Abstract
Besides the well-known use in supporting the non-invasive diagnosis of non-tumoral dermatoses (general dermatology), dermoscopy has been shown to be a promising tool also in predicting and monitoring therapeutic outcomes of such conditions, with the consequent improvement/optimization of their treatment. In the present paper, we sought to provide an up-to-date overview on the use of dermoscopy in highlighting response predictor factors and evaluating therapeutic results in the field of general dermatology according to the current literature data. Several dermatoses may somehow benefit from such applications, including inflammatory conditions (psoriasis, lichen planus, dermatitis, granulomatous conditions, erythro-telangiectatic rosacea, Zoon balanitis and vulvitis, cutaneous mastocytosis, morphea and extra-genital lichen sclerosus), pigmentary disorders (vitiligo and melasma) and infectious dermatoses (scabies, pediculosis, demodicosis and viral warts).
Keywords: Dermatoscopy, Dermoscopy, Monitoroscopy, Outcomes, Treatment, Therapy
Key Summary Points
| Why carry out the study? |
| Dermoscopy has recently been shown to be a promising tool in predicting and monitoring therapeutic outcomes of non-tumoral dermatoses (general dermatology). |
| Data on such a novel application is, however, sparse and no detailed review paper on this topic exists. |
| What was learned from the study? |
| We provided an up-to-date overview on the use of dermoscopy in highlighting response predictor factors and evaluating therapeutic results in the field of general dermatology according to the current literature data. |
| Dermoscopy may be of aid in the improvement/optimization of the management of several non-tumoral dermatoses by highlighting possible clinically subtle/invisible response predictors (positive or negative) and monitoring the lesions’ changes at a subclinical level. |
Digital Features
This article is published with digital features to facilitate understanding of the article. You can access the digital features on the article’s associated Figshare page. To view digital features for this article go to 10.6084/m9.figshare.12985496.
Introduction
Over the last few decades, the dermoscope has become an invaluable tool in dermatological daily clinical practice thanks to its ability to show findings not visible to the naked eye, thereby often being referred to as the dermatologist's “stethoscope” [1, 2]. Although it is traditionally used in the assessment of both melanocytic and non-melanocytic proliferative lesions as well as hair disorders [1–3], dermoscopy is increasingly gaining appreciation in the spectrum of non-tumoral skin conditions (i.e. general dermatology), including inflammatory, pigmentary, infectious and infiltrative dermatoses, with consequent reduction in the number of cases requiring biopsy [3–12]. In this regard, such a technique has been shown to have further applications apart from assisting the clinical diagnosis, including predicting and monitoring therapeutic outcomes, with the consequent improvement/optimization of the management of non-tumoral dermatoses [3–7].
The aim of this paper is to provide an up-to-date overview on the use of dermoscopy in highlighting response predictor factors and evaluating treatment results in the field of general dermatology according to the current literature data.
An electronic search was performed in August 2020 by using the PubMed database. In the first step the search terms were “predictors” AND “dermoscopy” (OR “dermatoscopy” OR “videodermoscopy”); “predictor factors” AND “dermoscopy” (OR “dermatoscopy” OR “videodermoscopy”); “monitoring” AND “dermoscopy” (OR “dermatoscopy” OR “videodermoscopy”); “follow-up” AND “dermoscopy” (OR “dermatoscopy” OR “videodermoscopy”). In the second step the search protocol included specific diagnoses i.e. “psoriasis” AND “dermoscopy” (OR “dermatoscopy” OR “videodermoscopy”). Additionally, pertinent references not identified by search engine and retrieved from articles/books were also taken into account. All studies written in English dealing with treatment outcomes prediction and monitoring of non-tumoral diseases (including inflammatory, pigmentary, infiltrative and infectious dermatoses) were analysed, including controlled studies, case series, case reports and reviews. Articles addressing hair, nail and mucosal diseases were excluded.
Tables 1 and 2 summarise dermoscopic data on response predictor factors and clues in treatment follow-up of non-tumoral dermatoses, including inflammatory, pigmentary and infectious diseases. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by the author.
Table 1.
Dermoscopic data on response predictor factors and clues in treatment follow-up of inflammatory diseases
| Disease | Main dermoscopic diagnostic clues | Dermoscopic response predictors | Clues in treatment follow-up | Type of supporting evidence |
|---|---|---|---|---|
| Psoriasis |
Uniform dotted/globular vessels Diffuse white scales |
Haemorrhagic dots appearing in the first 2/4 weeks of treatment with biologics are associated with a good outcome Baseline globular and dotted vessels are respectively associated with bad and good response to both narrowband ultraviolet B phototherapy and topical calcipotriol/betamethasone therapy Persistence of vessels at the end of topical calcipotriol/betamethasone therapy is related to an earlier relapse |
Reduction of vessels’ diameter and tortuosity at high magnification (videodermoscopy) during effective (topical or systemic) therapies Early (subclinical) detection of disease recurrence (appearance of dotted vessels) Early (subclinical) detection of steroid-induced skin atrophy (appearance of linear/reticular vessels) |
Review/book (mechanism-based reasoning) [3] |
| Lichen planus | Wickham striae | – |
Monitoring stage evolution of lesions under treatment Likelihood of post-inflammatory pigmentation persistence (dots and diffuse pigmentation are related to a longer and shorter course, respectively) |
Review/book (mechanism-based reasoning) [3–8] Case reports [27] |
| Dermatitis |
Clustered dotted vessels Diffuse white scales |
– |
Reduction of crusts and scales along with vascular pattern normalization during dupilumab treatment Early (subclinical) detection of steroid-induced skin atrophy (appearance of linear/reticular vessels) |
Case series [28] |
| Granulomatous dermatoses | Orange structureless areas | Indirect prediction of therapeutic outcomes in granuloma annulare by facilitating the recognition of the histological subtype | Disappearance of vessels and orange areas during effective therapies |
Review/book (mechanism-based reasoning) [29] Case series [30] |
| Rosacea (erythro-telangiectatic) | Linear vessels arranged in polygonal networks (vascular polygons) | Baseline protruding follicular plugs are associated with a better response to topical ivermectin therapy | Reduction of vessels as a marker of laser therapy efficacy | Case series (personal observation) |
| Zoon balanitis and vulvitis |
Orange areas Focused curved vessels |
– | Normalization of dermoscopic vascular pattern as a marker of therapeutic efficacy | Case report [37] |
| Cutaneous mastocytosis |
Coarse brown network (urticaria pigmentosa) Yellow-orange areas (mastocytoma) Diffuse light-brown discoloration and/or brown network (mastocytoma) |
“Reticular” vascular pattern (along with serum tryptase levels and plaque-type lesions) is associated with the need for a maintained antimediatior therapy | Monitoring stage evolution of lesions (mastocytoma) |
Case series [38] Review/book (mechanism-based reasoning) [39] |
| Morphea | Ill-defined dull white globules | – | Higher accuracy in monitoring inflammation and fibrosis regression by showing vessels and white areas reduction, respectively | Case-controlled study [41] |
| Lichen sclerosus (extra-genital) |
Bright white areas Follicular plugs |
– |
Higher accuracy in monitoring inflammation and fibrosis regression by showing vessels and white areas reduction, respectively |
Case-controlled study [41] |
Table 2.
Dermoscopic data on response predictor factors and clues in treatment follow-up of pigmentary and infectious diseases
| Disease | Main dermoscopic diagnostic clues | Dermoscopic response predictors | Clues in treatment follow-up | Type of supporting evidence |
|---|---|---|---|---|
| Vitiligo |
Milky/bright white structureless areas with sharp and convex margins White hairs (leukotrichia) Perifollicular pigmentation |
Baseline white hairs are related to poor response in both non-segmental vitiligo treated with excimer laser and segmental vitiligo treated with phototherapy, laser or surgery Baseline perifollicular pigmentation is associated with a good outcome in non-segmental vitiligo treated with narrowband ultraviolet B phototherapy Selection of the most appropriate therapy according to the lesion stage (active vs stable) |
Assessment of initial therapeutic response by showing subclinical pigmentation | Case series [42–48] |
| Melasma | Light/dark brown, grey or blue pseudonetwork |
Prediction of therapeutic response by facilitating the recognition of lesions deepness Significant dermoscopic vascular component supports the use of pulsed-dye laser |
Assessment of initial therapeutic response by showing attenuation/regression of pigmentary and vascular structures | Case series [49–54] |
| Scabies | “Delta-wing jet with contrail” sign | – |
Absence of mite migration 24 h after an effective treatment Progressive degradation of the mite with replacement with an amorphous material after an effective treatment |
Case series [55, 56] |
| Pediculosis | Evidence of louse and nits | – | Disappearance of parasites and viable nits after an appropriate therapy |
Case series [57] Case series [58] |
| Demodicosis | Protruding follicular plugs | – | Reduction of protruding follicular plugs as a marker of therapy efficacy | Case report [59] |
| Warts |
Dermatoglyphic/skin furrows interruption Thrombosed capillaries (common warts) Dotted vessels with white halos (plane warts) |
– | Assessment of complete healing by showing disappearance of subclinical findings | Review/book (mechanism-based reasoning) [60] |
Inflammatory Diseases
Psoriasis
Psoriasis is surely the most studied inflammatory condition from a dermoscopic point of view [13]. Its hallmarks on dermoscopy include uniform dotted/globular vessels and diffuse white scales; however, haemorrhagic dots/globules are also commonly seen and are often related to scratching [3–7], being found more frequently in areas in which psoriasis is typically itchy, such as scalp and legs [14]. Notably, at higher magnification (videodermoscopy), psoriatic dilated vessels usually show a bushy appearance [3–6].
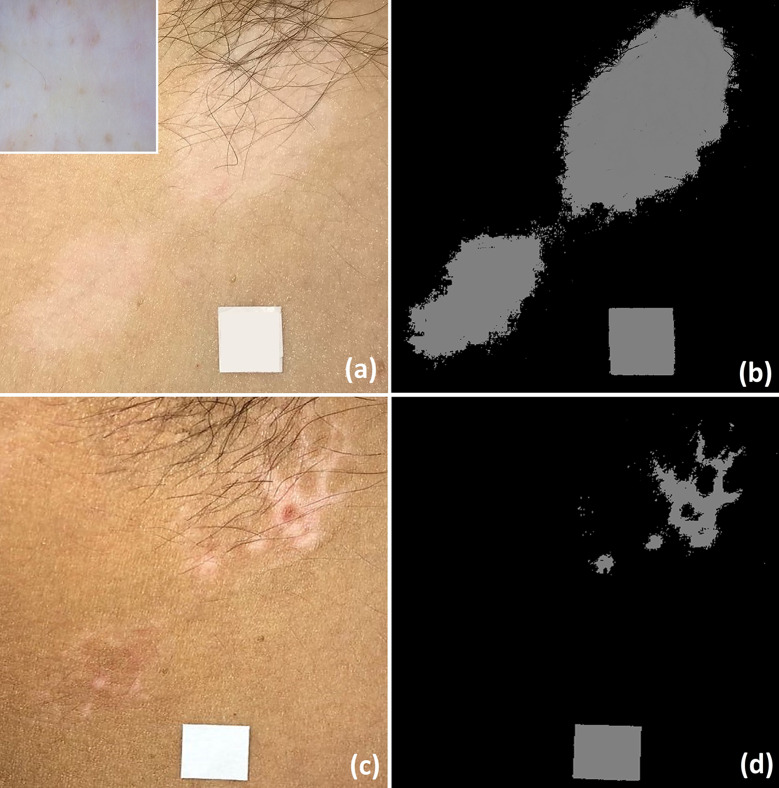
Several studies have demonstrated that videodermoscopic follow-up of psoriasis may facilitate treatment response assessment by showing changes in vessels morphology and diameter [15–23]. In particular, studies evaluating under high magnification (from ×100 to ×300) psoriatic plaques successfully treated with either topical [15–18] or systemic [19–23] therapies revealed a progressive reduction of vessels’ tortuosity and diameter over the time up to a complete normalization (i.e. capillaries with diameters ≤ 25 µm [23]), although this may sometimes not be reached despite clinical healing [23]. The possibility to evaluate vascular changes in psoriasis has a double practical implication: (I) early assessment of treatment response, as reduction in vessels’ diameter and tortuosity may already be seen in the first weeks of therapy [15–23], and (II) evaluation of persistence of vascular abnormalities in clinically healed lesions, as their presence has been supposed to be related to a higher tendency to relapse [22].
Interestingly, recent studies showed that even hand-held dermoscopy (×10 magnification) may be of aid in treatment outcomes assessment in plaque-type psoriasis [24–26]. Specifically, the appearance of haemorrhagic dots in the first 2/4 weeks of treatment is a predictor of subsequent response to biological agents [24], while the baseline evidence of globular and dotted vessels has respectively been related to a negative and positive response to both narrowband ultraviolet B phototherapy [25] and calcipotriene/betamethasone aerosol foam (Fig. 1) [26]. Additionally, regarding this last therapy, relapse is strongly associated with the persistence of vascular structures (either dotted or globular vessels) on dermoscopy at the end of the therapy when considering both lesions regardless of degree of improvement and lesions reaching a complete/nearly complete clinical healing [26]. Accordingly, it has been suggested that a “dermoscopic healing” would be more advisable than a mere “clinical healing” in order to ensure a longer response retention in psoriasis treated with calcipotriene/betamethasone aerosol foam [26].
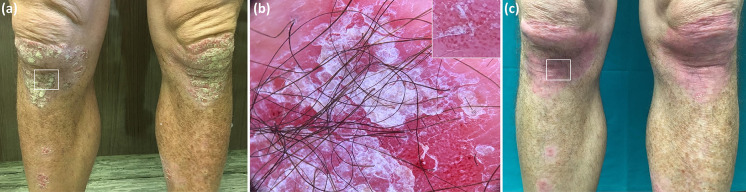
Fig. 1.
Clinical picture of a psoriatic plaque on the right leg (white square) at baseline (a), with corresponding dermoscopy image showing presence of uniform globular vessels (better visible in inset) and diffuse white scaling (b). Clinical view of the same lesion after 4 weeks of therapy with calcipotriene plus betamethasone dipropionate aerosol foam showing limited improvement (Local Psoriasis Severity Index improvement < 50%) (c).
(From Errichetti E, et al. Dermatol Ther (Heidelb) 2020;10:757–67)
Finally, hand-held dermoscopy is also helpful in early detection of both disease recurrence (as dermoscopic changes occur prior to clinical worsening) [24] and steroid-induced skin atrophy (by showing linear/reticular vessels before telangiectasias become clinically apparent) (Fig. 2) [3].
Fig. 2.
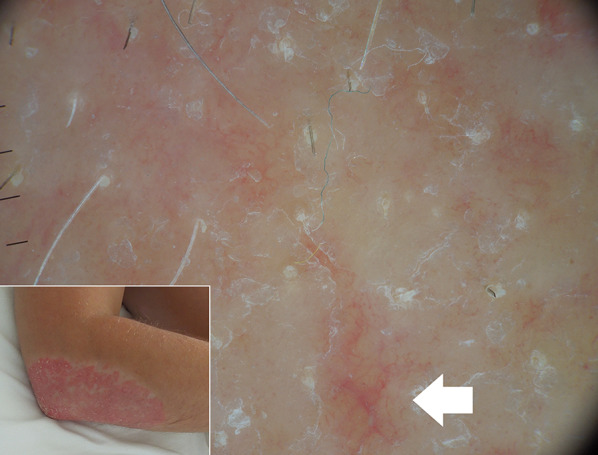
Dermoscopy of a psoriatic plaque after prolonged use of topical steroid reveals linear vessels (arrow) suggesting that skin atrophy has initiated, though it is not visible yet on clinical examination
Lichen Planus
The presence of Wickham striae on dermoscopy is a pathognomonic sign of lichen planus [3–8]. They usually appear as intersecting crossing white lines forming a sort of network, yet other morphologies (e.g. linear, radial, annular and round) and colours (i.e. yellow and blue-white) are also seen less commonly [3–8]. Additional findings include (I) dotted, globular and/or linear vessels, mainly detectable at the periphery of the lesion; (II) white/yellow dots; and (III) pigmented structures (dots, globules and/or reticular or cloud-like areas) [3–8].
Although all the aforementioned findings may sometimes coexist in a single lesion, dermoscopic patterns of lichen planus usually vary according to disease stage (Fig. 3), with early papules usually showing subtle Wickham striae over a reddish background and mature lesions displaying well-represented Wickham striae and peripheral vessels [3–8]. Both of these structures tend to fade over time, concomitantly to the gradual appearance of pigmented structures, and in long-standing lesions, pigmentary findings are often the only visible clue [3–8]. Accordingly, dermoscopy may be useful to accurately monitor disease stage evolution of lesions under treatment [8].
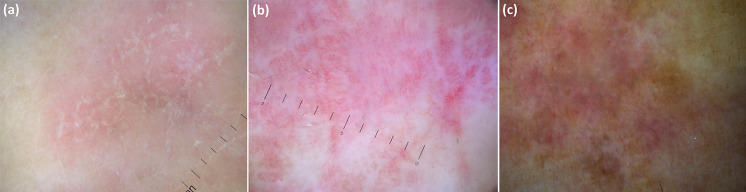
Fig. 3.
Dermoscopic pattern of lichen planus varies according to disease stage: subtle Wickham striae over a reddish background are seen in early stages (a), while mature and long-standing lesions respectively display well-represented Wickham striae along with peripheral dotted/linear vessels (b) and pigmented structures with Wickham striae and vascular remnants (c)
Additionally, dermoscopic examination may also be used to assess the likelihood of post-inflammatory pigmentation persistence, with homogeneous, structureless and light brown areas devoid of granularity being correlated with a shorter duration and granular pigmentation being associated with a longer course (Fig. 4) [27].
Fig. 4.
Post-inflammatory pigmentation in lichen planus may feature two dermoscopic patterns, i.e. granular pigmentation (a) and homogeneous brown area (b), respectively associated with a longer and shorter persistence
Dermatitis
The dermoscopic pattern of dermatitis varies according to the disease stage, even though overlaps are possible [3–8]. In particular, yellow serocrusts and dotted vessels distributed in clusters or randomly are typically seen in acute/subacute phases, while more or less uniform dotted vessels surrounded by a white halo are the main dermoscopic features in chronic phases (lichenification) [3–8]. Haemorrhages are also seen quite commonly as a result of intense itching [3–8].
A recent study demonstrated that atopic dermatitis treated with a 16-week course of dupilumab displayed a remarkable reduction of crusts and scales along with vascular pattern normalization on dermoscopic examination compared to baseline, thereby making dermoscopy a useful tool to assess subclinical improvement in this condition [28]. Notably, such dermoscopic changes seemed to occur concomitantly with objective [EASI (Eczema Area and Severity Index) score] and subjective [P-NRS (Peak Pruritus Numerical Rating Scale) and DLQI (Dermatology Life Quality Index) scores] clinical improvement [28].
Moreover, as a result of the common use of topical steroids in dermatitis, dermoscopy plays a significant role in preventing steroid-induced skin atrophy by showing subclinical early signs, i.e. linear and/or reticular vessels [3].
Granulomatous Dermatoses
The dermoscopic hallmark of granulomatous skin diseases is the presence of focal or diffuse orange structureless areas histologically corresponding to compact dermal granulomas, although such structures may be sometimes absent (i.e. deeply located granulomas or presence of remarkable epidermal changes) and are also seen in other conditions characterized by dense cell infiltrates or haemosiderin deposits in the dermis [29]. Additional dermoscopic clues may also be found in each granulomatous dermatosis (i.e. focused vessels in sarcoidosis and lupus vulgaris, follicular plugs and peripheral white striae in leishmaniasis, blurred vessels in granuloma annulare, yellow areas and serpiginous-branching vessels in necrobiosis lipoidica and vascular polygons in granulomatous rosacea) [29].
In general, dermoscopic examination may be of aid in treatment monitoring of granulomatous skin conditions by showing disappearance of vascular and non-vascular findings, especially orange areas as they are a marker for the presence of compact granulomatous infiltrate in the dermis (Fig. 5) [29, 30]. However, a recent study emphasized that such dermoscopic structures may persist despite treatment in sarcoidosis, likely because of an incomplete efficacy of therapies with persistence of some degree of subclinical inflammation [30].
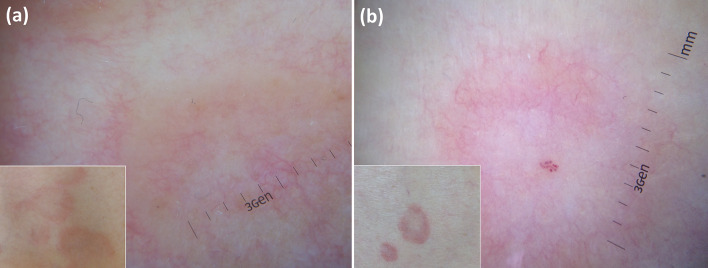
Fig. 5.
Dermoscopic assessment (a baseline; b after 8 weeks of oral doxycycline therapy) in a case of granulomatous rosacea of the forehead allows one to see the disappearance of orange areas (a arrows), histologically related to dermal granulomatous infiltrate fading
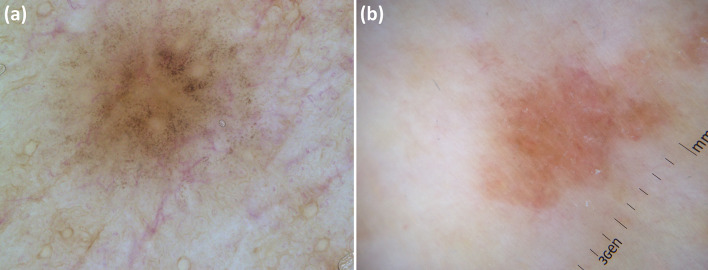
Finally, dermoscopy may indirectly help predict therapeutic outcomes in granuloma annulare by facilitating the recognition of the histological subtype, i.e. palisading vs interstitial, respectively characterized by an orange and pinkish background (Fig. 6) [31]. Indeed, it has been reported that the pathological pattern might influence the disease course/therapeutic response, with the palisading granuloma subtype being associated with a more protracted course/significant resistance to treatments than the interstitial form [31].
Fig. 6.
Granuloma annulare shows a different dermoscopic background according to histological subtype, i.e. orangish in “palisading” variant (a) and pinkish in “interstitial” variant (b), so dermoscopy may help in predicting therapeutic outcomes as the pathological pattern might influence the disease course/therapeutic response
Erythro-Telangiectatic Rosacea
Recognizing this facial dermatosis on dermoscopy is a very straightforward task owing to the presence of a highly specific feature, namely linear vessels characteristically arranged in polygonal networks (vascular polygons) [3–7]. Additional, less specific findings include rosettes, follicular plugs, white-yellowish scales, pigmentation structures and dilated follicle [3–7].
Besides diagnostic purposes, dermoscopic assessment in erythro-telangiectatic rosacea may also be helpful in monitoring post-treatment changes (with reduction of vascular and non-vascular findings, especially when treated with lasers) and predicting therapeutic response to topical treatments [32]. Indeed, according to a preliminary analysis on 20 patients suffering from erythro-telangiectatic rosacea, the presence of protruding follicular plugs is associated with a better response to an 8-week course of ivermectin 10 mg/g cream compared to metronidazole 1% gel used for the same time span (personal observations) (Fig. 7). This would be due to the possible active effect of the former therapy on Demodex folliculorum as there is a correlation between protruding follicular plugs on dermoscopy and a higher mite density that may be seen in rosacea (typically less than 5 mites/cm2) [33].
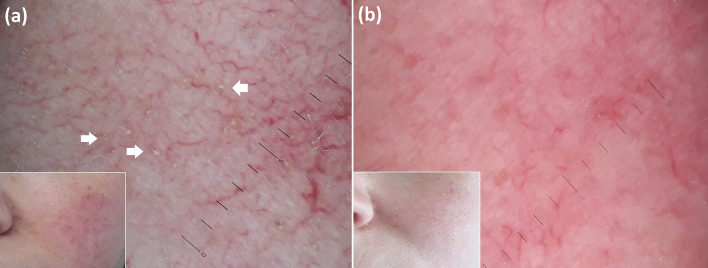
Fig. 7.
Erythro-telangiectatic rosacea. Besides typical vascular polygons, this patient also shows protruding follicular plugs (arrows) on baseline dermoscopic examination (a); an optimal clinical response is reached after an 8-week topical course of ivermectin, with disappearance of protruding plugs on dermoscopy (b)
Zoon Balanitis and Vulvitis
Such conditions are dermoscopically characterized by orange structureless areas and linear-curved vessels (including serpentine, chalice-shaped or convoluted) [34–37]. It has been reported that normalization of the dermoscopic vascular pattern may indicate resolution of the active phase despite the persistence of orange areas on both dermoscopy and clinical assessment (which may be evident for a long time) (Fig. 8), thus avoiding overtreatment of patients and its possible consequences (e.g. steroid-induced skin atrophy) [37].
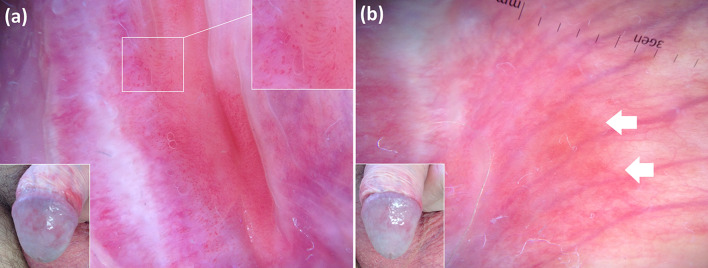
Fig. 8.
Zoon balanitis. Baseline dermoscopic assessment (a) reveals linear-curved vessels (better visible in the upper right inset). Dermoscopy after a 4-week course of a topical steroid (b) shows improvement of inflammation by displaying disappearance of the aforementioned vessels, though orange areas (arrows) are now evident (however, they are not a typical sign of disease activity as they correspond to dermal haemosiderin deposits on histology)
Cutaneous Mastocytosis
Apart from facilitating diagnosis (by showing coarse brown network or homogeneous brown areas), dermoscopy may also be of aid in therapeutic management of urticaria pigmentosa [38]. Indeed, although visible in a minority of cases, the presence of a “reticular” vascular pattern on dermoscopic assessment, along with serum tryptase levels and plaque-type lesions, was found to represent the best combination to predict the need for maintained antimediatior therapy in one study [38]. Consequently, the authors hypothesized that, in combination with other variables, dermoscopy could provide additional help in the identification of patients at risk for more severe symptoms [38].
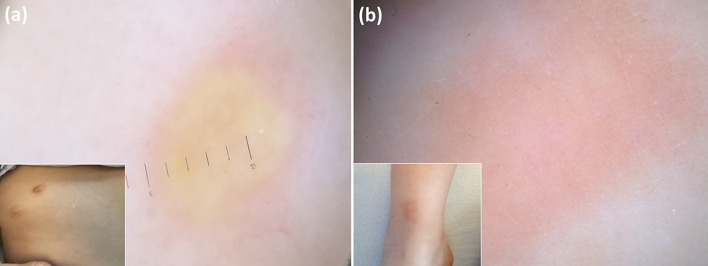
Additionally, dermoscopic examination may also be used in the follow-up of mastocytoma as lesions in regressing/healing phases usually do not display yellow-orange areas (which are more common in mature lesions) but only a diffuse light-brown discoloration and/or brown network (Fig. 9) [39].
Fig. 9.
Dermoscopy of a mature/well-developed mastocytoma reveals yellowish areas (a), while a diffuse light-brown discoloration is seen in a healing lesion (b)
Morphea and Extra-Genital Lichen Sclerosus
Dermoscopy has been speculated to be a possible effective technique for treatment monitoring of such conditions since it allows a more accurate assessment of inflammation regression and fibrotic process compared to clinical examination only [40, 41]. Indeed, dermoscopy shows a better correlation with pathologic findings, with erythema/vessels and white areas (ill-defined dull white globules in morphea and bright white areas in extra-genital lichen sclerosus) strictly related to dermal inflammation and sclerosis, respectively [41]. Consequently, it is possible to optimize the duration of therapy according to the persistence/resolution of subclinical inflammatory signs despite an “apparent” clinical healing, thus preventing fibrosis progression [41].
Pigmentary Dermatoses
Vitiligo
Milky/bright white structureless areas with sharp and convex margins represent the most common dermoscopic pattern of vitiligo [42, 43]; other possible findings include white hairs (leukotrichia), perifollicular pigmentation (more common in early and repigmenting lesions), perifollicular depigmentation, marginal pigmentation, marginal reticular pigmentation, reversed pigmentary network, and intralesional pigmentary (structureless or network-like) patches, with the last two features being more common in early stages of disease [42, 43].
Of note, margins may sometimes also be irregular and/or less defined giving rise to specific patterns (i.e. trichrome, “starburst” and “comet tail”), which have been related to disease activity [42, 43]. Additional signs of active/progressive lesions include the presence of small white globules in perilesional skin (described as satellites, confetti-like pattern and “tapioca sago” appearance) and micro-Koebner’s phenomenon (i.e. occurrence of isomorphic depigmented streaks along the line of trauma around the main vitiligo patch) [42, 43]. On the other hand, sharp borders and perilesional hyperpigmentation are considered signs of disease stability [42, 43]. From a management point of view, it is important to known if a vitiligo lesion is in a stable or active phase as some therapies are strictly related to disease stage (e.g. surgical treatments are preferentially done when disease is stable) [42, 43].
Additionally, dermoscopy has been reported to be a reliable tool to assess initial therapeutic response to treatments as it may show increases in perilesional and intralesional pigmentation that are not visible to the naked eye [44, 45].
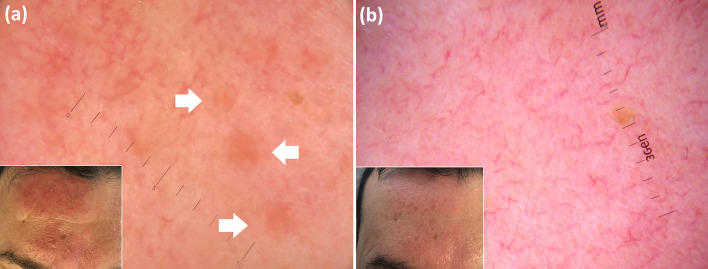
Finally, dermoscopic examination has also been shown to be useful to predict therapeutic outcomes [46–48]. In particular, a recent study showed a reduced response rate to excimer laser therapy of non-segmental vitiligous lesions displaying white hairs on baseline dermoscopy [46]. That finding was confirmed in a study on patients suffering from segmental vitiligo treated with either medical (phototherapy or laser therapy) or surgical treatments [47], yet it was not seen in another study investigating patients with non-segmental vitiligo treated with ultraviolet B phototherapy, which only found a correlation between positive response and dermoscopic evidence of perifollicular pigmentation at baseline (Fig. 10) [48].
Fig. 10.
Baseline clinical image of vitiligo involving right armpit (inset: dermoscopic examination shows presence of subclinical perifollicular pigmentation) (a); the same lesions seen by using a computer-aided method (b). Significant improvement at the end of the 30 sessions of narrowband ultraviolet B phototherapy is visible on clinical examination (c) and by using a computer-aided method (d).
(From Errichetti E, et al. Dermatol Ther (Heidelb) 2020. https://doi.org/10.1007/s13555-020-00431-6)
Melasma
Dermoscopic features of melasma include light/dark brown, grey or blue pseudonetwork (pigmentation with follicular and appendages ostia sparing) and, less commonly, telangiectasias (which are usually less thick than steroid overuse-induced vessels) as well as pigmented annular or arcuate structures, dots and globules [49–54].
The role of dermoscopy in therapeutic management of melasma has been investigated by several studies including patients treated with either topical treatments (tranexamic acid, hydroquinone, and combinations of hydroquinone, glycolic acid and hyaluronic acid) or laser therapies [49–54]. Besides the use of this technique in following up lesions changes during the treatment to see the attenuation/regression of pigmentary and vascular structures, dermoscopic examination also has a role in predicting therapeutic response in melasma as it allow one to establish the deepness of the lesions, i.e. epidermal (dark to light brown colour and sharp edges), dermal (grey to blue colour with ill-defined margins with or without annular/arcuate pigmented structures) or mixed (combination of dermoscopic findings), with the last two variants being notoriously more resistant to therapies [49–54].
Additionally, dermoscopy is useful in choosing the type of treatment since the presence of a significant vascular component on dermoscopic assessment (“telangiectatic melasma”) would support the use of a laser therapy targeting vessels (e.g. pulsed-dye laser) [54].
Infectious Dermatoses
Scabies
Scabies is one of the skin diseases that benefit most from dermoscopy in terms of both diagnosis and treatment monitoring [55, 56]. Hand-held dermoscopic examination typically displays the pathognomonic “delta-wing jet with contrail” sign, which represents the irregular burrow excavated by the mite, whose anterior part of the body is visible as a small black arrowhead/triangular area at the end of the whitish wavy line (corresponding to the burrow) [3–7]. At higher magnification (videodermoscopy—×20 to ×600), epimeres (chitinous internal structures attached to legs), anterior outlines, eating tools and both pairs of forelegs and hind legs of Sarcoptes scabiei are visible [55, 56].
Post-treatment follow-up of scabies may allow one to see the absence of migration of mites (after 24 h) and their progressive degradation with a gradual disappearance of their outlines and replacement with an amorphous material [55]. These last morphological changes have been reported to occur after mite immobilization, i.e. 48 h [55] to 2 weeks [56] after therapy administration. Notably, even hand-held dermoscopes (×10 magnification) may show the disappearance of the “arrowhead/triangular area”, although the accuracy of low magnification is obviously lesser than videodermoscopic evaluation [57].
Pediculosis
Dermoscopic examination of pediculosis may easily allow identification of parasites and eggs when these are not easy to identify by clinical inspection [57, 58]. Morphology of head and body lice is similar (though the latter is bigger) and consists of an elongated body, three pairs of clawed legs and narrow anterior mouthparts, while pubic lice differ from the presence of a short broad body [57, 58] (Fig. 11).
Fig. 11.

Dermoscopy of a case of pediculosis pubis shows a louse (arrowhead) as well as a viable nit having a brown colour and oval shape (arrow) (a); empty nits (arrows) in a patient successfully treated for pediculosis capitis are seen as translucent structures showing a plane free ending (b)
Additionally, besides demonstrating the absence of parasites after an appropriate therapy, dermoscopy may also help assess the presence of viable nits [57, 58]. Specifically, nits containing nymphs are ovoid brown structures, while empty nits are translucent and typically show a plane and fissured free ending [57, 58] (Fig. 11).
Demodicosis
Under dermoscopy, demodicosis typically shows white-yellow follicular plugs which may or not protrude from the skin surface (known as “Demodex tails” and “Demodex follicular openings”, respectively) [3–5]. Other unspecific dermoscopic findings may be observed, including diffuse erythema, scaling, pustules and reticular dilated vessels [3–5].
It has been speculated that dermoscopy may have a role in monitoring and optimizing treatment of this condition by showing the reduction of subclinical follicular plugs as they would be strictly related to the presence of a mixture of keratotic material and mites in the follicles [59].
Warts
Common and plantar warts are classically typified by dermatoglyphic/skin furrows interruption, dotted and/or linear vessels having white halos, finger-like projections containing elongated vessels surrounded by a white halo and thrombosed capillaries (seen as purple-black dots/lines), while plane warts usually feature dermatoglyphic/skin furrows interruption along with dotted vessels over a white background [5–7].
The main application of dermoscopy in therapeutic management of such lesions is represented by the assessment of a complete healing as it allows one to see subclinical persistence of infection (Fig. 12) that may be responsible for its spreading if not further treated [60].
Fig. 12.
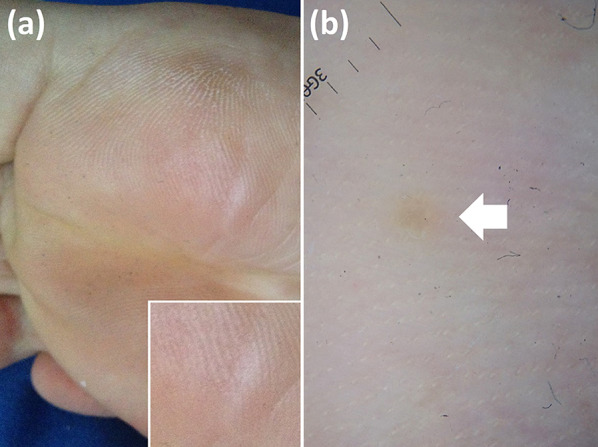
Plantar wart. Despite clinical examination indicating healing (a), dermoscopic examination still displays lesion remnant by revealing dermatoglyphics interruption (arrow) (b)
Conclusions
Dermoscopy may benefit therapeutic management of several non-tumoral skin conditions by highlighting possible clinically subtle/invisible response predictor factors (positive or negative) and monitoring the lesions changes at a subclinical level. The first possibility may help in choosing the most appropriate therapy and avoid unnecessary treatments, while the second advantage may allow one to appreciate early signs of response/recurrence (before clinical changes become evident) as well as ensure the disappearance of subclinical findings that may be related to inflammation/infection persistence, thus preventing disease spreading/recurrence.
Importantly, available supporting evidence has sometimes been drawn by using specific treatments or according to single case reports/mechanism-based reasoning. Therefore, future studies including dermoscopic-pathological correlation of subclinical changes during and after therapies are needed to support the existing promising data.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
The named author meets the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, takes responsibility for the integrity of the work as a whole, and has given their approval for this version to be published.
Authorship Contributions
All writers and contributors who participated in the preparation of the manuscript are listed as authors.
Disclosures
Enzo Errichetti is a member of the journal's Editorial Board.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by the author.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1.Zalaudek I, Lallas A, Moscarella E, Longo C, Soyer HP, Argenziano G. The dermatologist's stethoscope—traditional and new applications of dermoscopy. Dermatol Pract Concept. 2013;3:67–71. doi: 10.5826/dpc.0302a11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lallas A, Argenziano G. Dermatoscope–the dermatologist's stethoscope. Indian J Dermatol Venereol Leprol. 2014;80:493–494. doi: 10.4103/0378-6323.144141. [DOI] [PubMed] [Google Scholar]
- 3.Errichetti E. Dermoscopy of inflammatory dermatoses (inflammoscopy): an up-to-date overview. Dermatol Pract Concept. 2019;9:169–180. doi: 10.5826/dpc.0903a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Errichetti E, Stinco G. Dermoscopy in general dermatology: a practical overview. Dermatol Ther (Heidelb) 2016;6:471–507. doi: 10.1007/s13555-016-0141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vos MHE, Nguyen KP, Van Erp PEJ, Van de Kerkhof PCM, Driessen RJB, Peppelman M. The value of (video)dermoscopy in the diagnosis and monitoring of common inflammatory skin diseases: a systematic review. Eur J Dermatol. 2018;28:575–596. doi: 10.1684/ejd.2018.3396. [DOI] [PubMed] [Google Scholar]
- 6.Errichetti E, Stinco G. The practical usefulness of dermoscopy in general dermatology. G Ital Dermatol Venereol. 2015;150:533–546. [PubMed] [Google Scholar]
- 7.Errichetti E, Zalaudek I, Kittler H, et al. Standardization of dermoscopic terminology and basic dermoscopic parameters to evaluate in general dermatology (non-neoplastic dermatoses): an expert consensus on behalf of the International Dermoscopy Society. Br J Dermatol. 2020;182:454–467. doi: 10.1111/bjd.18125. [DOI] [PubMed] [Google Scholar]
- 8.Lallas A, Kyrgidis A, Tzellos TG, et al. Accuracy of dermoscopic criteria for the diagnosis of psoriasis, dermatitis, lichen planus and pityriasis rosea. Br J Dermatol. 2012;166:1198–1205. doi: 10.1111/j.1365-2133.2012.10868.x. [DOI] [PubMed] [Google Scholar]
- 9.Errichetti E, Piccirillo A, Viola L, Stinco G. Dermoscopy of subacute cutaneous lupus erythematosus. Int J Dermatol. 2016;55:e605–e607. doi: 10.1111/ijd.13331. [DOI] [PubMed] [Google Scholar]
- 10.Errichetti E, De Francesco V, Pegolo E, Stinco G. Dermoscopy of Grover's disease: variability according to histological subtype. J Dermatol. 2016;43:937–939. doi: 10.1111/1346-8138.13298. [DOI] [PubMed] [Google Scholar]
- 11.Jardim MML, Uchiyama J, Kakizaki P, Valente NYS. Dermoscopy of granuloma faciale: a description of a new finding. An Bras Dermatol. 2018;93:587–589. doi: 10.1590/abd1806-4841.20187017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bilgic SA, Cicek D, Demir B. Dermoscopy in differential diagnosis of inflammatory dermatoses and mycosis fungoides. Int J Dermatol. 2020;59:843–850. doi: 10.1111/ijd.14925. [DOI] [PubMed] [Google Scholar]
- 13.Grajdeanu IA, Statescu L, Vata D, et al. Imaging techniques in the diagnosis and monitoring of psoriasis. Exp Ther Med. 2019;18:4974–4980. doi: 10.3892/etm.2019.7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stinco G, Trevisan G, Piccirillo F, et al. Pruritus in chronic plaque psoriasis: a questionnaire-based study of 230 Italian patients. Acta Dermatovenerol Croat. 2014;22:122–128. [PubMed] [Google Scholar]
- 15.Strumia R, Altieri E, Romani I, Bettoli V, Negrini A, Trimurti S. Tacalcitol in psoriasis: a video-microscopy study. Acta Derm Venereol Suppl (Stockh) 1994;186:85–87. [PubMed] [Google Scholar]
- 16.Vázquez-López F, Marghoob AA. Dermoscopic assessment of long-term topical therapies with potent steroids in chronic psoriasis. J Am Acad Dermatol. 2004;51:811–813. doi: 10.1016/j.jaad.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 17.Stinco G, Lautieri S, Piccirillo F, Valent F, Patrone P. Response of cutaneous microcirculation to treatment with mometasone furoate in patients with psoriasis. Clin Exp Dermatol. 2009;34:915–919. doi: 10.1111/j.1365-2230.2009.03298.x. [DOI] [PubMed] [Google Scholar]
- 18.Rosina P, Giovannini A, Gisondi P, Girolomoni G. Microcirculatory modifications of psoriatic lesions during topical therapy. Skin Res Technol. 2009;15:135–138. doi: 10.1111/j.1600-0846.2008.00336.x. [DOI] [PubMed] [Google Scholar]
- 19.Stinco G, Lautieri S, Valent F, Patrone P. Cutaneous vascular alterations in psoriatic patients treated with cyclosporine. Acta Derm Venereol. 2007;87:152–154. doi: 10.2340/00015555-0216. [DOI] [PubMed] [Google Scholar]
- 20.Campanati A, Goteri G, Simonetti O, et al. Angiogenesis in psoriatic skin and its modifications after administration of etanercept: videocapillaroscopic, histological and immunohistochemical evaluation. Int J Immunopathol Pharmacol. 2009;22:371–377. doi: 10.1177/039463200902200214. [DOI] [PubMed] [Google Scholar]
- 21.Stinco G, Buligan C, Maione V, Valent F, Patrone P. Videocapillaroscopic findings in the microcirculation of the psoriatic plaque during etanercept therapy. Clin Exp Dermatol. 2013;38:633–637. doi: 10.1111/ced.12036. [DOI] [PubMed] [Google Scholar]
- 22.Stinco G, Buligan C, Errichetti E, Valent F, Patrone P. Clinical and capillaroscopic modifications of the psoriatic plaque during therapy: observations with oral acitretin. Dermatol Res Pract. 2013;2013:781942. doi: 10.1155/2013/781942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Micali G, Lacarrubba F, Santagati C, Egan CG, Nasca MR, Musumeci ML. Clinical, ultrasound, and videodermatoscopy monitoring of psoriatic patients following biological treatment. Skin Res Technol. 2016;22:341–348. doi: 10.1111/srt.12271. [DOI] [PubMed] [Google Scholar]
- 24.Lallas A, Argenziano G, Zalaudek I, et al. Dermoscopic hemorrhagic dots: an early predictor of response of psoriasis to biologic agents. Dermatol Pract Concept. 2016;6:7–12. doi: 10.5826/dpc.0604a02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Errichetti E, Stinco G. Clinical and dermoscopic response predictors in psoriatic patients undergoing narrowband ultraviolet B phototherapy: results from a prospective study. Int J Dermatol. 2018;57:681–686. doi: 10.1111/ijd.13983. [DOI] [PubMed] [Google Scholar]
- 26.Errichetti E, Croatto M, Arnoldo L, Stinco G. Plaque-type psoriasis treated with calcipotriene plus betamethasone dipropionate aerosol foam: a prospective study on clinical and dermoscopic predictor factors in response achievement and retention. Dermatol Ther (Heidelb) 2020;10:757–767. doi: 10.1007/s13555-020-00406-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vázquez-López F, Maldonado-Seral C, López-Escobar M, Pérez-Oliva N. Dermoscopy of pigmented lichen planus lesions. Clin Exp Dermatol. 2003;28:554–555. doi: 10.1046/j.1365-2230.2003.01302.x. [DOI] [PubMed] [Google Scholar]
- 28.Ferrillo M, Patruno C, Villani A, et al. Dermoscopic assessment of long-term systemic therapy with dupilumab in adult atopic dermatitis. J Eur Acad Dermatol Venereol. 2020 doi: 10.1111/jdv.16409. [DOI] [PubMed] [Google Scholar]
- 29.Errichetti E, Stinco G. Dermatoscopy of granulomatous disorders. Dermatol Clin. 2018;36:369–375. doi: 10.1016/j.det.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Ramadan S, Hossam D, Saleh MA. Dermoscopy could be useful in differentiating sarcoidosis from necrobiotic granulomas even after treatment with systemic steroids. Dermatol Pract Concept. 2016;6:17–22. doi: 10.5826/dpc.0603a05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Errichetti E, Lallas A, Apalla Z, Di Stefani A, Stinco G. Dermoscopy of granuloma annulare: a clinical and histological correlation study. Dermatology. 2017;233:74–79. doi: 10.1159/000454857. [DOI] [PubMed] [Google Scholar]
- 32.Deshapande A, Ankad BS. Dermoscopic monitoring of response to intense pulsed light in rosacea: a case report. Dermatol Pract Concept. 2020;10:e2020058. doi: 10.5826/dpc.1003a58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Errichetti E, Stinco G. Pyoderma faciale as a possible form of demodicosis in a subset of patients? New insights from dermoscopic examination. G Ital Dermatol Venereol. 2020 doi: 10.23736/S0392-0488.19.06452-6. [DOI] [PubMed] [Google Scholar]
- 34.Errichetti E, Lacarrubba F, Micali G, Stinco G. Dermoscopy of Zoon's plasma cell balanitis. J Eur Acad Dermatol Venereol. 2016;30:e209–e210. doi: 10.1111/jdv.13538. [DOI] [PubMed] [Google Scholar]
- 35.Errichetti E, Lallas A, Di Stefani A, et al. Accuracy of dermoscopy in distinguishing erythroplasia of Queyrat from common forms of chronic balanitis: results from a multicentric observational study. J Eur Acad Dermatol Venereol. 2019;33:966–972. doi: 10.1111/jdv.15359. [DOI] [PubMed] [Google Scholar]
- 36.Corazza M, Toni G, Virgili A, Borghi A. Plasma cell vulvitis: further confirmation of the diagnostic utility of dermoscopy. Int J Dermatol. 2018;57:e164–e165. doi: 10.1111/ijd.14233. [DOI] [PubMed] [Google Scholar]
- 37.Corazza M, Virgili A, Minghetti S, Toni G, Borghi A. Dermoscopy in plasma cell balanitis: its usefulness in diagnosis and follow-up. J Eur Acad Dermatol Venereol. 2016;30:182–184. doi: 10.1111/jdv.12692. [DOI] [PubMed] [Google Scholar]
- 38.Vano-Galvan S, Alvarez-Twose I, Heras E, et al. Dermoscopic features of skin lesions in patients with mastocytosis. Arch Dermatol. 2011;147:932–940. doi: 10.1001/archdermatol.2011.190. [DOI] [PubMed] [Google Scholar]
- 39.Errichetti E, Lallas A. Other infiltrative conditions. In: Lallas A, Errichetti E, Ioannides D, editors. Dermoscopy in general dermatology. 1. Boca Raton: CRC; 2018. pp. 170–173. [Google Scholar]
- 40.Campione E, Paternò EJ, Diluvio L, Orlandi A, Bianchi L, Chimenti S. Localized morphea treated with imiquimod 5% and dermoscopic assessment of effectiveness. J Dermatolog Treat. 2009;20:10–13. doi: 10.1080/09546630802132668. [DOI] [PubMed] [Google Scholar]
- 41.Errichetti E, Lallas A, Apalla Z, Di Stefani A, Stinco G. Dermoscopy of morphea and cutaneous lichen sclerosus: clinicopathological correlation study and comparative analysis. Dermatology. 2017;233:462–470. doi: 10.1159/000484947. [DOI] [PubMed] [Google Scholar]
- 42.Kumar Jha A, Sonthalia S, Lallas A, Chaudhary RKP. Dermoscopy in vitiligo: diagnosis and beyond. Int J Dermatol. 2018;57:50–54. doi: 10.1111/ijd.13795. [DOI] [PubMed] [Google Scholar]
- 43.Jha AK, Sonthalia S, Lallas A. Dermoscopy as an evolving tool to assess vitiligo activity. J Am Acad Dermatol. 2018;78:1017–1019. doi: 10.1016/j.jaad.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 44.ElGhareeb MI, Metwalli M, AbdelMoneim N. Combination of oral methotrexate and oral mini-pulse dexamethasone vs either agent alone in vitiligo treatment with follow up by dermoscope. Dermatol Ther. 2020 doi: 10.1111/dth.13586. [DOI] [PubMed] [Google Scholar]
- 45.Wang LM, Lu WJ, Yuan JT, et al. Utility of dermoscopy for evaluating the therapeutic efficacy of tacrolimus ointment plus 308-nm excimer laser combination therapy in localized vitiligo patients. Exp Ther Med. 2018;15:3981–3988. doi: 10.3892/etm.2018.5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim MS, Cho EB, Park EJ, Kim KH, Kim KJ. Effect of excimer laser treatment on vitiliginous areas with leukotrichia after confirmation by dermoscopy. Int J Dermatol. 2016;55:886–892. doi: 10.1111/ijd.12972. [DOI] [PubMed] [Google Scholar]
- 47.Lee DY, Kim CR, Park JH, Lee JH. The incidence of leukotrichia in segmental vitiligo: implication of poor response to medical treatment. Int J Dermatol. 2011;50:925–927. doi: 10.1111/j.1365-4632.2011.04914.x. [DOI] [PubMed] [Google Scholar]
- 48.Errichetti E, Zelin E, Pinzani C, Kyrgidis A, Lallas A, Stinco G. Dermoscopic and clinical response predictor factors in nonsegmental vitiligo treated with narrowband ultraviolet B phototherapy: a prospective observational study. Dermatol Ther (Heidelb) 2020 doi: 10.1007/s13555-020-00431-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Badawi AM, Osman MA. Fractional erbium-doped yttrium aluminum garnet laser-assisted drug delivery of hydroquinone in the treatment of melasma. Clin Cosmet Investig Dermatol. 2018;11:13–20. doi: 10.2147/CCID.S147413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ibrahim ZA, Gheida SF, El Maghraby GM, Farag ZE. Evaluation of the efficacy and safety of combinations of hydroquinone, glycolic acid, and hyaluronic acid in the treatment of melasma. J Cosmet Dermatol. 2015;14:113–123. doi: 10.1111/jocd.12143. [DOI] [PubMed] [Google Scholar]
- 51.Agamia N, Apalla Z, Salem W, Abdallah W. A comparative study between oral tranexamic acid versus oral tranexamic acid and Q-switched Nd-YAG laser in melasma treatment: a clinical and dermoscopic evaluation. J Dermatolog Treat. 2020;1:1–8. doi: 10.1080/09546634.2019.1708847. [DOI] [PubMed] [Google Scholar]
- 52.Abdel Hay R, Mohammed FN, Sayed KS, Abd El Fattah NA, Ibrahim S. Dermoscopy as a useful tool for evaluating melasma and assessing the response to 1064-nm Q-switched Nd:YAG laser. Dermatol Ther. 2020 doi: 10.1111/dth.13629. [DOI] [PubMed] [Google Scholar]
- 53.El-Sinbawy ZG, Abdelnabi NM, Sarhan NE, Elgarhy LH. Clinical & ultrastructural evaluation of the effect of fractional CO2 laser on facial melasma. Ultrastruct Pathol. 2019;43:135–144. doi: 10.1080/01913123.2019.1673861. [DOI] [PubMed] [Google Scholar]
- 54.Kong SH, Suh HS, Choi YS. Treatment of melasma with pulsed-dye laser and 1,064-nm Q-switched Nd:YAG Laser: a split-face study. Ann Dermatol. 2018;30:1–7. doi: 10.5021/ad.2018.30.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Micali G, Lacarrubba F, Tedeschi A. Videodermatoscopy enhances the ability to monitor efficacy of scabies treatment and allows optimal timing of drug application. J Eur Acad Dermatol. 2004;18:153–154. doi: 10.1111/j.1468-3083.2004.00858.x. [DOI] [PubMed] [Google Scholar]
- 56.Haas N, Sterry W. The use of ELM to monitor the success of antiscabietic treatment. Arch Dermatol. 2001;137:1656–1657. [PubMed] [Google Scholar]
- 57.Lacarrubba F, Nardone B, Milani M, et al. Head lice: ex vivo videodermatoscopy evaluation of the pediculocidal activity of two different topical products. G Ital Dermatol Venereol. 2006;141:233–235. [Google Scholar]
- 58.Di Stefani A, Hofmann-Wellenhof R, Zalaudek I. Dermoscopy for diagnosis and treatment monitoring of pediculosis capitis. J Am Acad Dermatol. 2006;54:909–911. doi: 10.1016/j.jaad.2005.11.1083. [DOI] [PubMed] [Google Scholar]
- 59.Friedman P, Sabban EC, Cabo H. Usefulness of dermoscopy in the diagnosis and monitoring treatment of demodicidosis. Dermatol Pract Concept. 2017;7:35–38. doi: 10.5826/dpc.0701a06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lacarrubba F, Verzì AE, Micali G. Viral infections. In: Lallas A, Errichetti E, Ioannides D, editors. Dermoscopy in general dermatology. 1. Boca Raton: CRC; 2018. pp. 221–223. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.