Abstract
Uterine cancer is the most and second most common gynecological malignancy in developed and developing countries, respectively. The majority of endometrial cancers are diagnosed early due to the presence of abnormal uterine bleeding. The existing literature however contains only little data regarding the prevalence of such symptoms compared to patients with no or benign pathology. Therefore, a systematic review was conducted in order to determine the significance of various clinical signs and symptoms predicting uterine cancer.
Embase, Web of Science and Medline databases were searched from inception until 18 June 2019. Studies eligible for selection inclusion assessed the diagnostic accuracy of clinical signs and symptoms in pre- and postmenopausal women aged 18–99 years old with uterine malignancy. Case reports, case series and studies of which full text was not available, were excluded. The risk of bias was assessed using the QUADAS-2 tool by two independent reviewers. Results were visualized by forest plots using RevMan(5.3).
Forty-one studies were eventually included in this systematic review. Abnormal uterine bleeding occurring in pre-, post- and perimenopausal women was proven to be the most widely investigated symptom in relation to cancer of the uterus. Thirty-two articles examined patients with postmenopausal bleeding of which sensitivity and specificity varied between 0.28 to 0.86 and 0.63 to 0.84, respectively. Abnormal bleeding in pre- and perimenopausal women on the other hand showed a sensitivity ranging from 0.63 to 0.81. Its specificity could not be calculated due to missing data. Other symptoms appeared not sufficiently examined to assess their diagnostic accuracy range.
This review highlights the current lack of knowledge regarding the diagnostic accuracy of several signs and symptoms for uterine cancer. After a thorough in-depth review of the literature, meta-analysis could not be performed due to the absence of control populations in the majority of articles. Further research is needed to establish the rule-in or rule-out value of specific clinical signs to identify patients at risk for uterine malignancy prompting further clinical assessment.
Keywords: Quality of life, Obstetrics & gynecology, Oncology, Health promotion, Diagnostics, Uterine cancer, Sign, Symptom, Diagnostic accuracy
Quality of life; Obstetrics & gynecology; Oncology; Health promotion; Diagnostics; Uterine cancer; Sign; Symptom; Diagnostic accuracy.
1. Introduction
Uterine cancer is the most frequent gynecological neoplasm in developed countries [1]. Mean age at diagnosis is 61 years although 5–30% of cases appear in women younger than fifty [1]. Over the last ten years, its diagnosis has increased worldwide and a further rise is expected [2]. North America and Europe tend to have the highest incidence rates [1, 2, 3]. These findings might be explained by a simultaneous rise in specific risk factors including obesity, metabolic syndrome, sedentary lifestyle, long-term use of hormone replacement therapies and delaying childbirth [4, 5]. The development of cancer cells within the endometrium is mainly explained as a result of unopposed estrogen exposure especially with a concurrent lack of progesterone. This applies for the major type (80%) of uterine carcinoma -known as type I malignancies-constituting of low-grade endometrioid histology. They usually have an early diagnosis and an overall good prognosis [4, 5]. Type II malignancies (20%) on the other hand are less common. They have different histologic features. They comprise a group of grade 3 endometrioid and non-endometrioid pathologies. Their prognosis is poor and they are not estrogen-dependent. Endometrial cancer accounts for more than ninety percent of the uterine neoplasms whereas uterine sarcomas - tumors arising from the myometrium or endometrial connective tissue elements - account for less than ten percent of cancers from the uterine corpus [4, 5, 6]. Long-term use of Tamoxifen and a history of pelvic radiotherapy have been suggested risk factors, although the evidence is small. They have a poorer prognosis due to aggressive behaviour [4, 5, 6, 7].
Early stages of endometrial cancer are often curable with surgery. Stage IV endometrial cancer on the contrary has a five-year survival of approximately 22% [5, 8, 9]. Therefore, early detection remains the most important factor to increase the overall survival. In primary care it is essential to be aware of associated presenting symptoms since most of the diagnoses can be made at an early stage of disease due to their presence [10]. However, the existing literature contains only little data regarding the prevalence of such symptoms compared to patients with no or benign pathology.
Abnormal bleeding has been proven to be the cardinal symptom of all types of uterine cancer. However, its specificity is low and a large amount of benign pathologies might present in a similar fashion [9, 11]. Postmenopausal women with significant risk factors such as older age and obesity appear more likely to develop uterine malignancy [12, 13, 14].
Much less attention has been given to other potential warning signs. Few data exist on the significance of pelvic pressure, urinary symptoms, vaginal discharge and abdominal pain. We aim to summarize the currently available evidence regarding the spectrum of presenting signs and symptoms of uterine malignancies to identify candidate predictors for future risk prediction models deciding which patients require prompt clinical assessment.
2. Methods
2.1. Inclusion and exclusion criteria
Cohort, case-control and cross-sectional studies were eligible for inclusion. Considering the focus was diagnostic accuracy studies, we did not anticipate a large volume of randomized controlled trials in our systematic review search. Case reports and series were excluded. Our population of interest were nonpregnant pre- and postmenopausal women, aged 18 to 99, with no previous gynecological cancer, pelvic radiotherapy or pelvic surgery with exception of straightforward minor diagnostic procedures. Usage of medication interfering with clotting was another exclusion criterion. Articles mainly focusing on exploring different imaging techniques for the detection of uterine cancer were also excluded. The inability to construct complete 2x2-tables of test and outcome positivity was not an exclusion criterion. All types of uterine cancer were accepted for inclusion. Our review was prospectively registered on the international database Prospero.
2.2. Search strategy
The literature was searched by exploring the databases of MedLine, Web of Science (WoS) and Embase from inception until 18 June 2019. Individual search strategies were built using Medical Subject Headings (MeSH) and non-MeSH keyword terms referring to uterine cancer, its subtypes and a whole range of possible associated symptoms in collaboration with a biomedical librarian at KU Leuven. The details of our search strategies can be found in appendices A, B and C. Our search was purposively extensive in order to obtain a broad range of symptoms relevant to the process of diagnosis. No search limits regarding language were used.
2.3. Data abstraction
Each title and abstract obtained through the initial search were reviewed by two independent examiners (SB, SD). A remaining group of conflicts were resolved by a third person (JV). Attempts were also made to contact the original authors worldwide via email of which one responded. The available full texts of the remaining articles were subsequently screened by two reviewers independently in detail (SB, SD). Information regarding study characteristics and numbers required to construct 2 × 2 tables were extracted and categorized by menopausal status.
2.4. Assessment of methodological quality
One reviewer assessed the quality of included studies and this was checked independently by a second reviewer. The quality and risk of bias of each study were assessed using the QUADAS-2 tool [15].
2.5. Statistical analysis
Our aim was to deduce 2 × 2 tables based on the result of each article in order to calculate sensitivity, specificity, positive and negative likelihood ratios of each symptom for uterine cancer. Our detailed search revealed however that majority of articles had missing data to carry out these calculations. Either a patient population with a specific symptom was studied to determine how many patients actually had uterine cancer or not, resulting only in true and false positives, or a population already known with proven uterine cancer was investigated for the presence or absence of a specific symptom, resulting in only true positives and false negatives, respectively. This consistently resulted in incomplete 2 × 2 tables which were not suitable for subsequent meta-analysis. We used RevMan to construct 2x2-tables when possible and to visualize our results. Sensitivity and specificity were calculated with their 95% confidence intervals (95%CI).
3. Results
3.1. Study characteristics
The detailed characteristics of all included studies are presented in Tables 1 and 2 [1, 2, 3, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54]. These studies were performed in several continents including America, Europe, Asia and Australia. Sample size of the case-controls and cohort studies ranged from 32 to 43 [18] women. Overall the median age varied between 40 and 62 years. Postmenopausal and abnormal bleeding were the most frequently examined symptoms. Three articles mentioned the amount and timing of postmenopausal bleeding episodes [36, 45, 50]. Only two studies analyzed other potential presentations of uterine cancer such as abdominal complaints, vaginal discharge, uterine prolapse and urinary incontinence [47, 52]. Plausible neurologic manifestations occurring as a result of secondary brain metastases were addressed in one publication [29].
Table 1.
Summary of included articles with land of origin, investigated symptoms and number of patients: Part 1.
|
Author |
Country | Symptom | Number of patients |
|---|---|---|---|
| Abdullah 2011 | Saoudi-Arabia | abnormal uterine bleeding postmenopausal bleeding |
2295 |
| Abid 2014 | Pakistan | abnormal uterine bleeding postmenopausal bleeding |
241 |
| AlanisFuentes 2007 | Mexico | postmenopausal bleeding | 372 |
| Alexopoulos 1999 | United Kingdom | abnormal uterine bleeding postmenopausal bleeding |
2581 |
| Allen 1990 | Australia | intermenstrual bleeding menorrhagia postcoital bleeding |
4318 |
| Al-Turiahi 2016 | Syria | postmenopausal bleeding | 140 |
| Antunes 2007 | Brazil | postmenopausal bleeding | 475 |
| Antunes 2011 | Brazil | postmenopausal bleeding | 870 |
| Asghar 2016 | Pakistan | abnormal uterine bleeding postmenopausal bleeding |
132 |
| Ash 1996 | Canada | abnormal uterine bleeding | 310 |
| Bahadur 2017 | India | abnormal uterine bleeding postmenopausal bleeding |
192 |
| Burbos 2010 | United Kingdom | postmenopausal bleeding | 4194 |
| Burbos 2012 | United Kingdom | postmenopausal bleeding | 3047 |
| Clarke 2018 | United States Belgium |
postmenopausal bleeding | |
| Cormio 1996 | Italy | motor weakness seizures confusion diziness/vertigo visual disturbances headache |
1069 |
| Crissman 1981 | United States | abnormal uterine bleeding | 32 |
| Deeba 2016 | Pakistan | postmenopausal bleeding | 110 |
| Doraiswami 2011 | India | abnormal uterine bleeding postmenopausal bleeding |
620 |
| Dvalishvili 2006 | United States | postmenopausal bleeding | 104 |
| Fatima 2014 | Pakistan | postmenopausal bleeding | 36 |
| Genc 2015 | Turkey | postmenopausal bleeding | 283 |
| Ghoubara 2018 | United Kingdom | postmenopausal bleeding (recurrent or single episode) |
1902 |
Table 2.
Summary of included articles with land of origin, investigated symptoms and number of patients: Part 2.
|
Author |
Country | Symptom | Number of patients |
|---|---|---|---|
| Giannella 2019 | Italy | abnormal uterine bleeding | 240 |
| Gredmark 1995 | Sweden | postmenopausal bleeding | 457 |
| Holzl 1973 | Germany | postmenopausal bleeding | 1106 |
| Kansal 2018 | India | abnormal uterine bleeding postmenopausal bleeding |
192 |
| Kim 2015 | Korea | postmenopausal bleeding | 792 |
| Kumari 2018 | India | postmenopausal bleeding | 50 |
| Lidor 1986 | Israel | postmenopausal bleeding | 226 |
| Mandić 2013 | Serbia | postmenopausal bleeding | 122 |
| Musonda 2011 | United Kingdom | postmenopausal bleeding (light or heavy) (recurrent or single episode) |
3795 |
| Nargis 2014 | Bangladesh | abnormal uterine bleeding | 211 |
| Pakish 2016 | United States | postmenopausal bleeding irregular menses intermenstrual bleeding menorrhagia hypermenorrhoea postcoital bleeding pelvic pain, pressure urinary frequency abnormal vaginal discharge fatigue change in bowel habits |
429 |
| Potikul 2015 | Thailand | abnormal uterine bleeding postmenopausal bleeding |
46 |
| Sadaf 2014 | Pakistan | abnormal uterine bleeding | 100 |
| Salman 2013 | Turkey | postmenopausal bleeding (light or heavy) (recurrent or single episode) |
142 |
| Schindler 1980 | Germany | postmenopausal bleeding | 1019 |
| Schmeler 2005 | United States | irregular menses | 188 |
| Thomas 2013 | United States | Urinary incontinence pelvic floor prolapse |
549 |
| Van den Bosch 2015 | Belgium United Kingdom |
abnormal uterine bleeding postmenopausal bleeding |
1217 |
| Wong 2001 | China | postmenopausal bleeding | 199 |
3.2. Search results
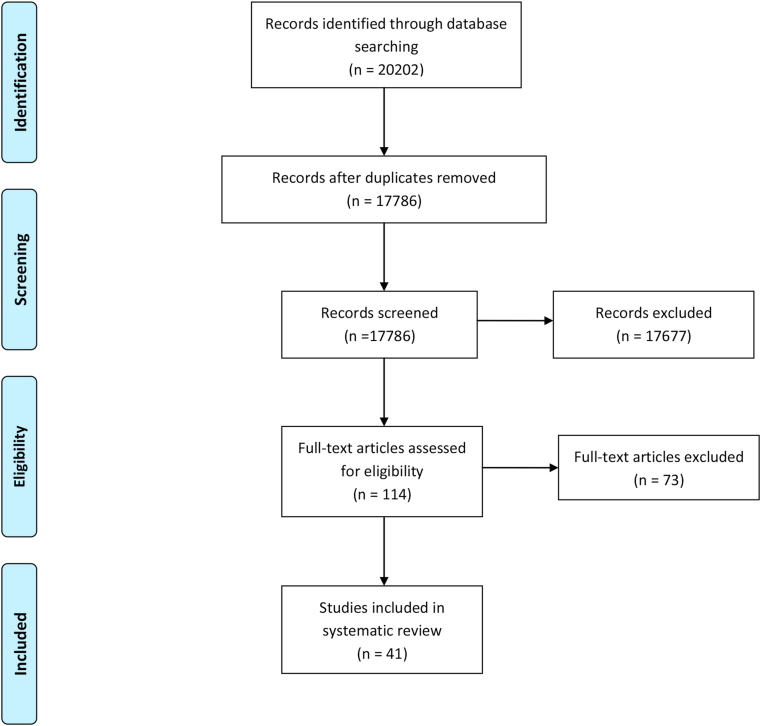
A total of 41 studies were eligible for inclusion [1, 2, 3, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54]. Figure 1 shows the flowchart of our search results with inclusion and exclusion of articles. Searching through the different databases, 20,202 references were identified. After removing duplicates, 17,786 were eligible for title-and-abstract screening. The full text of 114 publications was searched. Forty-two full text versions were not available despite extensive search. Seventy-two articles were screened for possible inclusion in our systematic review. This screening resulted in 41 studies meeting our inclusion criteria. None of the included studies were performed in primary care settings.
Figure 1.
PRISMA Flow chart.
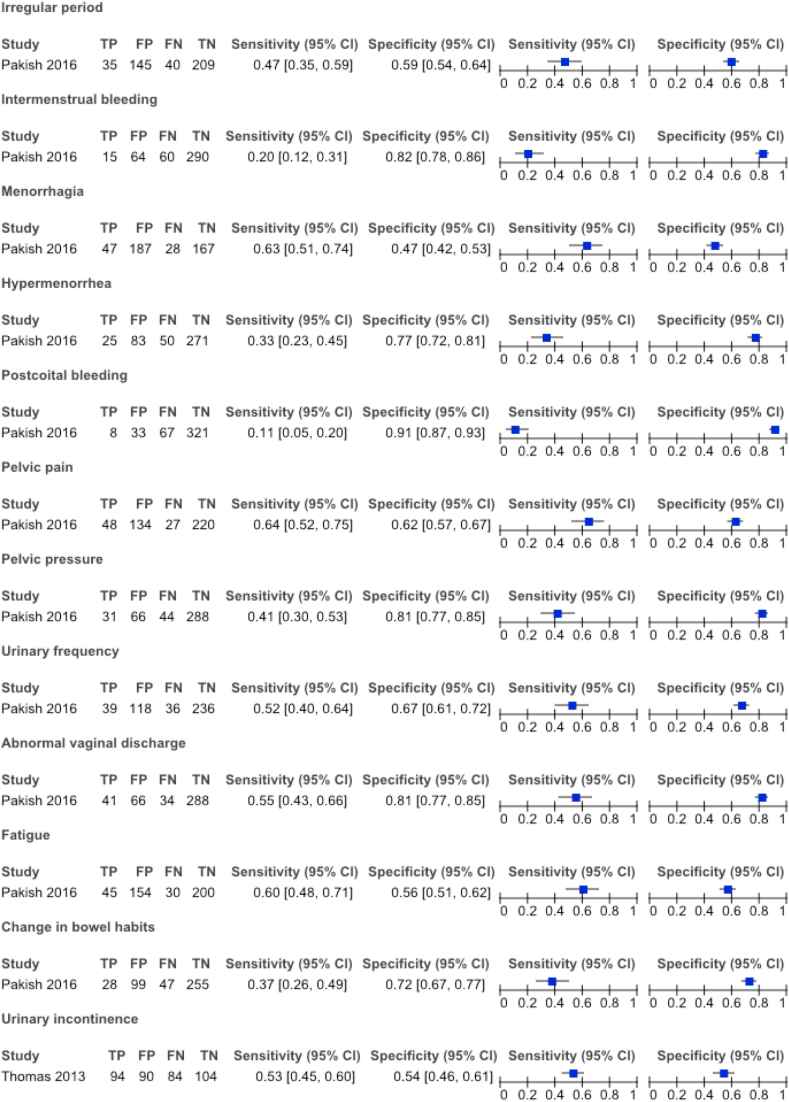
Twenty-two studies had a retrospective design whereas nineteen were conducted prospectively. A total of 21 symptoms were examined. Figures 2 and 3 show the sensitivity and specificity of these symptoms in case the extraction of complete 2 × 2 tables was possible. Only one publication addressed uterine sarcomas [48]. All the other articles analyzed endometrial carcinoma [1, 2, 3, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 49, 50, 51, 52, 53, 54].
Figure 2.
Summary of sensitivity & specificity of investigated symptoms and corresponding authors given 2 × 2 tables were fully reproducible.
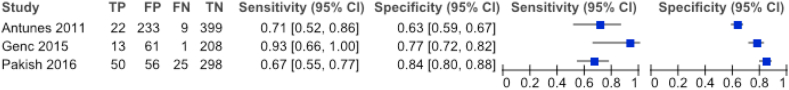
Figure 3.
Summary of sensitivity & specificity of postmenopausal bleeding and corresponding authors given 2 × 2 tables were fully reproducible.
Postmenopausal bleeding was clearly the most investigated presenting clinical complaint. Its accuracy was analyzed in 32 articles. 2 × 2 tables could only be extracted from three of these studies; Antunes et al. calculated a sensitivity and specificity of 0.71 [95% CI 0.52–0.86] and 0.63 [95% CI 0.59–0.67], respectively, whereas Pakish et al. generated a 0.67 [95% CI 0.55–0.77] sensitivity with 0.84 [95% CI 0.80–0.88] specificity. The publication of Genc et al. showed a sensitivity of 0.93 [95% CI 0.66–1.00] and a specificity of 0.77 [95% CI 0.72–0.82]. They all proved a significant correlation with uterine malignancy [23, 35, 47]. Three publications differentiated between the amount of postmenopausal bleeding or the number of episodes [36, 45, 50]. Salman et al. found a higher prevalence of uterine cancer correlating to an increased amount of bleeding though Musonda et al. did not find a similar effect. Both of them showed a significantly larger number of uterine malignancy in presence of recurrent bleeding which was not supported by a more recent publication by Ghoubara et al. [36, 45, 50]
The second most described symptom was abnormal uterine bleeding in pre- and perimenopausal women. A majority of fourteen studies used the term as a general description whilst not specifying in detail what types of abnormal bleeding were explored [16, 17, 19, 24, 25, 26, 30, 32, 37, 40, 46, 48, 49, 53]. Moreover, due to insufficient data, 2 × 2 tables could not be extracted from these articles. Pakish et al., Schmeler et al. and Allen et al. on the other hand, differentiated between the various types of abnormal bleeding, though only Pakish et al. was successful in providing complete data [1, 20, 47]. The latter examined intermenstrual and postcoital bleeding, menorrhagia, hypermenorrhoea and irregular menses, but none of these appeared significantly linked to uterine cancer [47]. Schmeler et al. on the other hand suggested a higher prevalence of irregular cycles in premenopausal women diagnosed with endometrial cancer [1].
Pelvic discomfort and abnormal vaginal discharge were studied by Pakish et al. [47] Abnormal vaginal discharge was found to be significantly associated with uterine cancer with a sensitivity of 0.55 [95% CI 0.43–0.66] and specificity of 0.81 [95% CI 0.77–0.85]. Overall, pelvic pain and pressure were not significant.
Urinary frequency and vaginal wall prolapse, stress and urge incontinence were explored by Pakish et al. and Thomas et al., respectively [47, 52]. Comparing women with benign pathology to patients with uterine malignancy, no significant difference was observed in prevalence or severity of these symptoms. Pakish et al. also mentioned fatigue with a sensitivity and specificity of 0.60 [95% CI 0.48–0.71] and 0.56 [95% CI 051–0.62] and changing bowel habits with 0.37 [95% CI 0.26–0.49] sensitivity and 0.72 [95% CI 0.67–0.77] specificity. Overall, they appeared not strongly correlated to cancer of the uterus [47].
Only one article examined the possible clinical picture of cerebral metastases secondary to endometrial cancer [29]. Their study included 1,069 women of whom only ten patients developed cerebral metastases. The most frequently seen symptom amongst them was headache.
3.3. Methodological quality of included studies
We assessed all included articles using the QUADAS-2 tool. The risk of bias for patient selection was low since most of the prospective articles included women suffering from the symptom in question and the retrospective articles only included women with a defined symptom or whom all were diagnosed with endometrial cancer. Overall, applicability concerns were low because all possible symptoms were relevant to our review question. Most articles were conducted in secondary care which raises question about the applicability to ambulatory care. Risk of bias for the index test was usually considered low. In most studies it was obvious what definition was used for the symptoms investigated. Reference standard, risk of bias and applicability concerns were low, because all articles used a well-defined reference standard. Risk of bias was low for flow and timing.
4. Discussion
4.1. Main findings
Out of 17,786 studies, only 41 publications were withheld being eligible for final inclusion in our systematic review [1, 2, 3, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54]. Overall, we addressed 21 potential symptoms of uterine cancer. Studies had either a cross-sectional or cohort design, except for the meta-analysis of Clarke et al. [3] Our purpose was an umbrella overview of plausible clinical features of uterine malignancies in order to facilitate the formation of patient groups at high risk requiring urgent referral for definite diagnosis-making based on the significance of their individual clinical picture.
A more or less analogous thought process was established in a systematic review by Raglan et al, although there are some differences [55]. In their work, they analyzed risk factors instead of symptoms. Furthermore, they solely focused on the most common type of uterine malignancy; endometrial carcinoma. Their objective however was identifying genuine risk factors which would also lead to a high-risk group aiding in the development of targeted strategies.
Raglan et al. investigated 171 meta-analyses comprising 53 risk factors [55]. Through a minute statistical evaluation, they discovered three main strongly associated risk factors. Body-mass-index and waist-to-hip ratio were positively associated with endometrial cancer in postmenopausal women and women of all ages, respectively, whereas parity was inversely related.
Whilst searching the literature, it has been obvious that risk factors relating to uterine malignancy, have been a popular and widely investigated topic with extensive comparison between disease-affected patients and controls. Our review on the contrary has revealed that this is not the case regarding patient's signs and symptoms, preventing us from reliably unveiling true and unbiased associations. A similar observation has been previously made by Pakish et al. when they highlighted the need for proper documentation of the prevalence of uterine cancer-related symptoms compared to a healthy population [47]. Notwithstanding these shortcomings, abnormal uterine bleeding and vaginal discharge in pre- and postmenopausal women have been often referred to as an established finding in uterine malignancy [47, 56, 57].
4.2. Postmenopausal bleeding versus uterine cancer
Postmenopausal bleeding has been an extensively evaluated symptom in its relationship to uterine cancer [3]. In our analysis as well, it was the most frequently investigated clinical complaint with a total number of 32 articles examining its importance. Only three studies however could provide complete 2 × 2 tables whilst also demonstrating a significant correlation with uterine malignancy [23, 35, 47]. Three articles scrutinized the severity and frequency of postmenopausal bleeding, but due to different conclusions their possible significance is not unequivocally clear [36, 45, 50].
“Beware of the weeping womb”, Notelovitz et al. warned [58]. Postmenopausal bleeding is the presenting symptom in more than 90% of confirmed endometrial cancer cases [45, 58, 59]. Nevertheless, only a minority of 5–10% of patients complaining of postmenopausal bleeding will be actually diagnosed with cancer of the endometrium [45, 50, 60, 61, 62, 63, 64]. This entails a low positive predictive value necessitating time- and money-consuming investigations of a large number of otherwise healthy women also presenting with postmenopausal bleeding. If in the future, a risk model would be constructed, such pathway can be used to guide a substantial low-risk group to primary and a smaller though more urgent high-risk group to secondary care. Furthermore, in those areas where health care access is rather limited, such model can be implemented as a triage tool to appropriately allocate restricted resources [50].
4.3. Abnormal uterine bleeding versus uterine cancer
Abnormal uterine bleeding in pre-and perimenopausal women was the second most reviewed symptom in our analysis. Although the majority of patients are postmenopausal at the time of diagnosis, 5–30% of women with endometrial cancer are younger than fifty [1, 65]. A high proportion of them have a body mass index (BMI) within the obesity range [1, 65]. Higher estrogen levels caused by anovulation might possibly explain the underlying mechanism between obesity and endometrial cancer, but it can also illustrate the phenomenon of menorrhagia due to an accelerated growth of endometrial lining [1, 14]. Pakish et al. stated that menorrhagia is subject to obesity itself and not to the presence of uterine cancer [47].
Only three publications clarified which specific subtypes of abnormal uterine bleeding were investigated, while a remaining group of fourteen studies used only a general description [1, 16, 17, 19, 20, 24, 25, 26, 30, 32, 37, 40, 46, 47, 48, 49, 53]. Furthermore, a complete 2 × 2 table could be extracted from of one study only, which did not show a significant link with endometrial cancer [47]. The group of Allen et al. also did not find a notable correlation [20]. They stated the incidence of endometrial carcinoma in women under 40 is rare and accounts for less than 3% of the total cases. Abnormal uterine bleeding under this age is mostly related to benign conditions [20, 30]. A publication by Schmeler et al. on the other hand claimed irregular cycles occur more often amongst endometrial cancer cases than within the general population [1]. Risk factors such as polycystic ovarian syndrome, hyperinsulinemia, chronic inflammatory state and obesity are known to ably dysregulate menstrual cycles while they can also contribute to the development of endometrial cancer in younger women [1, 14, 55]. This however does not imply a true relationship of irregular menses as symptom of cancer of the endometrium.
4.4. Abnormal vaginal discharge versus uterine cancer
After excluding studies with previous abdominal or pelvic radiotherapy, only one article remained eligible for analysis regarding abnormal vaginal discharge as presenting symptom for uterine cancer [47]. A strong association was voiced by Pakish et al., thereby also citing a study of Schmidt et al. that had evidenced a relation between intracavitary hemato- or pyometron on ultrasound and endometrial cancer [47, 66]. Nevertheless, the actual association with abnormal vaginal discharge arising as symptom because of this remains unclear [47, 66].
4.5. Pelvic (floor) symptoms versus uterine cancer
Pakish et al. found pelvic pain and pressure overall not significantly linked to endometrial cancer [47]. This finding might be explained by the fact that the major part of uterine malignancies are diagnosed at an early disease-stage often due to other earlier clinical symptoms such as postmenopausal bleeding [4, 5]. Therefore further tumor growth with possibly related pressure complaints is a rare presentation.
Many women with uterine cancer experience symptomatic pelvic floor dysfunction such as pelvic organ prolapse and bothersome urinary incontinence [52]. Thomas et al. could not observe a higher prevalence of these symptoms in women with uterine cancer compared to the general population [52]. Pakish et al. could also not prove a significant link with urinary frequency [47]. Both symptomatic floor disorders and cancer of the endometrium share high BMI and older age as precipitating factors. Therefore, occurring simultaneously can be expected and is not necessarily any proof of causality [55]. A higher parity on the other hand is a directly related risk factor to pelvic floor issues, but has been shown to have a protective influence against endometrial cancer. Raglan et al. suggested that increased progesterone production during pregnancy might provide endometrial protection by slowing down mitosis thus reducing the risk of cancer [55].
4.6. Symptoms caused by uterine cancer metastases
The article of Cormio et al. investigated central nervous system involvement caused by metastases from primary endometrial carcinoma [29]. Clinical manifestations of brain spread were headache, motor weakness, seizures, vertigo, confusion and visual disturbances [29].
Untreated or undiagnosed endometrial cancer extends by local, lymphatic or haematogenous spreading. If distant metastases occur, they usually occupy lungs, liver or bone [67]. Cormio's group analyzed 1,069 patients with endometrial cancer, but only ten developed brain metastases [29]. In other series reviewing secondary brain tumours, endometrial cancer was accountable for only 0.8% of the cases [68, 69]. Therefore, we can safely say that central nervous system symptoms caused by metastasising endometrial malignancy is uncommon.
Overall, Pakish et al. could not prove a significant correlation with non-specific symptoms such as fatigue and change in bowel habits [47].
During the phase of our title and abstract screening, we often came across publications about the clinical picture of bone metastases from primary endometrial cancer. These articles however were all case reports. Hence they were excluded.
5. Strengths and limitations of the study
This study provides an overview of the available literature on the relevance of signs and symptoms of uterine cancer. Due to its comprehensive search strategy a broad range of symptoms was assessed. However, a vast limitation of the analysis is that none of the studies were performed in ambulatory care. A gynecological clinic with a relatively high prevalence of uterine malignancy is not representative for the ambulatory care environment. Furthermore, certain symptoms such as incontinence and prolapse complaints, were analyzed fairly subjectively through questionnaires filled in by patients [52]. The main focus of the articles was laid on cancer of the endometrium with only one publication looking at clinical features of a different type of uterine cancer [48]. The most important limitation of our study was the lack of well-defined control populations, which significantly limited our statistical potential.
6. Comparison with existing literature
The systematic review from Clarke et al. clarifies the association between endometrial cancer and postmenopausal bleeding [3]. They searched Medline and EMBASE for English-language studies released up to 2017. Observational studies were selected, but case reports and series were excluded as well.
A number of 129 studies was included analyzing 40,790 patients with postmenopausal bleeding of which 6,358 had been diagnosed with endometrial cancer. Postmenopausal bleeding as an early symptom was able to detect up to 90% of the endometrial cancer, however its specificity was rather low and most patients suffering from postmenopausal bleeding were not diagnosed with cancer of the endometrium.
After detailed review of why our final group of included articles was significantly less compared to Clarke et al.' systematic review, we detected an important difference in inclusion criteria: articles mainly focussing on comparing various ultrasound markers within their diagnosis-making, had been excluded during our screening [3]. These studies would identify a specific risk group amongst patients determining whom would receive further biopsy and therefore final diagnosis based on certain ultrasound findings. A group of symptomatic patients below an examined ultrasound threshold, would have not underwent the golden standard histological pathology. Because our main focus was to point out the diagnostic accuracy of symptoms as opposed to ultrasound markers, we decided to exclude those studies concentrating rather on diagnostic prediction of imaging techniques possibly causing bias within the population groups.
Another difference is the fact that Clarke et al. investigated one of the main symptoms of endometrial cancer, whereas we aimed for an overview of a whole range of possible clinical presentations. Furthermore, Clarke et al. specified on endometrial cancer only, though we included all uterine cancers. Nevertheless, the main conclusions were similar to ours: further investigation is needed in order to identify a more specific risk group for cancer of the uterus.
7. Conclusion
Our review illustrates the current gap of knowledge regarding the range of symptoms uterine cancer might present with and their precise individual contribution. At present, a large number of women has to be examined in order to detect only a small group of patients with actual uterine malignancy. An enhanced risk model might expedite urgent evaluation specifically of high-risk patients, and hence their diagnosis, treatment and overall survival rate. Future research should carefully include study designs with appropriate and comparable control groups, allowing to extract the number of true positives, false positives, false negatives and true negatives. Both primary care clinics as well as more specialized centres ought to be involved for correctly obtaining representative large scale study populations to establish a refined clinical prediction model allowing reliable risk estimation of cancer of the uterus.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
I would like to thank I. Vanhooff for proofreading regarding grammar and spelling.
Appendix A.
Search strategy in Pubmed
("Uterine Neoplasms"[Mesh:NoExp] OR uterine-neoplasm∗[tiab] OR uterus-neoplasm∗[tiab] OR “Cancer∗ of Uterus”[tiab] OR “cancers of uterus”[tiab] OR uterine-cancer∗[tiab] OR uterus-cancer∗[tiab] OR “Cancer of the Uterus”[tiab] OR “Cancers of the uterus”[tiab] OR uterine-malignanc∗[tiab] OR "Endometrial Neoplasms"[Mesh] OR endometrial-neoplasm∗[tiab] OR endometrial-carcinoma∗[tiab] OR endometrial-cancer∗[tiab] OR “cancer of the endometrium”[tiab] OR “cancers of the endometrium”[tiab]OR “carcinoma of endometrium”[tiab] OR “carcinomas of endometrium”[tiab] OR endometrium-carcinoma∗[tiab] OR endometrial-carcinoma∗[tiab] OR “cancer of endometrium”[tiab] OR “cancers of endometrium” [tiab] OR endometrium-cancer∗[tiab] OR endometrium-malignanc∗[tiab] OR endometrial-stromal-tumor∗[tiab] OR endometrial-stromal-tumour∗[tiab] OR endometrial-stromal-sarcoma∗[tiab] OR uterine-sarcoma∗[tiab] OR uterine-carcinosarcoma∗[tiab] OR endometrial-adenocarcinoma∗[tiab] OR endometrial-adenosquamous-carcinoma∗[tiab] OR endometrial-papillary-serous-carcinoma∗[tiab] OR endometrial-clear-cell-carcinoma∗[tiab] OR leiomyosarcoma∗[tiab] OR uterine-mixed-mesodermal-tumor∗[tiab] OR uterine-mixed-mesodermal-tumour∗[tiab] OR uterine-rhabdomyosarcoma∗[tiab] OR uterine-chondrosarcoma∗[tiab])
AND
("Signs and Symptoms"[Mesh] OR sign[tiab] OR signs[tiab] OR symptom∗[tiab] OR Fatigue[tiab] OR “muscle weakness”[tiab] OR sleepiness[tiab] OR somnolence[tiab] OR weight-change∗[tiab] OR “weight loss”[tiab] OR “weight gain”[tiab] OR Edema[tiab] OR oedema[tiab] OR lymphedema[tiab] OR lymphoedema[tiab] OR swelling[tiab] OR swollen[tiab] OR Neurologic-manifestation∗[tiab] OR neurologic-deficit∗[tiab] OR neurologic-finding∗[tiab] OR neurologic-dysfunction∗[tiab] OR weakness[tiab] OR paralysis[tiab] OR “pins and needles”[tiab] OR confusion[tiab] OR seizure∗[tiab] OR epilepsy[tiab] OR memory[tiab] OR balance[tiab] OR vertigo[tiab] OR Fracture∗[tiab] OR pain[tiab] OR ache[tiab] OR anorexia[tiab] OR appetite[tiab] OR constipation[tiab] OR diarrhea[tiab] OR flatulence[tiab] OR nausea[tiab] OR vomiting[tiab] OR emesis[tiab] OR Jaundice[tiab] OR icterus[tiab] OR pallor[tiab] OR pruritus[tiab] OR itching[tiab] OR ascites[tiab] OR nausea[tiab] OR hepatomegaly[tiab] OR “enlarged liver”[tiab] OR “dark urine”[tiab] OR “light-colored stools”[tiab] OR cough[tiab] OR coughing[tiab] OR dyspnea[tiab] OR “shortness of breath”[tiab] OR breathlessness[tiab] OR hemoptysis[tiab] OR Urologic-manifestation∗[tiab] OR dysuria[tiab] OR nocturia[tiab] OR “overactive bladder”[tiab] OR “overactive detrusor”[tiab] OR incontinence[tiab] OR hesitancy[tiab] OR pollakisuria[tiab] OR urination[tiab] OR miction[tiab] OR “Uterine hemorrhage”[Mesh] OR “uterine hemorrhage”[tiab] OR “uterine bleeding”[tiab] OR “vaginal bleeding”[tiab] OR dyspareunia[tiab] OR Menorrhagia[Mesh] OR menorrhag∗[tiab] OR hypermenorrhoea[tiab] OR Metrorrhagia[Mesh] OR hypermenorrhea[tiab] OR heavy-period∗[tiab] OR “menstrual bleeding”[tiab] OR metrorrh∗[tiab] OR “intermenstrual bleeding”[tiab] OR spotting[tiab] OR “heavy bleeding”[tiab] OR “intermittent bleeding”[tiab] OR “abnormal uterine bleeding”[tiab]
OR “postmenopausal bleeding”[tiab] OR “postcoital bleeding”[tiab] OR Leukorrhea[tiab] OR “vaginal discharge”[Mesh] OR "Vaginal Discharge"[tiab] OR “vagina discharge”[tiab] OR “pelvic pressure”[tiab] OR “pelvic mass”[tiab] OR “abdominal mass”[tiab] OR “bloating”[tiab] OR “uterine enlargement”[tiab] OR “enlarged uterus”[tiab] OR “suprapubic pressure”[tiab] OR “suprapubic mass”[tiab] OR "Lymphadenopathy"[Mesh] OR lymphadenopathy[tiab] OR enlarged-lymph-node∗[tiab] OR swollen-lymph-node∗[tiab])
Appendix B.
Search strategy in Web of Science
“Uterus cancer” OR “uterine neoplasm$” OR “uterus neoplasm$” OR “Cancer$ of Uterus” OR “uterine cancer$” OR “uterus cancer$” OR “Cancer$ of the Uterus” OR “uterine malignanc∗” OR “endometrial neoplasm$” OR “endometrial carcinoma$” OR “endometrial cancer$” OR “cancer$ of the endometrium” OR “carcinoma$ of endometrium” OR “endometrium carcinoma$” OR “endometrial carcinoma$” OR “cancer$ of endometrium” OR “endometrium cancer$” OR “endometrium malignanc∗” OR “endometrial stromal tumo$r∗” OR “endometrial stromal sarcoma$” OR “uterine sarcoma$” OR “uterine carcinosarcoma$” OR “endometrial adenocarcinoma$” OR “endometrial adenosquamous carcinoma$” OR “endometrial papillary serous carcinoma$” OR “endometrial clear cell carcinoma$” OR leiomyosarcoma$ OR “uterine mixed mesodermal tumo$r∗” OR “uterine rhabdomyosarcoma$” OR “uterine chondrosarcoma$”
AND
“Physical disease by body function” OR sign$ OR symptom∗ OR fatigue OR “muscle weakness” OR sleepiness OR somnolence OR “weight change$” OR “weight loss” OR “weight gain” OR $Edema OR lymph$edema OR “swelling” OR “swollen” OR “neurologic manifestation$” OR “neurologic deficit$” OR “neurologic finding$” OR “neurologic dysfunction$” OR weakness OR paralysis OR “pins and needles” OR confusion OR seizure$ OR epilepsy OR memory OR balance OR vertigo OR Fracture$ OR “pain” OR ache OR anorexia OR appetite OR constipation OR diarrhea OR flatulence OR nausea OR vomiting OR emesis OR Jaundice OR icterus OR pallor OR pruritus OR itching OR ascites OR nausea OR hepatomegaly OR “enlarged liver” OR “dark urine” OR “light colored stools” OR cough OR coughing OR dyspnea OR “shortness of breath” OR breathlessness OR hemoptysis OR “Urologic manifestation$” OR dysuria OR nocturia OR “overactive bladder” OR “overactive detrusor” OR incontinence OR hesitancy OR pollakisuria OR urination OR miction OR “uterine hemorrhage” OR “Uterine bleeding” OR “vaginal bleeding” OR dyspareunia OR menorrhag∗ OR hypermenorrhe$a OR “heavy period$” OR “menstrual bleeding” OR metrorrh∗ OR “intermenstrual bleeding” OR spotting OR “heavy bleeding” OR “intermittent bleeding” OR “abnormal uterine bleeding” OR “postmenopausal bleeding” OR “postcoital bleeding” OR Leukorrhea OR “vaginal discharge” OR “vagina discharge” OR “pelvic pressure” OR “pelvic mass” OR “abdominal mass” OR “bloating” OR “uterine enlargement” OR “enlarged uterus” OR “suprapubic pressure” OR “suprapubic mass” OR lymphadenopathy OR “enlarged lymph node$” OR “swollen lymph node$”
Appendix C.
Search strategy in Embase
‘Uterus cancer’/de OR ‘endometrium tumor’/expOR‘uterine neoplasm$’:ti,ab,kw OR ‘uterus neoplasm$’:ti,ab,kw OR ‘Cancer$ of Uterus’:ti,ab,kw OR ‘uterine cancer$’:ti,ab,kw OR ‘uterus cancer$’:ti,ab,kw OR ‘Cancer$ of the Uterus’:ti,ab,kw OR ‘uterine malignanc∗’:ti,ab,kw OR ‘endometrial neoplasm$’:ti,ab,kw OR ‘endometrial carcinoma$’:ti,ab,kw OR ‘endometrial cancer$’:ti,ab,kw OR ‘cancer$ of the endometrium’:ti,ab,kw OR ‘carcinoma$ of endometrium’:ti,ab,kw OR ‘endometrium carcinoma$’:ti,ab,kw OR ‘endometrial carcinoma$’:ti,ab,kw OR ‘cancer$ of endometrium’:ti,ab,kw OR ‘endometrium cancer$’:ti,ab,kw OR ‘endometrium malignanc∗’:ti,ab,kw OR ‘endometrial stromal tumo$r$’:ti,ab,kw OR ‘endometrial stromal sarcoma$’:ti,ab,kw OR ‘uterine sarcoma$’:ti,ab,kw OR ‘uterine carcinosarcoma$’:ti,ab,kw OR ‘endometrial adenocarcinoma$’:ti,ab,kw OR ‘endometrial adenosquamous carcinoma$’:ti,ab,kw OR ‘endometrial papillary serous carcinoma$’:ti,ab,kw OR ‘endometrial clear cell carcinoma$’:ti,ab,kw OR leiomyosarcoma$:ti,ab,kw OR ‘uterine mixed mesodermal tumo$r$’:ti,ab,kw OR ‘uterine rhabdomyosarcoma$’:ti,ab,kw OR ‘uterine chondrosarcoma$’:ti,ab,kw
AND
‘Physical disease by body function’/exp OR ‘menorrhagia and metrorrhagia’/exp OR ‘vagina discharge’/exp OR ‘uterus bleeding’/exp OR ‘Lymphadenopathy’/exp OR ‘vagina discharge’/expORSign$:ti,ab,kw OR symptom∗:ti,ab,kw OR fatigue:ti,ab,kw OR ‘muscle weakness’:ti,ab,kw OR sleepiness:ti,ab,kw OR somnolence:ti,ab,kw OR ‘weight change$’:ti,ab,kw OR ‘weight loss’:ti,ab,kw OR ‘weight gain’:ti,ab,kw OR $Edema:ti,ab,kw OR lymph$edema:ti,ab,kw OR ‘swelling’:ti,ab,kw OR ‘swollen’:ti,ab,kw OR ‘neurologic manifestation$’:ti,ab,kw OR ‘neurologic deficit$’:ti,ab,kw OR ‘neurologic finding$’:ti,ab,kw OR ‘neurologic dysfunction$’:ti,ab,kw OR weakness:ti,ab,kw OR paralysis:ti,ab,kw OR ‘pins and needles’:ti,ab,kw OR confusion:ti,ab,kw OR seizure$:ti,ab,kw OR epilepsy:ti,ab,kw OR memory:ti,ab,kw OR balance:ti,ab,kw OR vertigo:ti,ab,kw OR Fracture$:ti,ab,kw OR ‘pain’:ti,ab,kw OR ache:ti,ab,kw OR anorexia:ti,ab,kw OR appetite:ti,ab,kw OR constipation:ti,ab,kw OR diarrhea:ti,ab,kw OR flatulence:ti,ab,kw OR nausea:ti,ab,kw OR vomiting:ti,ab,kw OR emesis:ti,ab,kw OR Jaundice:ti,ab,kw OR icterus:ti,ab,kw OR pallor:ti,ab,kw OR pruritus:ti,ab,kw OR itching:ti,ab,kw OR ascites:ti,ab,kw OR nausea:ti,ab,kw OR hepatomegaly:ti,ab,kw OR ‘enlarged liver’:ti,ab,kw OR ‘dark urine’:ti,ab,kw OR ‘light colored stools’:ti,ab,kw OR cough:ti,ab,kw OR coughing:ti,ab,kw OR dyspnea:ti,ab,kw OR ‘shortness of breath’:ti,ab,kw OR breathlessness:ti,ab,kw OR hemoptysis:ti,ab,kw OR ‘Urologic manifestation$’:ti,ab,kw OR dysuria:ti,ab,kw OR nocturia:ti,ab,kw OR ‘overactive bladder’:ti,ab,kw OR ‘overactive detrusor’:ti,ab,kw OR incontinence:ti,ab,kw OR hesitancy:ti,ab,kw OR pollakisuria:ti,ab,kw OR urination:ti,ab,kw OR miction:ti,ab,kw OR ‘uterine hemorrhage’:ti,ab,kw OR ‘Uterine bleeding’:ti,ab,kw OR ‘vaginal bleeding’:ti,ab,kw OR dyspareunia:ti,ab,kw OR menorrhag∗:ti,ab,kw OR hypermenorrhe$a:ti,ab,kw OR ‘heavy period$’:ti,ab,kw OR ‘menstrual bleeding’:ti,ab,kw OR metrorrh∗:ti,ab,kw ‘intermenstrual bleeding’:ti,ab,kw OR spotting:ti,ab,kw OR ‘heavy bleeding’:ti,ab,kw OR ‘intermittent bleeding’:ti,ab,kw OR ‘abnormal uterine bleeding’ OR ‘postmenopausal bleeding’:ti,ab,kw OR ‘postcoital bleeding’:ti,ab,kw OR Leukorrhea:ti,ab,kw OR ‘vaginal discharge’:ti,ab,kw OR ‘vagina discharge’:ti,ab,kw OR ‘pelvic pressure’:ti,ab,kw OR ‘pelvic mass’:ti,ab,kw OR ‘abdominal mass’:ti,ab,kw OR ‘bloating’:ti,ab,kw OR ‘uterine enlargement’:ti,ab,kw OR ‘enlarged uterus’:ti,ab,kw OR ‘suprapubic pressure’:ti,ab,kw OR ‘suprapubic mass’:ti,ab,kw OR lymphadenopathy:ti,ab,kw OR ‘enlarged lymph node$’:ti,ab,kw OR ‘swollen lymph node$’:ti,ab,kw
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Schmeler K.M. Endometrial cancer in young, normal-weight women. Gynecol. Oncol. 2005;99(2):388–392. doi: 10.1016/j.ygyno.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L. Cancer statistics 2019. CA A Cancer J. Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 3.Clarke M.A. Association of endometrial cancer risk with postmenopausal bleeding in women: a systematic review and meta-analysis. JAMA Inte. Med. 2018;178(9):1210–1222. doi: 10.1001/jamainternmed.2018.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Setiawan V.W. Type I and II endometrial cancers: have they different risk factors? J. Clin. Oncol. 2013;31(20):2607–2618. doi: 10.1200/JCO.2012.48.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L.M. Data from: SEER database for patients treated in 1988 through 2006, staging in these patients includes lymphadenectomy, staged according to the 2010 FIGO staging system. Obstet. Gynecol. 2010;116:1141. https://www-uptodate-com./contents/overview-of-endometrial-carcinoma UpToDate 2019 [Google Scholar]
- 6.Bokhman J.V. Two pathogenetic types of endometrial carcinoma. Gynecol. Oncol. 1983;15:10. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 7.Felix A.S. Factors associated with Type I and Type II endometrial cancer. Cancer Causes Control. 2010;21:1851. doi: 10.1007/s10552-010-9612-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abu-Rustm N.R. The revised 2009 FIGO staging system for endometrial cancer. Int. J. Gynecol. Canc. 2011;21(3) doi: 10.1097/IGC.0b013e31820cc305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American College of Obstetricians and Gynecologists ACOG practice bulletin, clinical management guidelines for obstetrician-gynecologists, number 65, August 2005: management of endometrial cancer. Obstet. Gynecol. 2005;106:413. doi: 10.1097/00006250-200508000-00050. [DOI] [PubMed] [Google Scholar]
- 10.Chen L.M. https://www-uptodate-com./contents/endometrial-carcinoma-clinical-features-diagnosis-prognosis-and-screening UpToDate 2020
- 11.Kimura T. Abnormal uterine bleeding and prognosis of endometrial cancer. Int. J. Gynaecol. Obstet. 2004;85:145. doi: 10.1016/j.ijgo.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Seebacher V. The presence of postmenopausal bleeding as prognostic parameter in patients with endometrial cancer: a retrospective multi-center study. BMC Canc. 2009;9:460. doi: 10.1186/1471-2407-9-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith R.A. American Cancer Society guidelines for the early detection of cancer: update of early detection guidelines for prostate, colorectal, and endometrial cancers. Ca - Cancer J. Clin. 2001;51:38. doi: 10.3322/canjclin.51.1.38. [DOI] [PubMed] [Google Scholar]
- 14.Chen L.M. https://www-uptodate-com/contents/endometrial-carcinoma-epidemiology-risk-factors-and-prevention UpToDate 2020
- 15.Whiting P.F. QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 16.Abdullah L.S. Histopathological pattern of endometrial sampling performed for abnormal uterine bleeding. Bahrain Med. Bull. 2011;33(4):195. [Google Scholar]
- 17.Abid M. Clinical pattern and spectrum of endometrial pathologies in patients with abnormal uterine bleeding in Pakistan: need to adopt a more conservative approach to treatment. BMC Women's Health. Nov 2014;14:132. doi: 10.1186/s12905-014-0132-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alanis Fuentes J. Hysteroscopy findings in patients with postmenopausal genital bleeding. Ginecolog. Obstet. Mex. 2007;75(5):253–258. [PubMed] [Google Scholar]
- 19.Alexopoulos E.D. A review of 2581 out-patient diagnostic hysteroscopies in the management of abnormal uterine bleeding. GynaEcol. Endosc. 1999;8(2):105–110. [Google Scholar]
- 20.Allen D.G. Abnormal uterine bleeding and cancer of the genital tract. Australian New Zealand J. Obstet. Gynaecol. Feb 1990;30(1):81–83. doi: 10.1111/j.1479-828x.1990.tb03204.x. [DOI] [PubMed] [Google Scholar]
- 21.Al-Turiahi A.M. Assessment of postmenopausal bleeding: a cohort case study. Am. J. BioMed. 2016;4(6):265–282. [Google Scholar]
- 22.Antunes A. Endometrial polyps in pre- and postmenopausal women: factors associated with malignancy. Maturitas. 2007;57(4):415–421. doi: 10.1016/j.maturitas.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 23.Antunes A. Risk of malignancy in endometrial polyps in premenopausal and postmenopausal women according to clinicalpathologic characteristics. Climacteric. 2011;14 doi: 10.1097/gme.0b013e31821e23a1. [DOI] [PubMed] [Google Scholar]
- 24.Asghar S. The prevalence of significant pathology in women presenting with abnormal uterine bleeding. Pak. J. Med. Health Sci. 2016;10(1):317–319. [Google Scholar]
- 25.Ash S.J. Endometrial biopsy in DUB. J. Reprod. Med. 1996;41(12):892–896. [PubMed] [Google Scholar]
- 26.Bahadur A. Spectrum of abnormal uterine bleeding: clinical pattern and endometrial pathology. Aspect. J. Gynaecol. Surg. 2017;34(1) [Google Scholar]
- 27.Burbos N. Age-related differential diagnosis of vaginal bleeding in postmenopausal women: a series of 3047 symptomatic postmenopausal women. Menopause Int. Mar 2010;16(1):5–8. doi: 10.1258/mi.2010.010005. [DOI] [PubMed] [Google Scholar]
- 28.Burbos N. Outcome of investigations for postmenopausal vaginal bleeding in women under the age of 50 years. Gynecol. Oncol. 2012;125(1):120–123. doi: 10.1016/j.ygyno.2011.12.453. [DOI] [PubMed] [Google Scholar]
- 29.Cormio G. Brain metastases from endometrial carcinoma. Gynecol. Oncol. 1996;61(1):40–43. doi: 10.1006/gyno.1996.0093. [DOI] [PubMed] [Google Scholar]
- 30.Crissman J.D. Endometrial carcinoma in women 40 years of age or younger. Obstet. Gynecol. 1981;57(6):699–704. [PubMed] [Google Scholar]
- 31.Deeba F. Histological pattern of endometrial samples in postmenopausal women with abnormal uterine bleeding. J. Ayub. Med. Coll. 2016;28(4):721–724. [PubMed] [Google Scholar]
- 32.Doraiswami S. Study of endometrial pathology in abnormal uterine bleeding. J. Obstet. Gynaecol. India. 2011;61(4):426–430. doi: 10.1007/s13224-011-0047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dvalishvili I. Clinical characteristics of prognostic factors in uterine endometrioid adenocarcinoma of various grade. Georgian Med. News. 2006;132:24–27. [PubMed] [Google Scholar]
- 34.Fatima S.S. Postmenopausal bleeding-an alarming symptom of endometrial carcinoma. J. Med. Sci. 2014;22(4):166–170. [Google Scholar]
- 35.Genc M. Endometrial pathology in postmenopausal women with no bleeding. Climacteric : J. Int. Menopause Soc. 2015;18(2):241–245. doi: 10.3109/13697137.2014.944152. [DOI] [PubMed] [Google Scholar]
- 36.Ghoubara A. Endometrial pathology in recurrent postmenopausal bleeding: observational study of 385 women. Climacteric:J. Int. Menopause Soc. 2018;21(4):391–396. doi: 10.1080/13697137.2018.1461825. [DOI] [PubMed] [Google Scholar]
- 37.Giannella L. Prediction of endometrial hyperplasia and cancer among premenopausal women with abnormal uterine bleeding. BioMed Res. Int. 2019;2019 doi: 10.1155/2019/8598152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gredmark T. Histopathological findings in women with postmenopausal bleeding. Br. J. Obstet. Gynaecol. 1995;102(2):133–136. doi: 10.1111/j.1471-0528.1995.tb09066.x. [DOI] [PubMed] [Google Scholar]
- 39.Holzl M. The aetiology of postmenopausal bleedings. DMW (Dtsch. Med. Wochenschr.) 1973;98(39):1796–1801. doi: 10.1055/s-0028-1107131. [DOI] [PubMed] [Google Scholar]
- 40.Kansal Y. Spectrum of abnormal uterine bleeding: clinical pattern and endometrial pathology aspects. J. Gynecol. Surg. 2018;34(1):12–17. [Google Scholar]
- 41.Kim M.K. Common causes of postmenopausal bleeding in Korean women: 10-year follow-up. Menopause. 2015;22(12):1386. [Google Scholar]
- 42.Kumari A. Clinicopathological study of postmenopausal bleeding in a tertiary care center. Indian J. Gynecol. Oncol. 2018;16(3) [Google Scholar]
- 43.Lidor A. Histopathological findings in 226 women with post-menopausal uterine bleeding. Acta Obstet. Gynecol. Scand. 1986;65(1):41–43. doi: 10.3109/00016348609158227. [DOI] [PubMed] [Google Scholar]
- 44.Mandić A. Clinical and histopathological characteristics in patients with postmenopausal bleeding. Arch. Oncol. 2013;21(1):5–10. [Google Scholar]
- 45.Musonda P. Comparing the performance of two clinical models in estimating the risk of endometrial cancer in symptomatic postmenopausal women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011;159(2):433–438. doi: 10.1016/j.ejogrb.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 46.Nargis N. Abnormal uterine bleeding in perimenopausal age: different causes and its relation with histopathology. Bangladesh J. Med. Sci. 2014;13(2):135–139. [Google Scholar]
- 47.Pakish J.B. Endometrial cancer associated symptoms: a case-control study. J. Wom. Health. 2016;25(11):1187–1192. doi: 10.1089/jwh.2015.5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Potikul C. Uterine sarcoma: clinical presentation, treatment and survival outcomes. Int. J. Gynecol. Canc. 2015;25(9):1148. doi: 10.7314/apjcp.2016.17.4.1759. [DOI] [PubMed] [Google Scholar]
- 49.Sadaf R. Frequency of outcomes of diagnostic dilatation & curettage in women with abnormal uterine bleeding. J. Med. Sci. (Peshawar) 2014;22(2):60–62. [Google Scholar]
- 50.Salman M.C. Role of postmenopausal bleeding pattern and women's age in the prediction of endometrial cancer. Aust. N. Z. J. Obstet. Gynaecol. 2013;53(5):484–488. doi: 10.1111/ajo.12113. [DOI] [PubMed] [Google Scholar]
- 51.Schindler A.E. Post-menopausal bleeding: a study of more than 1000 cases. Maturitas. 1980;2(4):269–274. doi: 10.1016/0378-5122(80)90028-6. [DOI] [PubMed] [Google Scholar]
- 52.Thomas S.G. Prevalence of symptomatic pelvic floor disorders among gynecologic oncology patients. Obstet. Gynecol. 2013;122(5):976–980. doi: 10.1097/AOG.0b013e3182a7ef3c. [DOI] [PubMed] [Google Scholar]
- 53.Van den Bosch T. Intra-cavitary uterine pathology in women with abnormal uterine bleeding: a prospective study of 1220 women. Facts Views Vision ObGyn. 2015;7(1):17–24. [PMC free article] [PubMed] [Google Scholar]
- 54.Wong S.F. Findings in women with postmenopausal bleeding investigated with hysteroscopy. J. Obstet. Gynaecol.: J. Inst. Obstetr. Gynaecol. 2001;21(4):392–395. doi: 10.1080/01443610120059978. [DOI] [PubMed] [Google Scholar]
- 55.Raglan O. Risk factors for endometrial cancer: an umbrella review of the literature. Int. J. Canc. 2019;145:1719–1730. doi: 10.1002/ijc.31961. [DOI] [PubMed] [Google Scholar]
- 56.Low E.L. Experience of symptoms indicative of gynaecological cancers in UK women. Br. J. Canc. 2013;109:882–887. doi: 10.1038/bjc.2013.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Balasubramaniam K. Specific and unspecific gynaecological alarm symtpoms. Prevalence estimated in different age groups: a population-based study. Acta Obstet. Gynecol. Scand. 2014;94:191–197. doi: 10.1111/aogs.12538. [DOI] [PubMed] [Google Scholar]
- 58.Notelovitz M. Beware of the weeping womb. S. Afr. Med. J. 1973;47:1653–1655. [PubMed] [Google Scholar]
- 59.The role of transvaginal ultrasonography in the evaluation of postmenopausal bleeding. Obstet. Gynecol. 2009;114:409–411. doi: 10.1097/AOG.0b013e3181b48feb. ACOG Committee Opinion No.440 American College of Obstetricians and Gynecologists. [DOI] [PubMed] [Google Scholar]
- 60.Alberico S. Clinical and epidemiological study of 245 postmenopausal metrorrhagia patients. Clin. Exp. Obstet. Gynecol. 1989;16(4):113–121. [PubMed] [Google Scholar]
- 61.Choo Y.C. Postmenopausal uterine bleeding of nonorganic cause. Obstet. Gynecol. 1985;66:225–228. [PubMed] [Google Scholar]
- 62.Gredmark T. Histopathological findings in women with postmenopausal bleeding. Br. J. Obstet. Gynaecol. 1995;102:133–136. doi: 10.1111/j.1471-0528.1995.tb09066.x. [DOI] [PubMed] [Google Scholar]
- 63.Emanuel M.H. An audit of true prevalence of intrauterine pathology: the hysteroscopic findings, controlled for patient selection in 1202 patients with abnormal uterine bleeding. Gynecol. Endosc. 1995;4:237–241. [Google Scholar]
- 64.Goldstein R.B. Evaluation of the woman with postmenopausal bleeding: society of Radiologists in Ultrasound-Sponsored Consensus Conference statement. Ultrasound Med. 2001;20:1025–1036. doi: 10.7863/jum.2001.20.10.1025. [DOI] [PubMed] [Google Scholar]
- 65.Soliman P.T. Risk factors for young premenopausal women with endometrial cancer. Obstet. Gynecol. 2005;105:575–580. doi: 10.1097/01.AOG.0000154151.14516.f7. [DOI] [PubMed] [Google Scholar]
- 66.Schmidt T. Differential indication for histological evaluation of endometrial fluid in postmenopause. Maturitas. 2005;50:177–181. doi: 10.1016/j.maturitas.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 67.Aalders J.C. Recurrent adenocarcinoma of the endometrium: a clinical and histopathological study of 379 patients. Gynecol. Oncol. 1984;17:85–103. doi: 10.1016/0090-8258(84)90063-5. [DOI] [PubMed] [Google Scholar]
- 68.Dhopesh V.P. Brain metastasis: analysis of patients with known cancer. South. Med. J. 1985;78:171–172. doi: 10.1097/00007611-198502000-00013. [DOI] [PubMed] [Google Scholar]
- 69.Winston K.R. Results of operative treatment of intracranial metastatic tumors. Cancer. 1980:2639–2645. doi: 10.1002/1097-0142(19800515)45:10<2639::aid-cncr2820451025>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.