Abstract
Severe coronavirus disease 2019 (COVID-19) causes an uncontrolled activation of the innate immune response, resulting in acute respiratory distress syndrome and systemic inflammation. The effects of COVID-19–induced inflammation on cancer cells and their microenvironment are yet to be elucidated. Here, we formulate the hypothesis that COVID-19–associated inflammation may generate a microenvironment favorable to tumor cell proliferation and particularly to the reawakening of dormant cancer cells (DCCs). DCCs often survive treatment of primary tumors and populate premetastatic niches in the lungs and other organs, retaining the potential for metastatic outgrowth. DCCs reawakening may be promoted by several events associated to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, including activation of neutrophils and monocytes/macrophages, lymphopenia and an uncontrolled production of pro-inflammatory cytokines. Among pro-inflammatory factors produced during COVID-19, neutrophil extracellular traps (NETs) released by activated neutrophils have been specifically shown to activate premetastatic cancer cells disseminated in the lungs, suggesting they may be involved in DCCs reawakening in COVID-19 patients. If confirmed by further studies, the links between COVID-19, DCCs reactivation and tumor relapse may support the use of specific anti-inflammatory and anti-metastatic therapies in patients with COVID-19 and an active or previous cancer.
Keywords: coronavirus disease 2019, cancer, dormancy, relapse, inflammation, disseminated tumor cells, tumor microenvironment
Introduction
Since the beginning of acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, several studies have investigated the susceptibility and mortality of COVID-19 in cancer patients. A recent meta-analysis reported a high mortality in patients with COVID-19 and cancer (1), with a worse prognosis in case of progressive cancer, hematological cancer, recent antineoplastic therapies or surgical interventions (2–5). While the impact of cancer and anticancer therapies on COVID-19 mortality is beginning to be understood, several questions concerning the pathophysiology of SARS-CoV-2 infection in cancer patients remain unanswered. Among these, potential long-term effects of COVID-19 on cancer outcome have not yet been explored. In this Perspective, we formulate the hypothesis that severe COVID-19 may increase the risk of subsequent cancer recurrence by inducing the reactivation of dormant cancer cells (DCCs). According to this viewpoint, the major events occurring during severe COVID-19 such as immune-mediated tissue inflammation, impairment of T-cell and natural killer (NK) cell activity, neutrophil hyperactivation and thrombocytosis may collectively generate a temporary pro-tumorigenic microenvironment favorable to DCCs reawakening. Understanding the effects of COVID-19–induced inflammation on tumor cells and their microenvironment will be crucial for a thorough evaluation of the potential long-term risks of COVID-19 in cancer patients and for the implementation of anti-inflammatory and anti-metastatic therapeutic schedules.
Inflammation, Metastatic Reawakening, and Cancer Recurrence
Cancer recurrence can occur after the apparently successful treatment of solid tumors, due to the presence of residual neoplastic cells in the primary tumor area or at metastatic sites. Metastatic recurrence is responsible for over 90% of cancer deaths and depends on the ability of tumor cells to migrate, seed other organs and restart to proliferate, often after an asymptomatic period named metastatic dormancy (6). During this period, pre-metastatic cells implement an array of strategies to ensure their survival and escape from immune surveillance (7). Among such strategies, adopting a non-proliferative state allows DCCs to persist for long time at metastatic sites, giving rise to tumor recurrence years or even decades after diagnosis. The events responsible for DCCs reactivation are only partly understood. Although it cannot be excluded that cell-intrinsic signals such as additional oncogenic mutations may cause DCCs reawakening, several studies have linked metastatic recurrence to microenvironmental cues such as inflammatory or immune-mediated signals (8). Metastatic reawakening has been reported to be triggered by disruption of tissue homeostasis that usually occurs during acute or chronic inflammation (8, 9). Inflammation has been linked to metastatic recurrence in a variety of conditions including obesity (10) or surgical removal of primary tumors (11). Also pathogen-induced infection has been reported to promote the migration of cancer cells to metastatic sites (12) and the reactivation of dormant metastatic cells (13–15). In addition to metastatic reawakening, acute infection by respiratory viruses has been shown to induce an exhaustion of CD8+ T cells that may contribute to release pre-metastatic cells from immune-mediated control (16). Altogether, these evidences indicate that inflammation plays an important role in dictating cancer recurrence and suggest that a defined pro-inflammatory event such as pathogen-induced inflammation seems sufficient to promote DCCs reawakening and metastasis (13–15).
Role of ACE2 in COVID-19 and in the Regulation of Inflammatory Pathways
Shortly after the beginning of the COVID-19 pandemic, ACE2 was identified as the entry receptor for SARS-CoV-2 and the serine protease TMPRSS2 as the responsible for spike (S) protein priming (17). Following entry, the S protein is cleaved by endosomal acid proteases, thereby releasing the viral genome. Subsequent steps of viral replication, assembly and release have been described in detail for SARS-CoV (18). ACE2 downregulation upon viral infection triggers a cascade of events that contribute to the catastrophic consequences of severe COVID-19 (19). Since ACE2 has been reported to exert multiple anti-tumor effects including inhibition of cancer angiogenesis and metastasis, its downregulation may per se promote tumor progression (20–22). Second, ACE2 is responsible for the conversion of angiotensin II (AngII) to angiotensin 1–7, a process that plays an important role in the control of inflammation and cardiovascular homeostasis by the renin-angiotensin system (RAS) (23). An alteration in the respective levels of AngII/Ang(1–7) can result in vasoconstrictive, proinflammatory, and prothrombotic effects, possibly contributing to the renal and cardiovascular complications observed in COVID-19 patients (24). RAS imbalance that follows SARS-CoV-2 infection has been also proposed to be responsible for an increased expression of TGF-β and pro-inflammatory cytokines that collectively promote lung fibrosis (25). Importantly, the AngII/AT1R axis acts on a variety of non-immune cells to activate nuclear factor-κB (NF-κB), a transcription factor essential for inflammatory responses (26). Moreover, AngII stimulates the release of soluble IL-6 and the subsequent activation of STAT3 (27), contributing to activate the IL-6 amplifier ( Figure 1 ). NF-κB hyperactivation consequent to ACE2 downregulation cumulates with NF-kB activation induced by MyD88 and pattern recognition receptors activated by viral particles, thus becoming a central molecular event in coronavirus clinical picture (28). NF-κB is the most important molecule linking inflammation to cancer. NF-κB activation in cancer cells promotes proliferation, chemoresistance, epithelial-to-mesenchymal transition, stemness and invasion, while in the tumor microenvironment (TME) it stimulates angiogenesis and immune suppression, collectively supporting the metastatic process (29).
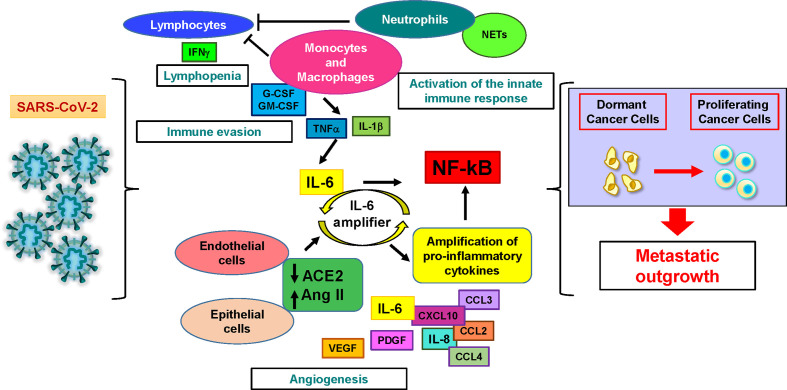
Figure 1.
SARS-CoV-2 infection may induce dormant cancer cell proliferation and metastatic relapse. Cellular and molecular factors involved in the pathogenesis of severe COVID-19 play also multiple roles in cancer. Lymphocytes are activated during the first phase of the disease and produce interferon-gamma (IFNγ), then their numbers and activity decrease, resulting in lymphopenia. Activated innate immune response cells (neutrophils and monocytes/macrophages) sustain immune evasion by depressing lymphocyte activity and hindering lymphocyte access to the tumor. They also trigger the production of interleukin-6 (IL-6), starting the systemic release of proinflammatory cytokines and chemoattractants by immune and non-immune cells. Interleukin-1β (IL-1β) and tumor necrosis factor α (TNF-α) further stimulate the production of IL-6. In virus-infected epithelial and endothelial cells, the downregulation of angiotensin-converting enzyme-2 (ACE2) that follows SARS-CoV-2 entry releases the brake from angiotensin II. This event stimulates additional IL-6 production by activating the IL-6 amplifier, a positive feedback loop leading to the uncontrolled production of pro-inflammatory factors. At the same time, neutrophil extracellular traps (NETs) generated by activated neutrophils physically obstruct the access of lymphocytes to inflamed tissues and promote the reawakening of dormant cancer cells. Additional cytokines increased during COVID-19 include granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF) (which stimulate neutrophil and monocyte expansion), platelet derived growth factor (PDGF) and vascular endothelial growth factor (VEGF) (which may contribute to tumor angiogenesis). All these events may generate a microenvironment favorable to the proliferation of dormant tumor cells and to subsequent metastatic outgrowth.
IL-6 and IL-1 Signaling in COVID-19 and Cancer
COVID-19 patients admitted to intensive care units usually develop acute respiratory distress and cytokine release syndrome (CRS), a life-threatening toxicity that may lead to sustained fever, edema, neurologic symptoms, organ failure and shock (26). Cytokines found to be elevated in the plasma of patients with severe COVID-19 include interleukin-1β (IL-1 β), IL-6, IL-7, IL-8, IL-9, IL-10, granulocyte colony stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon γ (IFN-γ), macrophage inflammatory proteins (MIP)-1α and β (also called CCL3 and CCL4), monocyte chemoattractant protein-1 (CCL2), C-X-C motif chemokine 10 (CXCL10), platelet derived growth factor (PDGF), tumor necrosis factor α (TNF-α), and vascular endothelial growth factor (VEGF) (30–32). IL-6 plays a central role in the pathophysiology of CRS. Accordingly, it was found to be elevated in hospitalized COVID-19 patients and associated with more severe form of the disease (30, 33). Uncontrolled IL-6 signaling may result in inflammation and tissue damage (34). During SARS-CoV-2 infection IL-6 signaling is activated in both immune and non-immune cells triggering the so-called IL-6 inflammation amplifier, a positive feedback loop resulting in the production of additional IL-6, of VEGF and of multiple chemoattractant proteins (35) ( Figure 1 ). In parallel to its key role in immune-mediated inflammation, IL-6 sustains tumorigenesis both through a direct stimulation of cancer cells and through an indirect action on the TME. IL-6 stimulates cancer growth, metastasis and immune evasion in several tumor types (36–42). Additionally, direct effects of IL-6 on cancer cells include an ability to promote stem cell characteristics (42–46), induction of mesenchymal traits (39) and resistance to therapies (47). IL-6 orchestrates the interactions between cancer cells and the TME by preparing the soil for cancer homing at target organs (36, 48) and has been also shown to stimulate immune evasion by inducing the stabilization of programmed death-ligand 1 (PD-L1) (38). A mechanistic link between IL-6 and DCCs reactivation is still missing, but elevated levels of IL-6 have been correlated with increased rates of tumor relapse in breast cancer and head and neck cancer (49, 50) while inhibition of IL-6/STAT3 signaling reduced cancer recurrence in preclinical models of breast, head and neck and hepatocellular carcinoma (51–54). IL-6 signaling is characterized by an extreme complexity, which derives at least in part by the promiscuous utilization of signaling components by multiple family members (55). In addition to its pro-tumorigenic effects, IL-6 has also been shown to exert anti-tumor effects by increasing T cell trafficking and adhesiveness to the tumor endothelium (56). Altogether, IL-6 may play multiple and possibly contrasting roles in patients with cancer and COVID-19, which will need to be addressed by future studies. Besides IL-6, also IL-1β has been reported to be elevated in COVID-19 patients as compared to controls (30). High doses of the recombinant IL-1R antagonist anakinra provided clinical improvement in COVID-19 patients (57). Similar to IL-6, also IL-1β has been reported to play a complex role in inflammation and cancer. The pro-tumorigenic role of IL-1β seems to be prevalent, as this cytokine drives chronic inflammation, recruits myeloid-derived suppressor cells (MDSCs), enhances neoangiogenesis and promotes invasion and metastasis (58). However, some anti-tumor effects of IL-1β have been reported (59). A mechanistic insight between IL-1β, inflammation and cancer was recently provided by Wellenstein and coworkers by showing that IL-1β is responsible for neutrophil expansion and neutrophilic inflammation that potentiates the metastatic progression of breast cancer (60). Future investigations will be essential to clarify whether IL-6, IL-1β, and other pro-inflammatory cytokines produced during COVID-19 may affect tumor cells and the TME, possibly supporting the use of anti-cytokine therapies in cancer patients with COVID-19.
Neutrophils and Neutrophil Extracellular Traps: Double Players in COVID-19 and Tumor Reawakening
Among cells of the innate immune system, neutrophils play a prominent role in fighting microbial infections but also in inflicting tissue damage. Furthermore, neutrophils are involved in a network of inflammatory reactions that promote all the stages of tumor initiation, progression, angiogenesis and metastasis (61, 62). Neutrophils generate reactive oxygen and nitrogen species, release proteases, arginase, ectonucleotidases, matrix metalloproteinases, prostaglandin E2, cyclooxygenases, IL-10 and TGFβ1 (62) and express Fas-ligand and PD-L1, which induce lymphocyte apoptosis and immune suppression (63, 64). Once they reach the tumor microenvironment, neutrophils may undergo transition to MDSCs that inhibit CD4+ and CD8+ tumor-infiltrating lymphocytes, as well as stimulating tumor growth, angiogenesis and metastasis (61). Besides releasing soluble pro-inflammatory factors, activated neutrophils produce Neutrophil Extracellular Traps (NETs), stretches of DNA and globular protein domains that aggregate into large 3D structures (65). NETs provide for a high local concentration of antimicrobial components and for a physical barrier preventing further pathogens spreading. However, NETs can also have a deleterious effect on host tissues, being involved in the pathogenesis of infectious, inflammatory and thrombotic disorders. An early study reported an intense neutrophilic infiltration of pulmonary interstitial spaces and alveoli, and proposed a role for NETs in COVID-19 (65). Lately, NET components have been detected in the sera of COVID-19 patients, being higher in cases requiring mechanical ventilation (66). Subsequently, the presence of NETs and of NET-specific marker myeloperoxidase/DNA complexes in COVID-19 patients was reported by other investigators (67–69) and was related to immunothrombosis (67). NETs have been also shown to play multiple roles in cancer. NETs contribute to tumor immune evasion by coating tumor cells and protecting them from CD8+ T cell- and NK-mediated cytotoxicity (70). Moreover, NETs have been reported to promote metastasis formation through several mechanisms including the capture of circulating tumor cells (71), the activation of pro-metastatic fibroblasts (72) and the proteolytic destruction of the antitumorigenic factor thrombospondin-1 (15). Importantly, NETs have been reported to awaken dormant breast cancer cells disseminated in the lungs. In fact, laminin destruction by NET-associated proteases can activate integrin signaling in lung-resident DCCs, triggering integrin-mediated activation of focal-adhesion kinase and ultimately resulting in tumor cell reactivation (13). In summary, neutrophils and their antimicrobial products may contribute not only to COVID-19-associated inflammation and immunothrombosis but also to the reawakening of DCCs disseminated in the lungs and possibly in other organs.
Potential Roles of Impaired T-Cell Responses in COVID-19 and DCCs Reawakening
Lymphopenia with drastically reduced numbers of circulating T cells (particularly striking for CD8+ T cells in patients requiring intensive care) and a functional impairment of NK cells have been consistently detected in severe COVID-19 cases (73). COVID-19–associated lymphopenia can be more severe and persistent as compared with other viral infections and seems to be more selective for T cell lineages (74). Also, it appears to impact prevalently CD8+ T cells, although also CD4+ T cells are affected (74). It is possible that the peripheral lymphopenia observed in COVID-19 patients reflects the recruitment of lymphocytes to the inflamed lungs. However, autopsy studies and single-cell RNA sequencing of bronchoalveolar lavage fluid did not highlight an excessive lymphocytic infiltration, suggesting that pulmonary sequestration of lymphocytes is not the main cause of lymphopenia in COVID-19 patients. More likely, the causes of lymphopenia during COVID-19 are multifactorial, possibly include extensive lymphocyte death, inhibition by the inflammatory cytokine milieu and indirect effects exerted by other cell types such as dendritic cells and neutrophils (73, 74). The impact of COVID-19–associated T cell and NK cell alterations on tumor cells is still unknown and will likely depend by context-specific and tumor-specific factors. However, both CD8+ T cells and NK cells have a crucial function in immune-mediated dormancy and their depletion has been shown to release the brakes from DCCs leading to metastatic outgrowth (75, 76). Latent cancer cells were also shown to persist long time by evading NK-mediated immune surveillance through downregulation of cell surface innate immune sensors (77). Therefore, lymphopenia may contribute, together with inflammation-related factors, to create a microenvironment favorable to metastatic reawakening.
Therapeutic Strategies for COVID-19 That Interfere With Pro-Tumorigenic Pathways
Hyper-inflammation crucially contributes to COVID-19 severity and patient death, and dexamethasone is the first drug shown to improve patient survival (78). Multiple anti-inflammatory agents are thus currently undergoing clinical evaluation for COVID-19, including not only corticosteroids but also biologicals that target inflammatory cytokines, such as anti-IL-6 or anti-IL-1β agents, and other immune-modulatory agents ( Table 1 ). Drugs that block IL-1β signaling may also inhibit the NET-IL-1β loop and decrease NETs formation (65). Additional drugs that interfere with NETs (although not specifically) are inhibitors of neutrophil elastase, recombinant DNases and colchicine (65). Trials evaluating the blockade of additional myeloid-derived inflammatory cytokines, such as TNF57 and granulocyte–macrophage colony- stimulating factor (GM–CSF) are also being considered and/or initiated (79). Other strategies to reduce hyperinflammation in patients include targeting common downstream mediators of cytokine signaling, such as JAK proteins (downstream of IL-6 receptor and several other cytokine receptors) (80, 81) or IRAK4 (that mediates Toll-like receptor (TLR) and IL-1β signaling). The Bruton’s tyrosine kinase inhibitor acalabrutinib, used for chronic lymphocytic leukemia (CLL) and involved in the inhibition of TLR7/8, has shown beneficial effects on CLL/COVID-19 patients (82) and is currently undergoing clinical trials for COVID-19. Drugs against chemokine receptors may reduce CRS in COVID-19 patients by inhibiting massive monocyte infiltration in the lungs or other organs. Accordingly, trials with anti-CCR5 (leronlimab) and anti CCR-2 (cenicriviroc) antibodies have been initiated in patients with COVID-19. Modulating the interferon response may also be useful in reducing inflammation during COVID-19. Clinical trials have been initiated testing the administration of type I interferons (IFNαβ) or type III interferon (IFNλ), which are potent activators of the antiviral response. By contrast, IFNγ production likely contributes to macrophage hyperactivation and tissue damage. Therefore, trials to evaluate IFNγ blockade with emapalumab are underway. In addition to short-term therapies aimed at reducing the deleterious consequences of COVID-19–associated hyperinflammation, long-term approaches to reduce the risks of inflammation-related metastasis may be evaluated by future clinical studies in cancer patients with COVID-19. Targeted approaches against critical receptors for metastatic niche components (such as integrin αvβ3) have been proposed as a feasible strategy to prevent DCCs reactivation (83). By contrast, long-term treatment of cancer patients with anti-inflammatory drugs should be carefully evaluated, as corticosteroids have been reported to increase breast cancer metastasis (84) while non-steroidal anti-inflammatory drugs increase the risk of venous thromboembolism (85). In all cases, strict adherence to recurrence monitoring schedules may be recommended for patients with COVID-19 and an ongoing or previous history of cancer.
Table 1.
Possible therapeutic targets linked to inflammation and cancer in COVID-19.
Clinical trials are indicated by clinicaltrials.gov identifier (NCT) number. For drugs whose clinical trials exceed three, the total number of trials available at the moment of submission is reported, with links leading to the relative trial list at clinicaltrials.gov. IL-6, interleukin-6; IL-1β, interleukin-1 beta; IL-1 βR, IL-1β receptor; CCR, CC- chemokine receptor; IFNγ, interferon gamma; JAK, Janus kinase; STAT, signal transducer and activator of transcription; NETs, neutrophil extracellular traps. N/A, not applicable.
Discussion
The hypothesis that severe COVID-19 may create a microenvironment favorable to cancer recurrence stems from the authors’ observation that several factors activated during coronavirus infection have been previously implicated in tumorigenesis and metastatic relapse. Recent studies on protein-protein interactions during COVID-19 revealed that common cancer pathways were targeted by SARS-CoV-2, including those involved in cell cycle progression, metabolism and epigenetics (86). However, the interactions between SARS-CoV-2, cancer cells and the immune system are currently unknown and will need to be investigated in detail, possibly with the use of complex in vitro models that reproduce multi-cellular microenvironments (87). By contrast, in vivo studies to explore COVID-19 and cancer recurrence would likely be challenging, as they should employ mice with multiple genetic modifications predisposing to both COVID-19 and cancer (such as mice transgenic for hACE2 and ErbB2/Neu). In parallel to preclinical studies, in our opinion it will be crucial to investigate all the clinical effects of COVID-19 in cancer patients. While the first large studies in this field have focused on the susceptibility and mortality of COVID-19 in cancer patients (3, 88, 89), new studies are investigating the relationships between anticancer therapies/interventions, single cancer types and immunological status. Additionally, it will be important to assess the long-term effects of severe COVID-19 in patients either with an active cancer, in remission or with a previous history of cancer. Understanding the links between COVID-19 and cancer recurrence may be a challenging task. For example, patients with severe COVID-19 often present concomitant clinical conditions predisposing to cancer recurrence (such as obesity or an immune-compromised state) that may complicate the evaluation of individual risk factors. Nevertheless, a recently launched observational study (CAPTURE, COVID-19 antiviral response in a pan-tumor immune monitoring study) (90) will assess long-term SARS-CoV-2 sequelae on cancer patients including the impact on cancer outcomes, helping to reveal potential effects of COVID-19 on cancer recurrence. In case future studies will confirm a link between severe COVID-19 and tumor recurrence, this finding may be used to schedule personalized treatments and follow-up programs for patients with both conditions. For example, prolonged anti-inflammatory therapies may be evaluated for cancer patients that experienced SARS-CoV-2 infection. Also, the use of drugs with double anticancer/anti-inflammatory action such as acalabrutinib (a Bruton’s tyrosine kinase inhibitor) or leronlimab (an antibody against CCR5 with anti-metastatic activity) is currently being evaluated in the COVID-19 setting ( Table 1 ) and in the future may find an increased use the treatment of cancer patients with COVID-19. Altogether, the observations presented in this Perspective suggest a possible link between COVID-19, inflammation and immune-mediated tumor reawakening that, if confirmed by future studies, may have important implications for the treatment and the long-term management of cancer patients.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author Contributions
AZ, FF, MLDA, and MBa wrote the manuscript. RR provided essential contribution in editing the manuscript. MBi provided essential expertise. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by an Italian Association for Cancer Research (AIRC) Investigator Grant to AZ (AIRC IG 2017 Ref: 20744).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1. Saini KS, Tagliamento M, Lambertini M, McNally R, Romano M, Leone M, et al. Mortality in patients with cancer and COVID-19: A systematic review and pooled analysis of 52 studies. Eur J Cancer (2020) 139:43–50. 10.1016/j.ejca.2020.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Archer JE, Odeh A, Ereidge S, Salem HK, Jones GP, Gardner A, et al. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet (2020) 396:27–38. 10.1016/S0140-6736(20)31182-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kuderer NM, Choueiri TK, Shah DP, Shyr Y, Rubinstein SM, Rivera DR, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet (2020) 395:1907–18. 10.1016/S0140-6736(20)31187-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee LY, Cazier J-B, Starkey T, Briggs SE, Arnold R, Bisht V, et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol (2020) 21:1309–16. 10.1016/S1470-2045(20)30442-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Robilotti EV, Babady NE, Mead PA, Rolling T, Perez-Johnston R, Bernardes M, et al. Determinants of COVID-19 disease severity in patients with cancer. Nat Med (2020) 26(8):1218–23. 10.1038/s41591-020-0979-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Seyfried TN, Huysentruyt LC. On the origin of cancer metastasis. Crit Rev Oncogen (2013) 18(1-2):43. 10.1615/CritRevOncog.v18.i1-2.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Phan TG, Croucher PI. The dormant cancer cell life cycle. Nat Rev Cancer (2020) 20(7):398–411. 10.1038/s41568-020-0263-0 [DOI] [PubMed] [Google Scholar]
- 8. Goddard ET, Bozic I, Riddell SR, Ghajar CM. Dormant tumour cells, their niches and the influence of immunity. Nat Cell Biol (2018) 20(11):1240–9. 10.1038/s41556-018-0214-0 [DOI] [PubMed] [Google Scholar]
- 9. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell (2010) 140(6):883–99. 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Quail DF, Olson OC, Bhardwaj P, Walsh LA, Akkari L, Quick ML, et al. Obesity alters the lung myeloid cell landscape to enhance breast cancer metastasis through IL5 and GM-CSF. Nat Cell Biol (2017) 19(8):974–87. 10.1038/ncb3578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hiller JG, Perry NJ, Poulogiannis G, Riedel B, Sloan EK. Perioperative events influence cancer recurrence risk after surgery. Nat Rev Clin Oncol (2018) 15(4):205. 10.1038/nrclinonc.2017.194 [DOI] [PubMed] [Google Scholar]
- 12. Yan L, Cai Q, Xu Y. The ubiquitin-CXCR4 axis plays an important role in acute lung infection-enhanced lung tumor metastasis. Clin Cancer Res (2013) 19(17):4706–16. 10.1158/1078-0432.CCR-13-0011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Albrengues J, Shields MA, Ng D, Park CG, Ambrico A, Poindexter ME, et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science (2018) 361(6409):eaao4227 10.1126/science.aao4227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De Cock JM, Shibue T, Dongre A, Keckesova Z, Reinhardt F, Weinberg RA. Inflammation Triggers Zeb1-Dependent Escape from Tumor Latency. Cancer Res (2016) 76(23):6778–84. 10.1158/0008-5472.CAN-16-0608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. El Rayes T, Catena R, Lee S, Stawowczyk M, Joshi N, Fischbach C, et al. Lung inflammation promotes metastasis through neutrophil protease-mediated degradation of Tsp-1. Proc Natl Acad Sci USA (2015) 112(52):16000–5. 10.1073/pnas.1507294112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Erickson JJ, Lu P, Wen S, Hastings AK, Gilchuk P, Joyce S, et al. Acute Viral Respiratory Infection Rapidly Induces a CD8+ T Cell Exhaustion-like Phenotype. J Immunol (2015) 195(9):4319–30. 10.4049/jimmunol.1403004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell (2020) 181(2):271–80 e8. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Du L, He Y, Zhou Y, Liu S, Zheng B-J, Jiang S. The spike protein of SARS-CoV—a target for vaccine and therapeutic development. Nat Rev Microbiol (2009) 7(3):226–36. 10.1038/nrmicro2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rivellese F, Prediletto E. ACE2 at the centre of COVID-19 from paucisymptomatic infections to severe pneumonia. Autoimmun Rev (2020) 19(6):102536. 10.1016/j.autrev.2020.102536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang Q, Lu S, Li T, Yu L, Zhang Y, Zeng H, et al. ACE2 inhibits breast cancer angiogenesis via suppressing the VEGFa/VEGFR2/ERK pathway. J Exp Clin Cancer Res (2019) 38(1):173. 10.1186/s13046-019-1156-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Feng Y, Wan H, Liu J, Zhang R, Ma Q, Han B, et al. The angiotensin-converting enzyme 2 in tumor growth and tumor-associated angiogenesis in non-small cell lung cancer. Oncol Rep (2010) 23(4):941–8. 10.3892/or_00000718 [DOI] [PubMed] [Google Scholar]
- 22. Yu C, Tang W, Wang Y, Shen Q, Wang B, Cai C, et al. Downregulation of ACE2/Ang-(1-7)/Mas axis promotes breast cancer metastasis by enhancing store-operated calcium entry. Cancer Lett (2016) 376(2):268–77. 10.1016/j.canlet.2016.04.006 [DOI] [PubMed] [Google Scholar]
- 23. Takimoto-Ohnishi E, Murakami K. Renin–angiotensin system research: from molecules to the whole body. J Physiol Sci (2019) 69(4):581–7. 10.1007/s12576-019-00679-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Franco R, Rivas-Santisteban R, Serrano-Marín J, Rodríguez-Pérez AI, Labandeira-García JL, Navarro G. SARS-CoV-2 as a Factor to Disbalance the Renin–Angiotensin System: A Suspect in the Case of Exacerbated IL-6 Production. J Immunol (2020) 205(5):1198–206. 10.4049/jimmunol.2000642 [DOI] [PubMed] [Google Scholar]
- 25. Delpino M, Quarleri J. SARS-CoV-2 Pathogenesis: Imbalance in the Renin-Angiotensin System Favors Lung Fibrosis. Front Cell Infect Microbiol (2020) 10:340. 10.3389/fcimb.2020.00340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hirano T, Murakami M. COVID-19: A New Virus, but a Familiar Receptor and Cytokine Release Syndrome. Immunity (2020) 52(5):731–3. 10.1016/j.immuni.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Murakami M, Kamimura D, Hirano T. Pleiotropy and Specificity: Insights from the Interleukin 6 Family of Cytokines. Immunity (2019) 50(4):812–31. 10.1016/j.immuni.2019.03.027 [DOI] [PubMed] [Google Scholar]
- 28. de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol (2016) 14(8):523–34. 10.1038/nrmicro.2016.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Taniguchi K, Karin M. NF-kappaB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol (2018) 18(5):309–24. 10.1038/nri.2017.142 [DOI] [PubMed] [Google Scholar]
- 30. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (2020) 395(10223):497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet (2020) 395(10224):565–74. 10.1016/S0140-6736(20)30251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu J, Li S, Liu J, Liang B, Wang X, Wang H, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine (2020) 55:102763. 10.1016/j.ebiom.2020.102763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (2020) 395(10229):1054–62. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kang S, Tanaka T, Narazaki M, Kishimoto T. Targeting Interleukin-6 Signaling in Clinic. Immunity (2019) 50(4):1007–23. 10.1016/j.immuni.2019.03.026 [DOI] [PubMed] [Google Scholar]
- 35. Atsumi T, Singh R, Sabharwal L, Bando H, Meng J, Arima Y, et al. Inflammation amplifier, a new paradigm in cancer biology. Cancer Res (2014) 74(1):8–14. 10.1158/0008-5472.CAN-13-2322 [DOI] [PubMed] [Google Scholar]
- 36. Lee JW, Stone ML, Porrett PM, Thomas SK, Komar CA, Li JH, et al. Hepatocytes direct the formation of a pro-metastatic niche in the liver. Nature (2019) 567(7747):249–52. 10.1038/s41586-019-1004-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li S, Wang N, Brodt P. Metastatic cells can escape the proapoptotic effects of TNF-alpha through increased autocrine IL-6/STAT3 signaling. Cancer Res (2012) 72(4):865–75. 10.1158/0008-5472.CAN-11-1357 [DOI] [PubMed] [Google Scholar]
- 38. Chan LC, Li CW, Xia W, Hsu JM, Lee HH, Cha JH, et al. IL-6/JAK1 pathway drives PD-L1 Y112 phosphorylation to promote cancer immune evasion. J Clin Invest (2019) 129(8):3324–38. 10.1172/JCI126022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sullivan NJ, Sasser AK, Axel AE, Vesuna F, Raman V, Ramirez N, et al. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene (2009) 28(33):2940–7. 10.1038/onc.2009.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huang L, Hu B, Ni J, Wu J, Jiang W, Chen C, et al. Transcriptional repression of SOCS3 mediated by IL-6/STAT3 signaling via DNMT1 promotes pancreatic cancer growth and metastasis. J Exp Clin Cancer Res (2016) 35:27. 10.1186/s13046-016-0301-7 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41. Itoh H, Kadomatsu T, Tanoue H, Yugami M, Miyata K, Endo M, et al. TET2-dependent IL-6 induction mediated by the tumor microenvironment promotes tumor metastasis in osteosarcoma. Oncogene (2018) 37(22):2903–20. 10.1038/s41388-018-0160-0 [DOI] [PubMed] [Google Scholar]
- 42. Wang YC, Wu YS, Hung CY, Wang SA, Young MJ, Hsu TI, et al. USP24 induces IL-6 in tumor-associated microenvironment by stabilizing p300 and beta-TrCP and promotes cancer malignancy. Nat Commun (2018) 9(1):3996. 10.1038/s41467-018-06178-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Balamurugan K, Mendoza-Villanueva D, Sharan S, Summers GH, Dobrolecki LE, Lewis MT, et al. C/EBPdelta links IL-6 and HIF-1 signaling to promote breast cancer stem cell-associated phenotypes. Oncogene (2019) 38(20):3765–80. 10.1038/s41388-018-0516-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gallo M, Frezzetti D, Roma C, Chicchinelli N, Barbieri A, Arra C, et al. RANTES and IL-6 cooperate in inducing a more aggressive phenotype in breast cancer cells. Oncotarget (2018) 9(25):17543–53. 10.18632/oncotarget.24784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rodrigues CFD, Serrano E, Patricio MI, Val MM, Albuquerque P, Fonseca J, et al. Stroma-derived IL-6, G-CSF and Activin-A mediated dedifferentiation of lung carcinoma cells into cancer stem cells. Sci Rep (2018) 8(1):11573. 10.1038/s41598-018-29947-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang T, Song P, Zhong T, Wang X, Xiang X, Liu Q, et al. The inflammatory cytokine IL-6 induces FRA1 deacetylation promoting colorectal cancer stem-like properties. Oncogene (2019) 38(25):4932–47. 10.1038/s41388-019-0763-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang Y, Zong X, Mitra S, Mitra AK, Matei D, Nephew KP. IL-6 mediates platinum-induced enrichment of ovarian cancer stem cells. JCI Insight (2018) 3(23):e122360. 10.1172/jci.insight.122360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gross AC, Cam H, Phelps DA, Saraf AJ, Bid HK, Cam M, et al. IL-6 and CXCL8 mediate osteosarcoma-lung interactions critical to metastasis. JCI Insight (2018) 3(16):e99791. 10.1172/jci.insight.99791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Meyer F, Samson É, Douville P, Duchesne T, Liu G, Bairati I. Serum prognostic markers in head and neck cancer. Clin Cancer Res (2010) 16(3):1008–15. 10.1158/1078-0432.CCR-09-2014 [DOI] [PubMed] [Google Scholar]
- 50. Semesiuk N, Zhylchuk A, Bezdenezhnykh N, Lykhova A, Vorontsova A, Zhylchuk V, et al. Disseminated tumor cells and enhanced level of some cytokines in bone marrow and peripheral blood of breast cancer patients as predictive factors of tumor progression. Exp Oncol (2013) 35,№ 4):295–302. [PubMed] [Google Scholar]
- 51. Chang T-S, Wu Y-C, Chi C-C, Su W-C, Chang P-J, Lee K-F, et al. Activation of IL6/IGFIR confers poor prognosis of HBV-related hepatocellular carcinoma through induction of OCT4/NANOG expression. Clin Cancer Res (2015) 21(1):201–10. 10.1158/1078-0432.CCR-13-3274 [DOI] [PubMed] [Google Scholar]
- 52. Finkel KA, Warner KA, Kerk S, Bradford CR, McLean SA, Prince ME, et al. IL-6 inhibition with MEDI5117 decreases the fraction of head and neck cancer stem cells and prevents tumor recurrence. Neoplasia (2016) 18(5):273–81. 10.1016/j.neo.2016.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lai S-C, Su Y-T, Chi C-C, Kuo Y-C, Lee K-F, Wu Y-C, et al. DNMT3b/OCT4 expression confers sorafenib resistance and poor prognosis of hepatocellular carcinoma through IL-6/STAT3 regulation. J Exp Clin Cancer Res (2019) 38(1):1–18. 10.1186/s13046-019-1442-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liao D, Liu Z, Wrasidlo WJ, Luo Y, Nguyen G, Chen T, et al. Targeted therapeutic remodeling of the tumor microenvironment improves an HER-2 DNA vaccine and prevents recurrence in a murine breast cancer model. Cancer Res (2011) 71(17):5688–96. 10.1158/0008-5472.CAN-11-1264 [DOI] [PubMed] [Google Scholar]
- 55. Jones SA, Jenkins BJ. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat Rev Immunol (2018) 18(12):773–89. 10.1038/s41577-018-0066-7 [DOI] [PubMed] [Google Scholar]
- 56. Fisher DT, Appenheimer MM, Evans SS. The two faces of IL-6 in the tumor microenvironment. Semin Immunol 26(1):38–47. 10.1016/j.smim.2014.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cavalli G, De Luca G, Campochiaro C, Della-Torre E, Ripa M, Canetti D, et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol (2020) 2:E325–31. 10.1016/S2665-9913(20)30127-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mantovani A, Barajon I, Garlanda C. IL-1 and IL-1 regulatory pathways in cancer progression and therapy. Immunol Rev (2018) 281(1):57–61. 10.1111/imr.12614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bent R, Moll L, Grabbe S, Bros M. Interleukin-1 beta—a friend or foe in malignancies? Int J Mol Sci (2018) 19(8):2155. 10.3390/ijms19082155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wellenstein MD, Coffelt SB, Duits DEM, van Miltenburg MH, Slagter M, de Rink I, et al. Loss of p53 triggers WNT-dependent systemic inflammation to drive breast cancer metastasis. Nature (2019) 572(7770):538–42. 10.1038/s41586-019-1450-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Leach J, Morton JP, Sansom OJ. Neutrophils: Homing in on the myeloid mechanisms of metastasis. Mol Immunol (2019) 110:69–76. 10.1016/j.molimm.2017.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rapoport BL, Steel HC, Theron AJ, Smit T, Anderson R. Role of the Neutrophil in the Pathogenesis of Advanced Cancer and Impaired Responsiveness to Therapy. Molecules (2020) 25(7):1618. 10.3390/molecules25071618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lu C, Redd PS, Lee JR, Savage N, Liu K. The expression profiles and regulation of PD-L1 in tumor-induced myeloid-derived suppressor cells. Oncoimmunology (2016) 5(12):e1247135. 10.1080/2162402X.2016.1247135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhu J, Powis de Tenbossche CG, Cane S, Colau D, van Baren N, Lurquin C, et al. Resistance to cancer immunotherapy mediated by apoptosis of tumor-infiltrating lymphocytes. Nat Commun (2017) 8(1):1404. 10.1038/s41467-017-00784-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. mkBarnes BJ, Adrover JM, Baxter-Stoltzfus A, Borczuk A, Cools-Lartigue J, Crawford JM, et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J Exp Med (2020) 217(6):e20200652. 10.1084/jem.20200652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA, et al. Neutrophil extracellular traps in COVID-19. JCI Insight (2020) 5:e138999. 10.1172/jci.insight.138999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Middleton EA, He XY, Denorme F, Campbell RA, Ng D, Salvatore SP, et al. Neutrophil Extracellular Traps (NETs) Contribute to Immunothrombosis in COVID-19 Acute Respiratory Distress Syndrome. Blood (2020) 136:1169–79. 10.1182/blood.2020007008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Radermecker C, Detrembleur N, Guiot J, Cavalier E, Henket M, d’Emal C, et al. Neutrophil extracellular traps infiltrate the lung airway, interstitial, and vascular compartments in severe COVID-19. J Exp Med (2020) 217(12):e20201012. 10.1084/jem.20201012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Veras FP, Pontelli M, Silva C, Toller-Kawahisa J, de Lima M, Nascimento D, et al. SARS-CoV-2 triggered neutrophil extracellular traps (NETs) mediate COVID-19 pathology. J Exp Med (2020) 217:Pe20201129. 10.1101/2020.06.08.20125823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Teijeira A, Garasa S, Gato M, Alfaro C, Migueliz I, Cirella A, et al. CXCR1 and CXCR2 Chemokine Receptor Agonists Produced by Tumors Induce Neutrophil Extracellular Traps that Interfere with Immune Cytotoxicity. Immunity (2020) 52:856–71. 10.1016/j.immuni.2020.03.001 [DOI] [PubMed] [Google Scholar]
- 71. Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest (2013) 123:3446–58. 10.1172/JCI67484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Takesue S, Ohuchida K, Shinkawa T, Otsubo Y, Matsumoto S, Sagara A, et al. Neutrophil extracellular traps promote liver micrometastasis in pancreatic ductal adenocarcinoma via the activation of cancerassociated fibroblasts. Int J Oncol (2020) 56(2):596–605. 10.3892/ijo.2019.4951 [DOI] [PubMed] [Google Scholar]
- 73. Vabret N, Britton GJ, Gruber C, Hegde S, Kim J, Kuksin M, et al. Immunology of COVID-19: Current State of the Science. Immunity (2020) 52(6):910–41. 10.1016/j.immuni.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chen Z, Wherry EJ. T cell responses in patients with COVID-19. Nat Rev Immunol (2020) 20:529–36. 10.1038/s41577-020-0402-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Eyles J, Puaux AL, Wang X, Toh B, Prakash C, Hong M, et al. Tumor cells disseminate early, but immunosurveillance limits metastatic outgrowth, in a mouse model of melanoma. J Clin Invest (2010) 120(6):2030–9. 10.1172/JCI42002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Romero I, Garrido C, Algarra I, Collado A, Garrido F, Garcia-Lora AM. T lymphocytes restrain spontaneous metastases in permanent dormancy. Cancer Res (2014) 74(7):1958–68. 10.1158/0008-5472.CAN-13-2084 [DOI] [PubMed] [Google Scholar]
- 77. Malladi S, Macalinao DG, Jin X, He L, Basnet H, Zou Y, et al. Metastatic Latency and Immune Evasion through Autocrine Inhibition of WNT. Cell (2016) 165(1):45–60. 10.1016/j.cell.2016.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Group RC. Dexamethasone in hospitalized patients with Covid-19—preliminary report. New Engl J Med (2020) NEJMoa2021436:1–11. 10.1056/NEJMoa2021436 [DOI] [Google Scholar]
- 79. Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol (2020) 20:355–62. 10.1038/s41577-020-0331-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yeleswaram S, Smith P, Burn T, Covington M, Juvekar A, Li Y, et al. Inhibition of cytokine signaling by ruxolitinib and implications for COVID-19 treatment. Clin Immunol (2020) 218:108517. 10.1016/j.clim.2020.108517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Stebbing J, Krishnan V, de Bono S, Ottaviani S, Casalini G, Richardson PJ, et al. Mechanism of baricitinib supports artificial intelligence-predicted testing in COVID-19 patients. EMBO Mol Med (2020) 12:e12697. 10.21203/rs.3.rs-23195/v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Thibaud S, Tremblay D, Bhalla S, Zimmerman B, Sigel K, Gabrilove J. Protective role of Bruton tyrosine kinase inhibitors in patients with chronic lymphocytic leukaemia and COVID-19. Br J Haematol (2020) 190:e73–6. 10.1111/bjh.16863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ghajar CM. Metastasis prevention by targeting the dormant niche. Nat Rev Cancer (2015) 15(4):238–47. 10.1038/nrc3910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Obradović MM, Hamelin B, Manevski N, Couto JP, Sethi A, Coissieux M-M, et al. Glucocorticoids promote breast cancer metastasis. Nature (2019) 567(7749):540–4. 10.1038/s41586-019-1019-4 [DOI] [PubMed] [Google Scholar]
- 85. Ungprasert P, Srivali N, Wijarnpreecha K, Charoenpong P, Knight EL. Non-steroidal anti-inflammatory drugs and risk of venous thromboembolism: a systematic review and meta-analysis. Rheumatology (2015) 54(4):736–42. 10.1093/rheumatology/keu408 [DOI] [PubMed] [Google Scholar]
- 86. Tutuncuoglu B, Cakir M, Batra J, Bouhaddou M, Eckhardt M, Gordon DE, et al. The Landscape of Human Cancer Proteins Targeted by SARS-CoV-2. Cancer Discovery (2020) 10:916–21. 10.1158/2159-8290.CD-20-0559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Fiorini E, Veghini L, Corbo V. Modeling Cell Communication in Cancer With Organoids: Making the Complex Simple. Front Cell Dev Biol (2020) 8:166. 10.3389/fcell.2020.00166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Garassino MC, Whisenant JG, Huang L-C, Trama A, Torri V, Agustoni F, et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol (2020) 21:914–22. 10.1016/S1470-2045(20)30314-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Group CC. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet (2020) 396:P27–38. 10.1016/S0140-6736(20)31182-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Au L, Boos LA, Swerdlow A, Byrne F, Shepherd ST, Fendler A, et al. Cancer, COVID-19, and antiviral immunity: the CAPTURE study. Cell (2020) 183:P4–10. 10.1016/j.cell.2020.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.