Abstract
Numerous studies have indicated that abnormal activation of the HGF/c-Met signaling pathway can lead to cell proliferation, invasiveness, and metastasis of cancers of the digestive system. Moreover, overexpression of c-Met has been implicated in poor prognosis of patients with these forms of cancer, suggesting the possibility for HGF/c-Met axis as a potential therapeutic target. Despite the large number of clinical and preclinical trials worldwide, no significant positive success in the use of anti-HGF/c-Met treatments on cancers of the digestive system has been achieved. In this review, we summarize advanced development of clinical research on HGF/c-Met antibody and small-molecule c-Met inhibitors of cancers of the digestive system and provide a possible direction for future research.
Keywords: hepatocyte growth factor, c-Met, molecular targeted therapy, digestive system cancer, inhibitors
Introduction
Cancers of the digestive system comprise hepatocellular carcinoma (HCC), gastric, pancreatic, esophageal, and colorectal cancers (CRCs) and have been reported to result in high morbidity and mortality all over the world (Zhang H. et al., 2018). A number of intervention methods including surgical treatment, chemotherapy, radiotherapy, and targeted drug treatment have been tried, but their overall effect on managing cancers of digestive system is still unsatisfactory (Ang et al., 2016). During advanced stages of development, systemic therapies based on targeted molecular drugs and cytotoxic chemotherapy holds the most promise to successful treatment of these cancers. For instance, rat sarcoma (RAS)-wild-type CRC was successfully treated with epidermal growth factor receptor (EGFR) monoclonal antibodies while human epidermal growth factor receptor 2 (HER2) high expression gastric cancer was managed in patients using the HER2 monoclonal antibody trastuzumab (Van Schaeybroeck et al., 2011; Gomez-Martín et al., 2014). Studies have shown that the hepatocyte growth factor (HGF)/mesenchymal epithelial transition (c-Met) signaling pathway plays a key role in growth, invasiveness, metastasis, and acquired drug resistance of cancers of digestive system. Based on this evidence, inhibition of HGF/c-Met axis may be a promising and revolutionary treatment method for these conditions.
HGF/C-Met Signaling Pathway
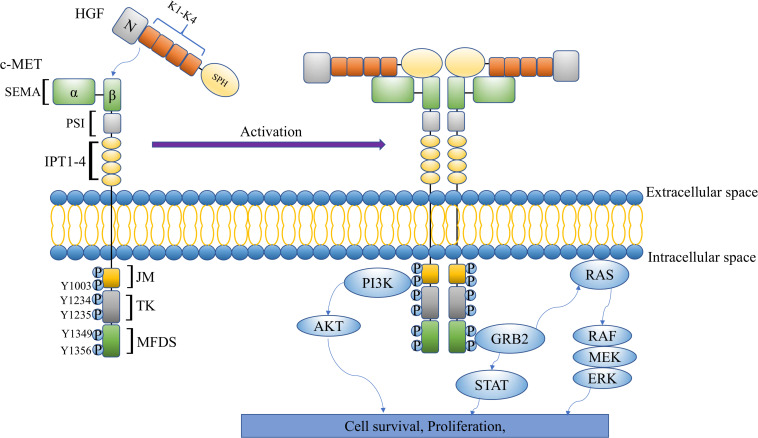
The c-Met proto-oncogene was originally identified in human osteosarcoma cells in the 1980s. It is located on chromosome 7q21-q31, encoding receptor tyrosine kinase c-Met (Cooper et al., 1984). Various differences between c-Met and other receptor tyrosine kinases can be found in the structure. Particularly, it is a heterodimer composed of a highly glycosylated 50-kDa α subunit and a transmembrane 145-kDa β subunit that are connected by a disulfide bond (Crepaldi et al., 1994). The basic structure of c-Met includes three parts: an extracellular structure, an intracellular structure, and a transmembrane domain. The extracellular part of c-Met is composed of a big N-terminal Sema, plexin-semaphorin-integrin (PSI), as well as four immunoglobulin-like IPT domains (IPT1–4) (Gherardi et al., 2006). On the other hand, the intracellular part is mainly composed of a juxtamembrane domain (JM), ser975, that plays a negative regulatory role in the signaling pathway, and a Y1003 residue that regulates degradation (Peschard et al., 2001). In addition, the Y1234 and Y1235 residues constitute the catalytic domain of c-Met where they positively regulate the pathway (Bradley et al., 2018). HGF is also located on chromosome 7q21.1 and is the only known c-Met ligand with high affinity (Parikh and Ghate, 2018). Mature HGF is an α-β heterodimer linked by disulfide bond. The α-chain (69 kDa) comprises an N-terminal (N) hairpin-loop and 4 kringle domains (K1–K4) while the β-chain (32–34 kDa) has a serine proteinase homology (SPH) domain (Holmes et al., 2007). It has been reported that autophosphorylation of Y1234 and Y1235 triggers further autophosphorylation of Y1349 and Y1356 residues in the multifunctional docking site (MFDS) (Miranda et al., 2018). The autophosphorylation of tyrosine residues can activate a multisubstrate docking site that comprises a variety of intercellular factors including Grb2-associated binding protein 1 (GAB1), Grb2-associated binding protein 1 (GAB2), phospholipase C (PLC), and sarcoma (SRC). With these, it can attract SH2 domain-containing tyrosine phosphatase 2 (SHP2), CT10 regulator of kinase-homolog-like (CRKL), and other docking molecules to activate Ras/mitogen-activated protein kinase (MAPK), PI3K/anaplastic lymphoma kinase (ALK), signal transducer and activator of transcription (STAT), and other signaling pathways (Eder et al., 2009; Gherardi et al., 2012). Activation of downstream signaling leads to regulation of cell proliferation, invasiveness, metastasis, and angiogenesis (Rong et al., 1994; Bladt et al., 1995; Michalopoulos and DeFrances, 1997; Xu et al., 2018; Figure 1).
FIGURE 1.
The multidomain structure and signaling pathway of HGF/c-Met.
Deregulation of HGF/C-Met Mediates Cancer
The strict regulation of HGF/c-Met signal transduction, observed in growth and regeneration, results in different degrees of maladjustment in various cancers, especially in the case of drug resistance or metastasis (Bradley et al., 2018). Failure to regulate the pathway leads to protein overexpression or, amplification, as well as gene mutations. In addition, a crosstalk between HGF/c-Met and other signaling pathways and increased paracrine and autocrine interaction have also been reported (Sierra and Tsao, 2011; Gherardi et al., 2012). All these eventually lead to oncogenic activation of c-Met. A previous study showed that overexpression of c-Met in mice led to eventual spontaneous development of HCC while inactivation of the transgene was found to initiate tumor regression (Wang et al., 2001). Studies have also shown that more c-Met mutations can be found at the site of metastasis, which indicates that such mutations are closely related to tumor progression (Parikh et al., 2014). In patients with gastroesophageal or CRCs, incidence of c-Met mutations is only 1–2% and 2–5% (Cancer Genome Atlas Network, 2012; Dulak et al., 2013; Cancer Genome Atlas Research Network, 2014). Amplification of the c-Met gene also accounts for a small proportion of gastric (3–6%) and CRCs (0.5–2%) (Cancer Genome Atlas Network, 2012; Cancer Genome Atlas Research Network, 2014; Gavine et al., 2015). Functionally, this amplification leads to overexpression of the c-Met protein, which brings about worse prognosis of the disease. Particularly, more c-Met gene amplifications can be found in patients with metastatic liver cancer (Zeng et al., 2008). A large number of solid tumors have been found to abundantly express HGF and c-Met, and this may be due to paracrine and autocrine interaction. In vivo studies have provided direct evidence that the effect of autocrine HGF/c-Met signaling is very important for proliferation and growth of lung adenocarcinoma cells (Yi and Tsao, 2000). In addition, expression of HGF can be detected all over the body. A study has shown that HGF is a systemic cytokine that also comes from tumor stroma (paracrine mode) (Sierra and Tsao, 2011). Another possible mechanism of c-Met activation is the crosstalk with other signaling pathways. For instance, while studying HCC, researchers showed that HGF triggered activation of c-Met, which resulted in simultaneous phosphorylation and expression of Caveolin1 (an integral membrane protein involved in signal transduction), while overexpression of Caveolin1 promoted the c-Met signaling pathway (Korhan et al., 2014). Similarly, EGFR stimulation also leads to signal transduction downstream of the c-Met pathway in bladder carcinoma cells that show moderate levels of EGFR and c-Met expression (Yamamoto et al., 2006). Some researchers found that insulin-like growth factor-I (IGF-I)-mediated progression of pancreatic cancer cells depended on c-Met, and the down-regulation of c-Met almost completely inhibited the tumorigenic effect of IGF-I (Yang et al., 2020). Several studies have shown that crosstalk also exists between c-Met and other receptor tyrosine kinase family members such as ERBB2 (also called HER2) and AXL (Khoury et al., 2005; Salian-Mehta et al., 2013). In general, these studies demonstrate that c-Met signaling can be activated in many ways. In non-small cell lung cancer (NSCLC), MET exon 14 (METex14) alterations are considered to be the primary driving mechanism of tumorigenesis. These alterations are closely related to MET overexpression and oncogenesis (Salgia et al., 2020). Previous data have shown that METex14 alterations can be detected in 3–4% of lung adenocarcinoma and 20–30% of pulmonary sarcomatoid carcinomas (Drilon et al., 2017). To date, there are few reports about the METex14 alterations in digestive system cancer. One study examined 230 solid tumor specimens (including 42 gastric and 43 colon cancer specimens) and found METex14 alterations in three gastric samples (7.1%) and four colon cancer samples (9.3%) (Lee et al., 2015). In addition, all the samples of positive METex14 were accompanied with overexpression of c-Met protein. This study preliminarily proved that METex14 alterations might play a driving role in digestive system cancer.
HGF/C-Met Signaling in Digestive System Cancers
HGF/c-Met Signaling Inhibitors in Digestive System Cancer
According to different mechanisms and structures, HGF/c-Met axis inhibitors are categorized into anti-HGF and anti-c-Met antibodies as well as small molecule c-Met kinase inhibitors. Based on chemical structures and different docking sites with kinases, there are three types of small-molecule c-Met kinase inhibitors, including selective, non-selective, and special structure c-Met inhibitors (Parikh and Ghate, 2018). So far, most of the HGF/c-Met inhibitors in digestive system cancer have been assessed in preclinical studies (Table 1) and phase I/II/III clinical trials (Table 2).
TABLE 1.
HGF/c-Met signaling inhibitors in preclinical studies.
| Cancer | Agent | Cell lines | Primary results | References |
| Hepatocellular carcinoma | Cabozantinib | MHCC97H | Cabozantinib inhibited tumor growth by decreasing angiogenesis, inhibiting proliferation, and promoting apoptosis | Xiang et al., 2014 |
| Gastric cancer | Savolitinib | Hs746t | Volitinib displayed a highly selective profile across a gastric cell line panel, potently inhibiting cell growth only in those lines with dysregulated c-Met | Gavine et al., 2015 |
| Pancreatic cancer | Crizotinib | Suit-2 | Crizotinib inhibits the peritoneal dissemination of Suit-2 cells | Takiguchi et al., 2017 |
| Crizotinib + gemcitabine | Orthotopic PDAC-FM-GC mouse models | Crizotinib decreased tumor dimension, prolonged survival, and increased blood and tissue concentrations of gemcitabine | Avan et al., 2013 | |
| Melanoma | SU11274 | Human malignant melanoma cell lines A375 (ATCC CRL-1619), M14 and M4Beu | SU11274 substantially decreased number of cells in adherent and spheroid cultures, but increased their tumorigenic potential | Kucerova et al., 2016 |
TABLE 2.
Summary of HGF/c-Met signaling inhibitors in clinical trials.
| Inhibitor name | Targets of inhibitor | Cancer type | Phase | Status | Clinical trial no. |
| Anti HGF antibody | |||||
| Rilotumumab (AMG 102) | HGF | Gastric cancer | Phase III | Terminated | NCT02137343 |
| Gastric cancer | Phase III | Terminated | NCT01697072 | ||
| Gastroesophageal adenocarcinoma | Phase II | Unknown | NCT01443065 | ||
| Colorectal and gastrointestinal cancer | Phase I/II | Completed | NCT00788957 | ||
| Gastric or esophagogastric junction cancer | Phase I/II | Completed | NCT00719550 | ||
| Gastric or GEJ cancer | Phase I | Completed | NCT01791374 | ||
| Ficlatuzumab (AV299) | HGF | Pancreatic cancer | Phase I | Recruiting | NCT03316599 |
| Anti c-Met antibody | |||||
| Onartuzumab (MetMAb) | c-Met | Gastric cancer | Phase III | Completed | NCT01662869 |
| Colorectal cancer | Phase II | Completed | NCT01418222 | ||
| Gastric cancer | Phase II | Completed | NCT01590719 | ||
| Hepatocellular carcinoma | Phase I | Completed | NCT01897038 | ||
| Emibetuzumab | c-Met | Advanced cancer | Phase I/II | Completed | NCT02082210 |
| Telisotuzumab–Vedotin | c-Met | Advanced solid tumors | Phase I | Recruiting | NCT02099058 |
| Small molecule c-Met kinase inhibitors | |||||
| Non-selective c-Met inhibitors (ATP competitive) | |||||
| Crizotinib | c-Met, ALK and ROS1 | c-Met positive gastric cancer | Phase II | Completed | NCT02435108 |
| Solid tumor and colorectal cancer | Phase I | Active, not recruiting | NCT02510001 | ||
| Diffuse gastric cancer or breast carcinoma | Phase II | Recruiting | NCT03620643 | ||
| Cabozantinib | c-Met, VEGFRs, RET, KIT and AXL | Hepatocellular carcinoma | Phase IV | Recruiting | NCT03963206 |
| Hepatocellular carcinoma | Phase III | Recruiting | NCT03755791 | ||
| Colorectal cancer | Phase II | Active, not recruiting | NCT03542877 | ||
| Colorectal cancer | Phase I | Completed | NCT02008383 | ||
| Pancreatic cancer | Phase I | Completed | NCT01663272 | ||
| Pancreatic adenocarcinoma | Phase II | Recruiting | NCT03213626 | ||
| Foretinib | c-Met, AXL, RON, VEGFR2 and TIE-2 | Gastric cancer | Phase II | Completed | NCT00725712 |
| Hepatocellular carcinoma | Phase I | Completed | NCT00920192 | ||
| Golvatinib (E7050) | c-Met, VEGFR-2 | Hepatocellular carcinoma | Phase I/II | Completed | NCT01271504 |
| Gastric cancer and solid tumors | Phase I/II | Terminated | NCT01355302 | ||
| Gastric cancer and solid tumors | Phase I | Completed | NCT01428141 | ||
| Selective c-Met inhibitors (ATP competitive) | |||||
| AMG 337 | c-Met | Stomach neoplasms | Phase II | Terminated | NCT02016534 |
| Phase I/II | Completed | NCT02096666 | |||
| Volitinib (Savolitinib) | c-Met | MET amplification gastric adenocarcinoma | Phase II | Recruiting | NCT02449551 |
| MET amplified metastatic or unresectable colorectal cancer | Phase II | Recruiting | NCT03592641 | ||
| Gastric adenocarcinoma with c-Met overexpression | Phase II | Recruiting | NCT02447380 | ||
| Gastric cancer | Phase I | Completed | NCT02252913 | ||
| Tepotinib (MSC2156119J) | c-Met | Hepatocellular carcinoma | Phase I/II | Completed | NCT02115373 |
| Hepatocellular carcinoma | Phase I/II | Active, not recruiting | NCT01988493 | ||
| Capmatinib | c-Met | Hepatocellular carcinoma | Phase II | Active, not recruiting | NCT01737827 |
| Hepatocellular carcinoma | Phase I/II | Active, not recruiting | NCT02795429 | ||
| Special structure c-Met inhibitors (Non-ATP competitive) | |||||
| Tivantinib (ARQ-197) | c-Met | Inoperable hepatocellular carcinoma | Phase III | Completed | NCT01755767 |
| Hepatocellular carcinoma | Phase III | Completed | NCT02029157 | ||
| Unresectable hepatocellular carcinoma | Phase II | Completed | NCT00988741 | ||
| Gastric cancer | Phase II | Completed | NCT01152645 | ||
| Pancreatic neoplasms | Phase II | Completed | NCT00558207 | ||
| Gastroesophageal cancer | Phase I/II | Completed | NCT01611857 | ||
| Hepatocellular carcinoma | Phase I | Completed | NCT00802555 | ||
| Advanced hepatocellular carcinoma | Phase I | Completed | NCT01656265 | ||
HGF/c-Met Signaling in HCC
Primary liver cancer is one of the most common malignant tumors in clinics. The condition ranks sixth and second based on incidence and mortality, respectively (Siegel and Miller, 2019). About 75–85% of primary liver cancer cases are HCC (Siegel and Miller, 2019). Sorafenib [a small-molecule multi-kinase inhibitor that targets vascular endothelial growth factor receptors (VEGFRs) rapidly accelerated fibrosarcoma (RAF) and other protein kinases] has been regarded as the standard of treatment for patients with advanced HCC since 2007 (Marisi et al., 2018). However, survival among patients even after treatment with sorafenib is still low, necessitating urgent development of new and effective treatment methods. Studies have demonstrated the role of HGF/c-Met axis in cell survival, cancer proliferation, and metastasis in HCC cases (Bahrami et al., 2017; García-Vilas and Medina, 2018; Ogunwobi et al., 2019) making anti-HGF/c-Met a promising therapeutic target for HCC treatment.
Tivantinib, a staurosporine derivative, is a non-ATP competitive selective small-molecule inhibitor and acts mainly as an anti c-Met inhibitor (Munshi et al., 2010). Previous studies that have targeted the molecule have, in turn, reported encouraging results in several phase I/II clinical trials. For instance, a randomized, controlled, phase II clinical trial evaluated the efficacy of tivantinib in patients with advanced HCC and Child–Pugh A cirrhosis with the results indicating that patients treated with the drug had a longer median disease progression time relative to the placebo (Santoro et al., 2013). Among patients with high c-Met expression, a more significant (2.7 months vs. 1.4 months; P = 0.03) curative effect was recorded (Santoro et al., 2013). However, some serious complications recorded in the tivantinib group included anemia and neutropenia, especially when a high dosage was administered. Unfortunately, in a phase III trial of METIV-HCC, tivantinib did not improve overall patient survival compared to the placebo (Rimassa et al., 2018). Nevertheless, there is evidence that a high expression of c-Met in HCC is related to a poor prognosis (Rimassa et al., 2016). Since the METIV-HCC trial selected patients with high c-Met levels, this does not necessarily mean that tivantinib has no role in targeted treatment in HCC patients. Therefore, determination of an accurate dosage coupled with guided patient stratification is key to the use of tivantinib as a target drug for HCC. Some scholars have pointed out that tivantinib cannot inhibit the c-Met tyrosine autophosphorylation, and its biological activity is mainly antimitotic. It kills any kind of cells regardless of the expression of c-Met protein, and the cytotoxicity of tivantinib is related to inhibit microtubule assembly (Basilico et al., 2013). This novel viewpoint may explain the results of phase III clinical trials.
Cabozantinib is a novel small-molecule multi-target tyrosine kinase inhibitor of c-Met, VEGFRs, RET, KIT, and AXL (Yakes et al., 2011). In mice xenotransplantation models, cabozantinib treatment has been shown to inhibit the growth and metastasis of HCC (Xiang et al., 2014). In a phase II placebo-controlled randomized discontinuation study, cabozantinib suppressed tumor growth, disease stability and reduced alpha fetoprotein (AFP) in HCC (Kelley et al., 2017). Recently, a phase III study assessment of the effect of cabozantinib on advanced HCC patients who had been previously treated showed a longer total survival time taken in the cabozantinib group compared to the controls (10.2 vs. 8 months; p = 0.005) (Abou-Alfa et al., 2018). The progression-free survival (PFS) period (5.2 vs. 1.9 months; p < 0.001) and objective response rate (ORR) (4 vs. < 1%; p = 0.009) also improved significantly. The main adverse events were erythrodysesthesia, hypertension, increased aspartate aminotransferase level, fatigue, and diarrhea.
Foretinib is a multi-target inhibitor for c-Met, AXL, RON, VEGFR2, and TIE-2 (Giordano and Columbano, 2014). A phase I/II clinical study evaluating the safety, pharmacodynamics, pharmacokinetics, and activity of foretinib in a late first-line trial reported 30 mg as the maximum dosage for foretinib in phase I. A total of 39 patients received foretinib treatment in phase II (38 patients were evaluable for efficacy), with a median overall survival time (OS) of 15.7 months and a median progression time of 4.2 months reported (Yau et al., 2017). The most common adverse reactions including hypertension (43.6%), loss of appetite (28.2%), ascites (25.6%), and fever (25.6%) were also recorded, although these were not considered serious. Overall, foretinib showed satisfactory antitumor activity in Asia in patients with advanced HCC.
Tepotinib is an effective and highly selective c-Met inhibitor, and its c-Met selectivity exceeds that of most kinase inhibitors with a cell-based 50% inhibitory concentration (IC50) of 1.7 nM (Falchook et al., 2020). In contrast, highly selective c-Met inhibitors have little effect on other targets and are expected to produce less toxicity. Therefore, a sufficient dose can guarantee effective c-Met inhibition. Tepotinib was first approved for the treatment of patients with advanced NSCLC in 2020 in Japan (Markham, 2020). A phase II clinical study on c-Met-positive advanced HCC showed that tepotinib had a longer progression time and PFS time than sorafenib (NCT01988493). The time to progression was 2.9 months in the tepotinib group and 1.4 months in the sorafenib group. The median PFS of the tepotinib group and sorafenib group was 2.8 and 1.4 months, respectively, and the median overall survival was 9.3 and 8.6 months, respectively. Another phase Ib/II study evaluated the efficacy and safety of tepotinib in patients with c-Met-positive HCC who have failed sorafenib treatment, and the PFS rate of 12 weeks was 63.3% (NCT02115373).
Capmatinib (INC280) is a highly selective c-Met inhibitor who has an IC50 of 0.13 nM for c-Met (Bouattour et al., 2018). A phase II study indicated that capmatinib is beneficial to tumor suppression in patients with c-Met-high HCC (Qin et al., 2019). The overall response rate was 30.0% and the disease control rate (DCR) was 50.0% in the c-Met high expression group. It even includes a long-term complete response of more than 600 days and two partial responses. Another phase Ib/II clinical trial is studying the safety and antitumor activity of capmatinib combined with PDR001 [a programmed death 1 (PD-1) inhibitor] in patients with HCC (NCT02795429). The clinical trial is anticipated to be completed at the end of 2020.
Several other similar c-Met inhibitors, such as golvatinib (E7050) and SU11274 have also been reported to have antitumor activity against HCC (Nakagawa et al., 2010; Inagaki et al., 2011). However, studies in relation to their effects in early clinical stages as well as their efficacy and safety are yet to be carried out. In view of the limited therapeutic effect of targeted drugs, a combination of therapies may be a more effective strategy for HCC treatment.
HGF/c-Met Signaling in Pancreatic Cancer
Pancreatic cancer (PC) is a highly malignant disease with the worst prognosis due to its strong invasion and metastasis ability. The disease’s 5-year survival rate is approximately 9%, the lowest of any cancer (Siegel and Miller, 2019). Pancreatic cancer patients with high c-Met and HGF expression levels tend to show poor prognosis and low survival rates (Yan et al., 2014). Studies have shown that c-Met expression levels in pancreatic tumors are related to tumor grades (Gardian et al., 2012). Similarly, it has been demonstrated that c-Met expression increased in pancreatic ductal adenocarcinoma, suggesting that c-Met might be a molecular marker for predicting prognosis in patients with pancreatic cancer (Zhu et al., 2011).
Crizotinib is an ATP competitive multi-target protein kinase inhibitor that targets c-Met, ALK, and ROS1 (Takiguchi et al., 2017). In an orthotopic mouse model experiment, crizotinib was found to increase the concentration of gemcitabine in blood and tissues, reduce sizes of tumors, inhibit peritoneal dissemination of highly Met-expressing pancreatic cancer, and prolong survival (Avan et al., 2013). Similarly, an in vitro experiment showed that crizotinib can be used to treat peritoneal spread of pancreatic cancer based on inhibition of cell proliferation and metastasis mediated by phosphorylation of the c-Met pathway (Takiguchi et al., 2017). However, it is not clear whether the above effects were mediated by the c-Met signaling pathway.
Reports have also indicated that long-term treatments involving cabozantinib induce less resistance and can improve the efficacy of gemcitabine and can overcome gemcitabine resistance in pancreatic cancer (Hage et al., 2013). The effects are achieved by suppressing total c-MET, controlling downstream phosphorylation of c-MET, and decreasing expression of transcription factor SRY-related HMG-box 2 (SOX2) (Hage et al., 2013). An experiment conducted to determine maximum tolerable concentrations of cabozantinib and gemcitabine in 10 advanced pancreatic cancer patients showed that more than 25% of the patients in all concentration groups had dose-limiting toxicity (≥grade 3 ALT or AST elevations or ≥grade 3 thrombocytopenia) (Zhen et al., 2016). Despite the small sample size used in the study, a certain level of significance for future research of cabozantinib can be still be derived from the findings.
HGF/c-Met Signaling in Gastric Cancer
Gastric cancer is the fifth most common form of cancer worldwide and the third leading cause of cancer-related deaths (Padmanabhan et al., 2017). Gastric cancer patients who have a high expression of c-Met show poor prognosis compared to those with c-Met negative tumors (Fuse et al., 2016). Numerous preclinical and clinical studies have generated data on monoclonal antibodies against HGF/c-Met axis, which provide possible targets for development of gastric cancer treatments.
Rilotumumab (AMG102), a monoclonal antibody of the human IgG2 targeting HGF, can block binding of HGF to c-Met. A randomized phase II clinical trial showed that this antibody in combination with epirubicin, cisplatin, and capecitabine (ECX) could prolong PFS in advanced gastric cancer patients relative to the control group, especially in patients with high c-Met expression (Iveson et al., 2014). Based on these results, a further study on the effects of rilotumumab combined with ECX in treatment of gastric cancer was conducted using two phase III trials (RILOMET-1 and 2) (Doi et al., 2015; Catenacci et al., 2017). Unfortunately, both trials were terminated because RILOMET-1 results showed an increase in the number of deaths due to complications in patients treated with rilotumumab compared to the placebo (Catenacci et al., 2017).
Onartuzumab is a recombinant humanized anti-c-Met monoclonal antibody, which is produced in Escherichia coli (Merchant et al., 2013). Evaluation of the safety and efficacy of onartuzumab combined with mFOLFOX6 in patients with HER2-negative gastric cancer showed that adding onartuzumab to mFOLFOX6 failed to improve efficacy of c-Met immunohistochemically positive population or even the whole population (Shah et al., 2016). Similarly, a phase III clinical trial revealed no significant improvements in clinical benefits in first-line chemotherapy for HER2-negative and c-Met-positive advanced gastroesophageal adenocarcinoma (GEC). Another small molecule, AMG 337, which is a selective inhibitor targeting c-Met has also been reported (Hughes et al., 2016). A phase II clinical study assessing 45 patients with gastric/gastroesophageal junction/esophageal tumor and AMG 337 monotherapy patients diagnosed with c-Met-amplified tumors showed an ORR of 18%, indicating that AMG 337 has certain antitumor activities (Van Cutsem et al., 2019).
Emibetuzumab (LY2875358) is a humanized immunoglobulin G4 monoclonal anti-Met antibody. The drug inhibits the activation of HGF/c-Met pathway by preventing HGF from binding to its receptor, c-Met, and by degrading c-Met (Liu et al., 2014). A phase II study evaluated the safety and efficacy of emibetuzumab in patients with advanced gastric cancer. The 8-week PFS rate is 47%, and the DCR is 40%. Although the sample size is small, monotherapy of emibetuzumab was well tolerated and showed certain anti-tumor activity (Sakai et al., 2017). A phase Ib/II study evaluated the efficacy of emibetuzumab combined with ramucirumab (a monoclonal anti-VEGFR-2 antibody) in 97 patients with solid tumors (including 16 gastric or gastroesophageal junction adenocarcinoma, 45 HCC). The results showed that the combination therapy had more significant antitumor activity.
Telisotuzumab (ABT-700) is a novel anti-c-Met antibody that binds c-Met with high affinity and inhibits c-Met signaling. Unlike most other c-Met antibodies, it destroys the productive dimerization and activation induced by HGF or c-Met on the cell surface independent of ligand (Wang et al., 2016). A phase I study evaluated the safety and efficacy of telisotuzumab in patients with advanced solid tumors. However, significant clinical antitumor activity was only observed in patients with MET-amplified gastroesophageal cancer (Strickler et al., 2020). Considering that primary MET genomic amplification a low-frequency event in most tumors, the researchers developed an antibody–drug conjugate, telisotuzumab–vedotin, also known as ABBV-399, which is composed of telisotuzumab and cytotoxic monomethyl auristatin E (MMAE) via a valine–citrulline linker (Wang et al., 2017). Telisotuzumab–vedotin does not require the tumors to be addicted to the oncogene. It delivers cytotoxin MMAE directly to c-Met-positive tumor cells and has demonstrated antitumor activity in tumors without increased copy number of the MET gene (Strickler et al., 2018). A phase I clinical trial is studying the safety and preliminary efficacy of telisotuzumab–vedotin in patients with advanced solid tumors (NCT02099058). The clinical trial is anticipated to be completed at the beginning of 2022.
Savolitinib is a novel and highly selective c-Met inhibitor, which has shown encouraging results in the phase III randomized clinical trial of papillary renal cell carcinoma (PRCC) (Choueiri et al., 2020). In a preclinical study, savolitinib showed tolerable side effects and significant antitumor effect in c-Met-amplified mice (Gavine et al., 2015). Recently, a multiple-arm clinical trial evaluated the efficacy of targeted therapy in 772 patients with metastatic gastric cancer (Lee et al., 2019). The results showed that there was the highest response rate in the fourth arm (c-Met amplification–savolitinib monotherapy). One patient developed malignant ascites after failure of capecitabine/oxaliplatin treatment. Following savolitinib treatment, the tumor was significantly reduced and the patient achieved curative resection.
Tivantinib, foretinib, and other c-Met tyrosine kinase inhibitors (TKIs), have previously resulted in no clear antitumor activity in gastric cancer patients (Shah et al., 2013; Kang et al., 2014). Although results from several recent clinical trials do not show promise as anti-c-Met drugs, a combined therapeutic strategy for multiple downstream signal transduction could have clinical potential, especially in c-Met-positive patients.
The Role of HGF/c-Met Signaling in Colorectal Cancer
Colorectal cancer is the third most common cancer in the world, although a decline in CRC mortality has been observed in the recent past (Siegel and Miller, 2019). Various studies have reported that overexpression or amplification of c-Met in early (I and II) and late CRC (III and IV) is closely related to its invasion and distant metastasis (Zeng et al., 2008; Garouniatis et al., 2013; Lee et al., 2018). For this reason, studies have hypothesized that inhibition of c-Met activation may decrease tumor activity in CRC.
Ficlatuzumab (AV-299) is a humanized, high-affinity monoclonal antibody that can block HGF/c-Met binding and mediate downstream phosphorylation of the signaling pathway (Mok et al., 2016). A study assessing tolerability and safety of ficlatuzumab in advanced CRC patients demonstrated that the antibody has the ability to regulate HGF/c-Met pathway and downstream signaling (Tabernero et al., 2014).
The therapeutic effect of rilotumumab in CRC has also been evaluated in a randomized phase Ib/II research where panitumumab (a monoclonal antibody targeting EGFR) was combined with rilotumumab or placebo in patients with kirsten rat sarcoma (KRAS) wild-type metastatic CRC who met the prespecified criterion for improvement in ORR (Van Cutsem et al., 2014). Although the combined inhibition of HGF/c-Met and EGFR showed some encouraging results, no further research findings have been reported with regard to rilotumumab and panitumumab development in CRC.
Another new compound, SU11274, that targets the c-Met ATP-binding site has also been reported and implicated in blocking of HGF-dependent c-Met activation (Gao W. et al., 2013). In vivo experiments showed that daily administration of SU11274 in mice resulted in inhibition of tumor growth in xenografts. It was also found that SU11274 could significantly inhibit survival and growth of colon cancer cells (Gao S. H. et al., 2013). However, a study of melanoma in vitro showed that the off-target action of SU11274 resulted in the increase of tumorigenic potential (Kucerova et al., 2016). Whether SU11274 can be used as a candidate drug to inhibit c-Met necessitates further clinical trials.
Analysis of clinical effects of tivantinib, following its gradual introduction into CRC, reveals negative results. For instance, a randomized controlled phase I/II clinical trial of tivantinib combined with irinotecan and cetuximab showed no significant improvement of PFS or OS in metastatic CRC patients with wild-type KRAS (Eng et al., 2016). Similarly, a new multicenter phase II study suggested that a combination of tivantinib and cetuximab did not achieve the expected efficacy in CRC patients who had high c-Met expression and acquired resistance to anti-EGFRs (Rimassa et al., 2019). However, a study indicated that inhibition of the HGF/c-MET pathway can improve the sensitivity of CRC to EGFR inhibitors, indicating that combination therapy could still be a future research direction (Liska et al., 2011).
Currently, relatively few reports about HGF inhibitors in CRC exist. In general, though the research on anti-HGF/c-Met axis for CRC is still in its infancy, they have great potential in the future treatment.
HGF/c-Met Signaling in Non-digestive System Cancer
In addition to digestive system cancers, the study of c-Met inhibitors is also very active for other cancer. A large number of clinical trials have been carried out on melanoma, breast cancer, PRCC, NSCLC, and medullary thyroid cancer (MTC), providing a lot of reliable experience for the treatment of digestive system cancers (Table 3).
TABLE 3.
The clinical research of c-Met inhibitors in non-digestive system cancers.
| Cancer | Agents | Phase | Primary results | References |
| Melanoma | Cabozantinib | II | Cabozantinib vs. temozolomide or dacarbazine: PFS: 60 vs. 59 days (P = 0.964; HR = 0.99). OS: 6.4 vs. 7.3 months (P = 0.580; HR = 1.21). Cabozantinib demonstrated no improvement in PFS but an increase in toxicity. | Luke et al., 2020 |
| Breast cancer with bone metastases | Cabozantinib | II | Bone scans improved in 38% of patients and remained stable in an additional 12% for a minimum duration of 12 weeks. PFS was 4.3 months and OS was 19.6 months. | Xu et al., 2020 |
| Papillary renal cell carcinoma | Savolitinib vs. sunitinib | III | PFS: 7.0 months (95% CI, 2.8–not calculated) for savolitinib and 5.6 months (95% CI, 4.1–6.9) for sunitinib. Savolitinib demonstrated encouraging efficacy with fewer grade 3 or higher adverse events. | Choueiri et al., 2020 |
| Non-Small Cell Lung Cancer | Tepotinib | II | The response rate of liquid-biopsy group (n = 66) and tissue-biopsy group (n = 60) were 48% and 50%, respectively. Median duration of response was 11.1 months. | Paik et al., 2020 |
| Tivantinib + erlotinib | III | Erlotinib + tivantinib vs. erlotinib + placebo; PFS: 13.0 vs. 7.5 months; OS: 25.5 vs. 20.3 months. Erlotinib + tivantinib was tolerable and showed improved efficacy over erlotinib monotherapy. | Scagliotti et al., 2018 | |
| Medullary thyroid cancer | Cabozantinib | III | Cabozantinib vs. placebo: PFS: 11.2 vs. 4.0 months; ORR: 28 vs. 0%. | Elisei et al., 2013 |
A phase II clinical trial evaluated the efficacy of cabozantinib compared with traditional chemotherapy in the treatment of uveal melanoma (Luke et al., 2020). The result showed that cabozantinib did not demonstrate improvement in PFS (cabozantinib vs. temozolomide or dacarbazine: 60 vs. 59 days). In another phase II study, cabozantinib showed bone-centric activity in patients of breast cancer with bone metastases (Xu et al., 2020). Savolitinib has shown encouraging results in the phase III randomized clinical trial of PRCC (Choueiri et al., 2020). In this clinical trial, 60 patients were randomly divided into group savolitinib (n = 33) and group sunitinib (n = 27). The primary end point was PFS; group savolitinib and group sunitinib were 7.0 and 5.8 months, respectively. Tivantinib and tepotinib have demonstrated significant antitumor activity in NSCLC. In a phase III study of tivantinib in patients with NSCLC, tivantinib combined with erlotinib led to a significant prolongation of PFS (13.0 vs. 7.5 months) and OS (25.5 vs. 20.3 months) compared to erlotinib monotherapy (Scagliotti et al., 2018). A phase II study evaluated the efficacy of tepotinib in patients with advanced NSCLC with METex14 alterations (Paik et al., 2020). The response rate of the liquid-biopsy group (n = 66) and the tissue-biopsy group (n = 60) were 48% and 50%, respectively. Median duration of response was 11.1 months. In a phase III clinical trial, 330 patients with progressive metastatic MTC were randomly assigned to the cabozantinib group (n = 220) or the placebo group (n = 110) (Elisei et al., 2013). Median duration of PFS was 11.2 and 4.0 months in cabozantinib and placebo, respectively. ORR was 28% in the cabozantinib group and 0% in the placebo group. The therapeutic effect of cabozantinib on MTC was statistically significant.
Prognostic Effect of HGF/C-Met Signaling Pathway in Digestive System Cancer
Previous clinical trials have indicated that that high expression of c-Met gene is involved in poor prognosis and high risk of many types of cancer. Consequently, scientists have extensively studied the relationship between overexpression of the c-Met gene and prognosis of a single tumor, mainly focusing on CRC, gastric cancer, breast cancer, and lung cancer (Bradley et al., 2018). A meta-analysis of 11 retrospective studies (including 1895 patients with CRC) suggested that OS (HR 1.33, 95% CI 1.06–1.59) and PFS duration (HR 1.47, 95% CI 1.03–1.91) in patients with positive c-Met was low (Liu et al., 2015). Similarly, another meta-analysis of 14 retrospective studies (including 2258 patients with gastric cancer) showed that high amplification and expression of the c-Met gene in gastric cancer is significantly related to poor prognosis (Peng et al., 2014). Furthermore, a meta-analysis of 1408 postoperative patients with liver cancer revealed a significant reduction in the relapse-free survival (HR 1.26; 95% CI, 1.02–1.56) and total survival (HR 1.16; 95% CI, 1.03–1.31) rates of patients overexpressing c-Met (Kim et al., 2017a). Similar studies have been carried out among patients diagnosed with pancreatic, biliary tract, esophageal, as well as other types of cancer (Kim et al., 2017b; Ren et al., 2017; Zhang Z. et al., 2018). All these results show that overexpression of the c-Met gene is significantly correlated with and can lead to the poor prognosis of these tumors.
Limitations of the Efficacy of C-Met Inhibitors
A large number of clinical trials have proved that the safety of HGF or c-Met inhibitors is reliable; however, there is no significant clinical benefit for patients. A variety of potential factors contribute to the regrettable result. The pathway of c-Met signal transduction is extremely complex, and there is extensive crosstalk between c-Met and other carcinogenic pathways, which may lead to the treatment of targeting HGF, or c-Met cannot fully block the downstream signal transduction. In addition, the inhibition of c-Met promotes the stability of PDL1 and makes tumor cells escape from immune surveillance. A study found that the expression of PDL1 is up-regulated in the HCC cells of mice treated with c-Met inhibitors, which induces the functional inactivation of T-cells and enables HCC cells to escape from the killing of T-cells (Li et al., 2019). Similar results were observed in an in vitro experiment of NSCLC (Sun et al., 2020). Therefore, the monotherapy of targeting c-Met may not bring significant clinical benefits to patients. Adopting novel combination strategy is the key of the research.
Combination Therapy
A study has shown that inhibiting the c-Met signaling pathway compensatively activates the EGFR pathway (Steinway et al., 2015). In addition, MET amplification is one of the mechanisms of drug resistance of EGFR TKI (Kumar et al., 2020). Therefore, combination therapy strategy targeting the c-Met and EGFR pathway may bring more significant anti-tumor effect. In particular, several studies have shown significant benefits of the combination therapy in NSCLC, melanoma, breast cancer, CRC, and HCC (Steinway et al., 2015; Zielonka et al., 2015; Dratkiewicz et al., 2019; Rimassa et al., 2019; Chu et al., 2020). An in vitro study showed that simultaneous inhibition of c-Met and EGFR pathways inhibits HCC tumor growth (Steinway et al., 2015). Similarly, the combined elimination of c-Met and EGFR significantly inhibited the proliferation of hepatocytes (Bhushan et al., 2019). A phase Ib study of patients with MET-positive CRC indicated that the treatment strategy of capmatinib in combination with cetuximab (an anti-EGFR monoclonal antibody) was tolerable and showed preliminary antitumor effects (Delord et al., 2020). Overall, the combined inhibition of c-Met and EGFR is a promising treatment strategy, warranting further exploration.
A study has indicated that the anti-tumor effect of the single drug of tivantinib or anti-PD1 is not as good as the combination of tivantinib and anti-PD1, and the combined treatment is more effective in inhibiting the growth of liver tumor (Li et al., 2019). Similarly, another study showed that anti-PD1 treatment can inhibit the expression of PDL1 induced by c-Met inhibitors, and the combination of c-Met inhibitor and anti-PD1 can better control the tumor progression (Glodde et al., 2017). A clinical case report evaluated a patient with advanced HCC. The patient was given 60 mg cabozantinib daily. One month later, nivolumab (100 mg per 21 days), a PD1 inhibitor, was added to the patient’s treatment regimen, and the patient showed a good tolerance response. Surprisingly, after the treatment of cabozantinib and nivolumab, the patient achieved a PFS of more than 25 months (Yang et al., 2020). A large number of clinical trials have been carried out to evaluate the combination regimens of c-Met inhibitors, anti-PD1 agents, and other drugs in the treatment of malignant tumors. For example, the combination of cabozantinib plus nivolumab is now being evaluated in a phase I study of patients with advanced HCC, and the primary endpoints are feasibility and efficacy (NCT03299946). Similarly, a clinical study is evaluating cabozantinib in combination with durvalumab (a PD1 inhibitor) in patients with advanced gastroesophageal cancer and other gastrointestinal malignancies (NCT03539822). Most noteworthy, the combination of cabozantinib and atezolizumab (a PD-L1 inhibitor) is now being evaluated in a phase III study of patients with advanced HCC (NCT03755791). The primary objectives of this study are OS and PFS. Experimental arm receives cabozantinib 40 mg once a day, plus atezolizumab 1200 mg i.v. every 3 weeks. Control arm will receive sorafenib 400 mg twice a day. The clinical study is anticipated to be completed at the end of 2021. In addition, the efficacy and safety of cabozantinib + nivolumab for patients with advanced HCC were first presented at 2020 ASCO Gastrointestinal Cancer Symposium. A total of 71 patients were included in the study. Thirty-five patients were treated with cabozantinib + nivolumab (doublet therapy) and 36 patients were treated with cabozantinib + nivolumab + ipilimumab (an antibody inhibiting CLTA-4) (triple therapy). ORR was 17% and 26% in the doublet therapy and triple therapy groups, respectively. DCR of the two groups was 81% and 83% and the median PFS was 5.5 months and 6.8 months, respectively. The clinically meaningful responses from these cabozantinib combinations are encouraging. Overall, these clinical studies likely portend the future of gastrointestinal tumors treatment.
Conclusion
An imbalance in HGF/c-Met signal transduction is a driving factor for cancers of the digestive system, and this promotes tumor growth, as well as its invasiveness and dissemination. Numerous studies have shown the wide application of c-Met in digestive system cancers with related clinical and preclinical evidences supporting the anti-HGF/c-Met signaling pathway as a reliable treatment for HCC, gastric, pancreatic, and CRC among others. In particular, c-Met-positive patients showed encouraging results in the phase II study, suggesting that finding the most appropriate subgroup of patients may make the most of HGF inhibitors. A crosstalk between c-Met and other signaling channels holds the key to future therapies. Particularly, single targets for inhibition of HGF/c-Met may not be the most ideal method for developing treatment against this condition. It will also be important for future research works to target other key signaling pathways during development of therapies.
In general, the HGF/c-Met axis is an excellent therapeutic target, but it needs a more in-depth and guided research approach. The key challenges for future research include the selection of patients who may benefit from the treatment, development of optimal therapeutic combinations, and identification of highly sensitive biomarkers for monitoring disease prognosis.
Author Contributions
ZS, HP, ST, and JZ drafted the manuscript. AS and SY reviewed and modified the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was funded by the China Postdoctoral Science Foundation (2017M612010) and the National Natural Science Foundation of China (81701144).
References
- Abou-Alfa G. K., Meyer T., Cheng A. L., El-Khoueiry A. B., Rimassa L., Ryoo B. Y., et al. (2018). Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N. Engl. J. Med. 379 54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang C., Doyle E., Branch A. (2016). Bisphosphonates as potential adjuvants for patients with cancers of the digestive system. World J. Gastroenterol. 22 906–916. 10.3748/wjg.v22.i3.906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avan A., Caretti V., Funel N., Galvani E., Maftouh M., Honeywell R. J., et al. (2013). Crizotinib inhibits metabolic inactivation of gemcitabine in c-Met-driven pancreatic carcinoma. Cancer Res. 73 6745–6756. 10.1158/0008-5472.can-13-0837 [DOI] [PubMed] [Google Scholar]
- Bahrami A., Shahidsales S., Khazaei M., Ghayour-Mobarhan M., Maftouh M., Hassanian S. M., et al. (2017). C-Met as a potential target for the treatment of gastrointestinal cancer: current status and future perspectives. Journal Cell. Physiol. 232 2657–2673. 10.1002/jcp.25794 [DOI] [PubMed] [Google Scholar]
- Basilico C., Pennacchietti S., Vigna E., Chiriaco C., Arena S., Bardelli A., et al. (2013). Tivantinib (ARQ197) displays cytotoxic activity that is independent of its ability to bind MET. Clin. Cancer Res. 19 2381–2392. 10.1158/1078-0432.ccr-12-3459 [DOI] [PubMed] [Google Scholar]
- Bhushan B., Stoops J. W., Mars W. M., Orr A., Bowen W. C., Paranjpe S., et al. (2019). TCPOBOP-induced hepatomegaly and hepatocyte proliferation are attenuated by combined disruption of MET and EGFR signaling. Hepatology 69 1702–1718. 10.1002/hep.30109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bladt F., Riethmacher D., Isenmann S., Aguzzi A., Birchmeier C. (1995). Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature 376 768–771. 10.1038/376768a0 [DOI] [PubMed] [Google Scholar]
- Bouattour M., Raymond E., Qin S., Cheng A. L., Stammberger U., Locatelli G., et al. (2018). Recent developments of c-Met as a therapeutic target in hepatocellular carcinoma. Hepatology 67 1132–1149. 10.1002/hep.29496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley C. A., Salto-Tellez M., Laurent-Puig P., Bardelli A., Rolfo C., Tabernero J., et al. (2018). Targeting c-MET in gastrointestinal tumours: rationale, opportunities and challenges. Nat. Rev. Clin. Oncol. 15:150. 10.1038/nrclinonc.2018.13 [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas Network (2012). Comprehensive molecular characterization of human colon and rectal cancer. Nature 487 330–337. 10.1038/nature11252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network (2014). Comprehensive molecular characterization of gastric adenocarcinoma. Nature 513 202–209. 10.1038/nature13480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catenacci D. V. T., Tebbutt N. C., Davidenko I., Murad A. M., Al-Batran S. E., Ilson D. H., et al. (2017). Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 18 1467–1482. 10.1016/s1470-2045(17)30566-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choueiri T. K., Heng D. Y. C., Lee J. L., Cancel M., Verheijen R. B., Mellemgaard A., et al. (2020). Efficacy of savolitinib vs sunitinib in patients with MET-driven papillary renal cell carcinoma: the SAVOIR phase 3 randomized clinical trial. JAMA Oncol. 6 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y. Y., Yam C., Chen M. K., Chan L. C., Xiao M., Wei Y. K., et al. (2020). Blocking c-Met and EGFR reverses acquired resistance of PARP inhibitors in triple-negative breast cancer. Am. J. Cancer Res. 10 648–661. [PMC free article] [PubMed] [Google Scholar]
- Cooper C. S., Park M., Blair D. G., Tainsky M. A., Huebner K., Croce C. M., et al. (1984). Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature 311 29–33. 10.1038/311029a0 [DOI] [PubMed] [Google Scholar]
- Crepaldi T., Prat M., Giordano S., Medico E., Comoglio P. M. (1994). Generation of a truncated hepatocyte growth factor receptor in the endoplasmic reticulum. J. Biol. Chem. 269 1750–1755. [PubMed] [Google Scholar]
- Delord J. P., Argilés G., Fayette J., Wirth L., Kasper S., Siena S., et al. (2020). A phase 1b study of the MET inhibitor capmatinib combined with cetuximab in patients with MET-positive colorectal cancer who had progressed following anti-EGFR monoclonal antibody treatment. Investig. New Drugs 10.1007/s10637-020-00928-z [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Doi T., Kang Y. K., Muro K., Jiang Y. Z., Jain R. K., Lizambri R. (2015). A phase 3, multicenter, randomized, double-blind, placebo-controlled study of rilotumumab in combination with cisplatin and capecitabine (CX) as first-line therapy for Asian patients (pts) with advanced MET-positive gastric or gastroesophageal junction (G/GEJ) adenocarcinoma: the RILOMET-2 trial. J. Clin. Oncol. 33:TPS226. [Google Scholar]
- Dratkiewicz E., Simiczyjew A., Pietraszek-Gremplewicz K., Mazurkiewicz J., Nowak D. (2019). Characterization of melanoma cell lines resistant to vemurafenib and evaluation of their responsiveness to EGFR- and MET-inhibitor treatment. Int. J. Mol. Sci. 21:113. 10.3390/ijms21010113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drilon A., Cappuzzo F., Ou S. I., Camidge D. R. (2017). Targeting MET in lung cancer: will expectations finally be MET? J. Thoracic Oncol. 12 15–26. 10.1016/j.jtho.2016.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulak A. M., Stojanov P., Peng S., Lawrence M. S., Fox C., Stewart C., et al. (2013). Exome and whole-genome sequencing of esophageal adenocarcinoma identifies recurrent driver events and mutational complexity. Nat. Genet. 45 478–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder J. P., Vande Woude G. F., Boerner S. A., LoRusso P. M. (2009). Novel therapeutic inhibitors of the c-Met signaling pathway in cancer. Clin. Cancer Res. 15 2207–2214. 10.1158/1078-0432.ccr-08-1306 [DOI] [PubMed] [Google Scholar]
- Elisei R., Schlumberger M. J., Müller S. P., Schöffski P., Brose M. S., Shah M. H., et al. (2013). Cabozantinib in progressive medullary thyroid cancer. J. Clin. Oncol. 31 3639–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng C., Bessudo A., Hart L. L., Severtsev A., Gladkov O., Müller L., et al. (2016). A randomized, placebo-controlled, phase 1/2 study of tivantinib (ARQ 197) in combination with irinotecan and cetuximab in patients with metastatic colorectal cancer with wild-type KRAS who have received first-line systemic therapy. Int. J. Cancer 139 177–186. 10.1002/ijc.30049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falchook G. S., Kurzrock R., Amin H. M., Xiong W., Fu S., Piha-Paul S. A., et al. (2020). Trial of the selective MET inhibitor tepotinib in patients with advanced solid tumors. Clin. Cancer Res. 26 1237–1246. 10.1158/1078-0432.ccr-19-2860 [DOI] [PubMed] [Google Scholar]
- Fuse N., Kuboki Y., Kuwata T., Nishina T., Kadowaki S., Shinozaki E., et al. (2016). Prognostic impact of HER2, EGFR, and c-MET status on overall survival of advanced gastric cancer patients. Gastr. Cancer 19 183–191. 10.1007/s10120-015-0471-6 [DOI] [PubMed] [Google Scholar]
- Gao S. H., Liu C., Wei J., Feng Y. (2013). Effect of c-Met inhibitor SU11274 on human colon cancer cell growth. Chinese Med. J. 126 2705–2709. [PubMed] [Google Scholar]
- Gao W., Bing X., Li M., Yang Z., Li Y., Chen H. (2013). Study of critical role of c-Met and its inhibitor SU11274 in colorectal carcinoma. Med. Oncol. 30:546. [DOI] [PubMed] [Google Scholar]
- García-Vilas J. A., Medina M. Á. (2018). Updates on the hepatocyte growth factor/c-Met axis in hepatocellular carcinoma and its therapeutic implications. World J. Gastroenterol. 24 3695–3708. 10.3748/wjg.v24.i33.3695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardian K., Janczewska S., Durlik M. (2012). Microenvironment elements involved in the development of pancreatic cancer tumor. Gastroenterol. Res. Pract. 2012:585674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garouniatis A., Zizi-Sermpetzoglou A., Rizos S., Kostakis A., Nikiteas N., Papavassiliou A. G. (2013). FAK, CD44v6, c-Met and EGFR in colorectal cancer parameters: tumour progression, metastasis, patient survival and receptor crosstalk. Int. J. Colorect. Dis. 28 9–18. 10.1007/s00384-012-1520-9 [DOI] [PubMed] [Google Scholar]
- Gavine P. R., Ren Y., Han L., Lv J., Fan S., Zhang W., et al. (2015). Volitinib, a potent and highly selective c-Met inhibitor, effectively blocks c-Met signaling and growth in c-MET amplified gastric cancer patient-derived tumor xenograft models. Mol. Oncol. 9 323–333. 10.1016/j.molonc.2014.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherardi E., Birchmeier W., Birchmeier C., Vande Woude G. (2012). Targeting MET in cancer: rationale and progress. Nat. Rev. Cancer 12 89–103. 10.1038/nrc3205 [DOI] [PubMed] [Google Scholar]
- Gherardi E., Sandin S., Petoukhov M. V., Finch J., Youles M. E., Ofverstedt L. G., et al. (2006). Structural basis of hepatocyte growth factor/scatter factor and MET signalling. Proc. Natl. Acad. Sci. U.S.A. 103 4046–4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano S., Columbano A. (2014). Met as a therapeutic target in HCC: facts and hopes. J. Hepatol. 60 442–452. 10.1016/j.jhep.2013.09.009 [DOI] [PubMed] [Google Scholar]
- Glodde N., Bald T., van den Boorn-Konijnenberg D., Nakamura K., O’Donnell J. S., Szczepanski S., et al. (2017). Reactive neutrophil responses dependent on the receptor tyrosine kinase c-MET limit cancer immunotherapy. Immunity 47 789.e9–802.e9. [DOI] [PubMed] [Google Scholar]
- Gomez-Martín C., Lopez-Rios F., Aparicio J., Barriuso J., García-Carbonero R., Pazo R., et al. (2014). A critical review of HER2-positive gastric cancer evaluation and treatment: from trastuzumab, and beyond. Cancer Lett. 351 30–40. 10.1016/j.canlet.2014.05.019 [DOI] [PubMed] [Google Scholar]
- Hage C., Rausch V., Giese N., Giese T., Schönsiegel F., Labsch S., et al. (2013). The novel c-Met inhibitor cabozantinib overcomes gemcitabine resistance and stem cell signaling in pancreatic cancer. Cell Death Dis. 4:e627. 10.1038/cddis.2013.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes O., Pillozzi S., Deakin J. A., Carafoli F., Kemp L., Butler P. J., et al. (2007). Insights into the structure/function of hepatocyte growth factor/scatter factor from studies with individual domains. J. Mol. Biol. 367 395–408. 10.1016/j.jmb.2006.12.061 [DOI] [PubMed] [Google Scholar]
- Hughes P. E., Rex K., Caenepeel S., Yang Y., Zhang Y., Broome M. A., et al. (2016). In vitro and in vivo activity of AMG 337, a potent and selective MET kinase inhibitor, in MET-dependent cancer models. Mol. Cancer Ther. 15 1568–1579. 10.1158/1535-7163.mct-15-0871 [DOI] [PubMed] [Google Scholar]
- Inagaki Y., Qi F., Gao J., Qu X., Hasegawa K., Sugawara Y., et al. (2011). Effect of c-Met inhibitor SU11274 on hepatocellular carcinoma cell growth. Biosci. Trends 5 52–56. 10.5582/bst.2011.v5.2.52 [DOI] [PubMed] [Google Scholar]
- Iveson T., Donehower R. C., Davidenko I., Tjulandin S., Deptala A., Harrison M., et al. (2014). Rilotumumab in combination with epirubicin, cisplatin, and capecitabine as first-line treatment for gastric or oesophagogastric junction adenocarcinoma: an open-label, dose de-escalation phase 1b study and a double-blind, randomised phase 2 study. Lancet Oncol. 15 1007–1018. 10.1016/S1470-2045(14)70023-3 [DOI] [PubMed] [Google Scholar]
- Kang Y. K., Muro K., Ryu M. H., Yasui H., Nishina T., Ryoo B. Y., et al. (2014). A phase II trial of a selective c-Met inhibitor tivantinib (ARQ 197) monotherapy as a second- or third-line therapy in the patients with metastatic gastric cancer. Investig. New Drugs 32 355–361. 10.1007/s10637-013-0057-2 [DOI] [PubMed] [Google Scholar]
- Kelley R. K., Verslype C., Cohn A. L., Yang T. S., Su W. C., Burris H., et al. (2017). Cabozantinib in hepatocellular carcinoma: results of a phase 2 placebo-controlled randomized discontinuation study. Ann. Oncol. 28 528–534. 10.1093/annonc/mdw651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury H., Naujokas M. A., Zuo D., Sangwan V., Frigault M. M., Petkiewicz S., et al. (2005). HGF converts ErbB2/Neu epithelial morphogenesis to cell invasion. Mol. Biol. Cell 16 550–561. 10.1091/mbc.e04-07-0567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. H., Kim H. S., Kim B. J., Jang H. J., Lee J. (2017a). Prognostic value of c-Met overexpression in hepatocellular carcinoma: a meta-analysis and review. Oncotarget 8 90351–90357. 10.18632/oncotarget.20087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. H., Kim H. S., Kim B. J., Lee J., Jang H. J. (2017b). Prognostic value of c-Met overexpression in pancreatic adenocarcinoma: a meta-analysis. Oncotarget 8 73098–73104. 10.18632/oncotarget.20392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhan P., Erdal E., Kandemis E., Cokakli M., Nart D., Yilmaz F., et al. (2014). Reciprocal activating crosstalk between c-Met and Caveolin 1 promotes invasive phenotype in hepatocellular carcinoma. PLoS One 9:e105278. 10.1371/journal.pone.0105278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucerova L., Demkova L., Skolekova S., Bohovic R., Matuskova M. (2016). Tyrosine kinase inhibitor SU11274 increased tumorigenicity and enriched for melanoma-initiating cells by bioenergetic modulation. BMC Cancer 16:308. 10.1186/s12885-016-2341-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Kumar V., Singh K., Kumar S., Kim Y. S., Lee Y. M., et al. (2020). Therapeutic advances for Huntington’s disease. Brain Sci. 10:43. 10.3390/brainsci10010043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Kim S. T., Kim K., Lee H., Kozarewa I., Mortimer P. G. S., et al. (2019). Tumor genomic profiling guides patients with metastatic gastric cancer to targeted treatment: the VIKTORY umbrella trial. Cancer Discov. 9 1388–1405. 10.1158/2159-8290.CD-19-0442 [DOI] [PubMed] [Google Scholar]
- Lee J., Ou S. H., Lee J. M., Kim H. C., Hong M., Kim S. Y., et al. (2015). Gastrointestinal malignancies harbor actionable MET exon 14 deletions. Oncotarget 6 28211–28222. 10.18632/oncotarget.4721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. J., Lee J., Park S. H., Park J. O., Lim H. Y., Kang W. K., et al. (2018). c-MET overexpression in colorectal cancer: a poor prognostic factor for survival. Clin. Colorect. Cancer 17 165–169. 10.1016/j.clcc.2018.02.013 [DOI] [PubMed] [Google Scholar]
- Li W., Feng G., Gauthier J. M., Lokshina I., Higashikubo R., Evans S., et al. (2019). Ferroptotic cell death and TLR4/Trif signaling initiate neutrophil recruitment after heart transplantation. J. Clin. Invest. 129 2293–2304. 10.1172/JCI126428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liska D., Chen C. T., Bachleitner-Hofmann T., Christensen J. G., Weiser M. R. (2011). HGF rescues colorectal cancer cells from EGFR inhibition via MET activation. Clin. Cancer Res. 17 472–482. 10.1158/1078-0432.CCR-10-0568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Zeng W., Wortinger M. A., Yan S. B., Cornwell P., Peek V. L., et al. (2014). LY2875358, a neutralizing and internalizing anti-MET bivalent antibody, inhibits HGF-dependent and HGF-independent MET activation and tumor growth. Clin. Cancer Res. 20 6059–6070. 10.1158/1078-0432.CCR-14-0543 [DOI] [PubMed] [Google Scholar]
- Liu Y., Yu X. F., Zou J., Luo Z. H. (2015). Prognostic value of c-Met in colorectal cancer: a meta-analysis. World J. Gastroenterol. 21 3706–3710. 10.3748/wjg.v21.i12.3706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke J. J., Olson D. J., Allred J. B., Strand C. A., Bao R., Zha Y., et al. (2020). Trial and tumor mutational spectrum analysis from cabozantinib versus chemotherapy in metastatic uveal melanoma (Alliance A091201). Clin. Cancer Res. 26 804–811. 10.1158/1078-0432.CCR-19-1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marisi G., Cucchetti A., Ulivi P., Canale M., Cabibbo G., Solaini L., et al. (2018). Ten years of sorafenib in hepatocellular carcinoma: are there any predictive and/or prognostic markers? World J. Gastroenterol. 24 4152–4163. 10.3748/wjg.v24.i36.4152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham A. (2020). Tepotinib: first approval. Drugs 80 829–833. 10.1007/s40265-020-01317-9 [DOI] [PubMed] [Google Scholar]
- Merchant M., Ma X., Maun H. R., Zheng Z., Peng J., Romero M., et al. (2013). Monovalent antibody design and mechanism of action of onartuzumab, a MET antagonist with anti-tumor activity as a therapeutic agent. Proc. Natl. Acad. Sci. U.S.A. 110 E2987–E2996. 10.1073/pnas.1302725110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos G. K., DeFrances M. C. (1997). Liver regeneration. Science 276 60–66. 10.1126/science.276.5309.60 [DOI] [PubMed] [Google Scholar]
- Miranda O., Farooqui M., Siegfried J. M. (2018). Status of agents targeting the HGF/c-Met axis in lung cancer. Cancers 10:280. 10.3390/cancers10090280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok T. S., Geater S. L., Su W. C., Tan E. H., Yang J. C., Chang G. C., et al. (2016). A randomized phase 2 study comparing the combination of ficlatuzumab and gefitinib with gefitinib alone in asian patients with advanced stage pulmonary adenocarcinoma. J. Thorac. Oncol. 11 1736–1744. 10.1016/j.jtho.2016.05.038 [DOI] [PubMed] [Google Scholar]
- Munshi N., Jeay S., Li Y., Chen C. R., France D. S., Ashwell M. A., et al. (2010). ARQ 197, a novel and selective inhibitor of the human c-Met receptor tyrosine kinase with antitumor activity. Mol. Cancer Ther. 9 1544–1553. 10.1158/1535-7163.MCT-09-1173 [DOI] [PubMed] [Google Scholar]
- Nakagawa T., Tohyama O., Yamaguchi A., Matsushima T., Takahashi K., Funasaka S., et al. (2010). E7050: a dual c-Met and VEGFR-2 tyrosine kinase inhibitor promotes tumor regression and prolongs survival in mouse xenograft models. Cancer Sci. 101 210–215. 10.1111/j.1349-7006.2009.01343.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogunwobi O. O., Harricharran T., Huaman J., Galuza A., Odumuwagun O., Tan Y., et al. (2019). Mechanisms of hepatocellular carcinoma progression. World J. Gastroenterol. 25 2279–2293. 10.3748/wjg.v25.i19.2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan N., Ushijima T., Tan P. (2017). How to stomach an epigenetic insult: the gastric cancer epigenome. Nat. Rev. Gastroenterol., Hepatol. 14 467–478. 10.1038/nrgastro.2017.53 [DOI] [PubMed] [Google Scholar]
- Paik P. K., Felip E., Veillon R., Sakai H., Cortot A. B., Garassino M. C., et al. (2020). Tepotinib in non-small-cell lung cancer with MET Exon 14 skipping mutations. N. Engl. J. Med. 383, 931–943. 10.1056/NEJMoa2004407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh P. K., Ghate M. D. (2018). Recent advances in the discovery of small molecule c-Met Kinase inhibitors. Eur. J. Med. Chem. 143 1103–1138. 10.1016/j.ejmech.2017.08.044 [DOI] [PubMed] [Google Scholar]
- Parikh R. A., Wang P., Beumer J. H., Chu E., Appleman L. J. (2014). The potential roles of hepatocyte growth factor (HGF)-MET pathway inhibitors in cancer treatment. OncoTargets Ther. 7 969–983. 10.2147/OTT.S40241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z., Zhu Y., Wang Q., Gao J., Li Y., Li Y., et al. (2014). Prognostic significance of MET amplification and expression in gastric cancer: a systematic review with meta-analysis. PLoS One 9:e84502. 10.1371/journal.pone.0084502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschard P., Fournier T. M., Lamorte L., Naujokas M. A., Band H., Langdon W. Y., et al. (2001). Mutation of the c-Cbl TKB domain binding site on the Met receptor tyrosine kinase converts it into a transforming protein. Mol. Cell 8 995–1004. 10.1016/S1097-2765(01)00378-1 [DOI] [PubMed] [Google Scholar]
- Qin S., Chan S. L., Sukeepaisarnjaroen W., Han G., Choo S. P., Sriuranpong V., et al. (2019). A phase II study of the efficacy and safety of the MET inhibitor capmatinib (INC280) in patients with advanced hepatocellular carcinoma. Ther. Adv. Med. Oncol. 11:1758835919889001. 10.1177/1758835919889001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J. L., Wu H. F., Wang W. J., Hu G. M., Gu B., Zhang M., et al. (2017). C-Met as a potential novel prognostic marker in squamous cell carcinoma and adenocarcinoma of esophagus: evidence from a meta-analysis. Panminerva Med. 59 97–106. [DOI] [PubMed] [Google Scholar]
- Rimassa L., Abbadessa G., Personeni N., Porta C., Borbath I., Daniele B., et al. (2016). Tumor and circulating biomarkers in patients with second-line hepatocellular carcinoma from the randomized phase II study with tivantinib. Oncotarget 7 72622–72633. 10.18632/oncotarget.11621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimassa L., Assenat E., Peck-Radosavljevic M., Pracht M., Zagonel V., Mathurin P., et al. (2018). Tivantinib for second-line treatment of MET-high, advanced hepatocellular carcinoma (METIV-HCC): a final analysis of a phase 3, randomised, placebo-controlled study. Lancet Oncol. 19 682–693. 10.1016/S1470-2045(18)30146-3 [DOI] [PubMed] [Google Scholar]
- Rimassa L., Bozzarelli S., Pietrantonio F., Cordio S., Lonardi S., Toppo L., et al. (2019). Phase II study of tivantinib and cetuximab in patients with KRAS wild-type metastatic colorectal cancer with acquired resistance to EGFR inhibitors and emergence of MET overexpression: lesson learned for future trials with EGFR/MET dual inhibition. Clin Colorect. Cancer 18 125.e2–132.e2. 10.1016/j.clcc.2019.02.004 [DOI] [PubMed] [Google Scholar]
- Rong S., Segal S., Anver M., Resau J. H., Vande Woude G. F. (1994). Invasiveness and metastasis of NIH 3T3 cells induced by Met-hepatocyte growth factor/scatter factor autocrine stimulation. Proc. Natl. Acad. Sci. U.S.A. 91 4731–4735. 10.1073/pnas.91.11.4731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai D., Chung H. C., Oh D. Y., Park S. H., Kadowaki S., Kim Y. H., et al. (2017). A non-randomized, open-label, single-arm, Phase 2 study of emibetuzumab in Asian patients with MET diagnostic positive, advanced gastric cancer. Cancer Chemother. Pharmacol. 80 1197–1207. 10.1007/s00280-017-3445-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgia R., Sattler M., Scheele J., Stroh C., Felip E. (2020). The promise of selective MET inhibitors in non-small cell lung cancer with MET exon 14 skipping. Cancer Treat. Rev. 87:102022. 10.1016/j.ctrv.2020.102022 [DOI] [PubMed] [Google Scholar]
- Salian-Mehta S., Xu M., Wierman M. E. (2013). AXL and MET crosstalk to promote gonadotropin releasing hormone (GnRH) neuronal cell migration and survival. Mol. Cell. Endocrinol. 374 92–100. 10.1016/j.mce.2013.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro A., Rimassa L., Borbath I., Daniele B., Salvagni S., Van Laethem J. L., et al. (2013). Tivantinib for second-line treatment of advanced hepatocellular carcinoma: a randomised, placebo-controlled phase 2 study. Lancet Oncol. 14 55–63. 10.1016/S1470-2045(12)70490-4 [DOI] [PubMed] [Google Scholar]
- Scagliotti G. V., Shuster D., Orlov S., von Pawel J., Shepherd F. A., Ross J. S., et al. (2018). Tivantinib in combination with erlotinib versus erlotinib alone for EGFR-mutant NSCLC: an exploratory analysis of the phase 3 MARQUEE study. J. Thorac. Oncol. 13 849–854. 10.1016/j.jtho.2017.12.009 [DOI] [PubMed] [Google Scholar]
- Shah M. A., Cho J. Y., Tan I. B., Tebbutt N. C., Yen C. J., Kang A., et al. (2016). A randomized phase II study of FOLFOX with or without the MET inhibitor onartuzumab in advanced adenocarcinoma of the stomach and gastroesophageal junction. Oncologist 21 1085–1090. 10.1634/theoncologist.2016-0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah M. A., Wainberg Z. A., Catenacci D. V., Hochster H. S., Ford J., Kunz P., et al. (2013). Phase II study evaluating 2 dosing schedules of oral foretinib (GSK1363089), cMET/VEGFR2 inhibitor, in patients with metastatic gastric cancer. PLoS One 8:e54014. 10.1371/journal.pone.0054014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R. L., Miller K. D. (2019). Cancer statistics, 2019. CA Cancer J. Clin. 69 7–34. 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- Sierra J. R., Tsao M. S. (2011). c-MET as a potential therapeutic target and biomarker in cancer. Ther. Adv. Med. Oncol. 3 S21–S35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinway S. N., Dang H., You H., Rountree C. B., Ding W. (2015). The EGFR/ErbB3 pathway acts as a compensatory survival mechanism upon c-Met inhibition in human c-Met+ hepatocellular carcinoma. PLoS One 10:e0128159. 10.1371/journal.pone.0128159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickler J., Weekes C., Nemunaitis J., Ramanathan R., Heist R., Morgensztern D., et al. (2018). First-in-human phase I, dose-escalation and -expansion study of telisotuzumab vedotin, an antibody-drug conjugate targeting c-Met, in patients with advanced solid tumors. J. Clin. Oncol. 36 3298–3306. 10.1200/jco.2018.78.7697 [DOI] [PubMed] [Google Scholar]
- Strickler J. H., LoRusso P., Salgia R., Kang Y. K., Yen C. J., Lin C. C., et al. (2020). Dose-escalation and -expansion study of telisotuzumab (ABT-700), an Anti-c-Met antibody, in patients with advanced solid tumors. Mol. Cancer Ther. 19 1210–1217. [DOI] [PubMed] [Google Scholar]
- Sun X., Li C. W., Wang W. J., Chen M. K., Li H., Lai Y. J., et al. (2020). Inhibition of c-MET upregulates PD-L1 expression in lung adenocarcinoma. Am. J. Cancer Res. 10 564–571. [PMC free article] [PubMed] [Google Scholar]
- Tabernero J., Elez M. E., Herranz M., Rico I., Prudkin L., Andreu J., et al. (2014). A pharmacodynamic/pharmacokinetic study of ficlatuzumab in patients with advanced solid tumors and liver metastases. Clin. Cancer Res. 20 2793–2804. [DOI] [PubMed] [Google Scholar]
- Takiguchi S., Inoue K., Matsusue K., Furukawa M., Teramoto N., Iguchi H. (2017). Crizotinib, a MET inhibitor, prevents peritoneal dissemination in pancreatic cancer. Int. J. Oncol. 51 184–192. [DOI] [PubMed] [Google Scholar]
- Van Cutsem E., Eng C., Nowara E., Swieboda-Sadlej A., Tebbutt N. C., Mitchell E., et al. (2014). Randomized phase Ib/II trial of rilotumumab or ganitumab with panitumumab versus panitumumab alone in patients with wild-type KRAS metastatic colorectal cancer. Clin. Cancer Res. 20 4240–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cutsem E., Karaszewska B., Kang Y. K., Chung H. C., Shankaran V., Siena S., et al. (2019). META multicenter phase II study of AMG 337 in patients with -amplified gastric/gastroesophageal junction/esophageal adenocarcinoma and other -amplified solid tumors. Clin. Cancer Res. 25 2414–2423. [DOI] [PubMed] [Google Scholar]
- Van Schaeybroeck S., Allen W. L., Turkington R. C., Johnston P. G. (2011). Implementing prognostic and predictive biomarkers in CRC clinical trials. Nat. Rev. Clin. Oncol. 8 222–232. 10.1038/nrclinonc.2011.15 [DOI] [PubMed] [Google Scholar]
- Wang J., Anderson M. G., Oleksijew A., Vaidya K. S., Boghaert E. R., Tucker L., et al. (2017). ABBV-399, A c-met antibody-drug conjugate that targets both -amplified and c-Met-overexpressing tumors, irrespective of pathway dependence. Clin. Cancer Res. 23 992–1000. 10.1158/1078-0432.ccr-16-1568 [DOI] [PubMed] [Google Scholar]
- Wang J., Goetsch L., Tucker L., Zhang Q., Gonzalez A., Vaidya K. S., et al. (2016). Anti-c-Met monoclonal antibody ABT-700 breaks oncogene addiction in tumors with MET amplification. BMC Cancer 16:105. 10.1186/s12885-016-2138-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Ferrell L. D., Faouzi S., Maher J. J., Bishop J. M. (2001). Activation of the Met receptor by cell attachment induces and sustains hepatocellular carcinomas in transgenic mice. J. Cell Biol. 153 1023–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Q., Chen W., Ren M., Wang J., Zhang H., Deng D. Y., et al. (2014). Cabozantinib suppresses tumor growth and metastasis in hepatocellular carcinoma by a dual blockade of VEGFR2 and MET. Clin. Cancer Res. 20 2959–2970. 10.1158/1078-0432.CCR-13-2620 [DOI] [PubMed] [Google Scholar]
- Xu J., Higgins M. J., Tolaney S. M., Come S. E., Smith M. R., Fornier M., et al. (2020). A phase II trial of cabozantinib in hormone-receptor positive breast cancer with bone metastases. Oncologist 25 652–660. 10.1634/theoncologist.2020-0127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Zhu C., Chen C., Zong Y., Feng H., Liu D., et al. (2018). CCL19 suppresses angiogenesis through promoting miR-206 and inhibiting Met/ERK/Elk-1/HIF-1α/VEGF-A pathway in colorectal cancer. Cell Death Dis. 9:974. 10.1038/s41419-018-1010-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakes F. M., Chen J., Tan J., Yamaguchi K., Shi Y., Yu P., et al. (2011). Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol. Cancer Ther. 10 2298–2308. 10.1158/1535-7163.MCT-11-0264 [DOI] [PubMed] [Google Scholar]
- Yamamoto N., Mammadova G., Song R. X. D., Fukami Y., Sato K. (2006). Tyrosine phosphorylation of p145(met) mediated by EGFR and Src is required for serum-independent survival of human bladder carcinoma cells. J. Cell Sci. 119 4623–4633. 10.1242/jcs.03236 [DOI] [PubMed] [Google Scholar]
- Yan H. H., Jung K. H., Son M. K., Fang Z., Kim S. J., Ryu Y. L., et al. (2014). Crizotinib exhibits antitumor activity by targeting ALK signaling not c-MET in pancreatic cancer. Oncotarget 5 9150–9168. 10.18632/oncotarget.2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Shi J., Chen X., Jiang Y., Zhao H. (2020). Efficacy of cabozantinib and nivolumab in treating hepatocellular carcinoma with RET amplification, high tumor mutational burden, and PD-L1 expression. Oncologist 25 470–474. 10.1634/theoncologist.2019-0563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau T. C. C., Lencioni R., Sukeepaisarnjaroen W., Chao Y., Yen C. J., Lausoontornsiri W., et al. (2017). A phase I/II multicenter study of single-agent foretinib as first-line therapy in patients with advanced hepatocellular carcinoma. Clin. Cancer Res. 23 2405–2413. 10.1158/1078-0432.CCR-16-1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi S., Tsao M. S. (2000). Activation of hepatocyte growth factor-met autocrine loop enhances tumorigenicity in a human lung adenocarcinoma cell line. Neoplasia 2 226–234. 10.1038/sj.neo.7900080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z. S., Weiser M. R., Kuntz E., Chen C. T., Khan S. A., Forslund A., et al. (2008). c-Met gene amplification is associated with advanced stage colorectal cancer and liver metastases. Cancer Lett. 265 258–269. 10.1016/j.canlet.2008.02.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Feng Q., Chen W. D., Wang Y. D. (2018). HGF/c-MET: a promising therapeutic target in the digestive system cancers. Int. J. Mol. Sci. 19:3295. 10.3390/ijms19113295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Yao Z., Wang L., Ding H., Shao J., Chen A., et al. (2018). Activation of ferritinophagy is required for the RNA-binding protein ELAVL1/HuR to regulate ferroptosis in hepatic stellate cells. Autophagy 14 2083–2103. 10.1080/15548627.2018.1503146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen D. B., Griffith K. A., Ruch J. M., Camphausen K., Savage J. E., Kim E. J., et al. (2016). A phase I trial of cabozantinib and gemcitabine in advanced pancreatic cancer. Invest. New Drugs 34 733–739. 10.1007/s10637-016-0376-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G. H., Huang C., Qiu Z. J., Liu J., Zhang Z. H., Zhao N., et al. (2011). Expression and prognostic significance of CD151, c-Met, and integrin alpha3/alpha6 in pancreatic ductal adenocarcinoma. Digestive Dis. Sci. 56 1090–1098. 10.1007/s10620-010-1416-x [DOI] [PubMed] [Google Scholar]
- Zielonka D., Mielcarek M., Landwehrmeyer G. B. (2015). Update on Huntington’s disease: advances in care and emerging therapeutic options. Parkinsonism Relat. Disord. 21 169–178. 10.1016/j.parkreldis.2014.12.013 [DOI] [PubMed] [Google Scholar]