Abstract
Background: Cerebral cavernous malformations (CCMs) presenting with seizures can be treated with neurosurgery or radiosurgery, but the ideal treatment remains unclear. Currently, there is no adequate randomized controlled trial comparing surgical treatment and radiotherapy for epileptogenic CCMs. Therefore, we conducted a systematic review and meta-analysis of available data from published literature to compare the efficacy and safety of neurosurgery and radiosurgery for epileptogenic CCMs.
Methods: We performed a comprehensive search of the Ovid MEDLINE, Web of Science, PubMed, China Biological Medicine and China National Knowledge Infrastructure databases for studies published between January 1994 and October 2019. The search terms were as follows: “epilepsy,” “seizures,” “brain cavernous hemangioma,” “cerebral cavernous malformation,” “cerebral cavernous hemangioma,” “hemangioma, cavernous, central nervous system.” Two researchers independently extracted the data and reviewed all the articles. We compared the advantages and disadvantages of the two treatments.
Results: A total of 45 studies were included in our analysis. Overall, the seizure control rate was 79% (95% CI: 75–83%) for neurosurgery and 49% (95% CI: 38–59%) for radiosurgery. In the neurosurgery studies, 4.4% of patients experienced permanent morbidity, while no patients in the radiotherapy studies had permanent morbidity. In addition, the results of subgroup analysis showed that ethnicity, CCMs location and average lesion number are likely significant factors influencing the seizure outcome following treatment.
Conclusions: The epilepsy control rate after neurosurgery was higher than that after radiosurgery, but neurosurgery also had a relatively higher rate of permanent morbidity.
Keywords: brain cavernous hemangioma, seizure, neurosurgery, radiosurgery, meta-analysis
Introduction
Cerebral cavernous malformations (CCMs), also known as cavernous angiomas, have an incidence of 0.1–0.5% and account for 5–10% of cerebral and spinal vascular malformations (1–3). CCMs are benign vascular lesions that can occur anywhere in the brain parenchyma or leptomeninges but mainly occur in the supratentorial region. They are abnormal low-flow blood vessels in the brain consisting of expanded, thin-walled capillary clusters filled with hemosiderin deposits. CCMs can manifest as central nervous system bleeding and other neurological defects based on their location, and 40–70% of supratentorial cavernous malformations tend to have seizures as the first symptom (2–4). A total of 35–40% of CCM patients develop medically refractory epilepsy. The vascular morphology of CCMs is fragile and prone to repeated microbleeds, leading to reactive gliosis and hemosiderin deposition in adjacent brain tissues (2, 5, 6). Thus, the resulting ischemia, venous hypertension, glial hyperplasia, and inflammatory responses can all induce seizures and involve the brain parenchyma near these lesions. Of all cerebral vascular malformations, CCMs are the most common epileptic substrate. Seizures are the most common symptoms of supratentorial CCMs (7, 8). Epilepsy is known to significantly reduce quality of life and cause severe morbidity, and antiepileptic drugs (AEDs) often have undesirable side effects (9–11). Therefore, eliminating epilepsy is an important and often underestimated therapeutic goal in managing these lesions.
However, the ideal treatment remains unclear. Microsurgery is considered the standard treatment for intractable epilepsy caused by CCMs. Surgical removal can prevent seizures in 50–90% of patients (12). In the past few decades, with the application of advanced technology such as diffusion tensor imaging (DTI) and electrophysiological monitoring, surgical intervention had produced better results (13). Additionally, recent studies had confirmed that microsurgery could exhibit great seizure control rate (14, 15). However, the risk of surgical morbidity and mortality is high when the lesion is located in deep or eloquent areas (16–19). Stereotactic radiosurgery is another option for the treatment of CCMs, especially in high-risk patients (20, 21). In the treatment of epileptogenic CCMs, several authors have indicated that gamma knife radiosurgery (GKRS) can provide good seizure control (22–24). Currently, there is no adequate randomized controlled trial comparing surgical treatment and radiotherapy for epileptogenic CCMs. Therefore, we conducted a systematic review and meta-analysis of available data from published literature to compare the efficacy and safety of neurosurgery and radiosurgery for epileptogenic CCMs.
Methods
The present study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA) (25).
Search Strategy
We performed a comprehensive search of the Ovid MEDLINE, Web of Science, PubMed, China Biological Medicine and China National Knowledge Infrastructure databases for studies published between January 1994 and October 2019. The search terms were as follows: “epilepsy,” “seizures,” “brain cavernous hemangioma,” “cerebral cavernous malformation,” “cerebral cavernous haemangioma,” “hemangioma, cavernous, central nervous system.” We retrieved the original articles of cohort studies published in peer-reviewed journals. We included eligible studies published in Chinese and English, while studies in other languages were excluded because we did not have translators (Figure 1).
Figure 1.
Flow chart of the data search.
Assessment of Eligibility
Two independent reviewers selected eligible studies based on the Patient, Intervention, Comparison, Outcome, and Study design (PICOS) guidelines (23): (1) Participants: patients' CCMs had to be confirmed by MRI or pathological examination; (2) Interventions: neurosurgery or radiosurgery; (3) Comparison: not applicable; (4) Outcome: seizure outcome estimated by Engel's classification; (5) Study designs: retrospective cohort study; the sample sizes of the studies had to be >20; studies must have described the follow-up time, and the follow-up rate had to be >80%. If the institution or author published multiple studies using the same cohort, only the report with the largest sample size was included for analysis. Case reports, reviews, meta-analyses, letters and conference articles were excluded.
Risk of Bias Assessment
The Newcastle-Ottawa Scale (NOS) was used to assess the quality of the included studies. The NOS score is used to assess three major components: selection, comparability, and exposure. Studies are defined as high quality when scoring ≥5. Two reviewers independently evaluated the quality of the studies and resolved disagreements by discussion.
Data Extraction
A total of 1,639 articles were retrieved in our initial search. Two researchers (Xiangyu Gao and Peng Luo) independently extracted the data and reviewed all the articles. First, two researchers screened the titles and abstracts of the retrieved literature. They then evaluated the full-texts of relevant articles to determine their eligibility. Opinion was sought from a senior investigator (Xiaofan Jiang) if the two researchers could not reach an agreement. Finally, 45 of 1,639 articles met the inclusion criteria. Two investigators extracted the following data from each eligible study: first author's last name, publication date, year of patients, total number of patients, number of female patients, mean follow-up time, mean age, mean duration of epilepsy, lesion location, post-operative seizure outcome, mortality, temporary morbidity and permanent morbidity (6, 24, 26–68) (Table 1). The term “mortality” is defined as patients' death attributed to CCMs or treatment. Temporary morbidity includes transient brain edema after surgery, new or worse neurological deficits, and a range of other complications, all of which can eventually be fully cured. Permanent morbidity includes memory deficits and persistent focal neurological deficits.
Table 1.
Basic patient characteristics of each included cohort.
|
First author, year of publication |
Year | Number of treated patients | Number of female patients (%) | Mean age (years) | Mean duration of seizure (years) | Mean duration of follow-up (years) | Engel class I (%) | Engel class II-IV (%) | Mortality(%) | Temporary morbidity(%) | Permanent morbidity(%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Neurosurgery (n = 37) | |||||||||||
| Cohen, 1995 | 1981–1992 | 51 | 29 (56.9) | 34.9 | 4.7 | 5.0 | NA | NA | 1.0 | NA | NA |
| Casazza, 1996 | 1988–1992 | 47 | 18 (38.3) | 32.4 | 5.3 | 4.0 | NA | NA | NA | 17.0 | NA |
| Zevgaridi, 1996 | 1984–1993 | 77 | 41 (53.2) | 32.3 | 4.8 | 2.5 | NA | NA | 0.0 | 36.4 | 2.6 |
| Cappabiar, 1997 | 1985–1994 | 35 | 21 (60.0) | 28.8 | NA | NA | NA | NA | NA | NA | NA |
| Baumann, 2006 | NA | 27 | NA | 36.3 | 12.0 | 3.0 | 8 (29.6) | 19 (70.4) | 0.0 | NA | 7.4 |
| D'Angelo, 2006 | 1992–2005 | 69 | NA | NA | NA | NA | 57 (82.6) | 12 (17.4) | 0.0 | 27.5 | NA |
| Ferroli, 2006 | 1988–2003 | 163 | NA | 33.4 | 4.5 | NA | NA | NA | 0.0 | 13.5 | NA |
| Hamen, 2007 | NA | 30 | 13 (43.3) | 39.4 | 10.8 | NA | 17 (56.7) | 12 (40.0) | NA | NA | NA |
| Huo, 2008 | 2003–2006 | 58 | NA | NA | NA | 1.8 | 42 (72.4) | 16 (17.6) | 0.0 | 7.0 | 0.0 |
| Stavrou, 2008 | 1981–2004 | 53 | 22 (41.5) | NA | 3.6 | 8.1 | 45 (84.9) | 8 (15.1) | 0.0 | 9.4 | 17.0 |
| Wang, 2008 | 1998–2005 | 25 | 10 (40.0) | 39.0 | NA | NA | 22 (88.0) | 3 (12.0) | 0.0 | 8.0 | NA |
| Chang, 2009 | 1996–2006 | 44 | NA | NA | NA | NA | 32 (72.7) | 12 (27.3) | NA | NA | NA |
| Yeon, 2009 | 1995–2005 | 60 | 23 (38.3) | NA | NA | NA | 50 (83.3) | 10 (16.7) | 0.0 | 1.7 | 15.0 |
| Guo, 2010 | 2003–2008 | 57 | 18 (31.6) | 27.4 | 2.6 | 1.8 | 45 (78.9) | 10 (17.5) | 0.0 | NA | 0.0 |
| Chen, 2011 | 2003–2008 | 27 | 11 (40.7) | 29.0 | NA | 3.2 | 16 (59.3) | 11 (40.7) | 0.0 | 11.1 | 0.0 |
| Hugelshofer, 2011 | 1974–2004 | 36 | NA | NA | NA | NA | 26 (72.2) | 10 (27.8) | NA | 8.3 | NA |
| Kivelev, 2011 | 1980–2009 | 39 | 29 (74.4) | NA | 3.0 | 6.0 | 30 (76.9) | 9 (23.1) | NA | 30.0 | NA |
| Gross, 2013 | 1997–2011 | 48 | NA | NA | NA | NA | 46 (95.8) | 2 (4.2) | NA | 6.3 | 8.3 |
| Kwon, 2013 | 1995–2008 | 56 | 29 (51.8) | 37.5 | NA | 7.3 | 46 (82.1) | 10 (17.9) | NA | NA | NA |
| Sommer, 2013 | 2002–2012 | 26 | 14 (53.8) | 39.1 | NA | 4.0 | 21 (80.8) | 5 (19.2) | 0.0 | 7.7 | 11.5 |
| Von der Brelie, 2013 | 1988–2010 | 118 | 47 (40.2) | 38.9 | 10.9 | NA | NA | NA | 6.8 | 17.8 | 0.0 |
| Wang, 2013 | 2000–2008 | 132 | 64 (48.5) | 39.3 | 2.3 | NA | NA | NA | 0.0 | 7.6 | 3.8 |
| Jin, 2014 | 2011–2012 | 36 | 15 (41.7) | 37.8 | 0.5 | 1.5 | 28 (77.8) | 8 (22.2) | 0.0 | NA | 0.0 |
| Kim, 2014 | 1989–2008 | 46 | 23 (50.0) | 31.2 | 3.6 | 8.0 | NA | NA | 0.0 | 2.2 | 0.0 |
| Wang, 2014 | 2009–2013 | 30 | 12 (40.0) | 34.6 | 2.3 | 1.3 | 25 (83.3) | 5 (16.7) | 0.0 | NA | 0.0 |
| Ge, 2015 | 2005–2013 | 25 | NA | NA | NA | NA | 23 (92.0) | 1 (4.0) | 4.0 | NA | NA |
| Meguins, 2015 | 2000–2012 | 21 | 8 (38.1) | 34.4 | 12.0 | 3.1 | 13 (61.9) | 8 (38.1) | 0.0 | 9.5 | 14.3 |
| Shan, 2015 | 2008–2012 | 52 | 21 (40.4) | 26.8 | NA | 3.2 | 42 (80.8) | 10 (19.2) | 0.0 | 0.0 | 0.0 |
| Sun, 2015 | 2008–2014 | 51 | 29 (56.9) | NA | NA | NA | 40 (78.4) | 11 (21.6) | 0.0 | 11.8 | 0.0 |
| Vale, 2015 | 1999–2011 | 34 | 18 (52.9) | 37.0 | 3.8 | 5.5 | 29 (85.3) | 5 (14.7) | 0.0 | 3.0 | 0.0 |
| Wu, 2015 | 2010–2014 | 27 | 9 (33.3) | 9.4 | 0.7 | 3.1 | 25 (92.6) | 2 (7.4) | 0.0 | 7.4 | 0.0 |
| Hou, 2016 | 2012–2015 | 56 | 26 (46.4) | 26.8 | NA | NA | 43 (76.8) | 13 (23.2) | 0.0 | 28.6 | 0.0 |
| Dammann, 2017 | NA | 41 | 18 (43.9) | 28.0 | 0.2 | 5.8 | NA | NA | NA | 19.5 | NA |
| He, 2017 | 2005–2009 | 181 | 81 (44.8) | 33.4 | 3.0 | 6.9 | 145 (80.1) | 36 (19.9) | 0.0 | 5.0 | 0.0 |
| Barzaghi, 2018 | 2010–2017 | 43 | NA | NA | NA | NA | 34 (79.1) | 9 (20.9) | 0.0 | 48.8 | 9.3 |
| Yang, 2018 | 2004–2014 | 47 | 20 (42.6) | 34.3 | 9.9 | 5.3 | 39 (83.0) | 8 (17.0) | 0.0 | 0.0 | 14.9 |
| Lin, 2018 | 2004–2016 | 27 | 15 (55.6) | 15.0 | 2.3 | 6.3 | 21 (77.8) | 6 (22.2) | 0.0 | 7.4 | 14.8 |
| GKRS (n = 9) | |||||||||||
| Regis, 2000 | 1991–1997 | 49 | 23 (46.9) | 36.0 | 7.5 | 2.0 | 26 (53.1) | 23 (46.9) | 0.0 | 4.1 | 0.0 |
| Wang, 2009 | 2002–2008 | 25 | NA | NA | NA | NA | 21 (84.0) | NA | NA | NA | 0.0 |
| Wang, 2010 | 1995–2005 | 44 | NA | NA | NA | NA | 24 (54.5) | 20 (45.5) | NA | NA | 0.0 |
| Chen, 2011 | 1997–2005 | 30 | 11 (36.7) | 35.0 | NA | 2.8 | 8 (26.7) | 22 (73.3) | 0.0 | 40 | 0.0 |
| Jia, 2014 | 1996–2010 | 48 | NA | NA | NA | 3.1 | 23 (47.9) | 25 (52.1) | 0.0 | NA | 0.0 |
| Kida, 2015 | 1991–2012 | 27 | NA | NA | NA | NA | 13 (48.1) | 14 (51.9) | NA | NA | NA |
| He, 2016 | 2008–2013 | 36 | 16 (44.4) | 30.0 | NA | 4.0 | 13 (36.1) | 23 (63.9) | 0.0 | 22.2 | 0.0 |
| Xu,2017 | 2012–2016 | 24 | NA | NA | NA | 3.6 | 11 (45.8) | 13 (54.2) | 0.0 | NA | 0.0 |
| Yang, 2019 | 2015–2017 | 60 | 28 (46.7) | 41.9 | 4.8 | 3.0 | 24 (40.0) | 36 (60.0) | 0.0 | 13.3 | 0.0 |
GKRS, gamma knife radiosurgery; NA, unknown.
Statistical Analysis
We pre-specified the following characteristics of the included cohorts as the baseline covariates of interest: mean duration of epilepsy, cohort midyear (defined as the middle of the year in which the treatment occurred), mean age of the patients, percentage of female patients, CCM location, percentage of patients who died, percentage of patients with temporary morbidity and percentage of patients with permanent morbidity. We used the Mann-Whitney U-test to evaluate the difference in the proportion of these characteristics between the neurosurgery and radiosurgery groups, with a p-value < 0.05 indicating a significant difference. The seizure outcome data were estimated by Engel's classification. Engel class I represented complete freedom from seizures since the operation, and Engel classes II-IV represented not seizure-free. To standardize the evaluation of the study results, we calculated the proportion of patients in Engel class I in each group. Meta-analysis software (version 14.2, Stata) was used to calculate the overall proportions. Statistical heterogeneity was evaluated by the I2 statistic. If I2 > 50%, we used a random effects model to analyze the assumption. Otherwise, we used a fixed effects model. Sensitivity analysis was performed to investigate the impact of an individual study on the overall risk assessment by omitting one study at a time. Publication bias was evaluated qualitatively examining the funnel plot and quantitatively by Egger's test, which was considered statistically asymmetrical when the p-value < 0.1.
Results
Systematic Literature Review
After screening, 45 studies (46 cohorts) involving 2,356 patients were identified. Thirty-seven studies described a total of 2013 patients who underwent neurosurgery, and nine studies described a total of 343 patients who underwent radiosurgery. Four (9%) cohorts examined patients from multiple centers, and the remaining 42 (91%) cohorts examined patients from a single center. Twenty-five (55%) cohorts were from Asia, 14 (30%) cohorts were from Europe, 5 (11%) cohorts were from North America, 1 (2%) cohort was from South America, and 1 (2%) cohort was from Oceania. All 45 studies were published between 1995 and 2019. Twenty-eight studies (62%) described the mean or median duration of follow-up. Thirty-seven studies (80%) described post-operative seizure outcomes. We found statistically significant differences in the CCM location and proportion of patients with permanent morbidity between the neurosurgery and radiosurgery groups. GKRS is more suitable for CCM lesions located in the parietal lobe and occipital lobe, while neurosurgery is more suitable for temporal lobe lesions. Compared with patients in the radiosurgery group, patients in the neurosurgery group had a higher incidence of permanent morbidity after surgery (Table 2).
Table 2.
Characteristics of the included cohorts.
| Overall (n = 46) | Neurosurgery (n = 37) | Radiosurgery (n = 9) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Study characteristics | Cohorts (%)a | Patients | Median (range) | Cohorts (%) | Patients | Median (range) | Cohorts (%) | Patients | Median (range) |
| Patients treated | 46 (100) | 2356 | 44 (21–181) | 37 (100) | 2013 | 46 (21–181) | 9 (100) | 343 | 36 (24–60) |
| Duration of epilepsy, y | 23 (50) | 1393 | 3.8 (0.2–12) | 21 (57) | 1284 | 3.6 (0.2–12) | 2 (22) | 109 | 6.2 (4.8–7.5) |
| Duration of follow–up, y | 28 (61) | 1307 | 3.4 (1.3–8.1) | 22 (59) | 1060 | 4 (1.3–8.1) | 6 (67) | 247 | 3.1 (2–4) |
| Midyear, y | 43 (93) | 2258 | 2004 (1987–2016) | 34 (92) | 1915 | 2005 (1987–2014) | 9 (100) | 343 | 2003 (1994–2016) |
| Age, y | 30 (65) | 1662 | 34.4 (9.4–41.9) | 26 (70) | 1487 | 33.9 (9.4–39.4) | 4 (44) | 175 | 35.5 (30.0–41.9) |
| Female, % | 32 (70) | 1675 | 44 (32–74) | 28 (76) | 1500 | 44 (32–74) | 4 (44) | 175 | 46 (37–47) |
| CMs location | |||||||||
| Frontal, % | 31 (74) | 1417 | 28 (0–100) | 26 (70) | 1180 | 28.5 (0–100) | 5 (56) | 237 | 25 (18–33) |
| Temporal,% | 35 (76) | 1817 | 47 (0–100) | 30 (81) | 1580 | 49 (0–100)* | 5 (56) | 237 | 28 (23–47)* |
| Parietal,% | 30 (65) | 1387 | 14 (0–39) | 25 (68) | 1150 | 10 (0–29)** | 5 (56) | 237 | 28 (10–39)** |
| Occipital,% | 29 (63) | 1341 | 5 (0–27) | 24 (65) | 1104 | 4 (0–25)* | 5 (56) | 237 | 7 (4–27)* |
| Others,% | 29 (63) | 1341 | 1.3 (0–26) | 24 (65) | 1104 | 2 (0–26) | 5 (56) | 237 | 0 (0–8) |
| Mortality,% | 34 (74) | 1884 | 0 (0–6.8) | 28 (76) | 1637 | 0 (0–7) | 6 (67) | 247 | 0 (0) |
| Temporary morbidity,% | 31 (67) | 1797 | 9 (0–49) | 27 (73) | 1622 | 8 (0–49) | 4 (44) | 175 | 18 (4–40) |
| Permanent morbidity,% | 32 (70) | 1668 | 0 (0–17) | 24 (65) | 1352 | 0 (0–17)* | 8 (89) | 316 | 0 (0)* |
The percentage is the number of cohorts reporting a particular study characteristic divided by the total number of cohorts.
P < 0.05 and
P < 0.01, showing a significant difference in the median ratio between the group describing neurosurgery and the group describing radiosurgery.
CMs, cavernous malformations.
Seizure Outcomes
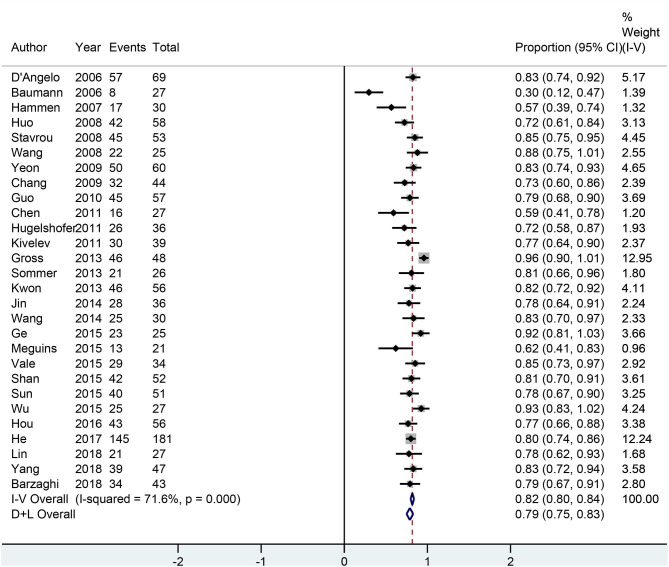
All 28 neurosurgery studies (except Baumann's study) showed that neurosurgery was an effective surgical treatment for seizures, with more than 50% of patients being classified as Engel class I. As shown in Figure 2, the overall proportion of patients in Engel class I was 0.79 (95% CI 0.75–0.83) across all 28 neurosurgery studies, which suggested that neurosurgery can significantly control seizures. Because I2 > 50%, we used a random effects model to analyze the data. The nine radiosurgery studies also demonstrated the efficacy of GKRS in the treatment of epileptogenic CCMs. As shown in Figure 3, the overall proportion of patients in Engel class I was 0.49 (95% CI 0.38–0.59). All radiosurgery studies were analyzed using a random effects model because I2 > 50%.
Figure 2.
Forest plot of neurosurgery studies.
Figure 3.
Forest plot of radiosurgery studies.
In addition, we performed subgroup analyses and the confounding factors in our studies were ethnicity, CCMs location and average lesion number (Table 3). Patients from North America (0.85, 95% CI 0.75–0.95), Asia (0.80, 95% CI 0.76–0.85) and Oceania (0.85, 95% CI 0.75–0.95) had higher proportions of favorable seizure outcomes in neurosurgery studies. When CCMs lesions were located in the frontal and temporal lobes, seizure outcomes of neurosurgery (0.78, 95% CI 0.40–0.99; 0.74, 95% CI 0.66–0.83; respectively) were significantly better than those of radiosurgery (0.56, 95% CI 0.39–0.73; 0.39, 95% CI 0.26–0.52; respectively). The effect of neurosurgery on single lesion (0.79, 95% CI 0.75–0.84) is better than that on multiple lesions (0.73, 95% CI 0.64–0.83). In contrast, the effect of neurosurgery on multiple lesions (0.47, 95% CI 0.36–0.59) is better than that on single lesion (0.35, 95% CI 0.27–0.44).
Table 3.
Subgroup analysis.
| Neurosurgery | Radiosurgery | |||
|---|---|---|---|---|
| Subgroup | Number of cohorts | Proportion of patients in Engel class I (95%CI) | Number of cohorts | Proportion of patients in Engel class I (95%CI) |
| Ethnicity | ||||
| European | 7 | 0.68 (0.54–0.82) | 1 | 0.53 (0.39–0.67) |
| North American | 4 | 0.85 (0.75–0.95) | - | - |
| South American | 1 | 0.62 (0.41–0.83) | - | - |
| Asian | 15 | 0.80 (0.76–0.85) | 8 | 0.48 (0.36–0.60) |
| Oceanian | 1 | 0.85 (0.75–0.95) | - | - |
| CCMs location | ||||
| Frontal | 4 | 0.78 (0.40–0.99) | 3 | 0.56 (0.39–0.73) |
| Temporal | 10 | 0.74 (0.66–0.83) | 3 | 0.39 (0.26–0.52) |
| Parietal | 4 | 0.62 (0.20–0.95) | 3 | 0.52 (0.37–0.67) |
| Occipital | 2 | 0.73 (0.00–1.00) | 3 | 0.72 (0.23–0.99) |
| Others | 1 | 1.00 (0.31–1.00) | 2 | 0.85 (0.16–1.00) |
| Average lesion number | ||||
| 1 | 4 | 0.79 (0.75–0.84) | 3 | 0.35 (0.27–0.44) |
| >1 | 11 | 0.73 (0.64–0.83) | 2 | 0.47 (0.36–0.59) |
Mortality and Morbidity
Of the 37 neurosurgery studies, thirty-three (89%) studies reported on the mortality or morbidity. Two (0.1%) patients died post-operatively, 212 (13.1%) patients experienced temporary morbidity, and 60 (4.4%) patients experienced permanent neurological symptoms. Eight (88.9%) of the nine radiosurgery studies reported on the mortality or morbidity. No deaths or permanent complications occurred. Thirty (17.1%) patients experienced temporary morbidity.
Sensitivity Analysis
We omitted one study at a time to investigate the influence of a single study on the pooled estimates. The comparison results in the radiosurgery group were not significantly altered, indicating that this group's results were statistically robust. In the neurosurgery group, Gross's study was shown to have a substantial influence on the pooled estimates due to its higher proportion of patients in Engel class I. However, Gross's study did not affect our conclusions (Figures 4, 5).
Figure 4.
Sensitivity analysis of neurosurgery studies.
Figure 5.
Sensitivity analysis of radiosurgery studies.
Publication Bias
Funnel plots and Egger's test were used to evaluate the publication bias. The p-values produced by Egger's test on the post-radiosurgery seizure outcomes and post-neurosurgery seizure outcomes were 0.778 and 0.000, respectively. Therefore, there was no publication bias in the radiosurgery studies, but publication bias might have influenced the results of the neurosurgery studies (Figures 6,7).
Figure 6.
Funnel plot of neurosurgery studies.
Figure 7.
Funnel plot of radiosurgery studies.
Discussion
Overall, our results indicate that the epilepsy control rate after neurosurgery was higher than that after radiosurgery, but neurosurgery also had a relatively higher rate of permanent morbidity. The effect of neurosurgery on multiple lesions is better than that on single lesion whereas radiotherapy was the opposite. The effect of neurosurgery on frontal lobe and temporal lobe lesions is significantly better than those of radiotherapy. Ethnicity affects the seizure outcome following the treatment. Radiosurgery is more suitable for CCM lesions located in the parietal lobe and occipital lobe, while neurosurgery is more suitable for temporal lobe lesions.
CCMs are low-flow vascular malformations that are usually static and can also bleed repeatedly and grow. CCMs are occult vascular malformations that are difficult to find on DSA. MRI has a high specificity and sensitivity for CCMs, which can be clearly diagnosed and characterized due to their nodular or circular appearance. There is generally no edema or placeholder effect around the lesion except when it is accompanied by bleeding (69). The mechanism of CCM-induced epilepsy is still not fully understood. CCMs do not contain nerve tissues and will not become the epilepsy initiation area by itself. Peripheral hemosiderin deposition and gliosis caused by recurrent microhemorrhage of malformed vessels are considered to be the main causes of epilepsy (70).
AEDs are the primary treatment for CCMs with epilepsy. For refractory epilepsy, neurosurgery or radiosurgery should be considered. Yang's research shows that surgery for intractable epilepsy can effectively control seizures. In addition, the appropriate operation scheme can be selected according to the location of CCMs and the responsiveness of patients to antiepileptic drugs to maximize the control of epilepsy and minimize post-operative neurological sequelae (68). He et al. also reported the effectiveness of neurosurgery for intractable epilepsy and pointed out that the shorter the duration of seizures before surgery, the better the control of seizures after surgery (67). Ruan et al. (14) conducted a meta-analysis and the result showed that patients who underwent surrounding hemosiderin excision could exhibit significantly improved seizure outcomes compared to patients without hemosiderin excision. Additionally, Shang-Guan's meta-analysis reported that extended lesionectomy does not contribute to better seizure control for patients with cerebral cavernous malformations with epilepsy (15). In addition, radiotherapy can also be used for the treatment of refractory epilepsy. There has been a considerable amount of research on its effectiveness. Regis et al. showed that GKRS can control seizures safely and effectively. When CCMs are located in a highly functional area, the risk of surgical treatment is higher, and GKRS treatment is more appropriate (24). However, the ideal treatment remains unclear.
To compare the efficacy and safety of neurosurgery and radiosurgery for epileptogenic CCMs, we conducted a systematic review and meta-analysis of available data from published literature. The results of our systematic review showed that neurosurgery is more likely to be used in refractory epilepsy patients with CCM lesions located in the temporal lobe, while radiosurgery is more likely to be used in patients with CCM lesions located in the parietal lobe and occipital lobe. In addition, there was no significant difference in mortality and post-operative transient morbidity between the two treatments, but the proportion of patients with permanent complications was significantly higher in the neurosurgery group than in the radiosurgery group. Additionally, the results showed that 4.4% of patients in the neurosurgery studies experienced permanent morbidity, while no patients in the radiosurgery studies had permanent morbidity. We also found that the proportion of patients with temporary morbidity in the radiosurgery group (17.1%) was greater than that in the neurosurgery group (13.1%). After consulting the literature, we found that radiosurgery could cause to post-operative brain edema in patients, leading to a significantly higher proportion of patients suffering from temporary morbidity; however, brain edema will eventually subside over time.
The results of our meta-analysis showed that the seizure control rate was 0.79 (95% CI 0.75–0.83) for neurosurgery and 0.49 (95% CI 0.38–0.59) for radiosurgery. In terms of controlling epilepsy, the effect of neurosurgery is significantly better than that of radiosurgery. In addition, CCMs multiplicity and CCMs location are important factors affecting the prognosis of CCMs. Englot et al. (71) had reported that individuals with a single lesion received neurosurgery were more likely to attain post-operative seizure freedom. Some of the neurosurgery studies found CCMs locations were not related to seizure outcomes (38, 71). Wang et al. believed that radiosurgery is more effective for seizure caused by CCMs in frontal and parietal lobe than that caused in temporal lobe (40). Therefore, we performed subgroup analyses to summarize the influence of these confounding factors on the results. We observed that the effect of neurosurgery on single lesion (0.79, 95% CI 0.75–0.84) is better than that on multiple lesions (0.73, 95% CI 0.64–0.83), which further supported the conclusions of Englot et al. (71). On the contrary, we found that the effect of radiosurgery on multiple lesions (0.47, 95% CI 0.36–0.59) is better than that on single lesion (0.35, 95% CI 0.27–0.44). These data revealed that average lesion number is likely a factor influencing seizure outcome which needs further case-control trials. Consistent with previous studies, our results showed that there is little difference in the effect of neurosurgery on each site and radiotherapy was more effective for frontal (0.56, 95% CI 0.39–0.73) and parietal (0.52, 95% CI 0.37–0.67) CCMs than for temporal (0.39, 95% CI 0.26–0.52) CCMs. we also found that for lesions located in the frontal lobe and temporal lobe, neurosurgery (0.78, 95% CI 0.40–0.99; 0.74, 95% CI 0.66–0.83; respectively) is significantly superior to radiosurgery (0.56, 95% CI 0.39–0.73; 0.39, 95% CI 0.26–0.52; respectively). For CCMs lesions at other locations, the differences in seizure outcome between the two treatments were not significant.
The difference of gene background in CCMs patients is closely related to clinical manifestation and prognosis. Different ethnic groups have different genetic backgrounds and different mutation sites (72). Previous cohort studies have not focused on this. Therefore, we did a subgroup analysis and our data indicated that North Americans (0.85, 95% CI 0.75–0.95), Asians (0.80, 95% CI 0.76–0.85) and Oceanians (0.85, 95% CI 0.75–0.95) benefited more from neurosurgery than Europeans (0.68, 95% CI 0.54–0.82) and South Americans (0.62, 95% CI 0.41–0.83). We speculated that ethnicity might be associated with prognosis and further random controlled trails were needed. Unfortunately, data on mortality and morbidity of the two treatment could not be subgroup analyzed as they were not provided in the majority of the included studies.
Lately, there is an emerging minimally invasive technique called stereotactic laser ablation (SLA) which is getting into focus. SLA could precisely ablate lesions with less collateral injury around lesions. A cohort study by Willie et al. (73) reported 17 patients receiving SLA, 14 (82%) of whom achieved Engel I after a year-long follow-up period. SLA has the same good seizure control rate as neurosurgery and is more tolerable for the patients. Therefore, SLA is expected to be a first-line minimally invasive therapy for CCMs-related epilepsy, but more case-control trials are still needed.
The NOS was used to assess the quality of the included studies, and each study had a moderate level of quality with an average score of 6. Our systematic review and meta-analysis has three limitations. First, all the included studies were retrospective studies. Therefore, randomized controlled trials are urgently needed. Second, neurosurgery was not consistent in all the included studies. Last, the experience of surgeons greatly affects the outcome of the operation.
Conclusion
In summary, our paper demonstrates that the epilepsy control rate after neurosurgery was higher than that after radiosurgery, but neurosurgery also had a relatively higher rate of permanent morbidity. Number of lesions, location and ethnicity are likely significant factors influencing the seizure outcome following treatment. Therefore, our data provide new ideas for clinical individualized precision medicine but further random controlled trials are still needed.
Data Availability Statement
All datasets presented in this study are included in the article/Supplementary Material.
Author Contributions
XG, KY, and JS contributed conception and design of the study. PL and XJ organized the database. YC performed the statistical analysis. XG wrote the first draft of the manuscript. BZ, HZ, SD, LZ, and PL wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by the National Natural Science Foundation of China (nos. 81871023, 81671303, and 81771322) and the Military Youth talent lifting project (no. 17-JCJQ-QT-037).
References
- 1.Amin-Hanjani S, Robertson R, Arginteanu MS, Scott RM. Familial intracranial arteriovenous malformations. Case report and review of the literature. Pediatr Neurosurg. (1998) 29:208–13. 10.1159/000028723 [DOI] [PubMed] [Google Scholar]
- 2.Awad I, Jabbour P. Cerebral cavernous malformations and epilepsy. Neurosurg Focus. (2006) 21:e7. 10.3171/foc.2006.21.1.8 [DOI] [PubMed] [Google Scholar]
- 3.Moriarity JL, Wetzel M, Clatterbuck RE, Javedan S, Sheppard JM, Hoenig-Rigamonti K, et al. The natural history of cavernous malformations: a prospective study of 68 patients. Neurosurgery. (1999) 44:1166–71. 10.1227/00006123-199906000-00003 [DOI] [PubMed] [Google Scholar]
- 4.Del Curling O, Kelly DL, Elster AD, Craven TE. An analysis of the natural history of cavernous angiomas. J Neurosurg. (1991) 75:702–8. 10.3171/jns.1991.75.5.0702 [DOI] [PubMed] [Google Scholar]
- 5.Bacigaluppi S, Retta SF, Pileggi S, Fontanella M, Goitre L, Tassi L, et al. Genetic and cellular basis of cerebral cavernous malformations: implications for clinical management. Clin Genetics. (2013) 83:7–14. 10.1111/j.1399-0004.2012.01892.x [DOI] [PubMed] [Google Scholar]
- 6.Cappabianca P, Alfieri A, Maiuri F, Mariniello G, Cirillo S, de Divitiis E. Supratentorial cavernous malformations and epilepsy: seizure outcome after lesionectomy on a series of 35 patients. Clin Neurol Neurosurg. (1997) 99:179–83. 10.1016/S0303-8467(97)00023-1 [DOI] [PubMed] [Google Scholar]
- 7.Attar A, Ugur HC, Savas A, Yüceer N, Egemen N. Surgical treatment of intracranial cavernous angiomas. J Clin Neurosci. (2001) 8:235–9. 10.1054/jocn.2000.0787 [DOI] [PubMed] [Google Scholar]
- 8.Porter PJ, Willinsky RA, Harper W, Wallace MC. Cerebral cavernous malformations: natural history and prognosis after clinical deterioration with or without hemorrhage. J Neurosurg. (1997) 87:190–7. 10.3171/jns.1997.87.2.0190 [DOI] [PubMed] [Google Scholar]
- 9.Cascino GD. When drugs and surgery don't work. Epilepsia. (2008) 49(Suppl. 9):79–84. 10.1111/j.1528-1167.2008.01930.x [DOI] [PubMed] [Google Scholar]
- 10.Cramer JA, Mintzer S, Wheless J, Mattson RH. Adverse effects of antiepileptic drugs: a brief overview of important issues. Expert Rev Neurother. (2010) 10:885–91. 10.1586/ern.10.71 [DOI] [PubMed] [Google Scholar]
- 11.Sheth RD. Adolescent issues in epilepsy. J Child Neurol. (2002) 17(Suppl. 2):2S23–7. 10.1177/08830738020170020801 [DOI] [PubMed] [Google Scholar]
- 12.Bertalanffy H, Gilsbach JM, Eggert HR, Seeger W. Microsurgery of deep-seated cavernous angiomas: report of 26 cases. Acta Neurochir. (1991) 108:91–9. 10.1007/BF01418515 [DOI] [PubMed] [Google Scholar]
- 13.Sandalcioglu IE, Wiedemayer H, Secer S, Asgari S, Stolke D. Surgical removal of brain stem cavernous malformations: surgical indications, technical considerations, and results. J Neurol Neurosurg Psychiatry. (2002) 72:351–5. 10.1136/jnnp.72.3.351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruan D, Yu X, Shrestha S, Wang L, Chen G. The role of hemosiderin excision in seizure outcome in cerebral cavernous malformation surgery: a systematic review and meta-analysis. PLoS ONE. (2015) 10:e0136619. 10.1371/journal.pone.0136619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shang-Guan H, Wu Z, Yao P, Chen G, Zheng S, Kang D. Is extended lesionectomy needed for patients with cerebral cavernous malformations presenting with epilepsy? A meta-analysis. World Neurosurg. (2018) 120:e984–90. 10.1016/j.wneu.2018.08.208 [DOI] [PubMed] [Google Scholar]
- 16.Gross BA, Batjer HH, Awad IA, Bendok BR. Brainstem cavernous malformations. Neurosurgery. (2009) 64:E805–18. 10.1227/01.NEU.0000343668.44288.18 [DOI] [PubMed] [Google Scholar]
- 17.Mathiesen T, Edner G, Kihlström L. Deep and brainstem cavernomas: a consecutive 8-year series. J Neurosurg. (2003) 99:31–7. 10.3171/jns.2003.99.1.0031 [DOI] [PubMed] [Google Scholar]
- 18.Monaco EA, Khan AA, Niranjan A, Kano H, Grandhi R, Kondziolka D, et al. Stereotactic radiosurgery for the treatment of symptomatic brainstem cavernous malformations. Neurosurg Focus. (2010) 29:E11. 10.3171/2010.7.FOCUS10151 [DOI] [PubMed] [Google Scholar]
- 19.Wang CC, Liu A, Zhang JT, Sun B, Zhao YL. Surgical management of brain-stem cavernous malformations: report of 137 cases. Surg Neurol. (2003) 59:444–54. 10.1016/S0090-3019(03)00187-3 [DOI] [PubMed] [Google Scholar]
- 20.Hasegawa T, McInerney J, Kondziolka D, Lee JY, Flickinger JC, Lunsford LD. Long-term results after stereotactic radiosurgery for patients with cavernous malformations. Neurosurgery. (2002) 50:1190–7. 10.1227/00006123-200206000-00003 [DOI] [PubMed] [Google Scholar]
- 21.Karlsson B, Kihlström L, Lindquist C, Ericson K, Steiner L. Radiosurgery for cavernous malformations. J Neurosurg. (1998) 88:293–7. 10.3171/jns.1998.88.2.0293 [DOI] [PubMed] [Google Scholar]
- 22.Bartolomei F, Régis J, Kida Y, Kobayashi T, Vladyka V, Liscàk R, et al. Gamma Knife radiosurgery for epilepsy associated with cavernous hemangiomas: a retrospective study of 49 cases. Stereotact Funct Neurosurg. (1999) 72(Suppl. 1):22–8. 10.1159/000056435 [DOI] [PubMed] [Google Scholar]
- 23.Liu KD, Chung WY, Wu HM, Shiau CY, Wang LW, Guo WY, et al. Gamma knife surgery for cavernous hemangiomas: an analysis of 125 patients. J Neurosurg. (2005) 102:81–6. 10.3171/sup.2005.102.s_supplement.0081 [DOI] [PubMed] [Google Scholar]
- 24.Regis J, Bartolomei F, Kida Y, Kobayashi T, Vladyka V, Liscak R, et al. Radiosurgery for epilepsy associated with cavernous malformation: retrospective study in 49 patients. Neurosurgery. (2000) 47:1091–7. 10.1097/00006123-200011000-00013 [DOI] [PubMed] [Google Scholar]
- 25.Liberati A, Altman D, Tetzlaff J, Mulrow C, Gøtzsche P, Ioannidis J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) 339:b2700. 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen DS, Zubay GP, Goodman RR. Seizure outcome after lesionectomy for cavernous malformations. J Neurosurg. (1995) 83:237–42. 10.3171/jns.1995.83.2.0237 [DOI] [PubMed] [Google Scholar]
- 27.Casazza M, Broggi G, Franzini A, Avanzini G, Spreafico R, Bracchi M, et al. Supratentorial cavernous angiomas and epileptic seizures: preoperative course and postoperative outcome. Neurosurgery. (1996) 39:26–32. 10.1097/00006123-199607000-00007 [DOI] [PubMed] [Google Scholar]
- 28.Zevgaridis D, van Velthoven V, Ebeling U, Reulen HJ. Seizure control following surgery in supratentorial cavernous malformations: a retrospective study in 77 patients. Acta Neurochir. (1996) 138:672–7. 10.1007/BF01411470 [DOI] [PubMed] [Google Scholar]
- 29.Baumann CR, Schuknecht B, Lo Russo G, Cossu M, Citterio A, Andermann F, et al. Seizure outcome after resection of cavernous malformations is better when surrounding hemosiderin-stained brain also is removed. Epilepsia. (2006) 47:563–6. 10.1111/j.1528-1167.2006.00468.x [DOI] [PubMed] [Google Scholar]
- 30.D'Angelo VA, De Bonis C, Amoroso R, Cali A, D'Agruma L, Guarnieri V, et al. Supratentorial cerebral cavernous malformations: clinical, surgical, and genetic involvement. Neurosurg Focus. (2006) 21:e9. 10.3171/foc.2006.21.1.10 [DOI] [PubMed] [Google Scholar]
- 31.Ferroli P, Casazza M, Marras C, Mendola C, Franzini A, Broggi G. Cerebral cavernomas and seizures: a retrospective study on 163 patients who underwent pure lesionectomy. Neurol Sci. (2006) 26:390–4. 10.1007/s10072-006-0521-2 [DOI] [PubMed] [Google Scholar]
- 32.Hammen T, Romstock J, Dorfler A, Kerling F, Buchfelder M, Stefan H. Prediction of postoperative outcome with special respect to removal of hemosiderin fringe: a study in patients with cavernous haemangiomas associated with symptomatic epilepsy. Seizure. (2007) 16:248–53. 10.1016/j.seizure.2007.01.001 [DOI] [PubMed] [Google Scholar]
- 33.Huo L, Wu L, Zhang MY, Hou YH, Ding XP, Fang JS. Electrocorticography monitoring in microsurgical treatment of solitary cavernous angiomas. Zhong Nan Da Xue Xue Bao Yi Xue Ban. (2008) 33:448–51. 10.3321/j.issn:1672-7347.2008.05.014 [DOI] [PubMed] [Google Scholar]
- 34.Stavrou I, Baumgartner C, Frischer JM, Trattnig S, Knosp E. Long-term seizure control after resection of supratentorial cavernomas: a retrospective single-center study in 53 patients. Neurosurgery. (2008) 63:888–96. 10.1227/01.NEU.0000327881.72964.6E [DOI] [PubMed] [Google Scholar]
- 35.Wang ZZ, zhuge QC, Ye S, Lin C, Zhang Y, Wu ZB, et al. Microsurgical treatment of supratentorial intracranial cavernous malformation associated with epilepsy. Chin J Neurosurg. (2008) 8:593–6. 10.3321/j.issn:1001-2346.2008.08.011 [DOI] [Google Scholar]
- 36.Chang EF, Gabriel RA, Potts MB, Garcia PA, Barbaro NM, Lawton MT. Seizure characteristics and control after microsurgical resection of supratentorial cerebral cavernous malformations. Neurosurgery. (2009) 65:31–8. 10.1227/01.NEU.0000346648.03272.07 [DOI] [PubMed] [Google Scholar]
- 37.Wang HW, Zhang GR, Zhu LF, Qi Y, Li MS. Diagnosis of cerebral cavernous angioma and its treatment by gamma knife. Inn Mong Med J. (2009) 41:553–5. 10.3969/j.issn.1004-0951.2009.05.017 [DOI] [Google Scholar]
- 38.Yeon JY, Kim JS, Choi SJ, Seo DW, Hong SB, Hong SC. Supratentorial cavernous angiomas presenting with seizures: surgical outcomes in 60 consecutive patients. Seizure. (2009) 18:14–20. 10.1016/j.seizure.2008.05.010 [DOI] [PubMed] [Google Scholar]
- 39.Guo Q, Zhu D, Wu J, Hua G, Tan JL, Jin X. Surgical strategy of supratentorial cavernous angiomas associated with epilepsy (a report of 57 cases). Chin J Stereotact Funct Neurosurg. (2010) 1:20–2. 10.3210/j.issn:1008-2425.2010.01.020 [DOI] [Google Scholar]
- 40.Wang P, Zhang FC, Zhang HY, Zhao HY. Gamma knife radiosurgery for intracranial cavernous malformations. Clin Neurol Neurosurg. (2010) 112:474–7. 10.1016/j.clineuro.2010.03.012 [DOI] [PubMed] [Google Scholar]
- 41.Chen GX, Xu LS, Xu MH, Shen GJ. Therapeutic effect of two methods on seizures induced by supratentorial cavernous hemangioma. Chin J Clin Neurosurg. (2011) 16:420–1. 10.3969/j.issn.1009-153X.2011.07.012 [DOI] [Google Scholar]
- 42.Hugelshofer M, Acciarri N, Sure U, Georgiadis D, Baumgartner RW, Bertalanffy H, et al. Effective surgical treatment of cerebral cavernous malformations: a multicenter study of 79 pediatric patients. J Neurosurg Pediatr. (2011) 8:522–5. 10.3171/2011.8.PEDS09164 [DOI] [PubMed] [Google Scholar]
- 43.Kivelev J, Niemela M, Blomstedt G, Roivainen R, Lehecka M, Hernesniemi J. Microsurgical treatment of temporal lobe cavernomas. Acta Neurochir. (2011) 153:261–70. 10.1007/s00701-010-0812-5 [DOI] [PubMed] [Google Scholar]
- 44.Gross BA, Smith ER, Goumnerova L, Proctor R, Madsen JR, Scott RM. Resection of supratentorial lobar cavernous malformations in children. J Neurosurg Pediatr. (2013) 12:367–73. 10.3171/2013.7.PEDS13126 [DOI] [PubMed] [Google Scholar]
- 45.Kwon CS, Sheth SA, Walcott BP, Neal J, Eskandar EN, Ogilvy CS. Long-term seizure outcomes following resection of supratentorial cavernous malformations. Clin Neurol Neurosurg. (2013) 115:2377–81. 10.1016/j.clineuro.2013.08.024 [DOI] [PubMed] [Google Scholar]
- 46.Sommer B, Kasper BS, Coras R, Blumcke I, Hamer HM, Buchfelder M, et al. Surgical management of epilepsy due to cerebral cavernomas using neuronavigation and intraoperative MR imaging. Neurol Res. (2013) 35:1076–83. 10.1179/016164113X13801151880551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.von der Brelie C, Malter MP, Niehusmann P, Elger CE, von Lehe M, Schramm J. Surgical management and long-term seizure outcome after epilepsy surgery for different types of epilepsy associated with cerebral cavernous malformations. Epilepsia. (2013) 54:1699–706. 10.1111/epi.12327 [DOI] [PubMed] [Google Scholar]
- 48.Wang X, Tao Z, You C, Li Q, Liu Y. Extended resection of hemosiderin fringe is better for seizure outcome: a study in patients with cavernous malformation associated with refractory epilepsy. Neurol India. (2013) 61:288–92. 10.4103/0028-3886.115070 [DOI] [PubMed] [Google Scholar]
- 49.Jia G, Zhang JM, Ma ZM, Qiu B, Hou YH. Therapeutic effect of gamma knife on intracranial cavernous angioma. Zhong Nan Da Xue Xue Bao Yi Xue Ban. (2014) 39:1320–5. 10.11817/j.issn.1672-7347.2014.12.016 [DOI] [PubMed] [Google Scholar]
- 50.Jin Y, Zhao C, Zhang S, Zhang X, Qiu Y, Jiang J. Seizure outcome after surgical resection of supratentorial cavernous malformations plus hemosiderin rim in patients with short duration of epilepsy. Clin Neurol Neurosurg. (2014) 119:59–63. 10.1016/j.clineuro.2014.01.013 [DOI] [PubMed] [Google Scholar]
- 51.Kim J, Kim CH, Chung CK. Longitudinal changes in seizure outcomes after resection of cerebral cavernous malformations in patients presenting with seizures: a long-term follow-up of 46 patients. Acta Neurochir. (2014) 156:1539–47. 10.1007/s00701-014-2121-x [DOI] [PubMed] [Google Scholar]
- 52.Wang FL, Wang QH, Jin P. Microsurgical treatment of intracerebral cavernous angioma with epilepsy as first symptom in 30 cases. Chin J Stereotact Funct Neurosurg. (2014) 27:208–11. 10.3210/j.issn:1008-2425.2014.04.004 [DOI] [Google Scholar]
- 53.Ge X. Surgical and non-surgical treatment of epilepsy associated with cavernous hemangioma. Chin J Mod Drug Appl. (2015) 9:98–9. 10.14164/j.cnki.cn11-5581/r.2015.19.071 [DOI] [Google Scholar]
- 54.Kida Y, Hasegawa T, Iwai Y, Shuto T, Satoh M, Kondoh T, et al. Radiosurgery for symptomatic cavernous malformations: a multi-institutional retrospective study in Japan. Surg Neurol Int. (2015) 6(Suppl. 5):S249–57. 10.4103/2152-7806.157071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meguins LC, Rocha da Cruz Adry RA, da Silva Junior SC, Pereira CU, de Oliveira JG, de Morais DF, et al. Microsurgical treatment of patients with refractory epilepsy and mesial temporal cavernous malformations: clinical experience of a tertiary epilepsy center. Surg Neurol Int. (2015) 6:169. 10.4103/2152-7806.169552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shan YZ, Fan XT, Meng L, An Y, Xu JK, Zhao GG. Treatment and outcome of epileptogenic temporal cavernous malformations. Chin Med J. (2015) 128:909–13. 10.4103/0366-6999.154289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun Z, Xie YF, Shi QH, Dan W, Yan Y, Lu B, et al. Treatment of supratentorial cerebral cavernous angioma associated with epilepsy as first symptom. Chin J Clin Neurosurg. (2015) 20:709–11. 10.13798/j.issn.1009-153X.2015.12.002 [DOI] [Google Scholar]
- 58.Vale FL, Vivas AC, Manwaring J, Schoenberg MR, Benbadis SR. Temporal lobe epilepsy and cavernous malformations: surgical strategies and long-term outcomes. Acta Neurochir. (2015) 157:1887–95. 10.1007/s00701-015-2592-4 [DOI] [PubMed] [Google Scholar]
- 59.Wu HJ, Yu Z, Zhao YL. Surgical treatment of intracranial cavernous angioma with epilepsy in children. J Int Neurol Neurosurg. (2015) 42:342–5. 10.16636/j.cnki.jinn.2015.04.009 [DOI] [Google Scholar]
- 60.He ZB, Wang RJ, Wang HW. Factors influencing the curative effect of gamma knife on intracranial cavernous hemangioma with epilepsy as the first symptom. Chin J Nerv Ment Dis. (2016) 42:291–4. 10.3969/j.issn.1002-0152.2016.05.008 [DOI] [Google Scholar]
- 61.Hou Z, Li W, An N, Shi XJ, Liu SY. Efficacy of surgical treatment for cerebral cavernous malformation related epilepsy: report of 56 cases. Acta Acad Med Militaris Tertiae. (2016) 38:1987–90. 10.16016/j.1000-5404.201601171 [DOI] [Google Scholar]
- 62.Dammann P, Wrede K, Jabbarli R, Neuschulte S, Menzler K, Zhu Y, et al. Outcome after conservative management or surgical treatment for new-onset epilepsy in cerebral cavernous malformation. J Neurosurg. (2017) 126:1303–11. 10.3171/2016.4.JNS1661 [DOI] [PubMed] [Google Scholar]
- 63.Xu WD, Quan JH, Gao JM. The curative effect of gamma knife therapy for intracranial cavernous hemangioma. Chin J Woman Child Health Res. (2017) 28:4 10.3316/j.issn.1673-5293.2017.03.001 [DOI] [Google Scholar]
- 64.Barzaghi LR, Capitanio JF, Giudice L, Panni P, Acerno S, Mortini P. Usefulness of ultrasound-guided microsurgery in cavernous angioma removal. World Neurosurg. (2018) 116:E414–20. 10.1016/j.wneu.2018.04.217 [DOI] [PubMed] [Google Scholar]
- 65.Lin Q, Yang PF, Jia YZ, Pei JS, Xiao H, Zhang TT, et al. Surgical treatment and long-term outcome of cerebral cavernous malformations-related epilepsy in pediatric patients. Neuropediatrics. (2018) 49:173–9. 10.1055/s-0038-1645871 [DOI] [PubMed] [Google Scholar]
- 66.Yang ZX, He ZB, Wang HW, Zhao XD, Wang YD, Zhang GR. The effect of gamma knife treatment on intracranial epileptogenic solitary cavernous angiomas. Chin J Stereotact Funct Neurosurg. (2019) 32:193–6. 10.3210/j.issn:1008-2425.2019.04.193 [DOI] [Google Scholar]
- 67.He K, Jiang S, Song J, Wu Z, Chen L, Mao Y. Long-term outcomes of surgical treatment in 181 patients with supratentorial cerebral cavernous malformation-associated epilepsy. World Neurosurg. (2017) 108:869–75. 10.1016/j.wneu.2017.08.095 [DOI] [PubMed] [Google Scholar]
- 68.Yang PF, Pei JS, Jia YZ, Lin Q, Xiao H, Zhang TT, et al. Surgical management and long-term seizure outcome after surgery for temporal lobe epilepsy associated with cerebral cavernous malformations. World Neurosurg. (2018) 110:e659–70. 10.1016/j.wneu.2017.11.067 [DOI] [PubMed] [Google Scholar]
- 69.Wang KY, Idowu OR, Lin DDM. Radiology and imaging for cavernous malformations. Handb Clin Neurol. (2017) 143:249–66. 10.1016/B978-0-444-63640-9.00024-2 [DOI] [PubMed] [Google Scholar]
- 70.Botterill JJ, Brymer KJ, Caruncho HJ, Kalynchuk LE. Aberrant hippocampal neurogenesis after limbic kindling: relationship to BDNF and hippocampal-dependent memory. Epilepsy Behav. (2015) 47:83–92. 10.1016/j.yebeh.2015.04.046 [DOI] [PubMed] [Google Scholar]
- 71.Englot D, Han S, Lawton M, Chang E. Predictors of seizure freedom in the surgical treatment of supratentorial cavernous malformations. J Neurosurg. (2011) 115:1169–74. 10.3171/2011.7.JNS11536 [DOI] [PubMed] [Google Scholar]
- 72.Davenport WJ, Siegel AM, Dichgans J, Drigo P, Mammi I, Pereda P, et al. CCM1 gene mutations in families segregating cerebral cavernous malformations. Neurology. (2001) 56:540–3. 10.1212/WNL.56.4.540 [DOI] [PubMed] [Google Scholar]
- 73.Willie J, Malcolm J, Stern M, Lowder L, Neill S, Cabaniss B, et al. Safety and effectiveness of stereotactic laser ablation for epileptogenic cerebral cavernous malformations. Epilepsia. (2019) 60:220–32. 10.1111/epi.14634 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets presented in this study are included in the article/Supplementary Material.