disclose.
Compliance with Ethics Guidelines
The ethics committee of the Sleep Clinic Chofu approved the study protocol (approval number, SC-IRB20200403a) and allowed all institutions belonging to us to participate in this study. All patients provided written informed consent before enrollment. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Data Availability
All data generated or analyzed during this study are included in this published article or as supplementary information files.
Footnotes
Digital Features
To view digital features for this article go to 10.6084/m9.figshare.12958025.
References
- 1.Bieber T. Atopic dermatitis. N Engl J Med. 2008;358(14):1483–1494. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- 2.Thomsen SF. Atopic dermatitis: natural history, diagnosis, and treatment. ISRN Allergy. 2014;2014:354250. doi: 10.1155/2014/354250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simpson EL, Bruin-Weller M, Flohr C, et al. When does atopic dermatitis warrant systemic therapy? Recommendations from an expert panel of the International Eczema Council. J Am Acad Dermatol. 2017;77(4):623–633. doi: 10.1016/j.jaad.2017.06.042. [DOI] [PubMed] [Google Scholar]
- 4.Silverberg JI, Gelfand JM, Margolis DJ, et al. Patient burden and quality of life in atopic dermatitis in US adults: a population-based cross-sectional study. Ann Allergy Asthma Immunol. 2018;121(3):340–347. doi: 10.1016/j.anai.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Czech W, Brautigam M, Weidinger G, Schopf E. A body-weight-independent dosing regimen of cyclosporine microemulsion is effective in severe atopic dermatitis and improves the quality of life. J Am Acad Dermatol. 2000;42(4):653–659. [PubMed] [Google Scholar]
- 6.Bemanian MH, Movahedi M, Farhoudi A, et al. High doses intravenous immunoglobulin versus oral cyclosporine in the treatment of severe atopic dermatitis. Iran J Allergy Asthma Immunol. 2005;4(3):139–143. [PubMed] [Google Scholar]
- 7.Granlund H, Erkko P, Remitz A, et al. Comparison of cyclosporin and UVAB phototherapy for intermittent one-year treatment of atopic dermatitis. Acta Derm Venereol. 2001;81(1):22–27. doi: 10.1080/000155501750208137. [DOI] [PubMed] [Google Scholar]
- 8.Haeck IM, Knol MJ, Ten Berge O, van Velsen SG, de Bruin-Weller MS, Bruijnzeel-Koomen CA. Enteric-coated mycophenolate sodium versus cyclosporin A as long-term treatment in adult patients with severe atopic dermatitis: a randomized controlled trial. J Am Acad Dermatol. 2011;64(6):1074–1084. doi: 10.1016/j.jaad.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 9.Pacor ML, Di Lorenzo G, Martinelli N, Mansueto P, Rini GB, Corrocher R. Comparing tacrolimus ointment and oral cyclosporine in adult patients affected by atopic dermatitis: a randomized study. Clin Exp Allergy. 2004;34(4):639–645. doi: 10.1111/j.1365-2222.2004.1907.x. [DOI] [PubMed] [Google Scholar]
- 10.Chang YS, Chiang BL. Sleep disorders and atopic dermatitis: a 2-way street? J Allergy Clin Immunol. 2018;142(4):1033–1040. doi: 10.1016/j.jaci.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Nakazaki K, Kitamura S, Motomura Y, et al. Validity of an algorithm for determining sleep/wake states using a new actigraph. J Physiol Anthropol. 2014;33:31. doi: 10.1186/1880-6805-33-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuo M, Masuda F, Sumi Y, et al. Comparisons of portable sleep monitors of different modalities: potential as naturalistic sleep recorders. Front Neurol. 2016;7:110. doi: 10.3389/fneur.2016.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shibata S, Tsutou A, Shiotani H. Relation between sleep quality and daily physical activity in hemodialysis outpatients. Kobe J Med Sci. 2014;59(5):E161–E166. [PubMed] [Google Scholar]
- 14.Bender BG, Ballard R, Canono B, Murphy JR, Leung DY. Disease severity, scratching, and sleep quality in patients with atopic dermatitis. J Am Acad Dermatol. 2008;58(3):415–420. doi: 10.1016/j.jaad.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Hanifin J, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol (Stockh) 1980;92(suppl.):44–47. [Google Scholar]
- 16.Silverberg JI, Garg NK, Paller AS, Fishbein AB, Zee PC. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135(1):56–66. doi: 10.1038/jid.2014.325. [DOI] [PubMed] [Google Scholar]
- 17.Li JC, Fishbein A, Singam V, et al. Sleep disturbance and sleep-related impairment in adults with atopic dermatitis: a cross-sectional study. Dermatitis. 2018;29(5):270–277. doi: 10.1097/DER.0000000000000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramirez FD, Chen S, Langan SM, et al. Association of atopic dermatitis with sleep quality in children. JAMA Pediatr. 2019;173(5):e190025. doi: 10.1001/jamapediatrics.2019.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaaz K, Szepietowski JC, Matusiak L. Influence of itch and pain on sleep quality in atopic dermatitis and psoriasis. Acta Derm Venereol. 2019;99(2):175–180. doi: 10.2340/00015555-3065. [DOI] [PubMed] [Google Scholar]
- 20.Simpson EL, Guttman-Yassky E, Margolis DJ, et al. Association of inadequately controlled disease and disease severity with patient-reported disease burden in adults with atopic dermatitis. JAMA Dermatol. 2018;154(8):903–912. doi: 10.1001/jamadermatol.2018.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huet F, Faffa MS, Poizeau F, Merhand S, Misery L, Brenaut E. Characteristics of pruritus in relation to self-assessed severity of atopic dermatitis. Acta Derm Venereol. 2019;99(3):279–283. doi: 10.2340/00015555-3053. [DOI] [PubMed] [Google Scholar]
- 22.Sowden JM, Berth-Jones J, Ross JS, et al. Double-blind, controlled, crossover study of cyclosporin in adults with severe refractory atopic dermatitis. Lancet. 1991;338(8760):137–140. doi: 10.1016/0140-6736(91)90134-B. [DOI] [PubMed] [Google Scholar]
- 23.Khattri S, Shemer A, Rozenblit M, et al. Cyclosporine in patients with atopic dermatitis modulates activated inflammatory pathways and reverses epidermal pathology. J Allergy Clin Immunol. 2014;133(6):1626–1634. doi: 10.1016/j.jaci.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ko KC, Tominaga M, Kamata Y, et al. Possible antipruritic mechanism of cyclosporine A in atopic dermatitis. Acta Derm Venereol. 2016;96(5):624–629. doi: 10.2340/00015555-2318. [DOI] [PubMed] [Google Scholar]
- 25.Besedovsky L, Lange T, Haack M. The sleep-immune crosstalk in health and disease. Physiol Rev. 2019;99(3):1325–1380. doi: 10.1152/physrev.00010.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Opp MR. Cytokines and sleep. Sleep Med Rev. 2005;9(5):355–364. doi: 10.1016/j.smrv.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Xerfan EMS, Tomimori J, Andersen ML, Tufik S, Facina AS. Sleep disturbance and atopic dermatitis: a bidirectional relationship? Med Hypotheses. 2020;140:109637. doi: 10.1016/j.mehy.2020.109637. [DOI] [PubMed] [Google Scholar]
- 28.Roekevisch E, Spuls PI, Kuester D, Limpens J, Schmitt J. Efficacy and safety of systemic treatments for moderate-to-severe atopic dermatitis: a systematic review. J Allergy Clin Immunol. 2014;133(2):429–438. doi: 10.1016/j.jaci.2013.07.049. [DOI] [PubMed] [Google Scholar]
- 29.Haw S, Shin MK, Haw CR. The efficacy and safety of long-term oral cyclosporine treatment for patients with atopic dermatitis. Ann Dermatol. 2010;22(1):9–15. doi: 10.5021/ad.2010.22.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article or as supplementary information files.