Summary
Biofilms are the habitat of 95% of bacteria successfully protecting bacteria from many antibiotics. However, inhibiting biofilm formation is difficult in that it is a complex system involving the physical and chemical interaction of both substrate and bacteria. Focusing on the substrate surface and potential interactions with bacteria, we examined both physical and chemical properties of substrates coated with a series of phenyl acrylate monomer derivatives. Atomic force microscopy (AFM) showed smooth surfaces often approximating surgical grade steel. Induced biofilm growth of five separate bacteria on copolymer samples comprising varying concentrations of phenyl acrylate monomer derivatives evidenced differing degrees of biofilm resistance via optical microscopy. Using goniometric surface analyses, the van Oss-Chaudhury-Good equation was solved linear algebraically to determine the surface energy profile of each polymerized phenyl acrylate monomer derivative, two bacteria, and collagen. Based on the microscopy and surface energy profiles, a thermodynamic explanation for biofilm resistance is posited.
Subject Areas: Polymer Chemistry, Thermodynamics, Microbiology
Graphical Abstract
Highlights
-
•
Surface energy components affect bacterial adhesion to substrates
-
•
Bacterial adhesion is directly related to the substrate's polar component
-
•
Bacterial adhesion is inversely related to the substrate's base component
-
•
Polarizable substrates may allow bacterial association, and not adhesion
Polymer Chemistry; Thermodynamics; Microbiology
Introduction
Biofilms allow the vast majority of microorganisms—infectious or otherwise—to persist in our world of antibiotics and antivirals. Biofilms are a complex, communicative aggregation of microorganisms in which 99% of all microorganisms persist (Flemming, 2002). Because of the ubiquitous nature of biofilms, their influence is similarly widespread—from biofouling of naval vessels, drinking and wastewater treatment facilities, medical implants and inserts to persistent pathogenic pathways in health care including nosocomial infections (Abbott et al., 2000; Cavitt and Faulkner, 2015, 2017; Cleaveland, 2005; Cooney and Tang, 1999; Flemming, 2002; Iwamoto and Matsutomo, 2018; Kenawy et al., 2007; Monroe, 2007; Montanaro et al., 2007; O'Flaherty et al., 2004; Vertes et al., 2012). Likewise, biofilms are non-trivial in remediation methods and often require multiple modalities to simply reduce and impede biofilm growth. With the inherent difficulty of biofilm remediation, research is moving toward inhibiting biofilm growth and/or formation.
Developing methods to inhibit biofilm growth and/or formation requires an intimate knowledge of the growth mechanism, which is parsed into five distinct categories: (1) primary colonization with reversible attachment, (2) aggregation and irreversible attachment, (3) growth and division, (4) maturation, and (5) dispersion (Monroe, 2007; Vertes et al., 2012). The most logical moment to disrupt biofilm formation is primary colonization in which microorganisms initially contact and reversibly adhere to a substrate's surface. Primary colonization is a multi-determinant system based on the thermodynamic (i.e., static) and kinetic (i.e., dynamic) nature of both microorganism and substrate.
One commonly investigated kinetic determinant involves the concentration dependence of biofilm inhibition. At a critical concentration (i.e., minimum inhibitory concentration [MIC]), biofilm growth is inhibited without introducing a cidal mechanism. The MIC likely addresses the kinetic effect based on the reversible attachment at primary colonization. At larger critical concentrations (i.e., minimum biocidal concentration [MBC]), a thermodynamic, cidal effect introduces disruptors to basic cellular function and, over time, shifts the microbial equilibrium away from homeostasis. Most commonly, biofilm inhibitory concentrations are in excess of the MBC with the thought that both kinetic and thermodynamic effects would be simultaneously addressed. As a motile species, Pseudomonas aeruginosa is likely most affected by the MIC-related kinetic effect, whereas the non-motile Staphylococcus aureus would be more affected by the MBC-related thermodynamic effect.
Introducing antimicrobial components to the substrate is a very common method to inhibit biofilm formation; however, such focuses on the previously described concentration determinant. Substrates are often functionalized with quaternary ammonium compounds (e.g., polymeric, polymer-grafted) in excess of MBC that penetrate the cell wall causing leakage and subsequent apoptosis (Kandiyote et al., 2019; Namivandi-Zangeneh et al., 2020; Valáriková et al., 2020). Upon apoptosis and in the absence of interfacial shear flow, the apoptotic fluids often remain associated with the substrate surface dynamically altering the nature of the substrate by concealing the quaternary ammonium under cellular debris. Furthermore, upon cell death, subsequent activation of lysosomes, and release of proteases, putrefaction of cellular proteins will produce free amines that neutralize protonated quaternary ammonium salts used in some of the aforementioned examples. The use of phenolic compounds to denature proteins necessary for cellular function are also common; unfortunately, like protonated quaternary ammonium compounds, the phenolic compounds are biocidal for a time until cellular debris and/or neutralization by free amines render them inert pending surface treatment (Li et al., 2020; Namivandi-Zangeneh et al., 2020). Other more robust compounds are also used to disrupt biofilm growth such as sugar alcohols to prevent dental caries and substituted (e.g., fluoro, chloro, nitro, cyano) aryl hydrazones (Kõljalg et al., 2020; Lu et al., 2020).
Using metals associated with the substrate to disrupt cellular function is also a common method of biofilm inhibition. Nanoparticles (e.g., silver) are of peculiar interest for thermodynamically mediated biofilm inhibition and have enhanced efficacy when employed with a secondary antimicrobial component (Moola et al., 2019; Namasivayam et al., 2019). However, nanoparticles often cannot endure rigorous surface treatments to recondition surfaces after eventual biofilm formation and/or fouling. Metal salts (e.g., Ce(IV)) have been shown to disrupt saccharide-dependent biofilm formation during the kinetically controlled reversible adhesion of primary colonization (Bhatt et al., 2020). However, metal salts often do not persist long term in aqueous environments thereby rendering them inactive.
Because of (1) significant genetic differentiation between microorganisms and (2) the colloidal nature of microorganisms, even adhesion is a complex process that is phenotypically heterogeneous and not a single determinant process (Vissers et al., 2019). For example, the surface protein SdrC of S. aureus has been shown to use Ca2+-mediated chelation of the N2 domains as a primary contributor to biofilm formation; the use of a metal salt associated with the substrate may disrupt the aforementioned chelation illustrating how metal salts effectively inhibit biofilm formation (Pi et al., 2020). To further illustrate the diversity in the adhesion process, McLay et al. were able to genetically alter Escherichia coli to demonstrate that the amount of fimbriation contributes to adhesion of the bacterium (McLay et al., 2018). The concentration dependence of adhesion is probably a kinetic effect unique to each bacterium.
In a paper examining P. aeruginosa and its interfacial behavior, Deng et al. noted that most bacteria align parallel to the oil-water interface (Deng et al., 2020). The parallel alignment is likely thermodynamically driven, whereas non-parallel alignment is kinetically controlled. The kinetic (i.e., dynamic) component has been modeled using complex algorithms and applied theories to describe bacterial attachment (Conrad and Poling-Skutvik, 2018; McLay et al., 2018; Vissers et al., 2019).
In this paper, we primarily focus on the thermodynamic components that drive the interfacial interaction of substrates with microbes present in primary colonization. Our fundamental assumption throughout our biological experimentation is that biofilm growth cannot occur without primary colonization. Although we are not explicitly studying primary colonization, we are examining the results of microbial colonization that are possible only if primary colonization occurs via a significant substrate-bacteria interfacial interaction, an interaction that is limited in the latter stages of biofilm growth. Therefore, seven phenyl acrylate monomers, including six halogenated monomers, were synthesized and characterized. The substrate was coated with a formulation that included 5–20 weight percent of a phenyl acrylate derivative and subsequently polymerized. Using atomic force microscopy (AFM), the surface smoothness was determined for representative samples and compared with surgical grade steel. Induced single species biofilm growth and separate exposure to clarified sewage provided biological evidence of biofilm inhibition relative to each of the seven phenyl acrylate derivatives at varying concentrations. After solving the van Oss-Chaudhury-Good equation via a linear algebraic method for relevant samples, surface energy analyses and comparison of each polymerized phenyl acrylate derivative to collagen and two representative bacteria (e.g., P. aeruginosa and S. aureus) inform potential thermodynamic efficacy. Thereafter, a thermodynamic explanation for the observed behavior was posited based on the evidence gathered.
Results and Discussion
Development of Potential Biofilm-Resistant Polymer Materials
Given evidence that covalently bound halogenated moieties have demonstrated efficacy for biofilm resistance, we designed a series of monomers based on phenyl acrylate (an internal control) that are likely biofilm-resistant candidates (Pickens, 2009). Table 1 details the reaction scheme and phenyl acrylate derivatives synthesized (see Transparent Methods). Impurities in acryloyl chloride (1), technical grade (70% purity), contributed in part to the low to moderate average isolated yields (Cavitt and Faulkner, 2015, 2017).
Table 1.
Employing Different Phenols
| Entry | R1/R2/R3(2) | 3 | Yield (%)a |
|---|---|---|---|
| 1 | H/H/H(2a) | 3a | 59 |
| 2 | H/Cl/H(2b) | 3b | 33 |
| 3 | H/H/Cl(2c) | 3c | 36 |
| 4 | Cl/H/Cl(2d) | 3d | 30. |
| 5 | H/H/Br(2e) | 3e | 21 |
| 6 | Br/H/Br(2f) | 3f | 39 |
| 7 | H/H/I(2g) | 3g | <10 |
Monomers were shown to be stable upon exposure to broad spectrum UV radiation indicating that the aryl–halogen bonds persist upon irradiation thereto. Photo-differential scanning calorimetry (photo-DSC) was used to confirm that the aryl-halogen bond does not undergo homolytic cleavage to initiate polymerization at 10 weight percent of each monomer as compared with 1,6-hexanediol diacrylate. The aforementioned stability allowed for UV curing of the monomer to form both homo- and copolymeric coatings on several substrates (e.g., stainless steel, glass slides, and plastic slides) for subsequent analyses. The monomers were incorporated at 5, 10, 15, and 20 weight percent into a standard copolymer formulation. Each liquid coating was manually drawn down to a wet thickness of 100 μm and polymerized thereafter.
Samples of the cured 20% monomer formulation were examined for methanol extractable monomer content using gas chromatographic (GC)-mass spectrometry (MS). No detectable monomer was observed in any of the samples to a detection limit of 100 μg/mL.
Atomic Force Microscopy
The average plate roughness (Ra) and average peak-valley height (Rz) was determined via AFM, contact scanning mode. The two controls included the uncoated stainless steel and cured acrylic formulation with no additional phenyl acrylate derivative; both yielded Rz values of 0.819 μm. The Ra and Rz were determined for each formulation at varying concentrations of representative monomers (3a, 3b, 3d, 3e, and 3f). With the exception of 3a, the smoothness as determined by the Ra and Rz generally increases as the concentration of the monomer increases owing to the increased dipole-dipole interactions of the coating (Table 2).
Table 2.
Surface Roughness Measured via AFM
| Monomer | 5 wt % |
5 wt % |
10 wt % |
10 wt % |
15 wt % |
15 wt % |
20 wt % |
20 wt % |
|---|---|---|---|---|---|---|---|---|
| Rz (μm) | Ra (μm) | Rz (μm) | Ra (μm) | Rz (μm) | Ra (μm) | Rz (μm) | Ra (μm) | |
| 3a | 0.7185 | 0.1532 | 2.5917 | 0.1562 | 4.4568 | 0.1575 | 5.1233 | 0.1567 |
| 3b | 2.265 | 0.1575 | 1.6857 | 0.1383 | 1.1517 | 0.107 | 0.8888 | 0.1077 |
| 3d | 1.1553 | 0.1098 | 1.2905 | 0.1122 | 0.7758 | 0.1072 | 0.6703 | 0.105 |
| 3e | 0.785 | 0.1532 | 1.3348 | 0.1552 | 0.819 | 0.1548 | 1.5117 | 0.1548 |
| 3f | 1.4265 | 0.155 | 1.192 | 0.1545 | 0.7897 | 0.1537 | 0.7102 | 0.1528 |
Based on cantilever deflection values measured during the contact scanning mode.
Rz is the average peak-valley height of the cured coating. Values meeting minimum surgical grade steel requisites are italicized.
Ra is the average surface roughness of the cured coating.
See also Figure S1.
Comparing Rz of the cured coating formulations to the peak-valley requisite for surgical grade steel (Rz ≤ 1 μm, 320 grit, electropolished), several of the cured coating formulations were well within the requisite value for surgical grade steel with 3a as a notable exception, providing evidence of smoothness capable of inhibiting many types of microbial growth by reducing the available surface area for attachment (Gillis and Gillis, 1996; Mei et al., 2011).
Biofilm Resistance Studies
Each bacterium (e.g., E. coli, P. aeruginosa, S. aureus, Streptococcus pneumoniae, and Salmonella typhimurium) was specifically chosen for its contribution to common infectious pathways including, but not limited to, food poisoning and life-threatening and/or nosocomial infections.
Having no halogenation, 3a was utilized as an internal standard having no inherent biofilm-resistant structural component. For reference, we also compared the standard control coating (no compound 3) to the uncoated portion of the slide. The motile bacteria examined (e.g., E. coli., P. aeruginosa, and S. typhimurium) had nominal or increased biofilm development on the control coating, whereas the non-motile cocci had nominal or decreased biofilm development on the control coating relative to the uncoated portion of the slide. As exhibited via the aforementioned variable biofilm development, the control coating likely has limited biofilm inhibitory effect.
Overall (see Figures S2–S7), biofilm formation was observed on the uncoated portions and the levels of growth clearly fall over a wide spectrum. As a non-halogenated internal standard, 3a did not exhibit any biofilm resistance. The monohalogenated derivatives (e.g., 3b, 3c, 3e, and 3g) showed some limited biofilm resistance especially at higher concentrations with 3e resisting biofilm formation best. Dihalogenated monomers (3d and 3f) seemed to impede biofilm formation better than their monohalogenated counterparts. Comparing chlorinated, brominated, and iodinated derivatives, the brominated monomers were the most effective biofilm-resistant monomers. Brominated coatings tended to perform better than chlorinated coatings with 40% of the brominated coatings passing our qualitative examination and 14% failing. Chlorinated coatings had a 15% pass rate with a 44% failure rate.
Laboratory conditions using lab-grown bacteria, which may have reduced immune functionality from multi-generational reproduction, may not provide an adequate environment for evaluating biofilm resistance. Therefore, we evaluated the coatings' biofilm resistance when exposed to 3.5 million gallons of raw clarified sewage for 2 days at the Abilene (Texas) Wastewater Reclamation Plant (see Figure S8). After preparing and evaluating the slides as before, the varying compositions' (3a-g) biofilm resistance produced comparatively consistent results to the previous bacterial studies (see Figure S8). Interestingly, visible algae growth was restricted solely to the BRApp (and not the slides) thereby indicating cursory resistance to algae growth.
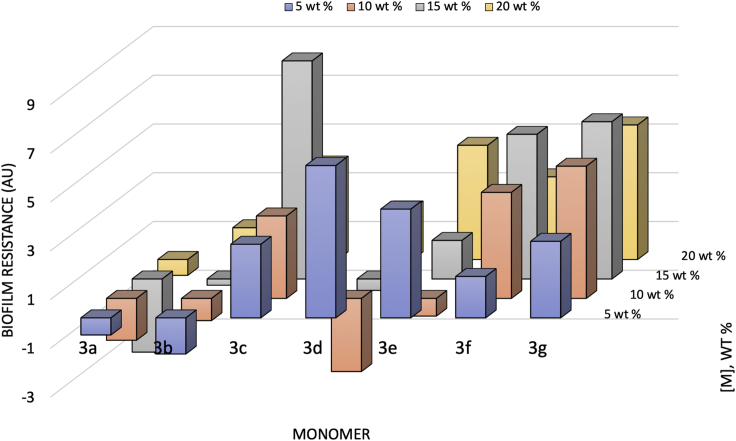
To evaluate the monomers and concentrations for optimal biofilm resistance, all biofilm resistance data (single and multiple species biofilm resistance studies) were aggregated and normalized relative to the equivalent of no biofilm resistance differential between uncoated control and coating (Figure 1).
Figure 1.
Normalized Quantitative Evaluation of Multiple Species Biofilm Resistance
Species evaluated include E. coli, P. aeruginosa, S. aureus, S. pneumoniae, and S. typhimurium.
Normalized relative to biofilm growth on control coating.
Reduced biofilm resistance relative to the control is negative.
Increased biofilm resistance relative to the control is positive.
See also Figures S2–S7.
In order to determine the most biofilm-resistant monomers, the normalized quantitative data seem to indicate that biofilm resistance is directly related to concentration of the monomers with the exception of the internal, non-halogenated control (3a). The normalized data demonstrate that 3b is not biofilm resistant at the incorporated amounts in the coating. 3d was more efficacious at lower concentrations indicating that the biostatic effect inherent to a MIC may be an important effect of biofilm resistance of this monomer. 3e also exhibited a biostatic effect overall at low concentration; however, biofilm resistance generally increased with concentration. Both 3f and 3g were the most consistently biofilm resistant with increasing efficacy up to 15 weight percent. Furthermore, halogenation at the para-position seems to produce a stereotypic effect for enhanced biofilm resistance. Finally, the presence of softer halogens (e.g., bromine and iodine) on the monomers seems to result in increased biofilm resistance.
Surface Energy Analyses
Surface energy analyses may be accomplished via many methods; however, we chose a goniometric method for its simplicity and affordability. Using three fully characterized liquids to obtain statistical contact angle averages, the van Oss-Chaudhury-Good Equation 1 was solved linear algebraically for the following surface energy components: nonpolar (), acid (), and base () components (van Oss et al., 1987).
| (Equation 1) |
The polar component () and overall surface energy () was then calculated via Equations 2 and 3, respectively (van Oss et al., 1988).
| (Equation 2) |
| (Equation 3) |
Table 3 tabulates the surface energy profiles for each polymerized phenyl acrylate derivative (3a-g, see Data S3 for homopolymer characterization).
Table 3.
Surface Energy Profile of Derivatized Phenyl Acrylate Polymers
| Substrate | |||||
|---|---|---|---|---|---|
| 3a | 35.14 ± 1.40 | 29.13 ± 1.20 | 6.01 ± 0.80 | 1.93 ± 0.50 | 6.51 ± 1.46 |
| 3ba | 41.44 ± 1.11 | 36.41 ± 0.70 | 5.04 ± 1.57 | 0.198 ± 0.077 | 51.39 ± 1.11 |
| 3c | 28.46 ± 0.67 | 25.64 ± 0.67 | 2.83 ± 0.60 | 2.73 ± 0.61 | 1.31 ± 0.43 |
| 3d | 39.08 ± 2.44 | 29.62 ± 0.63 | 9.46 ± 2.14 | 0.736 ± 0.230 | 32.47 ± 4.41 |
| 3e | 29.91 ± 0.70 | 24.41 ± 0.46 | 5.50 ± 0.27 | 0.745 ± 0.043 | 10.22 ± 0.61 |
| 3f | 36.06 ± 1.62 | 26.94 ± 0.56 | 9.12 ± 1.50 | 4.26 ± 0.91 | 9.70 ± 2.05 |
| 3g | 34.48 ± 0.43 | 33.15 ± 0.39 | 1.33 ± 0.28 | 1.15 ± 0.24 | 0.485 ± 0.130 |
All units are mJ/m2 ± SEM where number of samples (N) is 36 (3a), 24 (3b), 18 (3c), 18 (3d), 24 (3e), 36 (3f), and 18 (3g). See Transparent Methods.
Calculations based on contact angles from bromonaphthalene, formamide, and water.
Obtaining a smooth coating without smearing or orange peeling was difficult and may have contributed to an anomalous/inaccurate surface energy profile; however, for completeness, the surface energy profile for 3b was included in the dataset.
In order to compare the surface energy profiles of the polymerized phenyl acrylate derivatives (3a-g), the surface energy profile was similarly obtained for collagen (insoluble and soluble), S. aureus, and P. aeruginosa (Table 4).
Table 4.
Surface Energy Profile of Various Biologic Materials
| Substrate | |||||
|---|---|---|---|---|---|
| Collagen, insolublea | 45.75 ± 0.83 | 40.08 ± 0.46 | 5.67 ± 0.75 | 1.51 ± 0.39 | 6.90 ± 1.52 |
| Collagen, solubleb | 37.08 ± 2.15 | 30.13 ± 1.22 | 6.95 ± 1.70 | 0.806 ± 0.26 | 16.08 ± 3.99 |
| S. aureusc | 43.91 ± 0.50 | 39.63 ± 0.37 | 4.29 ± 0.49 | 0.066 ± 0.014 | 73.54 ± 0.69 |
| P. aeruginosac | 39.26 ± 0.77 | 34.82 ± 0.46 | 4.44 ± 0.78 | 0.089 ± 0.029 | 69.07 ± 1.99 |
All units are mJ/m2 ± SEM where number of samples (N) is 18 (collagen, insoluble), 36 (collagen, soluble), 21 (S. aureus), and 18 (P. aeruginosa). See Transparent Methods.
Calculations based on contact angles from bromonaphthalene, formamide, and water for insoluble collagen (100 μg/mL).
Calculations based on contact angles from bromonaphthalene, formamide, and water for soluble collagen (100 μg/mL) in phosphate buffer solution (1x, pH = 7.4).
Calculations based on contact angles from bromonaphthalene, dimethylsulfoxide, and water.
The surface energy component values for soluble collagen (e.g., , , and ) were slightly higher than established literature values with additional values for the acid () and base () components (Lewandowska et al., 2016; Skopińska-Wiśniewska et al., 2009). Insoluble collagen is noticeably differentiated from the soluble collagen per the base () components illustrating an increased substrate dipole for the soluble collagen. Often a quick comparison of the overall surface energy () of two interacting materials has been used to establish a degree of interfacial interaction between two materials.
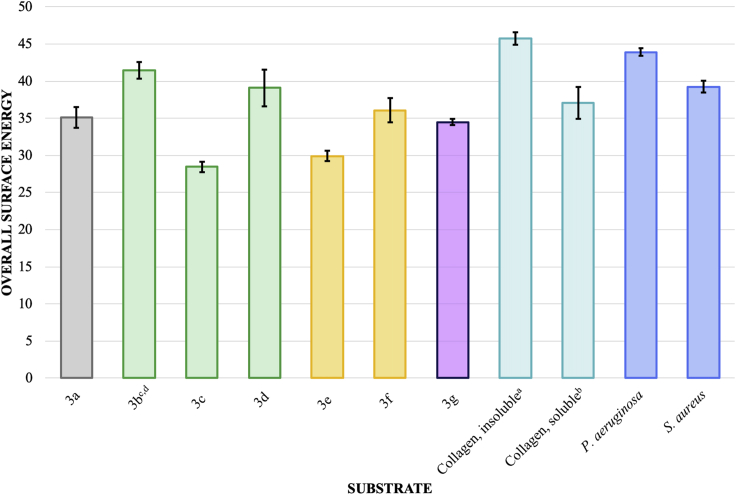
Based on the previous literature relating compositional variations to contact angles, the surface energy values for phenyl acrylate homopolymers could be inferred to impact the surface chemistry of copolymer formulations (Cassie, 1948; Drelich et al., 1996). In order to directly compare the surface energies of the phenyl acrylic coatings with collagen and select bacteria, the overall surface energy () of each was plotted; however, no clear trend is apparent (Figure 2).
Figure 2.
Overall Surface Energy Comparison () of Phenyl Acrylic Coatings, Collagens, and Bacteria
All units are mJ/m2 ± SEM where number of samples (N) is 36 (3a), 24 (3b), 18 (3c), 18 (3d), 24 (3e), 36 (3f), 18 (3g), 18 (collagen, insoluble), 36 (collagen, soluble), 21 (S. aureus), and 18 (P. aeruginosa). See Transparent Methods.
aCalculations based on contact angles from bromonaphthalene, formamide, and water for insoluble collagen (100 μg/mL).
bCalculations based on contact angles from bromonaphthalene, formamide, and water for soluble collagen (100 μg/mL) in phosphate buffer solution (1x, pH = 7.4).
cCalculations based on contact angles from bromonaphthalene, dimethylsulfoxide, and water.
dObtaining a smooth coating without smearing or orange peeling was difficult and may have contributed to an anomalous/inaccurate surface energy profile; however, for completeness, the surface energy profile for 3b was included in the dataset.
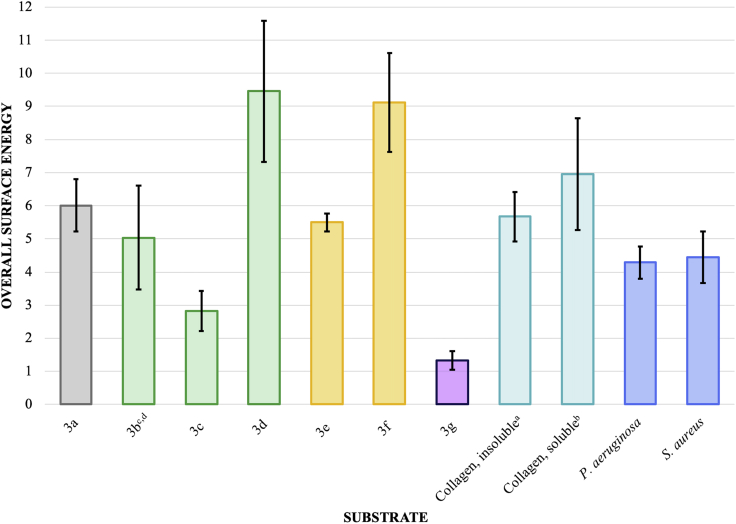
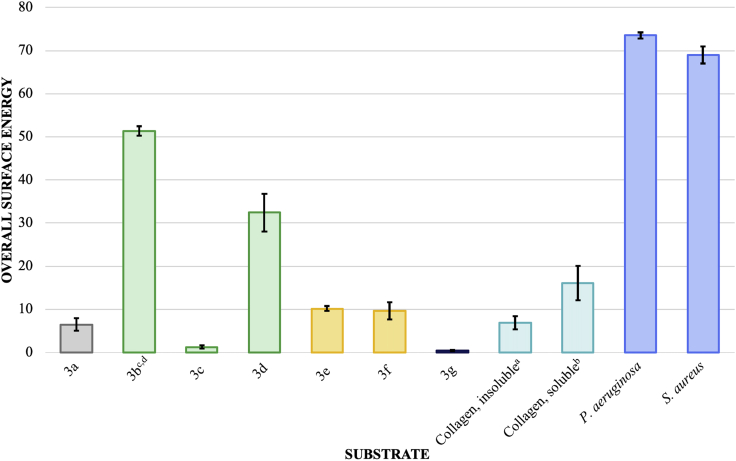
Therefore, each individual component was examined for all phenyl acrylate monomer derivatives, collagens, and bacteria. The most interesting individual component comparisons involved the polar () and base components () plotted in Figures 3 and 4, respectively.
Figure 3.
Surface Energy Polar Component () Comparison of Phenyl Acrylic Coatings, Collagens, and Bacteria
All units are mJ/m2 ± SEM where number of samples (N) is 36 (3a), 24 (3b), 18 (3c), 18 (3d), 24 (3e), 36 (3f), 18 (3g), 18 (collagen, insoluble), 36 (collagen, soluble), 21 (S. aureus), and 18 (P. aeruginosa). See Transparent Methods.
aCalculations based on contact angles from bromonaphthalene, formamide, and water for insoluble collagen (100 μg/mL).
bCalculations based on contact angles from bromonaphthalene, formamide, and water for soluble collagen (100 μg/mL) in phosphate buffer solution (1x, pH = 7.4).
cCalculations based on contact angles from bromonaphthalene, dimethylsulfoxide, and water.
dObtaining a smooth coating without smearing or orange peeling was difficult and may have contributed to an anomalous/inaccurate surface energy profile; however, for completeness, the surface energy profile for 3b was included in the dataset.
Figure 4.
Surface Energy Base Component () Comparison of Phenyl Acrylic Coatings, Collagens, and Bacteria
All units are mJ/m2 ± SEM where number of samples (N) is 36 (3a), 24 (3b), 18 (3c), 18 (3d), 24 (3e), 36 (3f), 18 (3g), 18 (collagen, insoluble), 36 (collagen, soluble), 21 (S. aureus), and 18 (P. aeruginosa). See Transparent Methods.
aCalculations based on contact angles from bromonaphthalene, formamide, and water for insoluble collagen (100 μg/mL).
bCalculations based on contact angles from bromonaphthalene, formamide, and water for soluble collagen (100 μg/mL) in phosphate buffer solution (1x, pH = 7.4).
cCalculations based on contact angles from bromonaphthalene, dimethylsulfoxide, and water.
dObtaining a smooth coating without smearing or orange peeling was difficult and may have contributed to an anomalous/inaccurate surface energy profile; however, for completeness, the surface energy profile for 3b was included in the dataset.
Excepting 3a (internal control) and 3b (inaccurate profile), the substrates with the most similar include 3d, 3e, 3f, insoluble collagen, P. aeruginosa, and S. aureus. Focusing on the 3d, 3e, 3f, and bacteria, the similarities in the polar component () likely indicates a more significant thermodynamic interaction. The single species biofilm resistance studies seem to qualitatively support a more significant interaction between 3d, 3e, 3f, and bacteria. The low values for both 3c and 3g likely result in reduced polar interactions explaining the observed biofilm resistance.
Because the magnitude of the acid components () is comparatively small for most monomers, the base components () shown in Figure 4 should be the most significant interaction.
3d is qualitatively less efficacious as a biofilm-resistant substrate perhaps owing to the larger nonpolar component, which may obfuscate the relatively hard (i.e., charge dense) chlorine atoms, especially when bound in an amorphous, cross-linked polymer matrix with little polar directionality. Furthermore, biofilm formation also seems to be more significantly inhibited by the softer halogens (e.g., bromine and iodine).
Based on Figures 1, 3c, 3e, 3f, and 3g were clearly the most biofilm-resistant substrates examined in this study with limited efficacy of 3d. Based on Figure 3, biofilm resistance of 3c and 3g have reduced polar components () and thus limited polar interactions with bacteria and adhesins. Figure 4 shows 3d, 3e, and 3f having appreciable base components (). A significant intermolecular (base-base) repulsion may be a causative agent of biofilm resistance for monomers with significantly large base components (). S. aureus, a non-motile bacterium, was most affected by 3c and 3g, whereas both 3e and 3f equally inhibited biofilm formation of the motile P. aeruginosa. With surface interactions being diffusion controlled, S. aureus adhesion is thermodynamically controlled. The parallel movement of P. aeruginosa along a surface interface would contribute a competing kinetic effect to the thermodynamic driving force for surface adhesion. Owing to kinetic competition, the biofilm resistance of 3e and 3f is slightly diminished for P. aeruginosa relative to S. aureus as observed.
The increased thermodynamic biofilm resistance may be 2-fold. First, as previously stated, polar interactions of the bacterium with the monomers contribute significantly to adhesion thereto. Diminished polar surface energy components of the substrate reduce the adhesive propensity for bacteria to bind to a substrate. Conversely, interacting base components () of a stationary substrate with a diffusing bacterium would have an increasing intermolecular charge repulsion as the distance between substrate and bacterium decreases. Such would especially be present in the non-chelated N2 domain of the surface protein SdrC of S. aureus (Pi et al., 2020). Second and likely to a lesser degree, a polarizable soft atom (e.g., bromine or iodine) or other polarizable moiety could allow the bacterium to remain associated with the substrate in the absence of reversible adhesion during primary colonization. The latter reasoning is used to explain, in part, limited bacterial attachment onto superhydrophilic substrates (Noorisafa et al., 2016; Yuan et al., 2017).
Conclusions
Seven monomers (i.e., phenyl acrylate and halogenated derivatives thereof) were successfully synthesized through a standard laboratory synthesis. Each monomer was added at variable concentrations (e.g., 5, 10, 15, and 20 weight percent) to a compatible industrial formulation that was subsequently UV cured onto various substrates. Examination via AFM illustrated that several of the cured coating formulations, including those of 3e and 3f, yielded exceptionally smooth coatings with limited surface areas evidenced by average peak to valley heights (Rz) less than 1.0 μm and very low roughness (Ra) measurements. 3f exhibited an inverse relationship of both Rz and Ra as concentration increased. The coatings were then analyzed for single (e.g., E. coli, P. aeruginosa, S. aureus, S. pneumoniae, and S. typhimurium) and multiple (e.g., clarified raw sewage) species biofilm resistance. After normalizing the biofilm resistance studies, coatings incorporating the brominated phenyl acrylate monomers (e.g., 3e and 3f), the monochlorinated 3c, and the monoiodinated 3g exhibited significant biofilm resistance. Because biofilm resistance is a symbiotic, multi-determinant system involving the physical and chemical interaction of both substrate and bacteria, we also examined the surface energies of the polymerized phenyl acrylate derivatives, collagen, and two representative bacteria (e.g., P. aeruginosa and S. aureus). Comparative analysis of each surface energy component demonstrated that the polar component () is likely the primary thermodynamic contributor to the observed biofilm resistance. Small polar components of the substrate reduce the adhesiveness of bacteria to the substrate, whereas a large base component () repels the bacterium. Repulsive intermolecular interactions between base components of both the substrate and bacteria prevent intimate bacterial association with the substrate. Secondarily, we posit that the presence of soft atoms (e.g., bromine and iodine) and/or polarizable moieties in the coating may allow bacterial association while inhibiting adhesion and biofilm formation during primary colonization.
Limitations of the Study
Potential caveats of this published work could include the following. First, as mentioned in the text, our fundamental assumption throughout our biological experimentation is that biofilm growth cannot occur without primary colonization. Although we are not explicitly studying primary colonization, we are examining the results of microbial colonization that are possible only if primary colonization occurs via a significant substrate-bacteria interfacial interaction, an interaction that is limited in the latter stages of biofilm growth. Also because we used non-virulent bacterial strains for researcher safety, biofilm formation and resistance thereto may differ from virulent strains of the same species. Finally, goniometric surface energy analyses, like those reported herein, have been shown to differ from other surface energy analyses that do not use contact angle measurements (e.g., density functional theory [DFT] and cleaving method); however, the non-contact angle methods are most effectively implemented with well-defined structures unlike those examined herein (Tran et al., 2016; Gilman, 1960; Jaccodine, 1963).
Resource Availability
Lead Contact
Further information and requests related to the research published herein should be directed to and fulfilled by the Lead Contact, T. Brian Cavitt (tbcavitt@lipscomb.edu).
Materials Availability
All unique/stable reagents generated in this study are available on request from the Lead Contact but may require a payment and/or Materials Transfer Agreement if there is potential for commercial application.
Data and Code Availability
The published article includes all datasets generated or analyzed during this study.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
The authors would like to thank the Albemarle Corporation for providing DMPA and Allied Photochemical for providing the standard industrial formulation used. We would also like to gratefully acknowledge the financial support of The Welch Foundation (Grant R-0021), the Abilene Christian University Office of Research and Sponsored Programs, and Lipscomb University's Office of the Provost and Department of Chemistry and Biochemistry.
Author Contributions
Conceptualization, T.B.C.; Methodology, T.B.C., J.G.C., R.A.F., C.J.H., J.R.L., D.S.O., and D.S.; Validation, T.B.C.; Formal Analysis, T.B.C., J.G.C., V.N.G., D.S.O., and P.R.P.; Investigation, J.G.C., A.R.D., R.A.F., T.C.G., V.N.G., P.R.H., E.B.H., C.J.H., J.R.L., J.A.L., D.S.O., P.R.P., D.S., and W.W.; Resources, T.B.C.; Data Curation, T.B.C., J.G.C., V.N.G., D.S.O., and P.R.P.; Writing – Original Draft, T.B.C.; Writing – Review & Editing, T.B.C.; Visualization, T.B.C.; Supervision, T.B.C.; Project Administration, T.B.C.; Funding Acquisition, T.B.C.
Declaration of Interests
The Lead Contact and a coauthor have two patents related to the research herein (Cavitt and Faulkner, 2015, 2017). The authors declare no other competing interest.
Published: November 20, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101702.
Supplemental Information
References
- Abbott A., Abel P.D., Arnold D.W., Milne A. Cost-benefit analysis of the use of TBT: the case for a treatment approach. Sci. Total. Environ. 2000;258:5–19. doi: 10.1016/s0048-9697(00)00505-2. [DOI] [PubMed] [Google Scholar]
- Bhatt L., Chen L., Guo J., Klie R.F., Shi J., Pesavento R.P. Hydrolyzed Ce(IV) salts limit sucrose-dependent biofilm formation by Streptococcus mutans. J. Inorg. Biochem. 2020;206:110997. doi: 10.1016/j.jinorgbio.2020.110997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassie A.B.D. Contact angles. Discuss. Faraday Soc. 1948;3:11–16. [Google Scholar]
- Cleaveland P. An evolution in coatings. Med. Des. Technol. 2005;6:18–22. [Google Scholar]
- Cavitt, T.B. and Faulkner, R.A.H. (2015). Biofilm resistant polymer materials. U.S. Patent No.: 9,072,292 B2.
- Cavitt, T.B. and Faulkner, R.A.H. (2017). Biofilm resistant polymer materials. U.S. Patent No.: 9,591,848 B2.
- Conrad J.C., Poling-Skutvik R. Confined flow: consequences and implications for bacteria and biofilms. Annu. Rev. Chem. Biomol. Eng. 2018;9:175–200. doi: 10.1146/annurev-chembioeng-060817-084006. [DOI] [PubMed] [Google Scholar]
- Cooney J.J., Tang R.J. Quantifying effects of antifouling paints on microbial biofilm formation. Methods Enzymol. 1999;310:637–644. doi: 10.1016/s0076-6879(99)10049-1. [DOI] [PubMed] [Google Scholar]
- Deng J., Molaei M., Chisholm N.G., Stebe K.J. Motile bacteria at oil-water interfaces: Pseudomonas aeruginosa. Langmuir. 2020;36:6888–6902. doi: 10.1021/acs.langmuir.9b03578. [DOI] [PubMed] [Google Scholar]
- Drelich J., Wilbur J.L., Miller J.D., Whitesides G.M. Contact angles for liquid drops at a model heterogeneous surface consisting of alternating and parallel hydrophobic/hydrophilic strips. Langmuir. 1996;12:1913–1922. [Google Scholar]
- Flemming H.–C. Biofouling in water systems–cases, causes and countermeasures. Appl. Microbiol. Biotechnol. 2002;59:629–640. doi: 10.1007/s00253-002-1066-9. [DOI] [PubMed] [Google Scholar]
- Gillis R.J., Gillis J.R. A comparative study of bacterial attachment to high-purity water system surfaces. Ultrapure Water. 1996;13:27–36. [Google Scholar]
- Gilman J.J. Direct measurements of the surface energies of crystals. J. Appl. Phys. 1960;31:2208–2218. [Google Scholar]
- Iwamoto, K. and Matsutomo, S. (2018). Biofilm amount determination method and a water treatment system comprising filtration membrane module. Japan Patent No.: 2018-202338 A.
- Jaccodine R.J. Surface energy of germanium and silicon. J. Electrochem. Soc. 1963;110:524–527. [Google Scholar]
- Kandiyote N.S., Avisdris T., Arnusch C.J., Kasher R. Grafted polymer coatings enhance fouling inhibition by an antimicrobial peptide on reverse osmosis membranes. Langmuir. 2019;35:1935–1943. doi: 10.1021/acs.langmuir.8b03851. [DOI] [PubMed] [Google Scholar]
- Kenawy el-R., Worley S.D., Broughton R. The chemistry and applications of antimicrobial polymers: a state-of-the-art review. Biomacromolecules. 2007;8:1359–1384. doi: 10.1021/bm061150q. [DOI] [PubMed] [Google Scholar]
- Kõljalg S., Smidt I., Chakrabarti A., Bosscher D., Mändar R. Exploration of singular and synergistic effect on xylitol and erythritol on causative agents of dental caries. Sci. Rep. 2020;10:6297–6304. doi: 10.1038/s41598-020-63153-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowska K., Sionkowska A., Grabska S., Kaczmarek B. Surface and thermal properties of collagen/hyaluronic acid blends containing chitosan. Int. J. Bio. Macromol. 2016;92:371–376. doi: 10.1016/j.ijbiomac.2016.07.055. [DOI] [PubMed] [Google Scholar]
- Li C., Jiang C., Jing H., Jiang C., Wang H., Du X., Lou Z. Separation of phenolics from peony flowers and their inhibitory activities and action mechanism on bacterial biofilm. Appl. Microbiol. Biotechnol. 2020;104:4321–4332. doi: 10.1007/s00253-020-10540-z. [DOI] [PubMed] [Google Scholar]
- Lu S., Zhang Z., Xu Y., Lu J., Tang W., Zhang J. Effect of new carbonyl cyanide aromatic hydrazones on biofilm inhibition against methicillin resistant Staphylococcus aureus. RSC Adv. 2020;10:17854–17861. doi: 10.1039/d0ra03124k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLay R.B., Nguyen H.N., Jaimes-Lizcano Y.A., Dewangan N.K., Alexandrova S., Rodrigues D.F., Cirino P.C., Conrad J.C. Level of fimbriation alters the adhesion of Escherichia coli bacteria to interfaces. Langmuir. 2018;34:1133–1142. doi: 10.1021/acs.langmuir.7b02447. [DOI] [PubMed] [Google Scholar]
- Mei L., Busscher H.J., Mei H.C.V.D., Ren Y. Influence of surface roughness on streptococcal adhesion forces to composite resins. Dent. Mater. 2011;27:770–778. doi: 10.1016/j.dental.2011.03.017. [DOI] [PubMed] [Google Scholar]
- Monroe D. Looking for chinks in the armor of bacterial biofilms. PLoS Biol. 2007;5:2458–2461. doi: 10.1371/journal.pbio.0050307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moola A.K., Balasubramanni S., Nithya C., Diana R.K.B. Preparation, characterization of silver-nanoparticles from seed coat exudates of celastrus paniculatus willd and their bactericidal and biofilm inhibition effects. Int. J. Pharm. Sci. Drug Res. 2019;11(5):164–173. [Google Scholar]
- Montanaro L., Campoccia D., Pirini V., Ravaioli S., Otto M. Antibiotic multiresistance strictly associated with IS256 and ica genes in Staphylococcus epidermidis strains from implant orthopedic infections. J. Biomed. Mater. Res. A. 2007;83:813–818. doi: 10.1002/jbm.a.31399. [DOI] [PubMed] [Google Scholar]
- Namasivayam S.K.R., Francis A.L., Bharani R.S.A., Nachiyar C.V. Bacterial biofilm or biofouling networks with numerous resilience factors from real water supplies of Chennai and their enhanced susceptibility to biocomposite nanoparticles. J. Clean. Prod. 2019;231:872–898. [Google Scholar]
- Namivandi-Zangeneh R., Yang Y., Xu S., Wong E.H.H., Boyer C. Antibiofilm platform based on the combination of antimicrobial polymers and essential oils. Biomacromolecules. 2020;21:262–272. doi: 10.1021/acs.biomac.9b01278. [DOI] [PubMed] [Google Scholar]
- Noorisafa F., Razmjou A., Emami N., Low Z.-X., Korayem A.H., Kajani A.A. Surface modification of polyurethane via creating a biocompatible superhydrophilic nanostructured layer: role of surface chemistry and structure. J. Exp. Nanosci. 2016;11:1087–1109. [Google Scholar]
- O'Flaherty S., Ross R.P., Meanry W., Fitzgerald G.F. Potential of the polyvalent anti-staphylococcus bacteriophage K for control of antibiotic-resistant staphylococci from hospitals. Appl. Environ. Microbiol. 2004;1:1836–1842. doi: 10.1128/AEM.71.4.1836-1842.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi Y., Chen W., Ji Q. Structural basis of Staphylococcus aureus surface protein SdrC. Biochemistry. 2020;59:1465–1469. doi: 10.1021/acs.biochem.0c00124. [DOI] [PubMed] [Google Scholar]
- Pickens, S.R. (2009). Method for inhibiting biofilm growth. US patent application Publication: US 2009/018062 A1.
- Skopińska-Wiśniewska J., Sionkowska A., Kaminska A., Kazinica A., Jachimiak R., Drewa T. Surface properties of collagen/elastin based biomaterials for tissue regeneration. Appl. Surf. Sci. 2009;225:8286–8292. [Google Scholar]
- Tran R., Xu Z., Radhakrishnan B., Winston D., Sun W., Persson K.A., Ong S.P. Surface energies of elemental crystals. Sci. Data. 2016;3:160080. doi: 10.1038/sdata.2016.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valáriková J., Čížová A., Račková L., Bystrický S. Anti-staphylococcal activity of quaternized mannan from the yeast Candida albicans. Carbohydr. Polym. 2020;240:116288. doi: 10.1016/j.carbpol.2020.116288. [DOI] [PubMed] [Google Scholar]
- van Oss C.J., Chaudhury M.K., Good R.J. Monopolar surfaces. Adv. Colloid Interf. Sci. 1987;28:35–64. doi: 10.1016/0001-8686(87)80008-8. [DOI] [PubMed] [Google Scholar]
- van Oss C.J., Good R.J., Chaudhury M.K. Additive and nonadditive surface tension components and the interpretation of contact angles. Langmuir. 1988;4:884–891. [Google Scholar]
- Vertes A., Hitchins V., Phillips K.S. Analytical challenges of microbial biofilms on medical devices. Anal. Chem. 2012;84:3858–3866. doi: 10.1021/ac2029997. [DOI] [PubMed] [Google Scholar]
- Vissers T., Koumakis N., Hermes M., Brown A.T., Schwarz-Linek J., Dawson A., Poon W.C.K. Dynamical analysis of bacteria in microscopy movies. PLoS One. 2019;14:1–15. doi: 10.1371/journal.pone.0217823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y., Hays M.P., Hardwidge P.R., Kim J. Surface characteristics influencing bacterial adhesion to polymeric substrates. RSC Adv. 2017;7:14254–14261. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The published article includes all datasets generated or analyzed during this study.