Abstract
Background
Severe early-onset erythroderma and gut inflammation, with massive tissue infiltration of oligoclonal activated T cells are the hallmark of Omenn syndrome (OS).
Objective
The impact of altered gut homeostasis in the cutaneous manifestations of OS remains to be clarified.
Methods
We analyzed a cohort of 15 patients with OS and the 129Sv/C57BL/6 knock-in Rag2R229Q/R229Q (Rag2R229Q) mouse model. Homing phenotypes of circulating lymphocytes were analyzed by flow cytometry. Inflammatory cytokines and chemokines were examined in the sera by ELISA and in skin biopsies by immunohistochemistry and in situ RNA hybridization. Experimental colitis was induced in mice by dextran sulfate sodium salt.
Results
We show that memory/activated T cells from patients with OS and from the Rag2R229Q mouse model of OS abundantly express the skin homing receptors cutaneous lymphocyte associated antigen and CCR4 (Ccr4), associated with high levels of chemokine C-C motif ligands 17 and 22. Serum levels of LPS are also elevated. A broad Th1/Th2/Th17 inflammatory signature is detected in the periphery and in the skin. Increased Tlr4 expression in the skin of Rag2R229Q mice is associated with enhanced cutaneous inflammation on local and systemic administration of LPS. Likewise, boosting colitis in Rag2R229Q mice results in increased frequency of Ccr4+ splenic T cells and worsening of skin inflammation, as indicated by epidermal thickening, enhanced epithelial cell activation, and dermal infiltration by Th1 effector T cells.
Conclusions
These results support the existence of an interplay between gut and skin that can sustain skin inflammation in OS.
Key words: RAG, T cells, skin inflammation, erythroderma, immune-mediated disease, cytokines, chemokines, gut-skin axis, LPS, dysbiosis
Abbreviations used: Actb, Housekeeping gene β-actin; AD, Atopic dermatitis; CCL, Chemokine C-C motif ligand; CLA, Cutaneous lymphocyte antigen; CXCL, C-X-C motif chemokine ligand; DC, Dendritic cell; DLN, Draining lymph node; DSS, Dextran sulfate sodium salt; EB, Evans blue dye; FACS, Fluorescence-activated cell sorting; HD, Healthy donor; H&E, Hematoxylin and eosin; MLN, Mesenteric lymph node; OS, Omenn syndrome; OT, OVA transgenic mouse; OVA, Ovalbumin; PLN, Peripheral lymph nodes; Rag2R229Q, 129Sv/C57BL/6 knock-in Rag2R229Q/R229Q; TLR, Toll-like receptor; WT, Wild type
Graphical abstract
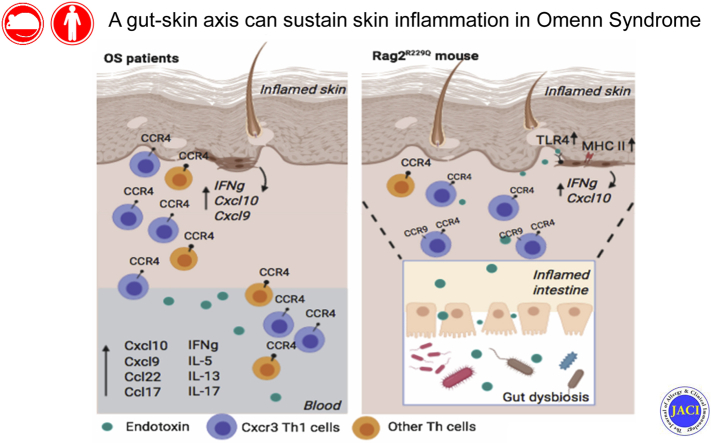
Maintenance of skin homeostasis relies on the crosstalk among epidermal keratinocytes, resident immune cells, and commensal microbiota. Breaching of the cutaneous barrier and inadequate or excessive immune response to microbial challenges potentially lead to chronic inflammation and autoimmunity.1 A large number of T cells normally populate the skin, providing immune surveillance. In addition to this resident population, a subset of recirculating memory T cells has been identified, contributing to widespread immune protection of distal lymphoid and cutaneous tissues.2,3 Migration of T cells into the skin requires ligands for E- and P-selectin, among which an important role is played by the P-selectin ligand CD162, also known as cutaneous lymphocyte antigen (CLA).4 Moreover, skin-homing T cells express the chemokine receptors CCR4 and CCR10, whose ligands—chemokine C-C motif ligands (CCL)17, CCL22, and CCL27—are upregulated in the dermal vasculature and in epidermal keratinocytes in many inflammatory conditions.5, 6, 7 Imprinting of skin-homing properties in T cells critically depends on skin-derived dendritic cells (DCs); T cells activated in skin-draining peripheral lymph nodes (PLNs) express higher levels of selectin ligands than those activated in mesenteric lymph nodes (MLNs).5 Conversely, the gut-homing receptors α4β7 and CCR9 are preferentially induced when T cells are activated in MLN.8 Thus, in general, effector T cells trafficking to the skin are devoid of gut-homing molecules and gut-tropic cells do not express skin-homing molecules. Nonetheless, evidence of plasticity in the homing phenotype has been accumulating in the context of inflammation.9
Cutaneous manifestations are common in patients with primary immunodeficiency disease.10,11 Omenn syndrome (OS) is a severe immunodeficiency most often caused by hypomorphic mutations in the RAG family, leading to generation of oligoclonal T cells that expand in the periphery and infiltrate various organs, particularly barrier tissues such as skin and gut.12 The most distinctive clinical feature of OS is a generalized exfoliative erythroderma with onset at neonatal age, associated with alopecia, lymphadenopathy, hepatosplenomegaly, recurrent and severe infections, and failure to thrive.12 Skin biopsies from patients with OS reveal massive T-cell infiltration and an aberrant distribution of Langerhans cells, indicating that immune abnormalities play an important role in the skin disease pathophysiology.13,14 Patients with OS also frequently suffer from chronic diarrhea, and biopsies of the gastrointestinal tract reveal prominent T-cell and eosinophil infiltrates in the lamina propria, villous flattening in the small bowel, and cryptitis in the colon.15 A close association between skin disease and microbiota (not limited to skin microbiota) has been demonstrated. For instance, atopic dermatitis (AD) and rosacea are both linked to changes in the gut barrier and intestinal microbiota.16 Reduced microbial diversity was reported in infants with eczema.17,18 Furthermore, a link between imbalances of the intestinal microbial communities and development of skin psoriasis has been documented in mice and patients.19 We have recently demonstrated that intestinal microbiota play a major role in the immune dysregulation of the 129Sv/C57BL/6 knock-in Rag2R229Q/R229Q (Rag2R229Q) mouse model of OS, leading to the systemic dissemination of gut-derived proinflammatory Th1/Th17 cells.20 Whether such alterations in gut homeostasis may also influence the cutaneous manifestations of OS is not known.
Here, we provide evidence supporting a role for gut-skin axis in the pathophysiology of OS. We found that compromised skin barrier integrity in Rag2R229Q mice results in altered microbial load and composition and dysregulated production of T cell–recruiting chemokines by activated epithelial cells. Importantly, intestinal inflammation and gut barrier leakage synergistically support the activation of skin epithelial cells and the recruitment of T cells to the skin. An increased proportion of circulating T cells expressing skin-homing receptors and elevated plasma levels of LPS and skin chemoattractants have been also documented in patients with OS, suggesting that mechanisms similar to those observed in the mouse model may also operate to induce skin inflammation in humans.
Methods
Human studies
Patient’s samples were collected with the informed consent of their parents, according to protocol 18-I-0041 approved by the National Institutes of Health institutional review board and protocols TIGET06 and TIGET09 approved by the San Raffaele Scientific Institute ethics committee. Table E1 (in the Online Repository available at www.jacionline.org) describes clinical, immunological, and molecular features of 15 unrelated patients with OS. A cohort of age-matched healthy donors (HDs), patients with acute graft-versus-host disease and patients with AD were also studied as controls. PBMCs from peripheral blood were purified on Ficoll gradient (Lympholyte cell separation media; Cedarlane Laboratories, Burlington, Ontario, Canada).
Mice
The Rag2R229Q mice were previously described.20 The colony was maintained in a specific pathogen–free facility in heterozygosity and littermates were kept in the same cage until weaning. Both male and female mice between 8 and 12 weeks old were used for experiments. OVA transgenic OT-I and OT-II mice were obtained from Charles River Laboratories (Wilmington, Mass). The animal procedures were approved by the Istituto Clinico Humanitas, according to the Italian Institutional Animal Care and Use Committee and European Union directive for animal experiments. Details about the experimental design, in vivo procedure, and treatments are described in the supplementary Methods in this article’s Online Repository at www.jacionline.org.
ELISA tests
Quantification of mouse Ccl17 and Ccl22 and human CCL17, CCL22, and C-X-C motif chemokine ligand (CXCL)-10 were determined with ELISA kits (R&D Systems, Minneapolis, Minn), according to manufacturer’s instructions. Human LPS-binding protein was determined in the sera of patients with OS and age-matched HDs using human LBP ELISA kit (MyBioSource, San Diego, Calif), used according to manufacturing’s instructions.
Quantification of adherent bacteria to cutaneous epithelium
One centimeter of skin biopsies was collected from age- and sex-matched mice using sterile conditions and digested in lysis buffer (10 mmol/L TRIS, 100 mmol/L NaCl, 10 mmol/L EDTA, 0.5% SDS, and 0.4 mg/mL proteinase K). Total DNA was extracted with the isopropanol-ethanol method. The microbial load was determined with real-time quantitative PCR (SYBR green) using 16S and 18S ribosomal RNA gene-specific probes.20
Mouse cutaneous cell preparation
Skin tissue was digested with Liberase TL (0.45 mg/mL; Roche, Basel, Switzerland) for 1 hour at 37°C. Cell suspension was washed, passed through a 100-μm cell strainer, and analyzed by flow cytometry,
Lymph flow assessment using EB
Three μL of 1% Evans blue dye (EB) solution in PBS was injected into the back of anesthetized mice using a Hamilton syringe (Hamilton Company, Reno, Nev). After 16 hours mice were sacrificed. EB was extracted from the skin biopsies of the back by incubating them at 55°C for 5.5 hours in formamide (Honeywell Fluka, Thermo Fisher Scientific, Waltham, Mass). The absorbance was measured with microplate reader at 620 nm. The dye concentration was calculated from a standard curve of EB in formamide (Sigma-Aldrich, St Louis, Mo) and is represented as absolute amount of dye remaining in the skin tissue.
Flow cytometry
Cell suspensions obtained from mouse organs were preincubated with FcγR-specific blocking antibodies (eBioscience, San Diego, Calif) and stained with the following antibodies from eBioscience: TCR-γδ (UC7-13D5), CD45 (30-F11), CD4 (RM4-5), CD8a (53-6.7), CCR9 (CW-1.2), CD103 (2E7), CD11C (N418), MHCII (M5/114.15.2), CCR4 (2G12), IL-17A (17B7) and IFN-γ (XMG1.2). Dead cells exclusion was performed with Aqua Cell Stain kit (Invitrogen, Thermo Fisher Scientific). For intracellular staining, cells were stimulated for 4 hours with phorbol 12-myristate 13-acetate (5 ng/ml; Sigma-Aldrich) and ionomycin (1 μg/ml; Sigma-Aldrich). Golgi stop (1000×; BD, Franklin Lakes, NJ) was added during the last 3 hours of stimulation. Cell fixation and permeabilization was performed using the Fixation and Permeabilization Buffer kit (eBioscience). Human cells were stained with the following antibodies from BioLegend (San Diego, Calif): CD4 (OKT4), CD8 (RPA-T8), CD45RA (HI100), CD45RO (UCHL1), CLA (HECA-452), CCR4 (L291H4). Fluorescence-activated cell sorting (FACS) data were acquired with FACSCanto II (BD) and analyzed with FlowJo software (version 9.9.6; Tree Star, BD).
Real-time PCR
Cutaneous tissues (1 cm2) were homogenized in PureZOL reagent (Bio-Rad Laboratories, Hercules, Calif) using TissueLyzer II (QIAGEN, Hilden, Germany). RNA was extracted using RNeasy Lipid Tissue kit (QIAGEN). A total of 1 μg of RNA was used to synthetize cDNA using the High-Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, Calif). Gene expression was investigated by SYBR green RT-qPCR using the CFX384 system (Bio-Rad). The housekeeping gene β-actin (Actb) ribosomal RNA was used as control. List of primers are available in Table E2 in this article’s Online Repository at www.jacionline.org.
Histology and immunohistochemistry
Mouse tissue samples were formalin-fixed and paraffin-embedded. Sections (1.5 μm) were used for routine hematoxylin and eosin (H&E) analysis and staining with primary antibodies. Arbitrary gut and skin histological score was calculated in a double-blind study by a pathologist. Scoring from 0 to 3 (where 0 is normal condition and 3 is severe structural changes) was adopted for evaluating different manifestations in each tissue. Detailed description of histological and morphometric analyses are described in the supplementary Methods in the Online Repository.
In situ RNA hybridization
In situ hybridization for IFN-γ, CXCL9, and CXCL10 on formalin-fixed paraffin-embedded samples was performed using the RNAscope 2.5 HD Assay-BROWN (Advanced Cell Diagnostics, Newark, Calif) according to the manufacturer's instructions. POLR2A mRNA labelling was used as positive control for the RNA quality in samples. Staining procedure is described in the supplementary Methods in the Online Repository.
Statistical analysis
Statistical analysis was conducted using GraphPad Prism software (GraphPad Software Inc, La Jolla, Calif). Results were analyzed using the nonparametric Mann-Whitney test or the ANOVA with multiple comparisons test. Data are generally shown as mean ± SEM unless otherwise stated. Value of P < .05 was considered significant.
Results
Enhanced skin tropism of PB memory T cells in patients with OS
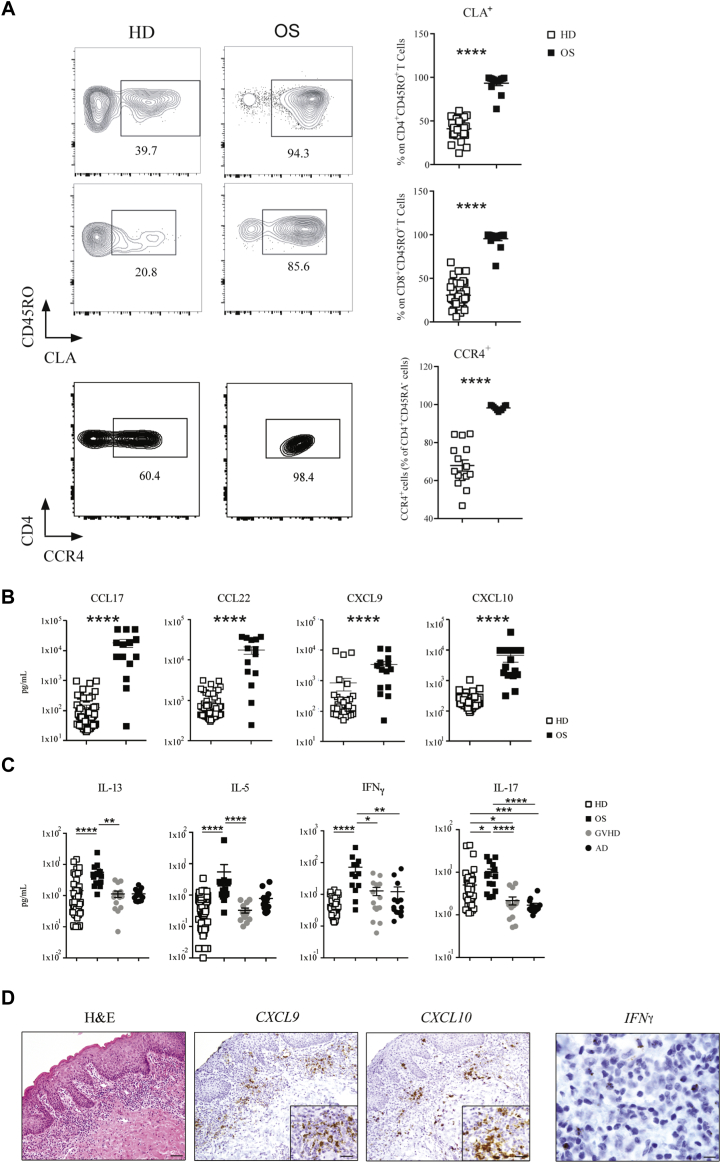
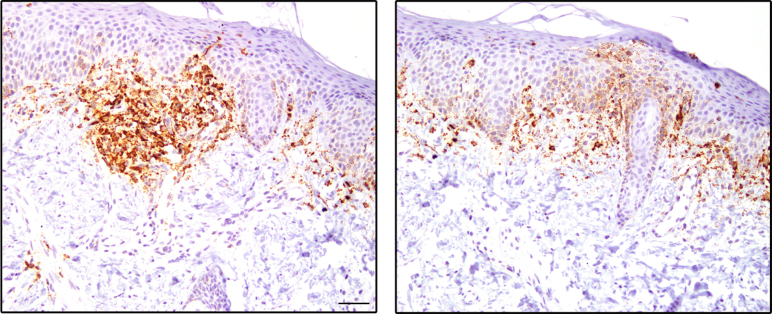
Erythroderma, with skin infiltration by autoreactive and oligoclonal autologous T cells, represents the clinical hallmark of OS. CLA controls the influx of memory T cells to cutaneous sites.21 We studied 15 patients with OS due to RAG mutations (Table E1) and 37 healthy infants (HDs) and examined CLA expression in PB T cells. In both CD4 and CD8 effector/memory CD45R0+ T cell subsets, the frequency of CLA+ cells was dramatically increased in patients with OS compared with age-matched HDs (Fig 1, A). Circulating CLA+ T cells coexpress the chemokine receptor CCR4 and rely on T-cell chemoattractant CCL22 and CCL17 to reach the inflamed skin.5,22 Indeed, CCR4 was expressed on almost the totality of CD4+ CD45RA− cells in patients with OS (Fig 1, A), and higher levels of the CCR4 ligands CCL17 and CCL22 were detected in the plasma, as compared to HDs (Fig 1, B). These features are consistent with the Th2 phenotype of OS, as also indicated by elevated serum levels of IL-5 and IL-13 (Fig 1, C). Surprisingly, we found that patients with OS exhibited also significantly high plasma levels of IFN-γ and IFN-γ–inducible chemokines CXCL9 and CXCL10, as well as of IL-13, IL-5, and IL-17 (Fig 1, B and C). Furthermore, plasma levels of Th1 and Th2 cytokines were significantly higher in patients with OS than in patients with graft-versus-host disease and AD, 2 prototypic conditions characterized by Th1 and Th2 skewing, respectively (Fig 1, C). Altogether, these data indicate that the inflammation of OS is characterized by a systemic and broad signature. Consistent with this, IFNG and to greater extent CXCL9 and CXCL10 mRNA signals were readily detected in skin biopsies from patients with OS (Fig 1, D and Fig E1 in this article’s Online Repository at www.jacionline.org).
Fig 1.
Preferential homing of T cells to the skin in patients with OS. A, Representative FACS plots and frequencies of CLA+ CD45RO+ T cells within the effector CD4+ and CD8+ T-cell populations isolated from PB of patients with OS (n = 13) and aged-matched HDs (n = 31). Frequencies of CCR4+ within the CD4+ CD45RA− T-cell population (n = 11-15). Plasma levels of chemokines (B) and cytokines (C) in HDs (n = 37), in patients with OS (n = 15) as well as in patients with acute graft-versus-host disease (GvHD) (n = 15) and patients with AD (n = 14), as controls of Th1- and Th2-mediated inflammation, respectively. D, Skin biopsies from patients with OS were investigated for IFNG, CXCL9, and CXCL10 mRNA expression using RNAscope. POLR2A mRNA labeling was used as control for the quality of the RNA on paraffin section. Values are mean ± SEM. ∗P < .05, ∗∗P < .01, ∗∗∗P < .005, ∗∗∗∗P < .001. Statistical significance determined by Mann-Whitney U test or by ANOVA. See also Table E1.
Fig E1.
Elevated CXCL9 expression in skin biopsies from patients with OS. Skin biopsies from 2 patients with OS were analyzed for CXCL9 mRNA expression using RNAscope. POLR2A mRNA labeling was used as control for the quality of the RNA on paraffin section.
Skin inflammation in Rag2R229Q mice is characterized by T-cell infiltration and a Th1-biased cytokine response
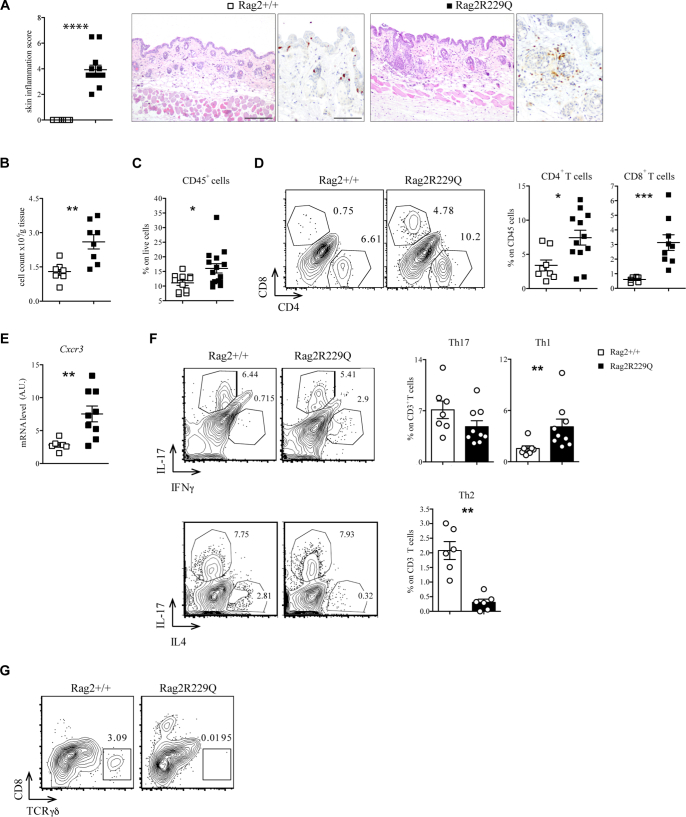
To investigate specifically the pathophysiology of skin disease in OS, we used the Rag2R229Q mouse model.14,23 Typically, mutant mice develop epidermal hyperplasia, hyperkeratosis, and dermal immune cell infiltration (Fig 2, A and B). Such alterations, associated with massive signs of subcutaneous hemorrhage and PLN enlargement, were much more pronounced in mice with overt skin erythroderma (Fig E2 in this article’s Online Repository at www.jacionline.org). Immunophenotypic analysis of the skin infiltrate confirmed an expansion of mononuclear CD45+ cells, particularly of CD4+ and CD8+ T cells (Fig 2, C and D). Moreover, the cutaneous expression of the chemokine receptor Cxcr3 was increased in mutant mice (Fig 2, E). Consistent with this, CD3+ T cells isolated from the skin of Rag2R229Q mice displayed much higher frequencies of Ifn-γ–producing cells on phorbol 12-myristate 13-acetate and ionomycin pulsing than those from wild-type (WT) control mice (Fig 2, F). Of note, the proportion of Il-17–producing cells in mutant mice was comparable to what observed in controls, despite lack of Tcrγδ+T cells, the main producers of this cytokine in the skin (Fig 2, F and G).
Fig 2.
Rag2R229Q mice exhibit severe T-cell–mediated inflammation in the skin. A, Representative skin sections from Rag2+/+ and Rag2R229Q mice (8-12 weeks old) stained with H&E and CD3 immunostaining. Histogram shows the inflammation score in the skin (n = 12-13). Bars = 200 μm (H&E) and 100 μm (CD3). B, Total cell count from skin suspension for g tissue (n = 7-8 mice/group). C, Frequencies of CD45+ cells in the skin suspension (n = 9-10 mice/group). D, Representative FACS plots and cumulative frequencies of CD4+ and CD8+ T cells inside the CD45+ population of skin suspension (n = 6-12 mice/group). E, Gene expression analysis of Cxcr3 in the skin tissue of Rag2+/+ and Rag2R229Q mice (n = 6-9 mice/group). Target mRNA was normalized to Actb mRNA. RNA contents are shown as arbitrary units (AUs). F, Representative FACS plots and frequencies of Th1, Th17, and Th2 cells inside the CD3+ T-cell population of skin suspension (n = 6-9 mice/group). G, Representative FACS plots showing TCRγδ T cells inside the CD45+ population in the skin of Rag2+/+ and Rag2R229Q mice. Values are mean ± SEM. ∗P < .05; ∗∗P < .01; ∗∗∗P < .005; ∗∗∗∗P < .001. Statistical analysis was performed using Mann-Whitney U test. See also Fig E1.
Fig E2.
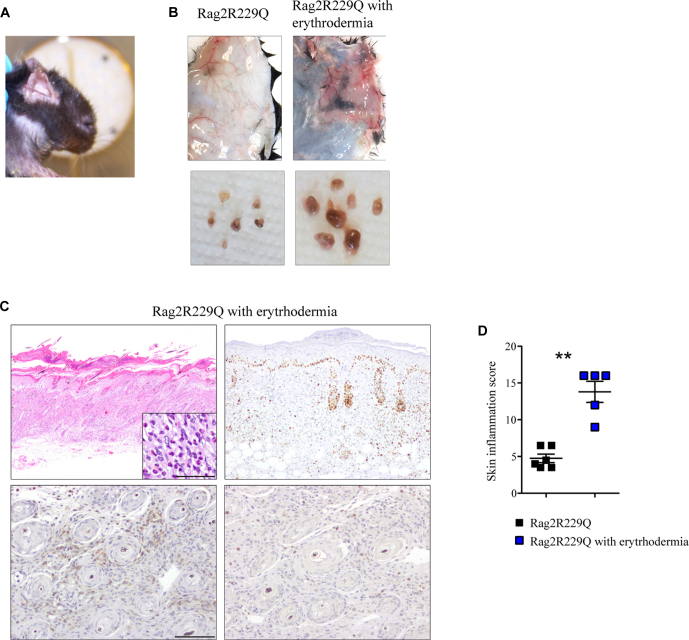
A small proportion of Rag2R229Q mice exhibits erythroderma-like phenotype. A, Cutaneous phenotype of Rag2R229Q mice affected by erythroderma. B, Macroscopic appearance of dorsal skin and lymph nodes of Rag2R229Q and Rag2R229Q with erythroderma-like phenotype. C, Representative skin sections from Rag2R229Q with evident skin erythroderma-like phenotype stained with H&E, KI67 (upper right; bar = 500 μm), CD3 (bottom left), and myeloperoxidase (bottom right; bar = 100 μm) immunostaining. Higher magnification shows eosinophils. D, Histogram shows the inflammation score of the skin (n = 5-6 mice/group). Values are mean ± SEM. ∗∗P < .01. Statistical analysis was performed using Mann-Whitney U test.
Rag2R229Q mice exhibit dysfunction of the epidermal barrier and increased microbial challenge
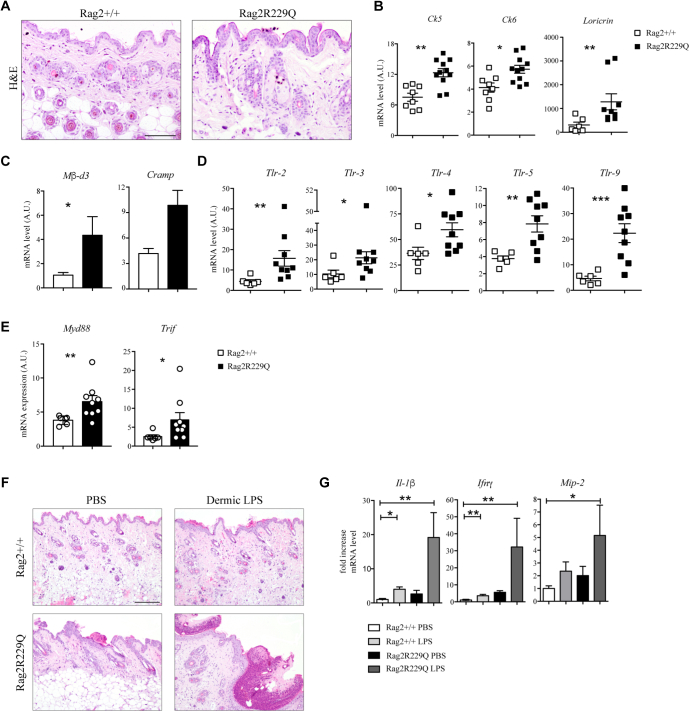
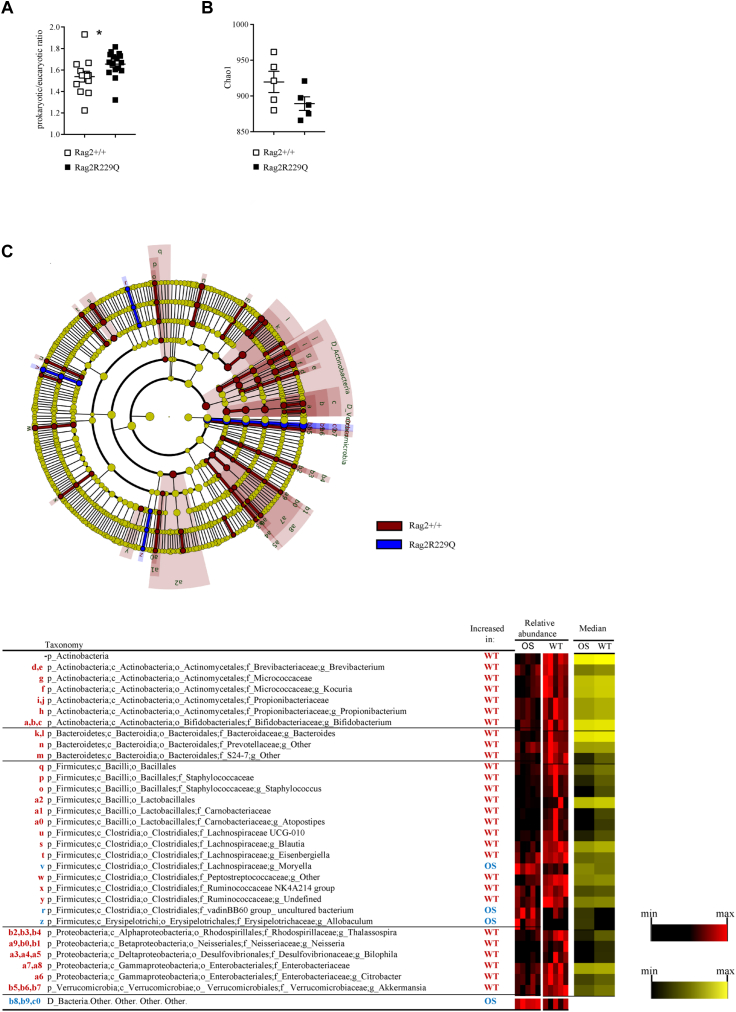
At steady state, the epidermis of Rag2R229Q mice appears thickened compared with the epidermis of WT mice (Fig 3, A). Expression of the epidermal differentiation markers, cytokeratin 5 (Ck5) and loricrin, was markedly increased (Fig 3, B). Similarly, the mRNA level of the hyperproliferative/wound-associated cytokeratin 6 (Ck6) was also augmented (Fig 3, B), overall indicating an altered keratinocyte differentiation program. Antimicrobial peptides are significantly upregulated in mouse models with altered skin barrier.24,25 We found that cutaneous expression of cathelin-related antimicrobial peptides (Cramp) and β-defensin 3 (mβ-d3) was increased in Rag2R229Q mice (Fig 3, C). Furthermore, mutant mice exhibited augmented tissue expressions of several Toll-like receptors (Tlrs) as well as of the downstream adaptor molecules, myeloid differentiation primary response 88 (MyD88) and TIR-domain–containing adapter-inducing IFN-β (Trif) (Fig 3, D and E), suggestive of disturbance in the cutaneous barrier integrity and increased microbial challenge. In line with this, skin bacterial load was increased in Rag2R229Q mice (Fig E3, A in this article’s Online Repository at www.jacionline.org), indicating a possible expansion of the overall number of bacteria compared with in their WT littermates. Notably, 16S ribosomal RNA gene sequencing revealed that bacterial taxonomic richness was generally reduced in the skin of Rag-mutant mice (Fig E3, B), with lower abundance of Propionibacterium (Padj < .028), Bacteroides (Padj < .009), and Staphylococcus (Padj < .008) (Fig E3, C) genera, which are cutaneous commensal bacteria contributing to maintenance of tissue homeostasis.26
Fig 3.
Altered barrier integrity and microbial load characterized the skin of Rag2R229Q mice. A, Representative skin sections from Rag2+/+ and Rag2R229Q mice stained with H&E. Bar = 100 μm. B, Cutaneous gene expression analysis of keratins (Ck5-Ck6) and Loricrin in Rag2+/+ and Rag2R229Q mice (n = 8-11 mice/group from 3 experiments). Target mRNA was normalized to Actb mRNA. RNA contents are shown as AUs. C, Gene expression analysis of Mβ-d3 and Cramp in the skin tissue (n = 4-8 mice/group). Gene expression analysis of Tlrs (D) and Myd88 and Trif (E) in the skin tissue of Rag2+/+ and Rag2R229Q mice (n = 6-9 mice/group). Target mRNA was normalized to Actb mRNA. RNA contents are shown as AUs. F,Rag2+/+ and Rag2R229Q mice received a single intradermal injection of 100 μg LPS on the left and PBS on the right portion of the back. Mice were then sacrificed 24 hours later. Representative skin sections from dermal LPS-treated and untreated Rag2+/+ and Rag2R229Q mice stained with H&E. Bar = 200 μm. G, Fold increase of Il-1β, Ifng, and macrophage inflammatory protein 2 (Mip-2, also known asCxcl2) on skin tissue. Data are representative results of 3 independent experiments with at least 6 mice/group. Values are mean ± SEM. ∗P < .05; ∗∗P < .01; ∗∗∗P < .005. Statistical analysis was performed using Mann-Whitney U test.
Fig E3.
The composition of skin microbiota differs between Rag2+/+ and Rag2R229Q mice. Skin tissue microbiota from adults Rag2+/+ (n = 10) and Rag2R229Q (n = 10) littermates was analyzed through 16s rRNA gene profiling. A, Quantitative PCR analysis of cutaneous adherent bacteria in Rag2+/+ and Rag2R229Q mice expressed as prokaryotic/eukaryotic DNA (ie, 16S/18S rRNA genes) ratios (n = 12 mice/group from 3 experiments). B, Scatter dot plot depicts the intrasample (α) diversity (Chao1 index) describing taxonomic richness of skin microbiota in Rag2+/+ and Rag2R229Q mice. C, A linear discriminant analysis effect size identifies the significant different abundance of bacterial taxa between Rag2R229Q mice and controls. The taxa with significantly different abundances among the genotypes are represented by colored shadows in the cladogram of the top panel and listed in the table below. From the center of the cladogram outward, kingdom, phylum, class, order, family, and genus levels are shown. Heat maps represent the relative abundance of significantly different taxa per sample (black-red heat map). Statistical analysis of data (A, C) was performed using Mann-Whitney U test. ∗P < .05.
Following the observation that cutaneous Toll-like receptor 4 (Tlr4) expression was markedly upregulated in Rag2R229Q mice, we examined further the effect of local Tlr4 activation. Mice received a single intradermal injection of 100-μg LPS on the right portion of the back and of PBS on the left and were sacrificed 24 hours later. Hemorrhage and dermal tissue necrosis were evident at the LPS injection site in almost all treated mutant mice (Fig 3, F). In particular, histopathology of skin sections revealed widespread epidermal thickening with hyperkeratosis and increased abscess formation and hemorrhage. By contrast, WT mice showed mild signs of hemorrhage and no microabscesses (Fig 3, F). We also analyzed the expression of the CK6-inducing cytokine Il-1β27 as well as of Ifng, involved in the pathogenesis of inflammatory skin diseases.28 Expression of both cytokines was strongly induced in the skin of Rag2R229Q mice in response to LPS (Fig 3, G). Moreover, the augmented expression of the keratinocyte-derived Cxcl2 chemokine correlated with a robust recruitment of innate cells to the skin of Rag2R229Q mice (Fig 3, G). Thus, Rag2R229Q mice are more susceptible to LPS-induced cutaneous inflammation.
Chronic skin inflammation sustains immune cell infiltration in Rag2R229Q mice
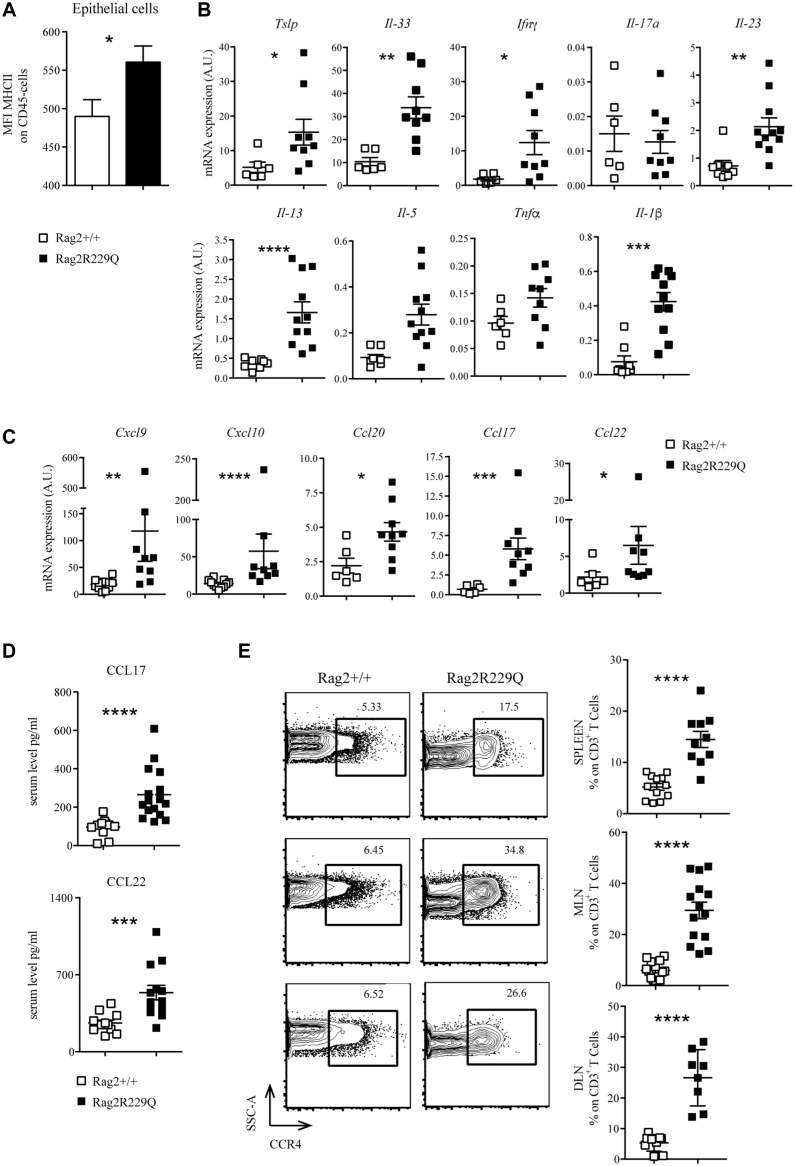
Chronic microbial stimulation may induce keratinocyte activation.29, 30, 31 Skin epithelial cells of Rag2R229Q mice expressed high levels of MHC class II molecules (Fig 4, A). Moreover, the expression of several cytokines, including Ifn-γ, Tnf-α, Il-23, Il-1β, Il-5, Il-13, Il-33, and thymic stromal lymphopoietin (Tslp), was significantly upregulated in the mutant skin (Fig 4, B), suggesting that these cytokines may contribute to skin inflammation, without an obvious skewing of Th responses. Consistent with this, levels of both Th1 (Cxcl9, Cxcl10) and Th2 (Ccl20, Ccl22, and Ccl17)-related chemokine transcripts were markedly increased in the skin of Rag2R229Q mice (Fig 4, C). Levels of Ccl17 and Ccl22 were also elevated in the serum of RagR229Q mice (Fig 4, D), correlating with a higher frequency of Ccr4-expressing skin homing CD3+ T cells detected in the PLNs, spleen, and MLNs (Fig 4, E). Overall, these data mirror what is observed in patients with OS (Fig 1) and suggest a broad inflammatory signature in this disease.
Fig 4.
Characterization of cutaneous inflammatory environment in Rag2R229Q mice. A, Mean fluorescence intensity (MFI) for MHCII expression among CD45− population of skin suspension (n = 6-7 mice/group). Transcriptional analysis of several cytokines (B) and chemokines (C) in the skin tissue (n = 6-9 mice/group from 3 experiments). Target mRNA was normalized to Actb mRNA. RNA contents are shown as AUs. D, Levels of the chemokines Ccl17 and Ccl22 in the serum of Rag2+/+ and Rag2R229Q mice (n = 12-13 mice/group from 3 experiments). E, Representative FACS plots and cumulative frequencies of CCR4+ inside the CD3+ T-cell population of spleen, MLN, and DLN (n = 14 mice/group from 3 experiments). Values are mean ± SEM. ∗P < .05; ∗∗P < .01; ∗∗∗P < .005; ∗∗∗∗P < .001. Statistical analysis was performed using Mann-Whitney U test. See also Fig E3. SSC-A, Side scatter.
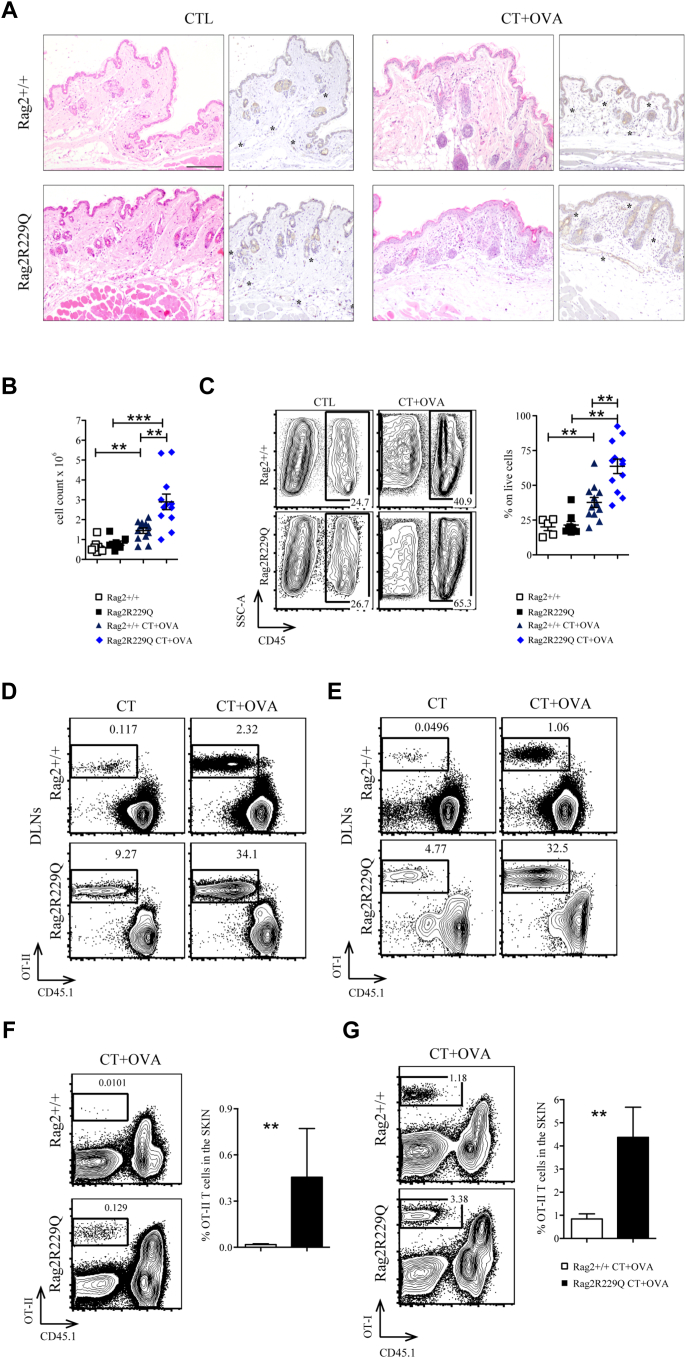
To test whether this inflammatory environment has a role in promoting antigen-driven cutaneous T-cell migration, we adoptively transferred CD4+ or CD8+ lymphocytes from OT-II or OT-I mice into Rag2R229Q and Rag2+/+ recipients. Then, we topically immunized the ear pinna once with ovalbumin (OVA) plus cholera toxin, and after 7 days, treated mice were sacrificed. Epicutaneous challenge caused marked epidermal changes in Rag2R229Q mice, with enhanced vascularization and dermal infiltration by immune cells (Fig E4, A-C in this article’s Online Repository at www.jacionline.org). Accumulation of transgenic T cells was significantly increased within the cervical LNs (Fig E4, D and E) and skin (Fig E4, F and G) of immunized Rag2R229Q mice compared with that of controls. Overall, these findings indicate that keratinocyte activation and cutaneous inflammatory environment in mutant mice contribute to T-cell skin homing and infiltration.
Fig E4.
Cutaneous inflammatory environment in mutant mice promote T-cell infiltration. A, Representative skin section from controls and CT+OVA-treated Rag2+/+ and Rag2R229Q mice stained with H&E and CD31 immunostaining (asterisks mark the CD31-positive vessels). Bar = 200 μm. B, Total skin counts from skin suspension (n = 5-12). C, Representative FACS plot and frequencies of CD45+ cell population of skin suspension (n = 5-12). D and E, CD4 or CD8 splenocytes from OT-II or OT-I mice were adoptively transferred into Rag2+/+ and Rag2R229Q recipients. Topical immunization was applied on ears once with OVA plus CT or only CT. Recipient mice were sacrificed after 7 days. Representative dot plots of donor-derived OT-II or OT-I cells within total live population of DLN suspension. F and G, Representative dot plots and frequencies of donor-derived OT-II or OT-I cells within total live population of skin suspension (n = 5-7 mice/group). Values are mean ± SEM. ∗∗P < .01; ∗∗∗P < .001. Statistical analysis was performed using Mann-Whitney U test.
Enhanced peripheral trafficking of skin-derived DCs in Rag2R229Q mice
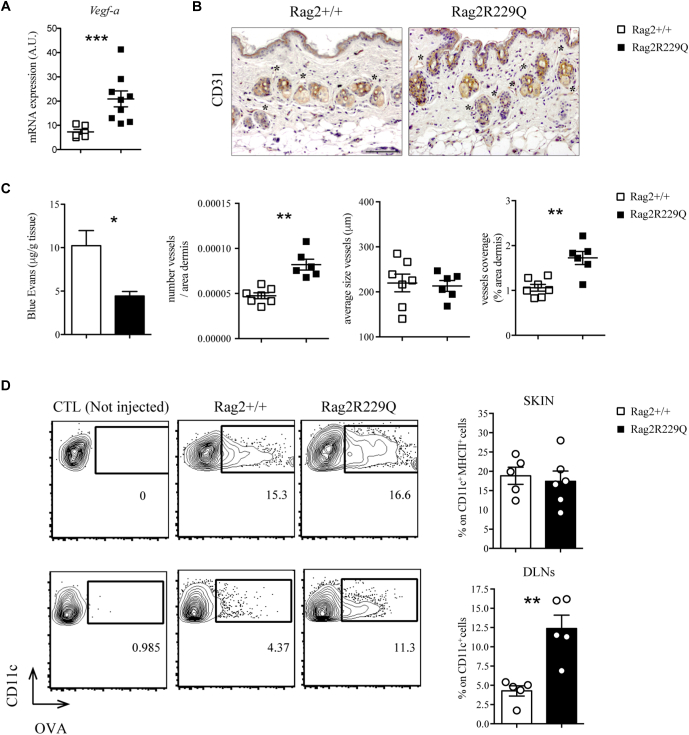
Cell trafficking to inflamed tissues is influenced by vascular remodeling.32 We found that levels of the angiogenic vascular endothelial growth factor A (Vegfa) were elevated, and CD31+ endothelial cells were present at higher number and density in the skin of Rag2R229Q mice (Fig 5, A and B). To investigate possible abnormalities of lymphatic vessels, we quantified the uptake and drainage of the lymphatic tracking dye EB after intradermal injection into the back skin.33 Significantly less EB remained localized within the skin of Rag2R229Q compared with in WT mice, indicating enhanced lymphatic clearance (Fig 5, C). Hence, trafficking of antigens and immune cells from the skin of Rag2R229Q mice could be facilitated by the expanded lymphatic network. To test this hypothesis, allophycocyanin-labeled OVA antigen was intradermally injected in the ear pinna of mice, and its uptake and transport by DCs was evaluated in the skin and draining lymph nodes (DLNs) after 6 hours. Comparable frequencies of OVA-loaded DCs were detected locally within the skin tissue of Rag2R229Q and Rag2+/+ mice (Fig 5, D). However, consistent with the enhanced EB dye transport, a higher proportion of OVA-positive DCs was recovered in the DLNs of Rag2R229Q mice compared with in controls (Fig 5, D).
Fig 5.
Increased DC trafficking from the skin of Rag2R229Q mice. A, Gene expression analysis of Vegfa in the skin tissue of Rag2+/+ and Rag2R229Q mice (n = 6-9 mice/group from 3 experiments). B, Representative skin sections stained with CD31. Bar = 100 μm. The evaluation of several parameters was performed by computer-assisted morphometric analysis of CD31-stained skin sections (n = 5 mice/group). C, Quantification of extracted EB from the back skin of Rag2+/+ and Rag2R229Q mice, normalized to tissue weight (g). Data are representative results of 3 independent experiments with at least 4 mice per group. D, Allophycocyanin-labeled OVA antigen was intradermally injected in the ears of Rag2+/+ and Rag2R229Q mice, and its uptake by DCs was evaluated in the skin and DLNs after 6 hours. Representative dot plots and frequencies of OVA-loaded DCs from skin (CD11c+ MHCII+) and DLNs (CD11c+) of OVA-injected Rag2+/+ and Rag2R229Q and negative control (not injected). Data are representative results of 3 independent experiments with at least 5 mice per group. Values are mean ± SEM. ∗P < .05; ∗∗P < .01; ∗∗∗P < .005. Statistical analysis was performed using unpaired Student’s t test and Mann-Whitney U test. CTL, Control.
Skin disease in Rag2R229Q mice is exacerbated by intestinal inflammation
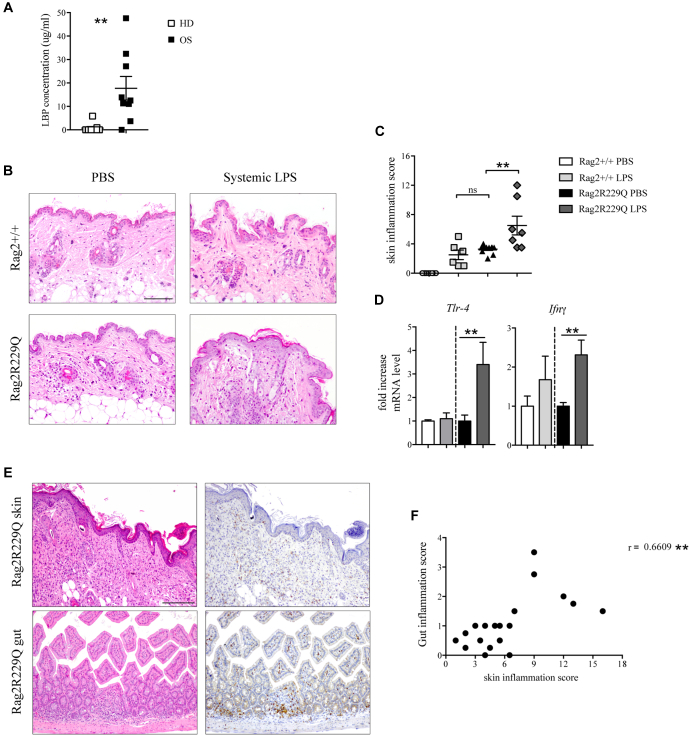
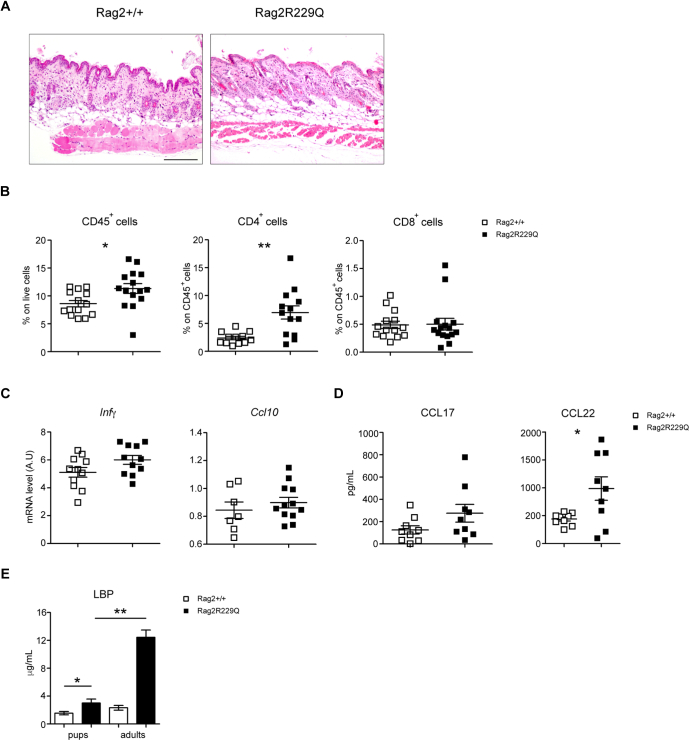
The aforementioned results indicate that endogenous Tlr4 ligands could have a role in cutaneous inflammation of Rag2R229Q mice. We previously reported high levels of endotoxin (LPS) in the circulation of Rag2R229Q mice correlating with gut dysbiosis and intestinal inflammation.20 Increased levels of LPS-binding protein, marker of microbial load and translocation, were detected also in sera of patients with OS (Fig 6, A), with the highest levels found in patients exhibiting intestinal inflammation and profuse diarrhea. To evaluate whether high serum endotoxin may contribute to skin inflammation in Rag2R229Q mice, we analyzed the cutaneous responses to systemic LPS. Mice received a single injection of 100 μg LPS intraperitoneally and were sacrificed 24 hours later. Histological examination of skin biopsies showed worsening of inflammation over baseline condition in Rag2R229Q mice, with increased epidermal thickening, hyperkeratosis, occasional abscesses, and more prominent dermal infiltrates (Fig 6, B and C). Signs of inflammation were evident also in the skin of LPS-treated Rag2+/+ mice, similar to those observed in untreated Rag2R229Q mice (Fig 6, C). Furthermore, on LPS challenge, levels of Tlr4 and Ifng transcripts were significantly upregulated in Rag2R229Q mice and, to a lesser extent, in Rag2+/+ mice (Fig 6, D), indicating that systemic endotoxin can play a role in the cutaneous inflammation of Rag2R229Q mice. Skin homeostasis was not markedly altered in mutant mice at weaning, likely reflecting the reduced LBP levels compared with LBP levels in adult mice and the time of exposure to endotoxin (Fig E5 in this article’s Online Repository at www.jacionline.org).
Fig 6.
Systemic LPS triggers cutaneous inflammation. A, LPS-binding protein (LBP) concentration in the sera of patients with OS and HDs (n = 8-9 from 2 experiments). B,Rag2+/+ and Rag2R229Q mice received a single injection of 100 μg LPS intraperitoneally and were sacrificed 24 hours later. Representative skin sections from systemic LPS-treated and untreated Rag2+/+ and Rag2R229Q mice stained with H&E. Bar = 200 μm. C, Histogram shows the inflammation score in the skin of n = 6-11 mice/group from 2 experiments. D, Fold increase of Ifng and Tlr4 on skin tissue (n = 6-8 mice/group from 2 experiments). E, Representative small intestinal and skin sections from Rag2R229Q mice with erythroderma stained with H&E and CD3 immunostaining. Bar = 200 μm. F, Correlation between intestinal and skin inflammation scores of Rag2R229Q mice manifesting or not evident cutaneous manifestations (black dots). The Spearman r value is indicated in the graph (n = 10 mice). Values are mean ± SEM. ∗∗P < .01. Statistical analysis was performed using Mann-Whitney U test.
Fig E5.
Analysis of skin homeostasis in young Rag2R229Q mice. A, Representative skin section from 20-days-old Rag2+/+ and Rag2R229Q mice stained with H&E. Bar = 200 μm. B, Cumulative frequencies of CD45+, CD4+, and CD8+ T cells infiltrating the skin. C, Gene expression analysis of Ifng and Cxcl10 in the skin tissue of Rag2+/+ and Rag2R229Q mice (n = 6-9 mice/group). Target mRNA was normalized to Actb mRNA. RNA contents are shown as AUs. Plasma levels of (D) CCL17 and CCL22 and (E) LPS-binding protein (LBP). Values are mean ± SEM. ∗P < .05; ∗∗P < .01. Statistical analysis was performed using Mann-Whitney U test.
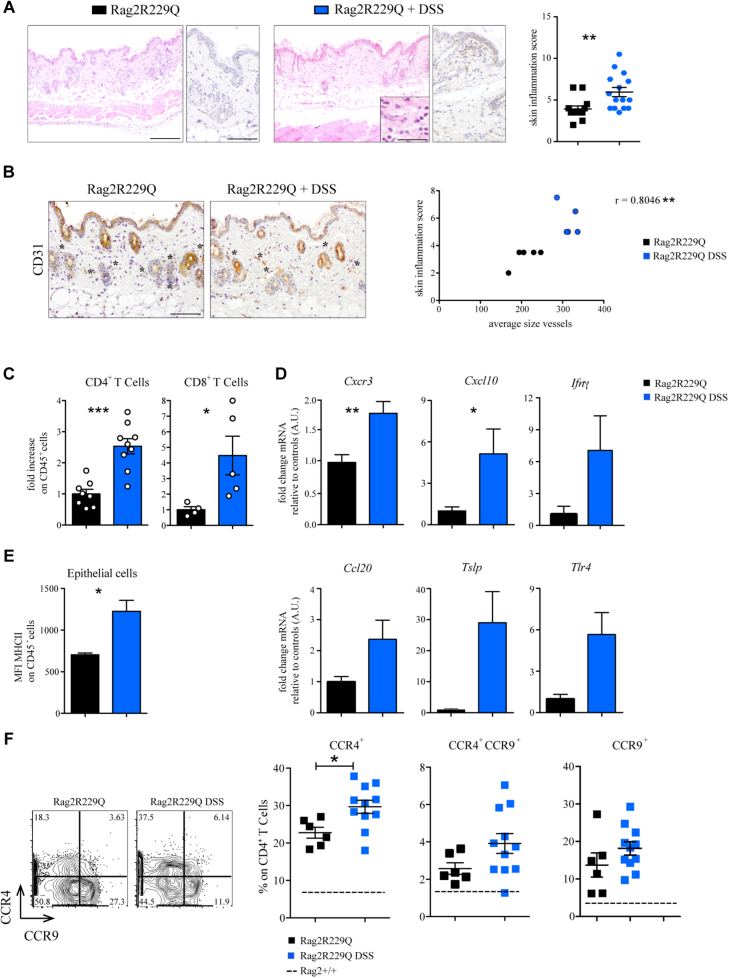
Moreover, we observed that Rag2R229Q mice in which the skin inflammation was more pronounced were those in which intestinal disease was also more severe (Fig 6, E and F). To determine the potential role of the gut-skin axis in the disease pathophysiology, we boosted intestinal inflammation in Rag2R229Q mice using chronic dextran sulfate sodium salt (DSS) treatment. Mice were sacrificed after 4 weeks. DSS-treated Rag2R229Q mice manifested worsened skin inflammation, as indicated by enhanced epidermal thickening, hyperkeratosis, and increased number of CD3+ T cells infiltrating the dermis (Fig 7, A). Furthermore, immunostaining for CD31 demonstrated enhanced cutaneous vascularization in DSS-treated Rag2R229Q mice (Fig 7, B). By contrast, DSS treatment did not induce similar skin abnormalities in Rag2+/+ mice (Fig E6, A and B in this article’s Online Repository at www.jacionline.org). FACS analysis of cutaneous cell suspensions confirmed that the proportion of skin-infiltrating CD4+ T cells and CD8+ T cells was increased by 2- to 4-fold in DSS-treated Rag2R229Q mice (Fig 7, C). Moreover, the frequency of circulating CD4+ Ccr4+ T cells was significantly increased in DSS-Rag2R229Q mice (Fig 7, F), with a significant proportion coexpressing also the gut-homing receptor Ccr9 (Fig 7, F), thus suggesting that memory T cells in Rag2R229Q mice may recirculate between skin and intestinal mucosa. Cutaneous levels of Cxcr3 and Ifng transcripts were upregulated in DSS-Rag2R229Q mice (Fig 7, D), leading to enhanced epithelial expression of MHC-II molecules and keratinocyte hyperactivation (Fig 7, E).34
Fig 7.
Boosted intestinal inflammation worsens skin inflammation in Rag2R229Q mice. A, Colitis in Rag2R229Q mice was induced by cyclic DSS administration. Representative skin sections stained with H&E and CD3 immunostaining. Bars = 200 μm (H&E) and 100 μm (CD3). Higher magnification shows eosinophils. Bar = 50 μm. Histogram shows the inflammation score in the skin (n = 12-15 mice/group from 3 experiments). B, Representative skin sections from untreated and DSS-treated Rag2R229Qmice stained with CD31 immunostaining (asterisks mark the CD31-positive vessels). Bar = 100 μm. Correlation between cutaneous inflammation and average vessels size of untreated Rag2R229Q (black dots) and DSS-treated Rag2R229Q (blue dots). The Spearman r value is indicated in the graph (n = 5 mice/group). C, Fold change of the CD4+ and CD8+ T-cell frequencies (n = 6-9 mice/group). D, Fold change of cutaneous Cxcr3, Cxcl10, Ifng, Ccl20, Tslp, and Tlr4 expressions (n = 6-9 mice/group). E, MFI for MHCII expression among CD45− population of Rag2R229Q and DSS-treated Rag2R229Q skin suspension (n = 5-6 mice/group). F, Representative FACS plots and cumulative frequencies of splenic Ccr4+, Ccr9+, and Ccr4+Ccr9+ CD4+ T cells (n = 6-11 mice/group from 3 experiments). Dotted line indicates WT averages. Values are mean ± SEM. ∗P < .05; ∗∗P < .01; ∗∗∗P < .005. Statistical significance determined by Mann-Whitney U test. See also Fig E4.
Fig E6.
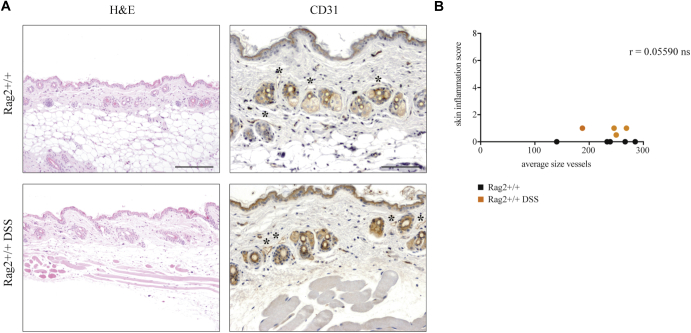
Cutaneous response to DSS treatment in Rag2+/+ mice. A, Representative skin sections from untreated and DSS-treated Rag2+/+ mice stained with CD31 immunostaining (asterisks mark the CD31-positive vessels). Bars = 100 μm. B, Correlation between cutaneous inflammation and average vessels size of untreated Rag2+/+ (black dots) and DSS-treated Rag2+/+ (blue dots). The Spearman r value is indicated in the graph (n = 5 mice/group).
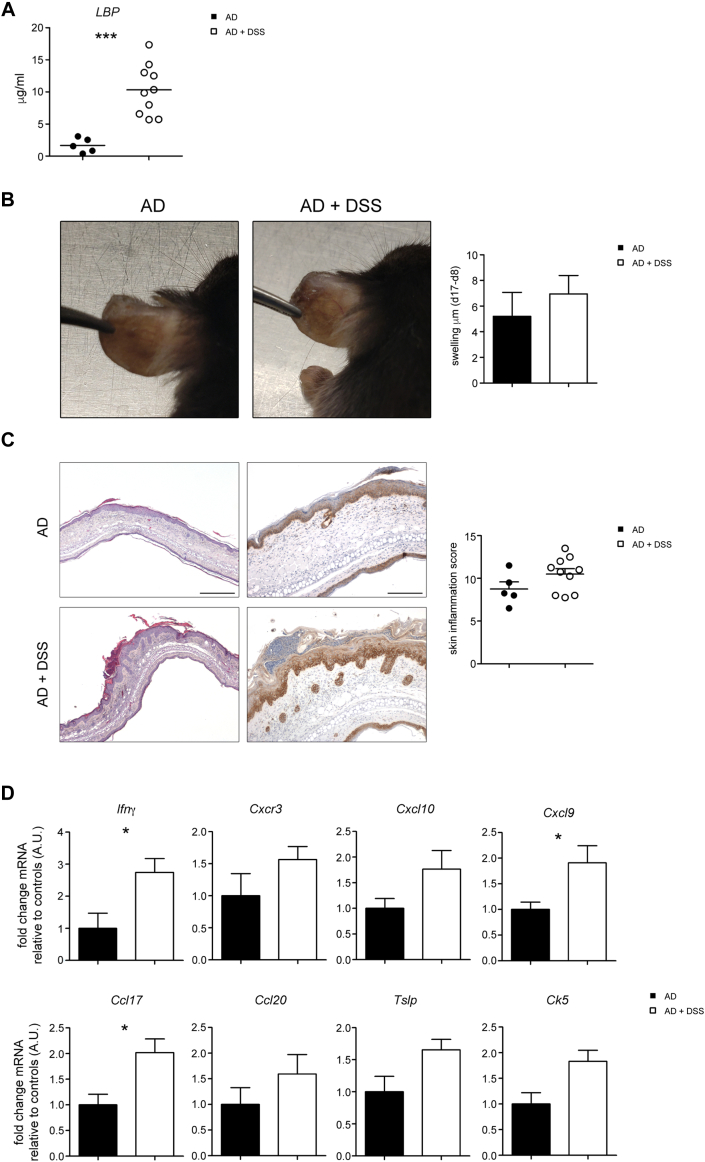
Finally, we investigated whether the mechanism for endotoxin-driven skin disease exacerbation is applicable to mice not displaying preexisting inflammatory bowel disease. To this end, we tested the effect of acute DSS in a mouse model of AD. Experimental AD was induced by daily topical application of the vitamin D3 analog MC903 on the ears. At day 8, groups of mice received for 7 days water with or without 2% DSS, followed by normal drinking water for an additional 2 days, and then euthanized. Plasma LBP was significantly augmented in mice receiving DSS administration (Fig E7, A in this article’s Online Repository at www.jacionline.org), which caused increased swelling, reddening, and scaling of the skin in the treated mice (Fig E7, B and C). Microscopic investigation revealed a stronger dermal inflammatory cell infiltration with epidermal hyperplasia and hyperkeratosis in DSS-treated AD mice (Fig E7, D). Consistent with increased inflammation, the expression of Th1 and Th2 inflammatory markers was augmented (Fig E7, E).
Fig E7.
Effect of DSS-induced intestinal inflammation on experimental model of AD. Mice were treated with MC903 for 8 days to induce AD-like dermatitis. Starting on day 8, a set of mice received 2% DSS water for 7 days, and then returned to normal drinking water for additional 2 days. A, Plasma LBP on day 17 is shown. B, Day 17 gross phenotype of AD mice treated or not with oral DSS. Bar graph shows the difference in ear thickness between day 17 and day 8 (n = 5-10 mice/group). C, Representative ear section from AD and AD+DSS mice stained with H&E (left; bar = 500 μm) and CK5 immunostaining (right; bar = 200 μm). Histogram shows the inflammation score in the ear skin (n = 5-10). D, Gene expression analysis of the ear tissue (n = 5-10 mice/group). Target mRNA was normalized to Actb mRNA. RNA contents are shown as AUs. Values are mean ± SEM. ∗P < .05. Statistical analysis was performed using Mann-Whitney U test.
Collectively, these results suggest that a leaky gut plays an important role in priming the skin disease for a hyperinflammatory response to systemic LPS.
Discussion
The cutaneous hallmark of OS is a severe exfoliative erythroderma, resulting in epidermal barrier defect and subsequent risk of life-threatening bacterial infections. The aberrant expression of skin-homing molecules CLA and CCR4 on T cells from patients with OS, together with the high CCL17/CCL22 serum levels, indicates that they are poised to migrate to the inflamed skin and have pathogenic potential.35 Along with high serum levels of IL-13 and IL-5, these observations may also sustain the notion that OS is a Th2-driven disease. Surprisingly, we detected high levels also of IFN-γ, CXCL9, and CXCL10 in the serum of patients with OS. Similar results have been demonstrated both in the skin and in circulation of Rag2R229Q mice, indicating that OS is not a pure Th2-driven disease, but rather manifests a broad inflammatory signature, with a prominent role also for Th1-mediated responses. Many studies reported promiscuous expression of chemokines in the serum of patients with cutaneous inflammation,36 proposing that the immunological phenotype in T-cell–mediated skin diseases can shift toward a Th2/Th1/Th17 mixed response as the duration of the disease increases.37 Furthermore, although expression of CCR4 is more commonly associated with Th2 responses, it has also been observed on Th17 cells, suggesting that the chemokines activating this receptor may have a broader impact on the inflammatory response than simply recruiting Th2 cells.38 Indeed, CCR4+ memory T cells also contain non-Th2 populations expressing CXCR3 and IFN-γ39 and CCL17 and CCL22 can be released not only by leukocytes, but also by activated dermal endothelial cells and keratinocytes, in response to both Th1 and Th2 cytokines.34 Both CCR4 and CXCR3 antagonists are available for the treatment of allergic disease and psoriasis40,41 and may represent a novel therapeutic target also in OS.
Increased expression of Tlr4 and downstream signaling molecules Myd88 and Trif in the skin of Rag2R229Q mice correlated with hyperinflammatory cutaneous responses on topical or systemic administration of LPS. Together with our previous demonstration of inflammatory bowel disease–like disease, altered gut microbiota, and elevated LPS serum levels in Rag2R229Q mice,20 these findings suggest that endogenous Tlr4 ligands play a role in the maintenance of keratinocyte activation and perpetuation of local inflammatory response.42 Consistently, DSS treatment markedly worsened cutaneous inflammation in Rag2R229Q mice. Chronic gastrointestinal diseases, such as inflammatory bowel disease or celiac disease, are often associated with skin inflammation.43,44 Likewise, marked changes in gut barrier and in intestinal microbiota are observed in AD and patients with rosacea.45 However, it cannot be excluded that inflammation in other organs may also contribute to increase the severity of skin inflammation in OS.46
High cutaneous expression of antimicrobial peptides and Tlrs in the Rag2R229Q mice is indicative of important alterations of skin barrier integrity and microbial challenge. Changes of the cutaneous microbiota have been correlated with several skin disorders.47, 48, 49 Here we have shown that Rag2R229Q mice manifested a selective shift in resident skin microbiota with decreased abundance of commensal bacteria belonging to Actinobacteria and Firmicutes phyla. The loss of some protective bacteria genera (Streptococcus, Staphylococcus, and Propionibacterium) might render the skin of Rag2R229Q mice more susceptible to development of local inflammation. These data indicate the importance of performing similar studies also in patients with OS, although the frequent use of immunosuppressive agents and antibiotics in these patients may affect interpretation of the results.
Similar to patients with OS, an increased proportion of Ccr4-expressing CD4+ T cells was detectable in lymphoid organs of Rag2R229Q mice, indicating that these cells are poised to migrate to the skin. Interestingly, we observed coexpression of skin-homing (Ccr4) and gut-homing (Ccr9) expression by a subset of splenic T cells in DSS-treated Rag2R229Q mice, suggesting that these cells might be potentially able to recirculate between gut and skin. Plasticity in the migratory property of immune cells has been suggested. Particularly, cutaneous exposure to food antigens can reprogram LN effector T cells expressing gut-homing receptors to express skin-homing receptors, thereby eliciting skin inflammation.50 Tissue-derived DCs have a key role in presenting local antigens and imprinting T cells with specific homing properties. Trafficking of skin-derived DCs to DLNs is increased in Rag2R229Q mice, correlating with abundant lymphatic network. Moreover, we previously described an augmented population of migratory CD103+ DCs in the spleen of Rag2R229Q mice,51 suggesting that reprogramming of T cell homing by tissue-derived DCs may also occur in secondary lymphoid organs other than DLNs. Intriguingly, skin-derived and antigen-bearing DCs were also identified in MLNs.50 Hence, breaches of skin and gut barriers in Rag2R229Q mice may promote systemic translocation of microbial antigens and induce activation of T cells expressing homing receptors for skin and/or gut. In summary, we have demonstrated that the cutaneous manifestations of OS are characterized by a broad inflammatory signature and can be severely influenced by gut inflammation, reinforcing the notion of an interplay between skin and gut in the pathophysiology of the disease.
Key messages.
-
•
A broad Th1/Th2/Th17 inflammatory signature is observed in the blood and skin of patients with OS and in the murine model.
-
•
Systemic LPS injection or boosting colitis in OS murine model aggravates cutaneous T-cell infiltration and skin inflammation.
-
•
High level of serum endotoxin is detected in patients with OS.
Footnotes
This work was partially supported by grants from the Italian Ministry of Health (GR-2011-02349759 to B. Cassani; PE-2016_02363691 to A. Villa, E. Fontana, and L.D. Notarangelo) and the Italian Fondazione Telethon (TIGET E2 Core grant). L.D. Notarangelo is supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Contributor Information
Luigi D. Notarangelo, Email: luigi.notarangelo2@nih.gov.
Anna Villa, Email: villa.anna@hsr.it.
Barbara Cassani, Email: barbara.cassani@humanitasresearch.it.
Methods
Histology and immunohistochemistry
Mouse tissue samples were formalin-fixed and paraffin-embedded. Sections (1.5 μm) were used for routine H&E staining. Moreover, sections were dewaxed and rehydrated; endogenous peroxidase activity was blocked by 0.1% H2O2; nonspecific background was reduced with Rodent Block (Biocare Medical, Pacheco, Calif) before microwaves or thermostatic bath treatment; and incubated with primary antibodies. The following primary antibodies were used: rabbit anti-CD3 (1:100; EDTA buffer pH 8.0; 1 hour at room temperature [RT]; Thermo Fisher Scientific), rabbit anti-Ki67 (1:350; citrate buffer pH 6.0; 1 hour RT; Cell Signaling Technology, Danvers, Mass), rabbit anti-keratin 5 (1:200; EDTA buffer pH 8.0; 1 hour RT; BioLegend), rabbit anti-myeloperoxidase (1:300; EDTA buffer pH 8.0; overnight; Abcam, Cambridge, Mass), and goat anti-CD31 (1:1000; R&D Systems; DIVA Decloaker 1×; Biocare Medical). Depending on the primary antibodies and tissue used, sections were incubated with Goat-on-Mouse HRP-Polymer (Biocare Medical), MACH 1 Universal HRP Polymer Kit (Biocare Medical); reactions were developed in Biocare's Betazoid DAB; and nuclei were counterstained with Hematoxylin (Dako, Agilent, Santa Clara, Calif). Digital images were acquired by an Olympus XC50 camera mounted on a BX51 microscope (Olympus, Tokyo, Japan) or by an Olympus DP70 camera mounted on a BX60 microscope (Olympus), with CellF Imaging software (Soft Imaging System GmbH, Münster, Germany).
Histological score
Arbitrary gut and skin histological score was calculated in a double-blind study by a pathologist. Scoring from 0 to 3 (where 0 is normal condition and 3 is severe structural changes) was adopted for evaluating different manifestations in each tissue. For gut analysis, a combined score—grade of inflammation, the structural changes of the glands, and the goblet cell depletion—was used. For skin analysis, abnormalities of keratinocytes, increased immune cells in the dermis, alterations of the epidermis, and presence of cutaneous abscesses were evaluated. Specifically, for DSS treatment, the number of CD3+ aggregates was also analyzed.
Morphometric analysis
Histological dermal thickness was determined, on H&E-stained sections, by measuring the distance between the epidermal-dermal junction and dermal–subcutaneous fat junction (60 fields for each sample). Morphometric analysis to evaluate coverage, number of vessels, and size was determined on CD31-stained sections. Both analyses were performed using Olympus Slide Scanner VS120-L100 to acquire digital images and Image-Pro software (Media Cybernetics, Rockville, Md).
Mice and in vivo treatments
OT-I and OT-II adoptive transfer
Splenic CD8+ OT-I cells or CD4+ OT-II cells were sorted from donor mice. T cells (3 × 106) were adoptively transferred intravenously into Rag2+/+ and Rag2R229Q mice (CD45.1). The day after, ears were immunized.E1 Briefly, both sides of each ear were gently stripped 10 times with Scotch Tape (3M, St Paul, Minn) and 25 μL of acetone was applied and evaporated. Then, 20 μL of cholera toxin (CT) or CT+ 50 μL of OVA (100 mg/mL; Sigma-Aldrich) was applied with a small paintbrush on the ears of recipient mice. Rag2+/+ and Rag2R229Q mice were sacrificed after 7 days from topical immunization.
Coculture DC-T cells
Topical immunization of ear skin of Rag2+/+ and Rag2R229Q mice was performed as described above. Briefly, CT plus OVA or CT alone was applied directly on ear skin. After 4 days, DCs from spleen and DLNs were sorted from OVA-immunized Rag2+/+ and Rag2R229Q mice and CT controls. DCs were put in coculture with CD4+ OT-II cells in 96-well round-bottom plates. A 1:1 DC–T cell ratio was used for the assay. Cocultures were maintained for 6 days and T-cell proliferation was evaluated by overnight incorporation of (3H)-thymidine.
In vivo treatments
Acute DSS treatment was induced by adding 2.5% of DSS (MP Biomedicals, Santa Ana, Calif) to drinking water ad libitum for 6 days. Treated and untreated mice were sacrificed at day 7, and transcriptional analyses were performed on skin tissue. Chronic colitis was induced by adding 1.5% of DSS to drinking water ad libitum for 4 cycles for a total of 4 weeks. Control mice received water continuously throughout all the experiment. For topical and systemic LPS administration, mice were anesthetized and injected intradermally into the back with 100 μg of LPS (right side) and PBS (left side) using Hamilton syringes. After 24 hours, LPS-treated mice were sacrificed and skin tissues were processed for histological analysis and quantitative RT-PCR analysis. For systemic LPS administration, mice were injected intraperitoneally with 100 μg of LPS. Systemic LPS-treated mice were sacrificed 24 hours later. Skin tissues were used for histological scores and transcriptional analysis. Mice were injected intradermally into the ear’s skin with 100 μg/mL OVA–Alexa Fluor (Thermo Fisher Scientific) with Hamilton syringe. After 6 hours, DLNs and ear tissues were collected. The analysis of OVA-loaded CD11C+ DCs from ears and DLNs was performed by FACS.
In situ RNA hybridization
Slides were deparaffinized in xylene (2×, 5 minutes each) and 100% ethyl alcohol (2×, 1 minute each) and air dried at RT, followed by H2O2 (Cat no 322330; ACDBio, Advanced Cell Diagnostics) at RT for 10 minutes. Slides were submerged in 700 mL fresh 1X Target Retrieval (Cat no 322000; ACDBio) at boiling for 15 minutes and immediately washed in deionized water followed by 100% ethyl alcohol and air dried. A hydrophobic barrier was drawn around tissue and protease plus (Cat no 322330; ACDBio) was applied for 15 minutes in a HybEZ oven (Cat no 322310; ACDBio) at 40°C. Slides were washed in deionized water and probes were added for 2 hours in the HybEZ oven. Signal amplification reagents antimicrobial peptide (AMP) 1 (30 minutes), AMP 2 (15 minutes), AMP 3 (30 minutes), AMP 4 (15 minutes), AMP 5 (75 minutes), and AMP 6 (15 minutes) were applied sequentially and incubated in the HybEZ oven. Before adding each AMP reagent, samples were washed twice with washing buffer (Cat no 310091; ACDBio). Diaminobenzidine chromogen detection reagent was applied and incubated for 10 minutes in the HybEZ oven. Samples were then counterstained, rinsed with tap water, and placed in 0.02% ammonia water followed by another tap water rinse. Samples were then dehydrated in graded alcohols followed by xylene treatment (2×, 5 minutes each) and cover slipped.
Cutaneous microbiota analysis
Weaned Rag2+/+ and Rag2R229Q littermates were maintained in the separated cages until analysis. Cutaneous tissues were weighed and digested in lysis buffer (10 mmol/L TRIS, 100 mmol/L NaCl, 10 mmol/L EDTA, 0.5% SDS, and 0.4 mg/mL proteinase K). Total DNA was extracted with the isopropanol-ethanol method. Bacterial DNA was analyzed by real-time PCR using specific primers for 16s ribosomal RNA (rRNA) gene. Microbiota taxonomic profiling was carried out at GenProbio Srl (Parma, Italy). In brief, primer pairs Probio Uni and Probio_RevE2 (GenProbio Srl) were used for the amplification of the region 16S rRNA gene encompassing the V3 variable region; amplicons were then processed and sequenced using an Illumina MiSeq sequencer (Illumina Inc, San Diego, Calif) with MiSeq Reagent Kit chemicals (Illumina). For taxonomic classification of taxonomic units, the resulting fastq files were then processed with the bioinformatic pipeline Quantitative Insights Into Microbial Ecology ([QIIME], version 1.7.0)E3 with the 16S rRNA gene database SILVA (version 128E4). To identify specific bacterial taxa distinguishing the skin microbiota of Rag2+/+ from Rag2R229Q mice, we used the linear discriminant analysis effect size method.E5 Specifically, linear discriminant analysis effect size analysis included Kruskal-Wallis and pairwise Wilcoxon tests considering significant a corrected P value of <.05.E5
Experimental model of AD
The induction of MC903 dermatitis was performed as previously described (6). Briefly, MC903 was applied to both ears from days 0 to 8, select cages were placed on 2% DSS water on days 8 to 15, and then all cages returned to normal drinking water for days 16 and 17. Ear thickness was measured as described on days 0, 8, and 17. On day 17, mice were euthanized, and ears and serum were harvested for further analysis.
Table E1.
Clinical features of RAG patients
| ID patient | Gene | Mutation | Erythroderma | Colitis/diarrhea | Autoimmunity | Diagnosis |
|---|---|---|---|---|---|---|
| SCID-400-MX | RAG2 | p. [L155P; R229W] | Yes | Yes | No | OS |
| SCID-417-UK | RAG1 | p. [R410Q/M1006V; M435V/M1006V] | Yes | No | No | OS |
| SCID-418-UK | RAG1 | p.[R396C; R404Q] | Yes | Yes | No | OS |
| SCID-419-UK | RAG1 | p. [R561C; R716W] | Yes | Yes | No | OS |
| SCID-420-UK | RAG1 | p.[M435V/R897∗; M1006V] | Yes | Infectious (rotavirus) | No | OS |
| SCID-421-UK | RAG1 | p.[K86Vfs∗33; R559S] | Yes | Infectious (enterovirus) | ITP, hepatitis | OS |
| SCID-426-KW | RAG1 | p. [L454Q; L454Q] | Yes | No | No | OS |
| SCID-454-IT | RAG1 | p. [E147fs∗27; E147fs∗27] | Yes | Yes | No | OS |
| 731-UK | RAG1 | p. [R829fs∗2; K86Vfs∗33] | Yes | Yes | No | OS |
| 769-AF | RAG1 | p. [K186Sfs∗15; K186Sfs∗15] | Yes | Yes | No | OS |
| 884-PA | RAG1 | p. [Q242R/R404Q; N766I] | Yes | Yes | No | OS |
| SCID-375-MX | RAG2 | p. [G35V; G35V] | Yes | Yes | No | OS |
| SCID-452-KW | RAG1 | p. [R394W; R394W] | Yes | Yes | No | OS |
| HIE-118-LB | RAG1 | p. [R404W; R737H] | Yes | No | ITP, AIHA | OS |
AIHA, Autoimmune hemolytic anemia; ITP, idiopathic thrombocytopenic purpura; SCID, severe combined immunodeficiency.
Table E2.
List of primers used in this study
| Primer ID | 5'-3' Sequence |
|---|---|
| Actb For | CTAAGGCCAACCGTGAAAAG |
| Actb Rev | ACCAGAGGCATACAGGGACA |
| IL-17A For | TCCAGAAGGCCCTCAGACTA |
| IL-17A Rev | TGAGCTTCCCAGATCACAGA |
| IFN-γ For | TCAAGTGGCATAGATGTGGAAGAA |
| IFN-γ Rev | TGGCTCTGCAGGATTTTCATG |
| IL-5 For | ATCCAGGAACTGCCTCGTC |
| IL-5 Rev | ATCCAGGAACTGCCTCGTC |
| IL-13 For | CCTCTGACCCTTAAGGAGCTTAT |
| IL-13 Rev | CGTTGCACAGGGGAGTCT |
| IL-33 For | TCCTTGCTTGGCAGT |
| IL-33 Rev | TGCTCAATGTGTCAA |
| TNF-α For | GACGTGGAACTGGCAGAAGAG |
| TNF-α Rev | TTGGTGGTTTGTGAGTGTGAG |
| IL-1β For | GCCCATCCTCTGTGACTCAT |
| IL-1β Rev | AGGCCACAGGTATTTTGTCG |
| CCL20 For | AACTGGGTGAAAAGGGCTGT |
| CCL20 Rev | GTCCAATTCCATCCCAAAAA |
| CXCL10 For | GCTGCCGTCATTTTCTGC |
| CXCL10 Rev | TCTCACTGGCCCGTCATC |
| CXCL9 For | CTTTTCCTCTTGGGCATCAT |
| CXCL9 Rev | GCATCGTGCATTCCTTATCA |
| 16s For | GTGSTGCAYGGYTGTCGTCA |
| 16s Rev | ACGTCRTCCMCACCTTCCTC |
| 18s For | CTCAACACGGGAAACCTCCTCAC |
| 18s Rev | CGCTCCACCAACTAAGAAGG |
| IL-23p19 For | AGCCAGTTCTGCTTGCAAAGG |
| IL-23p19 Rev | GGAGGTTGTGAAGTTGCTCCATG |
| Cramp For | AAGGAGACTGTATGTGGCAAGGCA |
| Cramp Rev | TTTCTTGAACCGAAAGGGCTGTGC |
| mBD-3 For | GTCAGATTGGCAGTTGTGGA |
| mBD-3 Rev | GCTAGGGAGCACTTGTTTGC |
| VEGF-A For | CAGGGCTTCATCGTTACAG |
| VEGF-A Rev | CATCTTCAAGCCGTCCTGT |
| TLR-2 For | AGCATCCTCTGAGATTTGA |
| TLR-2 Rev | GGGGCTTCACTTCTCTGCTT |
| TLR-3 For | TCCCCCAAAGGAGTACATT |
| TLR-3 Rev | GATACAGGGATTGCACCCA |
| TLR-4 For | GGACTCTGATCATGGCACTG |
| TLR-4 Rev | CTGATCCATGCATTGGTAGGT |
| TLR-5 For | CTGGAGCCGAGTGAGGTC |
| TLR-5 Rev | CGGCAAGCATTGTTCTCC |
| TLR-9 For | GAGAATCCTCCATCTCCCAAC |
| TLR-9 Rev | CCAGAGTCTCAGCCAGCAC |
| TSLP For | CAGCTTCGTCTCCTGA |
| TSLP Rev | AAATGTTTTGTCGGGGAGTG |
| CK5 For | CATTCTCAGCCGTGGTACG |
| CK5 Rev | CAGAGCTGAGGAACATGCAG |
| CK6 For | GTCCAGGACCTTGTTCTGCT |
| CK6 Rev | ATCCAGCGGGTCAGGACT |
| CCL22 For | CTGATGCAGGTCCTATGGT |
| CCL22 Rev | GGAGTAGCTTCTTCACCCAG |
| CCL17 Rev | TGCTTCTGGGGACTTTTCTG |
| CCL17 For | GAATGGCCCCTTTGAAGTAA |
| CXCR3 Rev | GGCATCTAGCACTTGACGTTC |
| CXCR3 For | GCAGCACGAGACCTGACC |
| Loricrin Rev | CATGAGAAAGTTAAGCCCATG |
| Loricrin For | GGTTGCAACGGAGACAACA |
| Involucrin Rev | TTGAGAGGTCCCTGAACCAC |
| Involucrin For | TCCTGTGAGTTTGTTTGGTC |
For, Forward; Rev, reverse.
References
- 1.Nestle F.O., Di Meglio P., Qin J.Z., Nickoloff B.J. Skin immune sentinels in health and disease. Nat Rev Immunol. 2009;9:679–691. doi: 10.1038/nri2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gebhardt T., Whitney P.G., Zaid A., Mackay L.K., Brooks A.G., Heath W.R. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature. 2011;477:216–219. doi: 10.1038/nature10339. [DOI] [PubMed] [Google Scholar]
- 3.Bromley S.K., Yan S., Tomura M., Kanagawa O., Luster A.D. Recirculating memory T cells are a unique subset of CD4+ T cells with a distinct phenotype and migratory pattern. J Immunol. 2013;190:970–976. doi: 10.4049/jimmunol.1202805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berg E.L., Yoshino T., Rott L.S., Robinson M.K., Warnock R.A., Kishimoto T.K. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J Exp Med. 1991;174:1461–1466. doi: 10.1084/jem.174.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell J.J., Haraldsen G., Pan J., Rottman J., Qin S., Ponath P. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature. 1999;400:776–780. doi: 10.1038/23495. [DOI] [PubMed] [Google Scholar]
- 6.Reiss Y., Proudfoot A.E., Power C.A., Campbell J.J., Butcher E.C. CC chemokine receptor (CCR)4 and the CCR10 ligand cutaneous T cell-attracting chemokine (CTACK) in lymphocyte trafficking to inflamed skin. J Exp Med. 2001;194:1541–1547. doi: 10.1084/jem.194.10.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Homey B., Alenius H., Muller A., Soto H., Bowman E.P., Yuan W. CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nat Med. 2002;8:157–165. doi: 10.1038/nm0202-157. [DOI] [PubMed] [Google Scholar]
- 8.Mora J.R., Von Andrian U.H. Specificity and plasticity of memory lymphocyte migration. Curr Top Microbiol Immunol. 2006;308:83–116. [PubMed] [Google Scholar]
- 9.Hart A.L., Ng S.C., Mann E., Al-Hassi H.O., Bernardo D., Knight S.C. Homing of immune cells: role in homeostasis and intestinal inflammation. Inflamm Bowel Dis. 2010;16:1969–1977. doi: 10.1002/ibd.21304. [DOI] [PubMed] [Google Scholar]
- 10.Lehman H. Skin manifestations of primary immune deficiency. Clin Rev Allergy Immunol. 2014;46:112–119. doi: 10.1007/s12016-013-8377-8. [DOI] [PubMed] [Google Scholar]
- 11.Oh J., Freeman A.F., Park M., Sokolic R., Candotti F., Holland S.M. for the NISC Comparative Sequencing Program. The altered landscape of the human skin microbiome in patients with primary immunodeficiencies. Genome Res. 2013;23:2103–2114. doi: 10.1101/gr.159467.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Notarangelo L.D., Kim M.S., Walter J.E., Lee Y.N. Human RAG mutations: biochemistry and clinical implications. Nat Rev Immunol. 2016;16:234–246. doi: 10.1038/nri.2016.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheimberg I., Hoeger P.H., Harper J.I., Lake B., Malone M. Omenn's syndrome: differential diagnosis in infants with erythroderma and immunodeficiency. Pediatr Dev Pathol. 2001;4:237–245. doi: 10.1007/s100240010171. [DOI] [PubMed] [Google Scholar]
- 14.Maina V., Marrella V., Mantero S., Cassani B., Fontana E., Anselmo A. Hypomorphic mutation in the RAG2 gene affects dendritic cell distribution and migration. J Leukoc Biol. 2013;94:1221–1230. doi: 10.1189/jlb.0713365. [DOI] [PubMed] [Google Scholar]
- 15.Smith P.D., MacDonald T.T., Blumberg R.S., Society for Mucosal Immunology . Garland Science/Taylor & Francis Group; London: 2013. Principles of mucosal immunology. [Google Scholar]
- 16.Parodi A., Paolino S., Greco A., Drago F., Mansi C., Rebora A. Small intestinal bacterial overgrowth in rosacea: clinical effectiveness of its eradication. Clin Gastroenterol Hepatol. 2008;6:759–764. doi: 10.1016/j.cgh.2008.02.054. [DOI] [PubMed] [Google Scholar]
- 17.Wang M., Karlsson C., Olsson C., Adlerberth I., Wold A.E., Strachan D.P. Reduced diversity in the early fecal microbiota of infants with atopic eczema. J Allergy Clin Immunol. 2008;121:129–134. doi: 10.1016/j.jaci.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Forno E., Onderdonk A.B., McCracken J., Litonjua A.A., Laskey D., Delaney M.L. Diversity of the gut microbiota and eczema in early life. Clin Mol Allergy. 2008;6:11. doi: 10.1186/1476-7961-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zanvit P., Konkel J.E., Jiao X., Kasagi S., Zhang D., Wu R. Antibiotics in neonatal life increase murine susceptibility to experimental psoriasis. Nat Commun. 2015;6:8424. doi: 10.1038/ncomms9424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rigoni R., Fontana E., Guglielmetti S., Fosso B., D'Erchia A.M., Maina V. Intestinal microbiota sustains inflammation and autoimmunity induced by hypomorphic RAG defects. J Exp Med. 2016;213:355–375. doi: 10.1084/jem.20151116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuhlbrigge R.C., Kieffer J.D., Armerding D., Kupper T.S. Cutaneous lymphocyte antigen is a specialized form of PSGL-1 expressed on skin-homing T cells. Nature. 1997;389:978–981. doi: 10.1038/40166. [DOI] [PubMed] [Google Scholar]
- 22.Biedermann T., Schwarzler C., Lametschwandtner G., Thoma G., Carballido-Perrig N., Kund J. Targeting CLA/E-selectin interactions prevents CCR4-mediated recruitment of human Th2 memory cells to human skin in vivo. Eur J Immunol. 2002;32:3171–3180. doi: 10.1002/1521-4141(200211)32:11<3171::AID-IMMU3171>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 23.Marrella V., Poliani P.L., Casati A., Rucci F., Frascoli L., Gougeon M.L. A hypomorphic R229Q Rag2 mouse mutant recapitulates human Omenn syndrome. J Clin Invest. 2007;117:1260–1269. doi: 10.1172/JCI30928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Guzman Strong C., Wertz P.W., Wang C., Yang F., Meltzer P.S., Andl T. Lipid defect underlies selective skin barrier impairment of an epidermal-specific deletion of Gata-3. J Cell Biol. 2006;175:661–670. doi: 10.1083/jcb.200605057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aberg K.M., Man M.Q., Gallo R.L., Ganz T., Crumrine D., Brown B.E. Co-regulation and interdependence of the mammalian epidermal permeability and antimicrobial barriers. J Invest Dermatol. 2008;128:917–925. doi: 10.1038/sj.jid.5701099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwarz A., Bruhs A., Schwarz T. The short-chain fatty acid sodium butyrate functions as a regulator of the skin immune system. J Invest Dermatol. 2017;137:855–864. doi: 10.1016/j.jid.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 27.Komine M., Rao L.S., Freedberg I.M., Simon M., Milisavljevic V., Blumenberg M. Interleukin-1 induces transcription of keratin K6 in human epidermal keratinocytes. J Invest Dermatol. 2001;116:330–338. doi: 10.1046/j.1523-1747.2001.01249.x. [DOI] [PubMed] [Google Scholar]
- 28.Nestle F.O., Turka L.A., Nickoloff B.J. Characterization of dermal dendritic cells in psoriasis: autostimulation of T lymphocytes and induction of Th1 type cytokines. J Clin Invest. 1994;94:202–209. doi: 10.1172/JCI117308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pasparakis M., Haase I., Nestle F.O. Mechanisms regulating skin immunity and inflammation. Nat Rev Immunol. 2014;14:289–301. doi: 10.1038/nri3646. [DOI] [PubMed] [Google Scholar]
- 30.Elias P.M. The skin barrier as an innate immune element. Semin Immunopathol. 2007;29:3–14. doi: 10.1007/s00281-007-0060-9. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L.J. Type 1 interferons potential initiating factors linking skin wounds with psoriasis pathogenesis. Front Immunol. 2019;10:1440. doi: 10.3389/fimmu.2019.01440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Randolph G.J., Ivanov S., Zinselmeyer B.H., Scallan J.P. The lymphatic system: integral roles in immunity. Annu Rev Immunol. 2017;35:31–52. doi: 10.1146/annurev-immunol-041015-055354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liao S., Ruddle N.H. Synchrony of high endothelial venules and lymphatic vessels revealed by immunization. J Immunol. 2006;177:3369–3379. doi: 10.4049/jimmunol.177.5.3369. [DOI] [PubMed] [Google Scholar]
- 34.Albanesi C., Scarponi C., Sebastiani S., Cavani A., Federici M., Sozzani S. A cytokine-to-chemokine axis between T lymphocytes and keratinocytes can favor Th1 cell accumulation in chronic inflammatory skin diseases. J Leukoc Biol. 2001;70:617–623. [PubMed] [Google Scholar]
- 35.Fierro M.T., Comessatti A., Quaglino P., Ortoncelli M., Osella Abate S., Ponti R. Expression pattern of chemokine receptors and chemokine release in inflammatory erythroderma and Sezary syndrome. Dermatology. 2006;213:284–292. doi: 10.1159/000096191. [DOI] [PubMed] [Google Scholar]
- 36.Lonsdorf A.S., Hwang S.T., Enk A.H. Chemokine receptors in T-cell-mediated diseases of the skin. J Invest Dermatol. 2009;129:2552–2566. doi: 10.1038/jid.2009.122. [DOI] [PubMed] [Google Scholar]
- 37.Di Cesare A., Di Meglio P., Nestle F.O. A role for Th17 cells in the immunopathogenesis of atopic dermatitis? J Invest Dermatol. 2008;128:2569–2571. doi: 10.1038/jid.2008.283. [DOI] [PubMed] [Google Scholar]
- 38.Lim H.W., Lee J., Hillsamer P., Kim C.H. Human Th17 cells share major trafficking receptors with both polarized effector T cells and FOXP3+ regulatory T cells. J Immunol. 2008;180:122–129. doi: 10.4049/jimmunol.180.1.122. [DOI] [PubMed] [Google Scholar]
- 39.Andrew D.P., Ruffing N., Kim C.H., Miao W., Heath H., Li Y. C-C chemokine receptor 4 expression defines a major subset of circulating nonintestinal memory T cells of both Th1 and Th2 potential. J Immunol. 2001;166:103–111. doi: 10.4049/jimmunol.166.1.103. [DOI] [PubMed] [Google Scholar]
- 40.Charo I.F., Ransohoff R.M. The many roles of chemokines and chemokine receptors in inflammation. New Engl J Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 41.Subramaniam J.M., Whiteside G., McKeage K., Croxtall J.C. Mogamulizumab: first global approval. Drugs. 2012;72:1293–1298. doi: 10.2165/11631090-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 42.Song P.I., Park Y.M., Abraham T., Harten B., Zivony A., Neparidze N. Human keratinocytes express functional CD14 and Toll-like receptor 4. J Invest Dermatol. 2002;119:424–432. doi: 10.1046/j.1523-1747.2002.01847.x. [DOI] [PubMed] [Google Scholar]
- 43.Huang B.L., Chandra S., Shih D.Q. Skin manifestations of inflammatory bowel disease. Front Physiol. 2012;3:13. doi: 10.3389/fphys.2012.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leffler D.A., Green P.H., Fasano A. Extraintestinal manifestations of coeliac disease. Nat Rev Gastroenterol Hepatol. 2015;12:561–571. doi: 10.1038/nrgastro.2015.131. [DOI] [PubMed] [Google Scholar]
- 45.Weinstock L.B., Steinhoff M. Rosacea and small intestinal bacterial overgrowth: prevalence and response to rifaximin. J Am Acad Dermatol. 2013;68:875–876. doi: 10.1016/j.jaad.2012.11.038. [DOI] [PubMed] [Google Scholar]
- 46.Orange J.S. Congenital immunodeficiencies and sepsis. Pediatr Crit Care Med. 2005;6(3 Suppl):S99–S107. doi: 10.1097/01.PCC.0000164488.19810.DB. [DOI] [PubMed] [Google Scholar]
- 47.Naik S., Bouladoux N., Wilhelm C., Molloy M.J., Salcedo R., Kastenmuller W. Compartmentalized control of skin immunity by resident commensals. Science. 2012;337:1115–1119. doi: 10.1126/science.1225152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao Z., Tseng C.H., Strober B.E., Pei Z., Blaser M.J. Substantial alterations of the cutaneous bacterial biota in psoriatic lesions. PLoS One. 2008;3:e2719. doi: 10.1371/journal.pone.0002719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kong H.H., Oh J., Deming C., Conlan S., Grice E.A., Beatson M.A. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012;22:850–859. doi: 10.1101/gr.131029.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oyoshi M.K., Elkhal A., Scott J.E., Wurbel M.A., Hornick J.L., Campbell J.J. Epicutaneous challenge of orally immunized mice redirects antigen-specific gut-homing T cells to the skin. J Clin Invest. 2011;121:2210–2220. doi: 10.1172/JCI43586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rigoni R., Grassi F., Villa A., Cassani B. RAGs and BUGS: an alliance for autoimmunity. Gut Microbes. 2016;7:503–511. doi: 10.1080/19490976.2016.1228517. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- Nizza S.T., Campbell J.J. CD11b+ migratory dendritic cells mediate CD8 T cell cross-priming and cutaneous imprinting after topical immunization. PLoS One. 2014;9 doi: 10.1371/journal.pone.0091054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milani C., Hevia A., Foroni E., Duranti S., Turroni F., Lugli G.A. Assessing the fecal microbiota: an optimized ion torrent 16S rRNA gene-based analysis protocol. PLoS One. 2013;8 doi: 10.1371/journal.pone.0068739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segata N., Izard J., Waldron L., Gevers D., Miropolsky L., Garrett W.S. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myles I.A., Williams K.W., Reckhow J.D., Jammeh M.L., Pincus N.B., Sastalla I. Transplantation of human skin microbiota in models of atopic dermatitis. JCI Insight. 2016;1 doi: 10.1172/jci.insight.86955. [DOI] [PMC free article] [PubMed] [Google Scholar]