Abstract
Magnesium is essential in plants where it is associated with chlorophyll pigments and serves as a cofactor of enzymes implicated in photosynthesis and metabolism. It is an essential nutrient for animals, involved in hundreds metabolic reaction and crucial for the biological activity of ATP. Not surprisingly, magnesium deficiency is detrimental for the health of plants and animals. In humans, subclinical magnesium deficiency is common and generates chronic inflammation, which is the common denominator of a wide range of mental and physical health problems from metabolic diseases to cognitive impairment, from osteopenia and sarcopenia to depression. It is ascertained that magnesium content in fruits and vegetables dropped in the last fifty years, and about 80% of this metal is lost during food processing. As a consequence, a large percentage of people all over the world does not meet the minimum daily magnesium requirement. In this scoping review, we summarize how agronomic and environmental factors, including global warming, affect magnesium content and availability in the soil and, consequently, in the food chain, with the aim of attracting the interest of botanists, agronomists, animal and human nutritionists and physicians to work on a strategy that grants adequate magnesium intake for everybody.
Keywords: Magnesium, Hypo-magnesemia, Magnesium bioavailability, Food quality, Food chain, Chemical composition of food, Dairy product, Meat, Nutrient availability, Nutrition, Plant products
Magnesium; Hypo-magnesemia; Magnesium bioavailability; Food quality; Food chain; Chemical composition of food; Dairy product; Meat; Nutrient availability; Nutrition; Plant products.
1. Introduction
Magnesium (Mg), an alkaline earth metal, has been essential for the origin of life on our planet. As the central structural element of chlorophyll, Mg is fundamental in photosynthesis, the process that removes carbon dioxide and leads to the accumulation of gaseous oxygen in the atmosphere, thus creating the environment that allowed life to arise and evolve to highly complex biological systems. Mg is central in both plants and animals because it is implicated in hundreds of enzymatic reactions. In plants, Mg is the second most abundant cation. Uptaken from the soil by the roots through the intervention of several transporters, Mg is then translocated to the sprouts and the leaves, where it is accumulated in chloroplasts [1]. In animals, Mg is required for the synthesis of all the macromolecules and the antioxidant glutathione [2]. In addition, Mg has a role in controlling the transport of calcium and potassium ions, crucial event in nervous conduction, muscle contraction and in maintaining heart rhythm. In the bone, beyond its biochemical functions, Mg has also a structural role in shaping apatite crystals [2]. In an adult, body Mg content is around 20–28 g. About 55 % is located in the bone, bound to apatite, 25 % is in the muscle, and 19.3 % in soft tissues [2]. Less than 1% is found in the serum, mainly as free cation, which is the bioactive form, and also conjugated to proteins [3]. Even if measuring serum Mg concentrations does not reflect total body content, this is a simple and widely used method to investigate Mg status [4]. The normal range of serum Mg falls within 0.75 and 0.95 mmol/L. Levels below 0.75 mmol/L define hypomagnesemia. Malabsorption syndromes, disorders of the kidney tubules, alcohol abuse, rare genetic diseases and some therapeutic regimens cause Mg deficiency. However, an insufficient dietary intake is the frequent root of an overlooked low Mg status in western populations [5, 6, 7, 8]. The intent of this scoping review is to offer insights into the mechanisms involved in reducing Mg availability in the food chain. Mg depletion in the soil results in lower concentrations in plants, therefore altering animal intake and resulting in human Mg deficient intake.
2. A glimpse of the relation between Mg intake and diseases
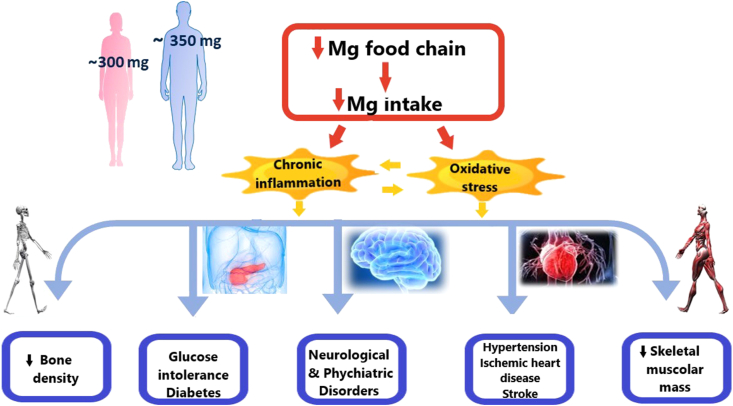
The recommended dietary allowance (RDA) for Mg in North America is 310–320 and 410–420 mg per day for females and males, respectively [9], while the European Food Safety Authority (EFSA) recommends 300 mg for women and 350 mg for men as the adequate daily intake of the mineral [10, 11]. Regardless of these indications, Mg intake has decreased in most individuals [12] and this is aggravated by the concomitant use of medications that inhibit Mg absorption (i.e. proton pump inhibitors) or increase its excretion in the urine (e.g. diuretics) [13]. Low Mg intake has been linked to chronic inflammation and increased oxidative stress [2], since several surveys have demonstrated that dietary Mg is inversely related with the plasma levels of the C reactive protein, a marker of inflammation, and thiobarbituric acid reactive substances, which reflect the content of lipid peroxides [14]. It is well known that subclinical, latent chronic inflammation and oxidative stress are two pathogenic factors in many common pathologies, from cardiovascular diseases to diabetes, from neurological and psychiatric disorders to sarcopenia and osteoporosis (Figure 1).
Figure 1.
A summary of the relationships existing between low Mg intake and various diseases. The recommendations of the European Food Safety Authority about adequate daily intake are reported [10].
Metanalyses have demonstrated an inverse relationship between Mg intake and hypertension, fatal ischemic heart disease and stroke [15, 16, 17, 18]. At the cellular and molecular levels, these results might be explained by the detrimental effects of low Mg on vascular cells, which results in endothelial dysfunction and altered smooth muscle cell behavior [19].
A significant inverse correlation between Mg intake and the risk for diabetes has been reported [20, 21]. Moreover, recent systematic reviews and metanalyses associate hypomagnesemia with the diagnosis and progression of type 1 and 2 diabetes [22, 23]. Significant lower levels of erythrocyte Mg were observed in gestational diabetes [24]. To get insights into the connection between Mg and glucose homeostasis, it is noteworthy that Mg is important in regulating the activity of enzymes involved in glucose metabolism. It is also required for the proper activation of insulin receptor, whose kinase domains bind Mg. In addition, chronic Mg deficiency induces oxidative stress and the release of inflammatory cytokines such al IL-1, IL-6 and TNFα, conditions that contribute to insulin resistance [2]. It is challenging to propose that a Mg rich diet might ease glycemic control in diabetic patients.
A systematic review and metanalysis reports a link between Mg intake and bone mineral density in total hip and femoral neck [25]. Recently, a metanalysis has demonstrated lower amounts of serum magnesium in postmenopausal women [26]. These results are in line with the evidence that Mg is involved in regulating bone development and constructing bone mineral matrix [27].
Mg is also crucial for healthy and performing skeletal muscles. A recent study demonstrates a strong association between dietary Mg intake and indices of skeletal muscle mass in middle to older-aged adults [28] and another shows that sarcopenic elderly consume significantly reduced amounts of Mg [29]. Interestingly, transcriptomic data from skeletal muscle biopsies indicate a substantial difference between young and old men in terms of Mg transport and homeostasis [30].
In the nervous system, Mg has a relevant role in nerve transmission, shelters from neuroinflammation and protects against excessive excitation, which could lead to neuronal cell death [2]. Consequently, Mg deficiency is implicated in many neurological disorders. A recent metanalysis discloses a negative correlation between dietary intake of Mg and depression, and a slight inverse relationship was reported also between dietary Mg intake and subjective anxiety [31, 32]. These data might be due to the action of Mg on the N-methyl-D-aspartate (NMDA) receptor and, therefore, on glutamatergic transmission.
Because reduced Mg intake is frequent and associated with common diseases, we addressed some important, albeit overlooked, questions: why are our foods Mg depleted? Are there food interactions that might lead to a low Mg status? What about Mg bioavailability in food? The following paragraphs will offer an overview on the roots of reduced Mg intake, which is the first step toward a low Mg status, a sneaky trigger of inflammatory and oxidative stress and, therefore, diseases.
3. Soil and agronomic factors affecting magnesium availability in food plant
Mg is the 8th most abundant mineral element of earth's continental crust [33]. Rocks are the source of all soil mineral materials and the origin of all plant nutrients except for nitrogen, hydrogen, and carbon. The mineral material from which the soil is formed is called the parent material. Mg in soils originates from parent material containing various types of silicates. The Mg content of different silicates varies considerably and most soil Mg (90–98%) is not directly available for plant uptake [34]. Parent material is transformed into secondary minerals through chemical-physical alterations induced by atmospheric agents. Secondary minerals found in the clay and fine silt fractions of soil are the sources of Mg available for plant nutrition [35]. The extent of these sources is influenced by several factors, including the pH and the interactions of Mg with other ions, especially K+, Ca 2+ and ammonium. Higher amounts of H+ reduce Mg absorption from plant roots and increase the release of exchangeable alluminium from the soil, which is harmful to plants, exacerbates Mg deficiency and reduces the yield and the quality of agricultural products [34]. Notably, higher soil concentration of K+, Ca2+ and ammonium results in lower Mg availability to plant roots because of competition during the uptake process. K+ in the soil is a potent Mg antagonist, because the root cells have specific K-transport systems, while Mg is uptaken by non-specific transporters that are shared with several different ions, including K+ itself [34].
Since the second half of the last century, the increase in food demand consequent to the impressive population growth has led to an exponential increase in the exploitation of fertile soil: it is the so-called Green Revolution.
This evolution of agriculture allowed a dramatic increase in agricultural production of the main species of food interest (corn, rice, wheat, etc.) through the use of new hybrid varieties created by artificial selection techniques, new agricultural technologies, pesticides, and fertilization based on N, K, and P, but not Mg. However, large amounts of Mg are necessary to sustain intensive crop forage and harvest, so that the soil is in a negative balance with respect to Mg unless Mg fertilizers do not compensate them. Furthermore, the massive use of K fertilizer has determined a progressive reduction in the ability of plant roots to absorb Mg from the soil. Over time, this has resulted in a gradual widespread decline of Mg in soils [34, 36] and consequently in cereals, fruits, and vegetables [37]. Therefore, while the green revolution has led to indisputable benefits by increasing the availability of food energy per capita per day by 35%, on the other hand, the consumption of cereals increasingly poor in Mg has contributed to a growing deficiency of this nutrient in the world population.
4. The effects of global warming on Mg content in crop
The change in earth climate is occurring faster than ever, endangering plant growth and productivity and significantly impacting on the quantity and quality of plant nutrients. Crop yields, nutritional values and safety of foods are particularly dependent on climate, therefore a large amount of the food produced for human nutrition is under threat [38, 39]. Even though large efforts have been undertaken to increase global food availability, the worldwide problem of malnutrition and micronutrient deficiencies remains worrisome and strongly linked to climate changes, especially in low income communities [40]. Several factors derived from climate changes, such as water scarcity, soil waterlogging, elevated CO2 and elevated temperature, impact directly plant nutrition and physiology [39]. In particular, the CO2-induced increase of growth rates and soil acidity has promoted significant losses of Mg in the soil [41]. In addition, climate change has different effects on plant physiology at the level of molecular function, developmental processes, and morphological traits. The release of anthropogenic greenhouse gases to the atmosphere, which is one of the principal causes of climate change, has gradually increased from the 280 ppm preindustrial levels [42, 43] to the current levels which are above 400 ppm [44]. The increase of atmospheric CO2 will accelerate warming and exacerbate changes of the climate system [42]. There are increasing evidences on the influence of climatic changes and elevated CO2 on crop plants mineral concentrations [45]. In particular, it has been shown that an elevated CO2 decreases N, Mg, Fe, and Zn, but not P, K, S, Cu, Mn concentrations in the edible part of vegetables. A challenging study deals with the effect of elevated CO2 on the growth and nutrient composition of Triticum durum under normal or Mg deficient conditions. Low Mg plants respond to high CO2 by decreasing biomass, particularly in roots. Elevated CO2 increases photosynthesis in adequate-Mg plants, but not in Mg deficient plants [46]. The same authors report that low plant Mg and elevated CO2 decrease carbohydrate transport from source to sink tissues.
There are several studies showing that Mg deficiency increases the susceptibility of plants toward heat stress [47, 48, 49]. Plants tolerate heat stress conditions if supplied with an adequate amount of Mg, while when heat stress is accompanied by Mg deficiency the tolerance is reduced. In particular, Mg deficiency increases susceptibility of wheat and maize plants to heat stress [49], and the effect was hypothesized to be caused by an increased oxidative cellular damage caused by free radicals. Interestingly, an in silico study showed that Mg induced thermostability of the Mg-dependent phosphatases PP2C heterodimer and this was ascribed as one of the mechanisms behind the heat stress response by plants [47]. Even more stimulating is the finding that Mg deficiency elicits an alteration of circadian clock gene expression in the roots of Arabidopsis thaliana [48]. This finding provides a further evidence on the influence of Mg on circadian rhythms in different organisms, since treatments affecting intracellular Mg concentration influence key clock parameters in human cells, mouse fibroblasts, marine unicellular alga, and a filamentous fungus [50]. The clear message is that Mg rhythms are crucial to cellular circadian timekeeping. It is therefore extremely fascinating to emphasize the concept that intracellular Mg oscillations provide an amazingly effective way to dynamically tune cellular biochemistry and time energy. It is therefore evident that an alteration of a fundamental physiologic characteristic of living organisms, evolved over a billion years ago, would have a profound impact on the entire cycle of life.
5. Food sources of magnesium
Rich Mg food sources are whole grains and grain products, green vegetables (e.g. spinach and broccoli), nuts and seeds. Legumes (soybeans and black beans), fruit (e.g. banana), berries, meat and fish have an intermediate Mg content. Lastly, dairy products have a low Mg content [5, 10, 51]. It is important to point out that all these food categories are essential not only to have a balanced diet, but also to satisfy Mg needs. However, the contribution to the diet of each of these food groups is very different. Cereal grains are the most important source of calories to most of the world population. In particular, rice is the single most important source of calories for humans, followed by wheat [52]. The results of a worldwide longitudinal study based on country-level data of 124 countries indicate that the major source of energy in the diet is represented by cereals (40.3%), followed by sugar and sweeteners (10.8 %), vegetables (9.3%), meats (7.5%), starchy roots (6.4%), dairy products (6.3%), fruit (3.7%), animal fats (3.5%), legumes (2.1%) and vegetable oils (1.9%) [53].
5.1. Plant sources
Plant sources can interact with Mg absorption, reducing its bioavailability due to their non-fermentable fibres (lignin, cellulose), oxalate (OA), and phytate (PA) contents. These latter are two anti-nutrients that limit the absorption of mineral salts, including Mg [51]. It is known that Mg forms insoluble, non-absorbable complexes with oxalic acid in the gastrointestinal tract. In 2004, Bohn and collaborators evaluated the absorption of Mg from two vegetable sources characterized by different oxalic acid content, i.e. spinach (6.6 mmol oxalate) and kale (0.1 mmol oxalate). Their results demonstrate that the mean Mg absorption from spinach is about 35 % lower than Mg absorption from kale because of the high OA content of spinach. However, since native Mg content of spinach is 26 % higher than kale's, the significantly lower Mg absorption from spinach can be counterbalanced by the high Mg content [54]. In relation to the amounts of Mg and OA in the same food, particularly in cereals and beans, it should be noted that OA is present in soluble and insoluble forms. OA becomes more soluble under gastric acid condition (pH 1.5–2) and forms a sparingly soluble complex with Mg in the intestine [55]. However, at intestinal pH, only a small part of soluble OA-Mg complex may be absorbed in the small bowel. Besides, PA is present with OA and Mg in the same food, in a higher concentration than OA. PA chelates Mg reducing its bioavailability in a dose-dependent manner [56], but at the same time increases OA bioavailability [55]. Consequently, PA and OA highly reduce the bioavailability of Mg in Mg-rich foods. Germination and soaking of seeds, legumes and grains and sour-dough methods in the bakery process activate endogenous phytase that reduces PA levels [57]. Therefore, these treatments seem to restrict the negative effects of PA on Mg bioavailability. Conversely, the presence of low or indigestible carbohydrates like short-chain fructo-oligosaccharides, inulin, lactulose, mannitol and fructose in plant foods improves Mg uptake and balance [6, 51]. Finally, food processing is another important factor influencing Mg content of plant food, especially of cereal grains (Table 1). In fact, the milling process removes important nutrients from the grains, including Mg. Unfortunately, most of wheat and rice used for human nutrition are milled to remove the bran and germ, mainly to satisfy the taste of the consumers [58, 59]. However, in recent years, in many countries, the growing number of scientific publications confirming the health effects of whole grain consumption has led to dietary guidelines with recommendations to replace refined grain foods with foods derived from whole grains [52]. Finally, it is important to note that although the whole grains contain more PA and fibre than refined, the reduced bioavailability of their Mg is considerably compensated by its higher concentration [60].
Table 1.
| Cereal food | Mg (mg/100 g) |
|
|---|---|---|
| Whole | Refined | |
| Wheat flour | 120 | 20 |
| Rice | 107.8 ± 6.6 | 37.7 ± 15 |
| Corn | 127 | |
| flour | 93 | 18 |
| masa flour | 93 | 93 |
5.2. Animal sources
5.2.1. Mg in meat and seafood products
As reported above, seeds, nuts and green vegetables are characterized by a high Mg content; but, due to their low consumption rates, they do not constitute a remarkable source of dietary Mg. In the literature, a few studies have been conducted in Europe to evaluate Mg content and intake in various meat and fish products. In Spain, Jodral-Segado and co-workers [65] determined Mg content in 243 food, 69 drinks and 11 potable water samples. Focusing on Mg in meat and fish, they found the highest Mg levels in molluscs and crustaceans (up to 2300 mg/kg), which, however, do not contribute significantly to Mg intake because of the low consumption rate of these foods and their low edible portion. By contrast in Spain, chicken meat and milk have been identified as the main contributors to Mg daily intake. This was mainly due to their high consumption rate that compensates their intermediate Mg content [65]. In Poland, meats and seafood contribute to 13.1% of Mg in the diet. In particular, the main contributors were meat products, namely poultry and red meat [66]. These results are in line with other studies [67] in which the highest Mg levels were found in dried meat products obtained from pork and poultry. Specifically, pork cuts and products contain higher Mg levels than chicken cuts and chicken meat products [67]. These data are summarised in Table 2. Therefore, even though seafood seems to be good source of Mg in western diets, their limited consumption hinders their potential, while pork and poultry meat, thanks to their higher consumption rates, are among the main contributors to Mg intake in human diets (Figure 2). In this respect, it is important to underline that Mg supplementation is nowadays a routine practice in animal nutrition to guarantee an adequate Mg concentration in animal products [68].
Table 2.
| Mg (mg/kg fresh wt) | |
|---|---|
| Chicken (range) | 196–210 |
| Breast | 210 |
| Drumstick | 196 |
| Chicken meat products |
135–142 |
| Pork (range) | 195–290 |
| Loin | 207 |
| Neck | 212 |
| Hind leg | 237 |
| Shoulder | 195 |
| Pork products (e.g. sausages) | 117–289 |
| Fish seafoods (range) | 171–2371 |
| Fish | 282 |
| Molluscs and crustaceans | 1040 |
| Cephalopoda | 278 |
| Milk (range) | 86–100 |
| Colostrum | 400 |
| Whole (3.25% fat) | 98–110 |
| Reduced Fat (2% fat) | 98–111 |
| Low fat (1% fat) | 98–112 |
| Skim | 98–113 |
| Goat | 139 |
| Sheep | 180 |
| Water Buffalo | 311 |
| Dairy products | |
| Cream | 60 |
| Butter | 20 |
| Cheese (range) | 130–425 |
| Parmesan | 380 |
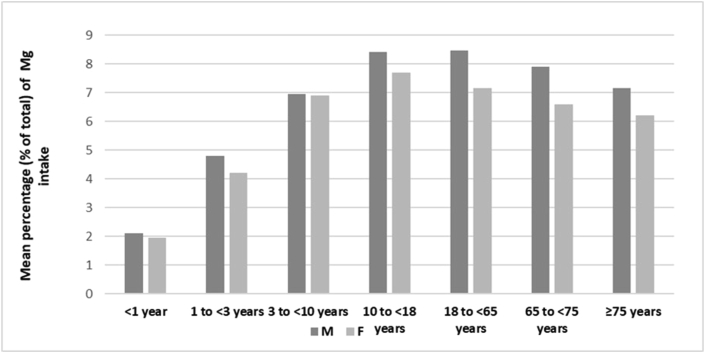
Figure 2.
Mean percentage contribution of meat and meat products to total Mg intake in males and females at different age [10].
5.2.2. Mg in milk and dairy products
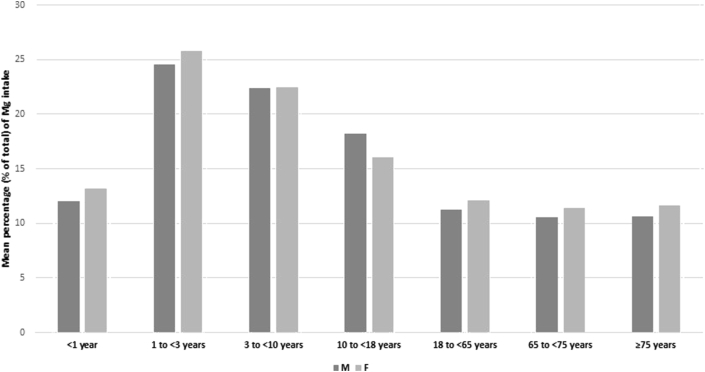
Although there are few studies about the bioavailability of Mg from milk in humans, milk and dairy products are one of the main dietary sources of Mg that contribute approximately 15–40% of the total Mg intake and in particular to 16–21% of the total Mg intake in Western countries [69] (Figure 3). The importance of milk and dairy products in meeting the daily Mg intake requirement is often overshadowed by the fact that milk is mainly known as a significant food source for calcium and the content of Mg in dairy products is lower than in green vegetables, nuts and whole grains [3, 70]. In general, milk mineral content is not constant but is influenced by stage of lactation, nutritional status of the animal, environmental and genetic factors. The average content of Mg in cows' milk is 110 mg/L. Mg concentration in colostrum is 2–3 times higher and decreases within the first 1–5 days of lactation, remaining relatively constant thereafter. Milk Mg content is unaffected by cow dietary intake of Mg [69, 70], as well as by the milk fat removal (Table 2). In cow's milk, about 98% of Mg is found in the skim milk phase and this explains why Mg content is not affected by fat removal (Table 2). The total Mg content is much lower than the total calcium level and the distribution between the serum and colloidal phases of milk is different. About two thirds of Mg are in the serum phase, while a similar proportion of calcium is in the colloidal phase, associated with the casein micelles [70]. In particular, in skim milk phase, 65% of the Mg is in a soluble form (40% as Mg citrate, 7% as Mg phosphate, and 16% as free ion Mg2+), while the remainder is associated with the casein micelles (about one half associated with colloidal calcium phosphate and the other half bound directly to phosphoserine residues in caseins) [69]. Several studies have shown that lactose contained in dairy products improves intestinal absorption of minerals including Mg in human infants and in animals. For example, Ziegler and Fomon observed an enhanced intestinal absorption of calcium, Mg and manganese in human infants thanks to the effect of milk lactose content [71]. In 1993 Heijnen and collaborators evaluated the effect of lactose on the intestinal absorption of Mg and other minerals among which calcium. They demonstrate that lactose fermentation lowers the pH in the ileal lumen and prevents the formation of insoluble Ca–Mg-phosphate complexes, an event that usually occur in the ileum, thus improving ileal solubility of the minerals involved [72]. The bioavailability of Mg in dairy products containing different quantities of lactose has been investigated also in rats fed with cheese, skim milk and yogurt [73]. Bioavailability was evaluated by apparent absorption and bone mineralization. The results showed that cheese provided a lower apparent absorption and a lower bone deposition of Mg if compared with the other milk products. These results can be explained considering that cheese contains lower levels of lactose than other dairy products, due to maturing. Since differences in the intestinal absorption of Mg were positively related to the dietary lactose content [71, 72], this may explain the different absorptions of Mg observed in this study from the different dairy products [73].
Figure 3.
Mean percentage contribution of milk and dairy foods to total Mg intake in males and females at different age [10].
6. Conclusions
Even though Mg is one of the most important nutrients, it has been overlooked by botanists, agronomists, nutritionists, and clinicians. It is now emerging that Mg deficiency is an alarming problem for plant and human health. In the past several decades, a significant decline of Mg concentration in cereals, which represent the fundamental food for billions of people, has been reported, due to heavy chemical fertilization and the effects of the Green Revolution, worsened by global warming and high concentrations of CO2.. No easy solution is foreseen. For people on an omnivorous diet, animal sources, starting with milk and dairy products, can help in compensating for the reduced Mg content of cereals; however, their recommended daily intake should not be exceeded as these foods are high in saturated fat, cholesterol, and calories. For all people, increased consumption of no-refined or unprocessed foods and whole grains is undoubtedly an essential first step. In the short term, the consumption of whole grains can be made more effective by a soil fertilization program that includes adequate amounts of Mg. In the long term, stopping deforestation, switching to alternative energy sources, and promoting more environmentally friendly agronomic techniques are some useful countermeasures to reduce the greenhouse effect and tame global warming and, consequently, Mg soil depletion. In Paleolithic societies daily Mg intake was about 600 mg, significantly higher than today's [74]. However, we share the same homeostatic mechanisms with our Paleolithic ancestors, thereby indicating that human metabolism is tuned on a higher Mg intake than ours. Therefore, Mg should not remain the forgotten cation with the urgent aim to prevent subclinical Mg deficiency.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
This work was developed as part of the PhD program in Nutrition Sciences, University of Milan.
References
- 1.Sun Y., Yang R., Li L., Huang J. The magnesium transporter MGT10 is essential for chloroplast development and photosynthesis in Arabidopsis thaliana. Mol. Plant. 2017 Dec 4;10(12):1584–1587. doi: 10.1016/j.molp.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 2.de Baaij J.H.F., Hoenderop J.G.J., Bindels R.J.M. Magnesium in man: implications for health and disease. Physiol. Rev. 2015;95:1–46. doi: 10.1152/physrev.00012.2014. [DOI] [PubMed] [Google Scholar]
- 3.Jahnen-Dechent W., Ketteler M. Magnesium basics. Clin. Kidney J. 2012;5:3–14. doi: 10.1093/ndtplus/sfr163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Witkowski M., Hubert J., Mazur A. Methods of assessment of magnesium status in humans: a systematic review. Magnes. Res. 2011;24:163–180. doi: 10.1684/mrh.2011.0292. [DOI] [PubMed] [Google Scholar]
- 5.Razzaque M.S. Magnesium: are we consuming enough? Nutrients. 2018 Dec 2;10(12):1863. doi: 10.3390/nu10121863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nielsen F.H. The problematic use of dietary reference intakes to assess magnesium status and clinical importance. Biol. Trace Elem. Res. 2019;188:52–59. doi: 10.1007/s12011-018-1573-x. [DOI] [PubMed] [Google Scholar]
- 7.Kumssa D.B., Joy E.J.M., Ander E.L., Watts M.J., Young S.D., Rosanoff A., White P.J., Walker S., Broadley M.R. Global magnesium supply in the food chain. Crop Pasture Sci. 2015;66:1278. [Google Scholar]
- 8.Fulgoni V.L., Keast D.R., Bailey R.L., Dwyer J. Foods, fortificants, and supplements: where do Americans get their nutrients? J. Nutr. 2011;141:1847–1854. doi: 10.3945/jn.111.142257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moshfegh A.J., Goldman J.D., Ahuja J.K., Rhodes D.G., Lacomb R.P. 2009. What We Eat In America, NHANES 2005–2006, Usual Nutrient Intakes from Food and Water Compared to 1997 Dietary Reference Intakes for Vitamin D, Calcium, Phosphorus, and Magnesium.http://www.ars.usda.gov/ba/bhnrc/fsrg Available: [Google Scholar]
- 10.N. and A. EFSA Panel on dietetic products, scientific opinion on dietary reference values for magnesium. EFSA J. 2015;13:1–63. [Google Scholar]
- 11.Tarleton E.K. Factors influencing magnesium consumption among adults in the United States. Nutr. Rev. 2018;76:526–538. doi: 10.1093/nutrit/nuy002. [DOI] [PubMed] [Google Scholar]
- 12.Flynn A., Hirvonen T., Mensink G.B.M., Ocke M.C., Serra-Majem L., Stos K., Szponar L., Tetens I., Turrini A., Fletcher R., Wildemann T. Intake of selected nutrients from foods, from fortification and from supplements in various European countries. Food Nutr. Res. 2009;53:10.3402. doi: 10.3402/fnr.v53i0.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bateman S.W. A quick reference on magnesium. Vet. Clin. North Am. - Small Anim. Pract. 2017;47:235–239. doi: 10.1016/j.cvsm.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Nielsen F.H. Effects of magnesium depletion on inflammation in chronic disease. Curr. Opin. Clin. Nutr. Metab. Care. 2014;17:525–530. doi: 10.1097/MCO.0000000000000093. [DOI] [PubMed] [Google Scholar]
- 15.Del Gobbo L.C., Imamura F., Wu J.H.Y., De Oliveira Otto M.C., Chiuve S.E., Mozaffarian D. Circulating and dietary magnesium and risk of cardiovascular disease: a systematic review and meta-analysis of prospective studies. Am. J. Clin. Nutr. 2013;98:160–173. doi: 10.3945/ajcn.112.053132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang X., Han H., Li M., Liang C., Fan Z., Aaseth J., He J., Montgomery S., Cao Y. Dose-response relationship between dietary magnesium intake and risk of type 2 diabetes mellitus: a systematic review and meta-regression analysis of prospective cohort studies. Nutrients. 2016;8:739. doi: 10.3390/nu8110739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nie Z.L., Wang Z.M., Zhou B., Tang Z.P., Wang S.K. Magnesium intake and incidence of stroke: meta-analysis of cohort studies. Nutr. Metabol. Cardiovasc. Dis. 2013;23:169–176. doi: 10.1016/j.numecd.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 18.Adebamowo S.N., Spiegelman D., Willett W.C., Rexrode K.M. Association between intakes of magnesium, potassium, and calcium and risk of stroke: 2 Cohorts of US women and updated meta-analyses. Am. J. Clin. Nutr. 2015;101:1269–1277. doi: 10.3945/ajcn.114.100354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maier J.A.M. Endothelial cells and magnesium: implications in atherosclerosis. Clin. Sci. 2012;122:397–407. doi: 10.1042/CS20110506. [DOI] [PubMed] [Google Scholar]
- 20.Larsson S.C., Wolk A. Magnesium intake and risk of type 2 diabetes: a meta-analysis. J. Intern. Med. 2007;262:208–214. doi: 10.1111/j.1365-2796.2007.01840.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhao B., Zeng L., Zhao J., Wu Q., Dong Y., Zou F., Gan L., Wei Y., Zhang W. Association of magnesium intake with type 2 diabetes and total stroke: an updated systematic review and meta-analysis. BMJ Open. 2020;10:32240. doi: 10.1136/bmjopen-2019-032240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodrigues A.K., Melo A.E., Domingueti C.P. Association between reduced serum levels of magnesium and the presence of poor glycemic control and complications in type 1 diabetes mellitus: a systematic review and meta-analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14:127–134. doi: 10.1016/j.dsx.2020.01.015. [DOI] [PubMed] [Google Scholar]
- 23.Esmeralda C.A.C., Ibrahim S.N.A., David P.E., Maldonado I.C., David A.S., Escorza M.A.Q., Dealmy D.G. Deranged fractional excretion of magnesium and serum magnesium levels in relation to retrograde glycaemic regulation in patients with type 2 diabetes mellitus. Curr. Diabetes Rev. 2020;16 doi: 10.2174/1573399816666200714150434. In press. [DOI] [PubMed] [Google Scholar]
- 24.Musavi H., Tahroodi F.M., Fesahat F., Bouzari Z., Esmaeilzadeh S., Elmi F., Yazdani S., Moazezi Z. Investigating the relationship between magnesium levels and diabetes mellitus in pregnant women. Int. J. Mol. Cell. Med. 2019;8:223–231. doi: 10.22088/IJMCM.BUMS.8.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farsinejad-Marj M., Saneei P., Esmaillzadeh A. Dietary magnesium intake, bone mineral density and risk of fracture: a systematic review and meta-analysis. Osteoporos. Int. 2016;27:1389–1399. doi: 10.1007/s00198-015-3400-y. [DOI] [PubMed] [Google Scholar]
- 26.Castiglioni S., Cazzaniga A., Albisetti W., Maier J.A.M. Magnesium and osteoporosis: current state of knowledge and future research directions. Nutrients. 2013;5:3022–3033. doi: 10.3390/nu5083022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang J., Yu D., Ji J., Wang N., Yu S., Yu B. The association between the concentration of serum magnesium and postmenopausal osteoporosis. Front. Med. 2020;7:381. doi: 10.3389/fmed.2020.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayhoe R.P.G., Lentjes M.A.H., Mulligan A.A., Luben R.N., Khaw K.T., Welch A.A. Cross-sectional associations of dietary and circulating magnesium with skeletal muscle mass in the EPIC-Norfolk cohort. Clin. Nutr. 2019;38:317–323. doi: 10.1016/j.clnu.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 29.Beaudart C., Locquet M., Touvier M., Reginster J.Y., Bruyère O. Association between dietary nutrient intake and sarcopenia in the SarcoPhAge study. Aging Clin. Exp. Res. 2019;31:815–824. doi: 10.1007/s40520-019-01186-7. [DOI] [PubMed] [Google Scholar]
- 30.Coudy-Gandilhon C., Gueugneau M., Taillandier D., Combaret L., Polge C., Roche F., Barthélémy J.C., Féasson L., Maier J.A., Mazur A., Béchet D. Magnesium transport and homeostasis-related gene expression in skeletal muscle of young and old adults: analysis of the transcriptomic data from the proof cohort study. Magnes. Res. 2019;32:72–82. doi: 10.1684/mrh.2019.0458. [DOI] [PubMed] [Google Scholar]
- 31.Jacka F., Overland S., Stewart R., Tell G., Bjelland I., Mykletun A. Association between magnesium intake and depression and anxiety in community-dwelling adults: the Hordaland health study. Aust. N. Z. J. Psychiatr. 2009;43:45–52. doi: 10.1080/00048670802534408. [DOI] [PubMed] [Google Scholar]
- 32.Li B., Lv J., Wang W., Zhang D. Dietary magnesium and calcium intake and risk of depression in the general population: a meta-analysis. Aust. N. Z. J. Psychiatr. 2017;51:219–229. doi: 10.1177/0004867416676895. [DOI] [PubMed] [Google Scholar]
- 33.Taylor S.R., McLennan S.M. Blackwell Scientific Pub.; Palo Alto, CA, United States: 1985. The continental Crust: its Composition and Evolution. [Google Scholar]
- 34.Senbayram M., Gransee A., Wahle V., Thiel H. Role of magnesium fertilisers in agriculture: plant–soil continuum. Crop Pasture Sci. 2015;66:1219. [Google Scholar]
- 35.Willy H. 2009. Verheye, LAND USE, LAND COVER and SOIL SCIENCES - Land Cover and Land Use. [Google Scholar]
- 36.Han W.X., Fang J.Y., Reich P.B., Ian Woodward F., Wang Z.H. Biogeography and variability of eleven mineral elements in plant leaves across gradients of climate, soil and plant functional type in China. Ecol. Lett. 2011;14:788–796. doi: 10.1111/j.1461-0248.2011.01641.x. [DOI] [PubMed] [Google Scholar]
- 37.Rosanoff A. Changing crop magnesium concentrations: impact on human health. Plant Soil. 2013;368:139–153. [Google Scholar]
- 38.Burritt D.J. Encycl. Food Chem. Elsevier; 2018. Crop plant adaption to climate change and extreme environments; pp. 196–201. [Google Scholar]
- 39.Soares J.C., Santos C.S., Carvalho S.M.P., Pintado M.M., Vasconcelos M.W. Preserving the nutritional quality of crop plants under a changing climate: importance and strategies. Plant Soil. 2019;443:1–26. [Google Scholar]
- 40.FAO’s Technical Departments . FAO; Rome: 2017. The Future of Food and Agriculture–Trends and Challenges. [Google Scholar]
- 41.Fakhri F.A., Bazzaz A., Sombroek W.G. Food and Agriculture Organization of the United Nations; 1996. Food and Agriculture Organization of the United Nations., Global Climate Change and Agricultural Production: Direct and Indirect Effects of Changing Hydrological, Pedological, and Plant Physiological Processes. [Google Scholar]
- 42.Myers S.S., Smith M.R., Guth S., Golden C.D., Vaitla B., Mueller N.D., Dangour A.D., Huybers P. Climate change and global food systems: potential impacts on food security and undernutrition. Annu. Rev. Publ. Health. 2017;38:259–277. doi: 10.1146/annurev-publhealth-031816-044356. [DOI] [PubMed] [Google Scholar]
- 43.Ainsworth E.A., Long S.P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2004;165:351–372. doi: 10.1111/j.1469-8137.2004.01224.x. [DOI] [PubMed] [Google Scholar]
- 44.IPCC . 2018. Summary for Policymakers of IPCC Special Report on Global Warming of 1.5oC Approved by Governments. [Google Scholar]
- 45.Loladze I. Hidden shift of the ionome of plants exposed to elevated CO2 depletes minerals at the base of human nutrition. Elife. 2014;3:e02245. doi: 10.7554/eLife.02245. Published 2014 May 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yilmaz O., Kahraman K., Ozturk L. Elevated carbon dioxide exacerbates adverse effects of Mg deficiency in durum wheat. Plant Soil. 2017;410:41–50. [Google Scholar]
- 47.Timucin E., Sezerman O.U. Thermostability of the PYL-PP2C heterodimer is dependent on magnesium: in silico insights into the link between heat stress response and magnesium deficiency in plants. J. Chem. Inf. Model. 2018;58:661–672. doi: 10.1021/acs.jcim.7b00655. [DOI] [PubMed] [Google Scholar]
- 48.Hermans C., Vuylsteke M., Coppens F., Craciun A., Inzé D., Verbruggen N. Early transcriptomic changes induced by magnesium deficiency in Arabidopsis thaliana reveal the alteration of circadian clock gene expression in roots and the triggering of abscisic acid-responsive genes. New Phytol. 2010;187:119–131. doi: 10.1111/j.1469-8137.2010.03258.x. [DOI] [PubMed] [Google Scholar]
- 49.Mengutay M., Ceylan Y., Kutman U.B., Cakmak I. Adequate magnesium nutrition mitigates adverse effects of heat stress on maize and wheat. Plant Soil. 2013;368:57–72. [Google Scholar]
- 50.Feeney K.A., Hansen L.L., Putker M., Olivares-Yañez C., Day J., Eades L.J., Larrondo L.F., Hoyle N.P., O’neill J.S., Van Ooijen G. Daily magnesium fluxes regulate cellular timekeeping and energy balance Europe PMC Funders Group. Nature. 2016;532:375–379. doi: 10.1038/nature17407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schuchardt J.P., Hahn A. Intestinal absorption and factors influencing bioavailability of magnesium-an update. Curr. Nutr. Food Sci. 2017;13:260–278. doi: 10.2174/1573401313666170427162740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Awika J.M. ACS Symp. Ser. 2011. Major cereal grains production and use around the world; pp. 1–13. [Google Scholar]
- 53.Green R., Sutherland J., Dangour A.D., Shankar B., Webb P. Global dietary quality, undernutrition and non-communicable disease: a longitudinal modelling study. BMJ Open. 2016 Jan 12;6(1):e009331. doi: 10.1136/bmjopen-2015-009331. PMID: 26758259; PMCID: PMC4716260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bohn T., Davidsson L., Walczyk T., Hurrell R.F. Fractional magnesium absorption is significantly lower in human subjects from a meal served with an oxalate-rich vegetable, spinach, as compared with a meal served with kale, a vegetable with a low oxalate content. Br. J. Nutr. 2004;91:601–606. doi: 10.1079/BJN20031081. [DOI] [PubMed] [Google Scholar]
- 55.Israr B., Frazier R.A., Gordon M.H. Effects of phytate and minerals on the bioavailability of oxalate from food. Food Chem. 2013;141:1690–1693. doi: 10.1016/j.foodchem.2013.04.130. [DOI] [PubMed] [Google Scholar]
- 56.Bohn T., Davidsson L., Walczyk T., Hurrell R.F. Phytic acid added to white-wheat bread inhibits fractional apparent magnesium absorption in humans. Am. J. Clin. Nutr. 2004;79:418–423. doi: 10.1093/ajcn/79.3.418. [DOI] [PubMed] [Google Scholar]
- 57.Agnoli C., Baroni L., Bertini I., Ciappellano S., Fabbri A., Papa M., Pellegrini N., Sbarbati R., Scarino M.L., Siani V., Sieri S. Position paper on vegetarian diets from the working group of the Italian Society of Human Nutrition. Nutr. Metab. Cardiovasc. Dis. 2017 Dec;27(12):1037–1052. doi: 10.1016/j.numecd.2017.10.020. Epub 2017 Oct 31. PMID: 29174030. [DOI] [PubMed] [Google Scholar]
- 58.Hu F.B., Liu Y., Willett W.C. Preventing chronic diseases by promoting healthy diet and lifestyle: public policy implications for China. Obes. Rev. 2011;12:552–559. doi: 10.1111/j.1467-789X.2011.00863.x. [DOI] [PubMed] [Google Scholar]
- 59.Jones J.M., Sheats D.B. Ref. Modul. Food Sci. Elsevier; 2016. Consumer trends in grain consumption. [Google Scholar]
- 60.Levrat-Verny M.-A., Coudray C., Bellanger J., Lopez H.W., Demigné C., Rayssiguier Y., Rémésy C. Wholewheat flour ensures higher mineral absorption and bioavailability than white wheat flour in rats. Br. J. Nutr. 1999;82:17–21. doi: 10.1017/s0007114599001075. [DOI] [PubMed] [Google Scholar]
- 61.Truswell A.S. Cereal grains and coronary heart disease. Eur. J. Clin. Nutr. 2002;56:1–14. doi: 10.1038/sj.ejcn.1601283. [DOI] [PubMed] [Google Scholar]
- 62.Zhang Z., Moon C., Watanabe T. Contents of pollutant and nutrient elements in rice and wheat grown on the neighboring fields. Biol. Trace Elem. Res. 1997;57:39–50. doi: 10.1007/BF02803868. [DOI] [PubMed] [Google Scholar]
- 63.Gwirtz J.A., Garcia-Casal M.N. Processing maize flour and corn meal food products. Ann. N. Y. Acad. Sci. 2014 Apr;1312(1):66–75. doi: 10.1111/nyas.12299. Epub 2013 Dec 11. PMID: 24329576; PMCID: PMC4260129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Elin R.J. Magnesium metabolism in health and disease. Disease-a-Month. 1988;34:166–218. doi: 10.1016/0011-5029(88)90013-2. [DOI] [PubMed] [Google Scholar]
- 65.Jodral-Segado A.M., Navarro-Alarcon M., Lopez-G De La Serrana H., Marıa ´ ´, Lopez-Martınez´´ C., Martınez´´ M. Magnesium and calcium contents in foods from SE Spain: influencing factors and estimation of daily dietary intakes. Sci. Total Environ. 2003;312:47–58. doi: 10.1016/s0048-9697(03)00199-2. [DOI] [PubMed] [Google Scholar]
- 66.Laskowski W., Górska-Warsewicz H., Kulykovets O. Meat, meat products and seafood as sources of energy and nutrients in the average polish diet. Nutrients. 2018;10:1412. doi: 10.3390/nu10101412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Djinovic-Stojanovic J.M., Nikolic D.M., Vranic D.V., Babic J.A., Milijasevic M.P., Pezo L.L., Jankovic S.D. Zinc and magnesium in different types of meat and meat products from the Serbian market. J. Food Compos. Anal. 2017;59:50–54. [Google Scholar]
- 68.Gaál K.K., Sáfár O., Gulyás L., Stadler P. Magnesium in animal nutrition. J. Am. Coll. Nutr. 2004;23:754S–757S. doi: 10.1080/07315724.2004.10719423. [DOI] [PubMed] [Google Scholar]
- 69.Cashman K.D. Encycl. Dairy Sci. second ed. Elsevier Inc.; 2011. Milk salts: macroelements, nutritional significance; pp. 925–932. [Google Scholar]
- 70.Oh H.E., Deeth H.C. Magnesium in milk. Int. Dairy J. 2017;71:89–97. [Google Scholar]
- 71.Ziegler E.E., Fomon S.J. Lactose enhances mineral absorption in infancy. J. Pediatr. Gastroenterol. Nutr. 1983;2:288–294. [PubMed] [Google Scholar]
- 72.Heijnen A.M.P., Brink E.J., Lemmens A.G., Beynen A.C. Ileal pH and apparent absorption of magnesium in rats fed on diets containing either lactose or lactulose. Br. J. Nutr. 1993;70:747–756. doi: 10.1079/bjn19930170. [DOI] [PubMed] [Google Scholar]
- 73.Delisle J., Amiot J., Doré F. Biological availability of calcium and magnesium from dairy products. Int. Dairy J. 1995;5:87–96. [Google Scholar]
- 74.Dinicolantonio J.J., O’keefe J.H., Wilson W. Subclinical magnesium deficiency: a principal driver of cardiovascular disease and a public health crisis Coronary artery disease. Open Heart. 2018;5:668. doi: 10.1136/openhrt-2017-000668. [DOI] [PMC free article] [PubMed] [Google Scholar]