Summary
The universal importance of epigenetic regulation has become explicit over the last decade. There is now a detailed understanding of the molecular signatures and chromatin-modifying enzymes determining epigenetic regulation. For example, the trimethylation of lysine 27 at histone H3 by Polycomb complexes is a hallmark of silenced gene expression conserved across animal and plant kingdoms. The repressive activity of Polycomb complexes is balanced by the histone demethylase activity of Jumonji C-domain proteins. There has been a lot of research on Polycomb functions and H3K27 methylation; however, until recently, little was known about the role of histone H3K27 demethylases. Here, we review the role of Jumonji C-domain proteins from the plant development perspective. We will recall the history of histone lysine demethylation and explore the recent advances on the H3K27 demethylases in plant biology. Conserved and novel genomic functions of these epigenetic regulators will be discussed.
Subject Areas: Biological Sciences, Plant Biology, Plant Development
Graphical Abstract
Highlights
-
•
Jumonji C-domain proteins demethylate the repressive epigenetic mark H3K27me3
-
•
Plant H3K27me3 demethylases include ELF6, REF6, JMJ13, JMJ30, and JMJ32
-
•
Plant H3K27me3 demethylases regulate a wide range of developmental processes
Biological Sciences; Plant Biology; Plant Development
Introduction
H3K27me3 is a Hallmark of Epigenetic Gene Silencing
In eukaryotic cells, chromatin is the basic unit of genomic regulation. The chromatin fiber is composed by the tight association of genomic DNA, nucleosome histones, and accessory proteins. The C-terminal globular domains of histones are wrapped by DNA but the hydrophobic N-terminal domains protrude from the double-helix surface. These N-terminal tails are subject to a myriad of post-translational modifications including acetylation, ubiquitination, or methylation of specific amino acid residues (Kouzarides, 2007). The genome-wide occurrence of these histone modifications results from the coordinated action of specific “writer” modifying enzymes, “readers” effector proteins, and “erasers” that remove these modifications. A large number of studies over the last decades show that some histone modifications are context dependent and may have regulatory functions. For example, the trimethylation of histone H3 lysine 27 (H3K27me3) is associated with gene silencing, whereas the trimethylation of histone H3 lysine 36 (H3K36me3) is coupled with transcriptional elongation (Li et al., 2007). In addition, some histone modifications are considered epigenetic marks because they are maintained through development or even through generations in the absence of the initial pioneer signal or stimuli. The best example is H3K27me3, a repressive epigenetic mark involved in the maintenance of cellular fates during development of multicellular eukaryotes like plants or animals (Grossniklaus and Paro, 2014; Xiao and Wagner, 2014).
In the model plant Arabidopsis thaliana (hereinafter referred to as Arabidopsis), H3K27me3 decorates about 15–60% of protein coding genes in a given tissue (You et al., 2017; Zhang et al., 2007). H3K27me3 is set by the methyltransferase activity present in the polycomb repressive complex 2 (PRC2), first characterized in Drosophila and conserved from animals to plants (Förderer et al., 2016; Grossniklaus and Paro, 2014). There are three related SET (su(var)3–9, enhancer-of-zeste, trithorax) domain proteins that catalyze H3K27me3 in Arabidopsis: CURLY LEAF, SWINGER, and MEDEA. The importance of H3K27me3 in plant development is highlighted by the fact that plants strongly impaired in PRC2 function leads to embryo abortion or results in the formation of amorphous almost non-viable callus-like structures. Plant Polycomb histone methyltransferase complexes are especially important for the regulation of developmental transitions like flowering or seed formation and play a key role in plant developmental transitions and other processes that require preservation of pluripotency or differentiation. For example, Polycomb activity regulates flowering time at several levels by modulating the expression of master regulators like the floral integrators FLOWERING LOCUS T (FT) and SUPPRESSOR OF OVEREXPRESSION OF CO 1 (SOC1) or the floral repressor FLOWERING LOCUS C (FLC). All these functions of H3K27me3 methylation in plants associated to Polycomb activity have been extensively reviewed over recent years (Costa and Dean, 2019; Förderer et al., 2016; Grossniklaus and Paro, 2014; Schatlowski et al., 2008; Xiao and Wagner, 2014). However, until recently, the function of histone demethylases, the “erasers”, was underestimated in plant development.
The History of Histone Demethylation
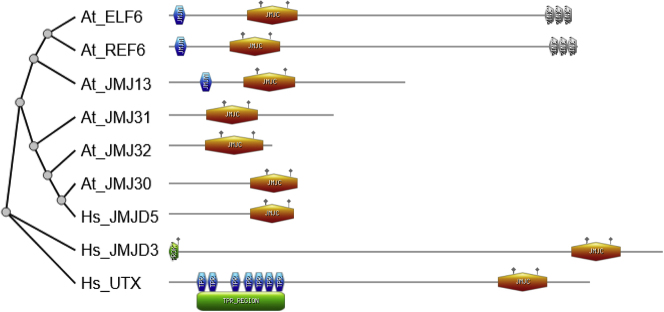
It was initially thought that histone methylation could be only passively removed through cell division. In 2004, a class of flavin adenine dinucleotide (FAD)-dependent amine oxidases were characterized as mono- and di-methylated histone demethylases in animals (Shi et al., 2004) and soon after homologs were characterized in plants (Liu et al., 2007). The final change in the dogma came with the discovery of the Jumonji C (JmjC) family of Fe(II)-dependent and 2-oxoglutarate-dependent dioxygenases as demethylases of tri-, di- and mono-methylated histones (Cloos et al., 2006; Klose et al., 2006; Tsukada et al., 2006). The protein Jumonji (meaning cruciform in Japanese) was named after the “cross-like” shape of neural grooves in jmj mutant mice (Takeuchi et al., 1995). The JmjC domains contain a conserved double-stranded B helix fold, and it is present in a large family of thousands of proteins (Markolovic et al., 2016). The JmjC protein family has a great complexity with an increased copy number and divergences in sequence and function that seems to contribute to the evolutionary success of animals and plants. There are 21 Arabidopsis JmjC proteins that can be classified in five groups according to phylogenetic information and protein domain architecture (Lu et al., 2008). A comprehensive phylogenetic analysis of the JmjC proteins in animals, plants, and fungi identified up to 14 monophyletic subfamilies including conserved and plant-specific groups (Qian et al., 2015). In animals, the main H3K27 demethylases are ubiquitously transcribed tetratricopeptide repeat gene on the X chromosome (UTX/KDM6A) and Jumonji domain-containing protein D3 (JMJD3/KDM6B) (Agger et al., 2007). These proteins regulate homeotic gene expression, embryonic development, cellular reprogramming, immune diseases, and, in particular, have a critical role in cancer (Arcipowski et al., 2016). JMJD3 and UTX homologs are not conserved in plants, but up to five proteins have been reported with H3K27me3 demethylase activity in Arabidopsis (Figure 1): the JmjC domain-only proteins JUMONJI 30 (JMJ30/AtJMJD5, AT3G20810) and JUMONJI 32 (JMJ32, AT3G45880) and the C2H2-type zinc-finger (ZnFn)-containing JmjC proteins EARLY FLOWERING 6 (ELF6/JMJ11, AT5G0424), RELATIVE OF ELF6 (REF6/JMJ12, AT3G48430), and JUMONJI 13 (JMJ13, AT5G46910). Here, we review the plant H3K27me3 demethylases with an emphasis on their functions as regulators of development.
Figure 1.
Phylogenetic Relationships among Histone H3K27 Demethylases
Left, the phylogenetic tree of selected Arabidopsis and human histone demethylases (ELF6 Q6BDA0, REF6 Q9STM3, JMJ13 F4KIX0, JMJ30 Q8RWR1, JMJ31 F4K2M8, JMJ32 Q0WVR4, UTX O15550, JMJD3 O15054, and JMJD5 A0A0S2Z5T1). The dendogram was obtained using PhyML + SMS/OneClick pipeline at https://ngphylogeny.fr. Right, graphical representations of the protein domains obtained from prosite (https://prosite.expasy.org/prosite.html). Legend: JMJN, Jumonji N domain; JMJC, Jumonji C-domain with metal catalytic sites; ZINC, zinc finger C2H2 type domain; PROK prokaryotic membrane lipoprotein lipid attachment site profile; TPR, tetratricopeptide repeat profile.
The JmjC Domain-Only Group
The Arabidopsis JmjC domain-only group consists of 3 members: JMJ30, JMJ31 (AT5G19840), and JMJ32 (Figure 1). These proteins belong to the monophyletic subfamily PKMD12 which is conserved in plants and animals (Qian et al., 2015). JMJ30 was first described as a circadian regulator that modulates the expression of the core clock components CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) and LATE ELONGATED HYPOCOTYL (LHY) (Jones et al., 2010). In due course, CCA1 and LHY directly repress JMJ30 gene expression, establishing a regulatory loop with this histone demethylase (Lu et al., 2011b). Consequently, jmj30 mutation affects the length of circadian output rhythms (Jones et al., 2010; Lu et al., 2011b). Interestingly, human cells deficient on the H3K36 demethylase Hs.JMJD5 (KDM8), the human homolog of JMJ30, show a short period phenotype as well (Jones et al., 2010).
In addition to the circadian cycle, Arabidopsis JMJ30 also influences several developmental processes. For example, JMJ30 promotes callus formation from leaf explants (Lee et al., 2018), contributes to abscisic acid-dependent growth arrest (Wu et al., 2019), and has been recently found important for plant heat acclimation (Yamaguchi et al., 2020). This chromatin factor is especially relevant in regulating flowering time. On one side, JMJ30 interacts with EARLY FLOWERING MYB PROTEIN (EFM) to regulate the expression of the floral integrator FT (Yan et al., 2014). On the other hand, JMJ30 and JM32 participate in the flowering thermosensory pathway (Gan et al., 2014). The jmj30 jmj32 double mutant was found to be early flowering at high ambient temperature (29°C) but not at standard growth conditions (22°C). This early flowering phenotype was associated with reduced expression and increased H3K27 methylation at FLC locus (Gan et al., 2014).
Nevertheless, the specificity of the JmjC domain-only demethylases is not totally clear. Gan and collaborators found that JMJ30 and JMJ32 are able to demethylate H3K27me3 and H3K27me2 in vitro and in vivo (Gan et al., 2014). However, other authors propose that JMJ30 can demethylate H3K36me3 (Yan et al., 2014), and another group found that jmj30 mutation alters H3K9me3 levels at target genes (Lee et al., 2018). In addition, JMJ31 function remains unknown and it may not be an active demethylase because it lacks conserved amino acids (Gan et al., 2014; Lu et al., 2011b). Further genomic and biochemical studies are needed to conclude the specificity of the Arabidopsis JmjC domain-only histone demethylases.
The ZnFn-Containing JmjC H3K27 Demethylases
The plant-specific monophyletic subfamily PKMD9 includes three Arabidopsis ZnFn-containing JmjC domain proteins (Qian et al., 2015). ELF6 and REF6 are plant-specific JmjC homolog proteins that carry four N-terminal C2H2-type ZnFn motifs (Figure 1). JMJ13 is phylogenetically related to ELF6 and REF6 but lacks the ZnFn N-terminal domain. ELF6, REF6, and JMJ13 are nuclear proteins with different expression profiles. REF6 is widely expressed across the plant, whereas ELF6 is expressed at lower levels and mainly at floral buds and during embryo development (Crevillén et al., 2014; Lu et al., 2008; Noh et al., 2004). Conversely, JMJ13 exhibits lower expression levels in vegetative tissues but it is the most expressed plant-specific H3K27me3 demethylases in pollen (Borg et al., 2020).
In vivo Nicotiana benthamiana leaf-based enzymatic activity assays have shown that ELF6 (Crevillén et al., 2014), REF6 (Lu et al., 2011a), and JMJ13 (Zheng et al., 2019) are able to demethylate H3K27me3; however. only ELF6 and REF6 were found to demethylate H3K27me2. None of these three enzymes seem to have any activity in vivo on histone H3 lysine K4, K9, or K36 methylation, although REF6 has been found to demethylate H3K4me3 and H3K36me3 using in vitro based assays (Ko et al., 2010). All these data suggest that these related epigenetic modifier enzymes have specialized functions in the genome.
REF6
REF6 was the first reported plant H3K27me3 demethylase, and it has a key role in a number of developmental processes. Initially, the ref6 mutant was described as late flowering because it has high levels of FLC expression (Noh et al., 2004). However, the exact mechanism of REF6 regulating floral transition remains elusive because REF6 does not bind to FLC locus (Cui et al., 2016; Li et al., 2016; Yang et al., 2016). REF6 acts on floral development at several levels. It has been proposed that REF6 interacts with Nuclear Factor Y proteins (NF-Y) to regulate SOC1 expression in inflorescences (Hou et al., 2014) and that SOC1 aids the recruitment of REF6 to regulate downstream targets such as FRUITFUL (FUL) (Hyun et al., 2016) and TARGET OF FLC AND SVP 1 (TFS1) (Richter et al., 2019). KNUCKLES (KNU), a repressor of the stem cell pool regulator WUSCHEL (WUS), is activated by REF6 during floral development as well (Yan et al., 2018). All these data illustrate the important role of REF6 orchestrating the derepression of H3K27me3-silenced genes during floral meristem development. Accordingly, REF6 overexpressing plants resembled Polycomb mutants and show pleiotropic developmental defects like small plant size and wrinkled and curled leaves due the upregulated expression of floral homeotic genes in seedlings (Lu et al., 2011a).
Further, REF6 has also a role in brassinosteroid (BR) signaling. REF6 and ELF6 proteins were isolated as interactors of BRI1-EMS-SUPPRESSOR 1 (BES1), and both elf6 and ref6 mutants have a reduced cell elongation phenotype, characterized by shorter leaf petioles compared to wildtype plants (Yu et al., 2008). It has been shown that REF6 directly regulates the expression of a number of BR-responsive genes including the cell wall-modifying enzyme TOUCH 4 (TCH4) and the flavin monooxygenase YUCCA 3 (YUC3) (Cui et al., 2016; Li et al., 2016; Lu et al., 2011a). In addition to flowering and BR signaling, REF6 participates in a number of other developmental processes. REF6 modulates the expression of ETHYLENE INSENSITIVE 2 (EIN2), a central regulator of the ethylene signaling pathway (Zander et al., 2019). REF6 also promotes lateral roots formation through H3K27me3 demethylation of PIN-FORMED auxin efflux carrier genes (Wang et al., 2018) and directly controls NONYELLOWING 1 (NYE1) expression, a regulator of chlorophyll degradation during leaf senescence (Wang et al., 2019). It has also been proposed that REF6 and HEAT SHOCK TRANSCRIPTION FACTOR A2 (HSFA2) establish a positive feedback loop to transmit the transgenerational epigenetic memory of heat (Liu et al., 2019).
All the data indicate that REF6 is a general histone demethylase which is able to engage in a wide number of H3K27me3-regulated processes. But how is REF6 able to recognize all its target regulatory regions? Chromatin immunoprecipitation followed by high-throughput sequencing (ChIP-seq) studies have shown that REF6 protein directly binds thousands of genomic regions in seedlings (Cui et al., 2016; Li et al., 2016) and flowers (Yan et al., 2018). In fact, biochemical studies indicate that the REF6 ZnFn domain can bind DNA in a sequence-specific manner. Genomic DNA motif discovery analyses defined that REF6 binds to a specific CTCTGYTY motif, where Y represents T or C (Cui et al., 2016; Li et al., 2016, 2018a). This DNA-protein interaction has been proposed to define a novel half-cross braced ZnFn-type domain (Tian et al., 2020). Interestingly, REF6 binding to DNA is inhibited by DNA methylation which explains why REF6 is depleted from heterochromatin regions in the genome (Qiu et al., 2019; Tian et al., 2020).
The REF6 DNA binding domain is crucial for flowering time regulation (Cui et al., 2016; Yan et al., 2018). However, the REF6ΔZnF truncated protein was found to bind to thousands of genomic sites when expressed in a ref6 elf6 jmj13 triple mutant background (Yan et al., 2018). Consistently, a full knock-out ref6 CRISPR/Cas9 mutant line showed a stronger phenotype than ref6-1 mutant (which carries a T-DNA insertion at the ZnFn domain) (Yan et al., 2018). Thus, although important for target recognition and binding stability, some REF6 functions rely on specific interactions with transcription factors or other chromatin modifiers. A well-known REF6 partner is BRHAMA (BRM), an ATP-dependent SWI/SNF-related chromatin remodeller. REF6 co-purified with BRM protein complexes and BRM co-occupied up to 40% of REF6 target genes in seedlings (Li et al., 2016). Consistent with these data, REF6 and BRM regulate together HSF2A and TFS1 gene expression (Hyun et al., 2016; Liu et al., 2019; Richter et al., 2019). REF6 also interacts with a number of transcription factors. For example, REF6 has been found to co-purify with a number of MADS-box transcription factors including SOC1, AGAMOUS (AG), SEPALLATA3 (SEP3), APETALA3 (AP3), and APATELA1 (AP1) in inflorescences (Smaczniak et al., 2012; Yan et al., 2018). Interestingly, some of these protein interactions seem to happen independently of the REF6 ZnFn domain (Yan et al., 2018).
ELF6
Mutations in the ELF6 gene resulted in early flowering plants in long and short day photoperiods (Noh et al., 2004). The elf6 mutant early flowering phenotype is due to the downregulation of the floral repressor FLC (Crevillén et al., 2014) and subsequent upregulation of the florigen FT expression (Jeong et al., 2009; Noh et al., 2004). ELF6 protein binds directly to the FLC locus and interacts with SET DOMAIN GROUP 8 (SDG8), an H3K36me3 methyltransferase that enhances gene expression by promoting transcriptional elongation (Shafiq et al., 2013; Yang et al., 2016). Thus, the concerted action of gene activation by SDG8 and derepression by ELF6 maintain an active transcriptional state at FLC locus (Yang et al., 2016). As mentioned earlier, ELF6 interacts with BES1 linking BR signaling to chromatin regulation (Yu et al., 2008). In fact, it has been proposed that BZR1 (BRASSINAZOLE-RESISTANT 1), a homolog of the transcription factor BES1, recruits ELF6 to a specific FLC intronic regulatory region facilitating FLC locus activation (Li et al., 2018b).
Some Arabidopsis ecotypes carry a functional FRIGIDA (FRI) allele, a potent activator of FLC expression. These winter accessions are extremely late flowering and require prolonged cold exposure to epigenetically silence FLC expression, and eventually flower, in a process called vernalization (Costa and Dean, 2019). The epigenetic silencing of FLC involves H3K27me3 Polycomb-mediated histone methylation and it is maintained until embryogenesis, when FLC expression is reset to ensure a vernalization requirement in the next generation (Schatlowski et al., 2008). Strikingly, an elf6 hypomorphic allele was found in a genetic screening looking for mutants impaired in the resetting of FLC expression after vernalization (Crevillén et al., 2014). The molecular characterization of this elf6 hypomorphic allele demonstrated that ELF6 is required for the epigenetic reprogramming of H3K27m3 at the FLC locus after vernalization (Crevillén et al., 2014). This is one of the few examples of an environmentally induced transgenerational inheritance.
In summary, it is well established how FLC is regulated by ELF6. In addition, it has been reported that ELF6 may bind the FT locus (Jeong et al., 2009). However, no other ELF6 direct target has been reported, and the precise role of ELF6 beyond FLC and flowering time regulation remains largely unexplored.
JMJ13
The third ZnFn-containing JmjC H3K27 demethylases is JMJ13, a protein phylogenetically related to ELF6 and REF6 that carries a C4HCHC-type helical-zinc finger cassette fused to the JmjC domain (Zheng et al., 2019). Mutations at JMJ13 do not cause strong developmental alterations but, as with its close homologs, JMJ13 modulates flowering time (Zheng et al., 2019). JMJ13 regulates FLC expression redundantly with ELF6 (Yang et al., 2016) and when mutated confers an early flowering phenotype under long day conditions (Yan et al., 2018; Zheng et al., 2019). Under short day photoperiod, the jmj13 mutant is early flowering only at high ambient temperature suggesting a temperature-dependent role in flowering time regulation (Zheng et al., 2019). Strikingly, a recent report shows that JMJ13 is the most abundant H3K27 demethylase in pollen sperm cells, suggesting that JMJ13 contributes to paternal H3K27me3 resetting (Borg et al., 2020). These and other data lead Borg and collaborators to propose that H3K27me3 demethylases play a key role in a multi-layered genome wide epigenetic reprogramming ensuring that epigenetic marks are erased and not passed to the next generation (Borg et al., 2020; Crevillén et al., 2014).
Redundant Roles of ZnFn-Containing JmjC H3K27 Demethylases
As discussed above, specific H3K27 demethylases play an important role in a number of developmental processes and plant responses to biotic and abiotic stresses. REF6 is a general H3K27me3 demethylase with roles in a number of developmental processes (Lu et al., 2011a); JMJ13 contributes to the genome wide H3K27me3 erasure during gamete formation (Borg et al., 2020); and ELF6 is the main H3K27 demethylases regulating the floral repressor FLC (Crevillén et al., 2014; Li et al., 2018b).
On the other hand, there is some functional redundancy among plant H3K27 demethylases. The elf6 jmj13 double mutant, in an FRI background, showed increased flowering time acceleration and reduced FLC mRNA levels compared to a single elf6 mutant, indicating that ELF6 and JM13 are partially redundant in the regulation of FLC locus expression (Yang et al., 2016). Recent reports also suggest that REF6 and ELF6 may have independent and partially redundant roles in the regulation of genomic H3K27me3 profiles (Antunez-Sanchez and Gutierrez-Marcos, 2020). In fact, elf6 ref6 double full knockout mutants show a dwarf phenotype and pleiotropic defects in leaf morphology not observed in single mutant plants (Antunez-Sanchez and Gutierrez-Marcos, 2020; Yan et al., 2018). Furthermore, this pleiotropic phenotype is enhanced in the elf6 ref6 jmj13 triple mutant, which shows a strong dwarf phenotype, wrinkled leaves, reduced plant size, and floral organ alterations (Yan et al., 2018). Interestingly, this pleiotropic phenotype is not additive with mutations in the JmjC domain-only proteins JMJ30 and JMJ32 suggesting independent roles of these two families of plant histone demethylases (Yan et al., 2018).
It could be expected that this functional redundancy may hide unexpected new roles of the histone demethylases. For example, it has been recently proposed that impaired ELF6 and REF6 activity during sexual reproduction may result in the transgenerational inheritance of ectopic H3K27me3 imprints (Antunez-Sanchez and Gutierrez-Marcos, 2020). These H3K27me3 imprints were formed in elf6 ref6 double mutant backcrossed to wildtype plants and were stably transmitted over generations even after wildtype histone demethylase function was restored (Antunez-Sanchez and Gutierrez-Marcos, 2020).
Genomic Functions of ZnFn-Containing JmjC H3K27 Demethylases
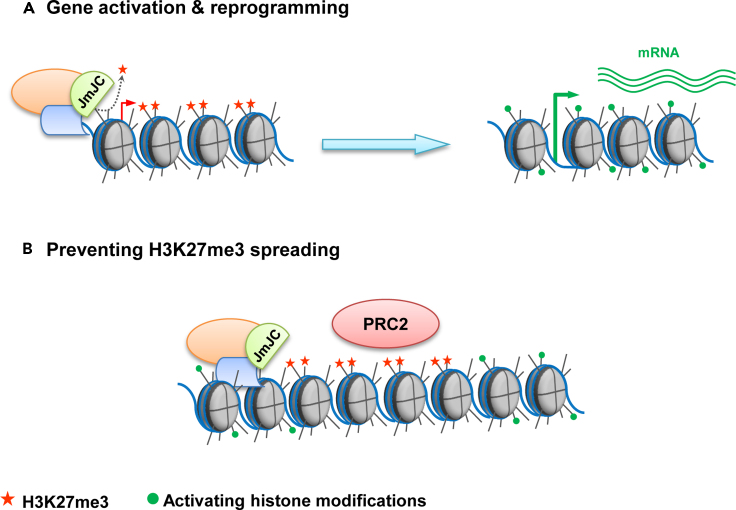
We have discussed that one of the main functions of H3K27 demethylases is to modulate gene expression and to reactivate H3K27m3 Polycomb-repressed genes in response to external or internal plant stimuli (Figure 2A). In fact, consistent with the myriad of REF6 functions, ref6 mutants show thousands of hypermethylated H3K27me3 regions across the genome (Antunez-Sanchez and Gutierrez-Marcos, 2020; Cui et al., 2016; Li et al., 2016; Yan et al., 2018). The number of hypermethylated regions compared to wildtype plants is reduced in epigenomic analyses of elf6 (Antunez-Sanchez and Gutierrez-Marcos, 2020) and it is much less prominent in jmj13 mutants (Zheng et al., 2019). Consistent with the partial functional redundancy among these proteins, H3K27me3 genomic analyses in the elf6 ref6 jmj13 triple mutant show an increased number of hypermethylated regions and misregulated genes compared to any single mutant (Yan et al., 2018).
Figure 2.
Genomic Functions of the Plant H3K27 Demethylases
The catalytic activity of H3K27 demethylases together with other promoting or repressing chromatin remodeling activities may fine-tune gene expression in different ways.
(A) The active removal of H3K27me3 from gene coding regions or regulatory regions across the genome is crucial for the re-activation of Polycomb-silenced genes during developmental transitions or in response to internal or external stimuli.
(B) Plant H3K27 demethylases also function delimiting Polycomb-silenced chromatin regions and preventing the uncontrolled spreading of the epigenetic silencing.
The characterization of the H3K27me3 landscape in the elf6 ref6 jmj13 triple mutant together with the study of REF6 genomic occupancy revealed another important role for plant H3K27me3 demethylases (Figure 2B). Interestingly, the binding patterns of Polycomb complex and the REF6 protein do not overlap. REF6 binding sites appear at the boundaries of H3K27me3 regions whereas Polycomb proteins cover H3K27me3 marked regions (Yan et al., 2018). In addition, most hypermethylated genes found in ref6 mutants appear at the boundary of gene coding regions. Thus, Yan and collaborators proposed that REF6 not only counteracts Polycomb silencing but also prevents H3K27me3 spreading and delimits Polycomb-silenced regions (Yan et al., 2018).
H3K27 Demethylation in Crops
JmjC proteins can be found in most plant lineages, and we are beginning to understand their role in some crop species. The best example is Os.JMJ705, the rice (Oriza sativa) REF6 homolog. Os.JMJ705 gene expression is stress induced, and during pathogen infection, Os.JMJ705 removes repressive H3K27 methylation from plant defense genes (Li et al., 2013). Consequently, an Os.jmj705 T-DNA insertion mutant was found more susceptible to pathogen infection, and transgenic Os.JMJ705 overexpression enhances the expression of jasmonic acid response genes (Li et al., 2013). In addition, it has been proposed that rice WOX11 recruits Os.JMJ705 to activate a number of genes involved in meristem identity, chloroplast biogenesis, and energy metabolism to promote shoot grow (Cheng et al., 2018). Therefore, Os.JMJ705 not only plays a role in abiotic stress but also is required for the proper development of the rice shoot meristem.
In other crop species, information is scarce, but H3K27 demethylases seem to influence important crop traits. For instance, a recent report has shown that tomato (Solanum lycopersicum) Sl.JMJ6 is an active H3K27me2/3 demethylase that is required for the activation of a number of fruit ripening-related genes (Li et al., 2020). Further research is needed to characterize the role of H3K27 demethylases in major crop species. It is envisaged that these combined insights into epigenetic regulators will have an impact on crop yield in the near future (Gallusci et al., 2017; Springer and Schmitz, 2017).
Concluding Remarks
The control of H3K27me3 genomic homeostasis may be achieved by the correct balance between the histone deposition, histone methylation, and histone demethylation machinery. We cannot rule out the existence of other active forms of H3K27 demethylation independently of the enzymes discussed here. However, it is quite remarkable that JmjC H3K27me3 demethylases play an important role in plant development. Despite the many studies, there are still many opened question: Are plant H3K27me3 demethylases part of multiprotein chromatin remodeling complexes? How is the activity of these epigenetic enzymes modulated? Do plant H3K27 demethylases have catalytic independent functions? For example, human UTX functions as a scaffold protein facilitating the binding of other regulatory factors to target loci (Arcipowski et al., 2016). Further biochemical studies, cell-type-specific genomic profiles, and the use of modern genome editing technologies will be required to understand the precise role of these chromatin regulators in plant biology. It is tempting to speculate that natural or induced histone demethylase activity alterations may lead to epigenetic variation that, eventually, could be genetically fixed. The ability to unlock this knowledge will potentially improve modern genomic breeding technologies (Gallusci et al., 2017; Springer and Schmitz, 2017).
Acknowledgments
Thanks to Mark Wilkinson for helpful criticism of the manuscript. This work was supported by grant RTI2018-097749-B-100 to P.C. (Ministerio de Ciencia, Innovación y Universidades, Spain; Agencia Estatal de Investigación,Spain; FEDER, EU). Research at Centro de Biotecnología y Genómica de Plantas is supported by grant SEV-2016-0672 (2017–2021) from the "Severo Ochoa Program for Centres of Excellence in R&D” (Agencia Estatal de Investigación, Spain)."
References
- Agger K., Cloos P.A.C., Christensen J., Pasini D., Rose S., Rappsilber J., Issaeva I., Canaani E., Salcini A.E., Helin K. UTX and JMJD3 are histone H3K27 demethylases involved in HOX gene regulation and development. Nature. 2007;449:731–734. doi: 10.1038/nature06145. [DOI] [PubMed] [Google Scholar]
- Antunez-Sanchez J., Gutierrez-Marcos J. A new role for histone demethylases in the maintenance of plant genome integrity. bioXriv. 2020 doi: 10.1101/2020.03.02.972752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcipowski K.M., Martinez C.A., Ntziachristos P. Histone demethylases in physiology and cancer: a tale of two enzymes, JMJD3 and UTX. Curr. Opin. Genet. Dev. 2016;36:59–67. doi: 10.1016/j.gde.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg M., Jacob Y., Susaki D., LeBlanc C., Buendía D., Axelsson E., Kawashima T., Voigt P., Boavida L., Becker J. Targeted reprogramming of H3K27me3 resets epigenetic memory in plant paternal chromatin. Nat. Cell Biol. 2020;22:621–629. doi: 10.1038/s41556-020-0515-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S., Tan F., Lu Y., Liu X., Li T., Yuan W., Zhao Y., Zhou D.-X. WOX11 recruits a histone H3K27me3 demethylase to promote gene expression during shoot development in rice. Nucleic Acids Res. 2018;46:2356–2369. doi: 10.1093/nar/gky017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloos P.A.C., Christensen J., Agger K., Maiolica A., Rappsilber J., Antal T., Hansen K.H., Helin K. The putative oncogene GASC1 demethylates tri- and dimethylated lysine 9 on histone H3. Nature. 2006;442:307–311. doi: 10.1038/nature04837. [DOI] [PubMed] [Google Scholar]
- Costa S., Dean C. Storing memories: the distinct phases of Polycomb-mediated silencing of Arabidopsis FLC. Biochem. Soc. Trans. 2019;47:1187–1196. doi: 10.1042/BST20190255. [DOI] [PubMed] [Google Scholar]
- Crevillén P., Yang H., Cui X., Greeff C., Trick M., Qiu Q., Cao X., Dean C. Epigenetic reprogramming that prevents transgenerational inheritance of the vernalized state. Nature. 2014;515:587–590. doi: 10.1038/nature13722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Xia, Lu F., Qiu Q., Zhou B., Gu L., Zhang S., Kang Y., Cui Xiekui, Ma X., Yao Q. REF6 recognizes a specific DNA sequence to demethylate H3K27me3 and regulate organ boundary formation in Arabidopsis. Nat. Genet. 2016;48:694–699. doi: 10.1038/ng.3556. [DOI] [PubMed] [Google Scholar]
- Förderer A., Zhou Y., Turck F. The age of multiplexity: recruitment and interactions of Polycomb complexes in plants. Curr. Opin. Plant Biol. 2016;29:169–178. doi: 10.1016/j.pbi.2015.11.010. [DOI] [PubMed] [Google Scholar]
- Gallusci P., Dai C., Génard M., Gauffretau A., Leblanc-Fournier N., Richard-Molard C., Vile D., Brunel-Muguet S. Epigenetics for plant improvement: current knowledge and modeling avenues. Trends Plant Sci. 2017;22:610–623. doi: 10.1016/j.tplants.2017.04.009. [DOI] [PubMed] [Google Scholar]
- Gan E.-S., Xu Y., Wong J.-Y., Geraldine Goh J., Sun B., Wee W.-Y., Huang J., Ito T. Jumonji demethylases moderate precocious flowering at elevated temperature via regulation of FLC in Arabidopsis. Nat. Commun. 2014;5:5098. doi: 10.1038/ncomms6098. [DOI] [PubMed] [Google Scholar]
- Grossniklaus U., Paro R. Transcriptional silencing by polycomb-group proteins. Cold Spring Harb. Perspect. Biol. 2014;6:a019331. doi: 10.1101/cshperspect.a019331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X., Zhou J., Liu C., Liu L., Shen L., Yu H. Nuclear factor Y-mediated H3K27me3 demethylation of the SOC1 locus orchestrates flowering responses of Arabidopsis. Nat. Commun. 2014;5:4601. doi: 10.1038/ncomms5601. [DOI] [PubMed] [Google Scholar]
- Hyun Y., Richter R., Vincent C., Martinez-Gallegos R., Porri A., Coupland G. Multi-layered regulation of SPL15 and cooperation with SOC1 integrate endogenous flowering pathways at the Arabidopsis shoot meristem. Dev. Cell. 2016;37:1–13. doi: 10.1016/j.devcel.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Jeong J.-H., Song H.-R., Ko J.-H., Jeong Y.-M., Kwon Y.E., Seol J.H., Amasino R.M., Noh B., Noh Y.-S. Repression of flowering locus T chromatin by functionally redundant histone H3 lysine 4 demethylases in Arabidopsis. PLoS One. 2009;4:e8033. doi: 10.1371/journal.pone.0008033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M.A., Covington M.F., DiTacchio L., Vollmers C., Panda S., Harmer S.L. Jumonji domain protein JMJD5 functions in both the plant and human circadian systems. Proc. Natl. Acad. Sci. U. S. A. 2010;107:21623–21628. doi: 10.1073/pnas.1014204108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose R.J., Yamane K., Bae Y., Zhang D., Erdjument-Bromage H., Tempst P., Wong J., Zhang Y. The transcriptional repressor JHDM3A demethylates trimethyl histone H3 lysine 9 and lysine 36. Nature. 2006;442:312–316. doi: 10.1038/nature04853. [DOI] [PubMed] [Google Scholar]
- Ko J.-H., Mitina I., Tamada Y., Hyun Y., Choi Y., Amasino R.M., Noh B., Noh Y.-S. Growth habit determination by the balance of histone methylation activities in Arabidopsis. EMBO J. 2010;29:3208–3215. doi: 10.1038/emboj.2010.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Lee K., Park O., Seo P.J. JMJ30-mediated H3K9me3 demethylation drives tissue identity changes to promote callus formation in Arabidopsis. Plant J. 2018;95:961–975. doi: 10.1111/tpj.14002. [DOI] [PubMed] [Google Scholar]
- Li B., Carey M., Workman J.L. The role of chromatin during transcription. Cell. 2007;128:707–719. doi: 10.1016/j.cell.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Li C., Chen C., Chen H., Wang S., Chen X., Cui Y. Verification of DNA motifs in Arabidopsis using CRISPR/Cas9-mediated mutagenesis. Plant Biotechnol. J. 2018;16:1446–1451. doi: 10.1111/pbi.12886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Gu L., Gao L., Chen C., Wei C.-Q., Qiu Q., Chien C.-W., Wang S., Jiang L., Ai L.-F. Concerted genomic targeting of H3K27 demethylase REF6 and chromatin-remodeling ATPase BRM in Arabidopsis. Nat. Genet. 2016;48:687–693. doi: 10.1038/ng.3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Chen X., Zhong X., Zhao Y., Liu X., Zhou S., Cheng S., Zhou D.-X. Jumonji C protein jmj705-mediated removal of histone H3 lysine 27 trimethylation is involved in defense-related gene activation in rice. Plant Cell. 2013;25:4725–4736. doi: 10.1105/tpc.113.118802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Jiang G., Liu X., Ding X., Zhang D., Wang X., Zhou Y., Yan H., Li T., Wu K. Histone demethylase SlJMJ6 promotes fruit ripening by removing H3K27 methylation of ripening-related genes in tomato. New Phytol. 2020;227:1138–1156. doi: 10.1111/nph.16590. [DOI] [PubMed] [Google Scholar]
- Li Z., Ou Y., Zhang Z., Li J., He Y. Brassinosteroid signaling recruits histone 3 lysine-27 demethylation activity to FLOWERING LOCUS C chromatin to inhibit the floral transition in Arabidopsis. Mol. Plant. 2018;11:1135–1146. doi: 10.1016/j.molp.2018.06.007. [DOI] [PubMed] [Google Scholar]
- Liu F., Quesada V., Crevillén P., Bäurle I., Swiezewski S., Dean C. The Arabidopsis RNA-binding protein FCA requires a lysine-specific demethylase 1 homolog to downregulate FLC. Mol. Cell. 2007;28:398–407. doi: 10.1016/j.molcel.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Liu J., Feng L., Gu X., Deng X., Qiu Q., Li Q., Zhang Y., Wang M., Deng Y., Wang E. An H3K27me3 demethylase-HSFA2 regulatory loop orchestrates transgenerational thermomemory in Arabidopsis. Cell Res. 2019;29:379–390. doi: 10.1038/s41422-019-0145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F., Cui X., Zhang S., Jenuwein T., Cao X. Arabidopsis REF6 is a histone H3 lysine 27 demethylase. Nat. Genet. 2011;43:715–719. doi: 10.1038/ng.854. [DOI] [PubMed] [Google Scholar]
- Lu F., Li G., Cui X., Liu C., Wang X.-J., Cao X. Comparative analysis of JmjC domain-containing proteins reveals the potential histone demethylases in Arabidopsis and rice. J. Integr. Plant Biol. 2008;50:886–896. doi: 10.1111/j.1744-7909.2008.00692.x. [DOI] [PubMed] [Google Scholar]
- Lu S.X., Knowles S.M., Webb C.J., Celaya R.B., Cha C., Siu J.P., Tobin E.M. The Jumonji C domain-containing protein JMJ30 regulates period length in the Arabidopsis circadian clock. Plant Physiol. 2011;155:906–915. doi: 10.1104/pp.110.167015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markolovic S., Leissing T.M., Chowdhury R., Wilkins S.E., Lu X., Schofield C.J. Structure-function relationships of human JmjC oxygenases - demethylases versus hydroxylases. Curr. Opin. Struct. Biol. 2016;41:62–72. doi: 10.1016/j.sbi.2016.05.013. [DOI] [PubMed] [Google Scholar]
- Noh B., Lee S., Kim H., Yi G., Shin E., Lee M., Jung K., Doyle M.R., Amasino R.M., Noh Y.-S. Divergent roles of a pair of homologous jumonji/zinc-finger-class transcription factor proteins in the regulation of Arabidopsis flowering time. Plant Cell. 2004;16:2601–2613. doi: 10.1105/tpc.104.025353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian S., Wang Y., Ma H., Zhang L. Expansion and functional divergence of the JmjC gene family: significance of duplications in ancestral angiosperms and vertebrates. Plant Physiol. 2015;168:1321–1337. doi: 10.1104/pp.15.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Q., Mei H., Deng X., He K., Wu B., Yao Q., Zhang J., Lu F., Ma J., Cao X. DNA methylation repels targeting of Arabidopsis REF6. Nat. Commun. 2019;10:2063. doi: 10.1038/s41467-019-10026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter R., Kinoshita A., Vincent C., Martinez-Gallegos R., Gao H., van Driel A.D., Hyun Y., Mateos J.L., Coupland G. Floral regulators FLC and SOC1 directly regulate expression of the B3-type transcription factor TARGET OF FLC AND SVP 1 at the Arabidopsis shoot apex via antagonistic chromatin modifications. PLoS Genet. 2019;15:e1008065. doi: 10.1371/journal.pgen.1008065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatlowski N., Creasey K., Goodrich J., Schubert D. Keeping plants in shape: polycomb-group genes and histone methylation. Semin. Cell Dev. Biol. 2008;19:547–553. doi: 10.1016/j.semcdb.2008.07.019. [DOI] [PubMed] [Google Scholar]
- Shafiq S., Berr A., Shen W.-H. Combinatorial functions of diverse histone methylations in Arabidopsis thaliana flowering time regulation. New Phytol. 2013;201:312–322. doi: 10.1111/nph.12493. [DOI] [PubMed] [Google Scholar]
- Shi Yujiang, Lan F., Matson C., Mulligan P., Whetstine J.R., Cole P.A., Casero R.A., Shi Yang. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Smaczniak C., Immink R.G.H., Muino J.M., Blanvillain R., Busscher M., Busscher-Lange J., Dinh Q.D., Liu S., Westphal A.H., Boeren S. Characterization of MADS-domain transcription factor complexes in Arabidopsis flower development. Proc. Natl. Acad. Sci. U S A. 2012;109:1560–1565. doi: 10.1073/pnas.1112871109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer N.M., Schmitz R.J. Exploiting induced and natural epigenetic variation for crop improvement. Nat. Rev. Genet. 2017;18:563–575. doi: 10.1038/nrg.2017.45. [DOI] [PubMed] [Google Scholar]
- Takeuchi T., Yamazaki Y., Katoh-Fukui Y., Tsuchiya R., Kondo S., Motoyama J., Higashinakagawa T. Gene trap capture of a novel mouse gene, jumonji, required for neural tube formation. Genes Dev. 1995;9:1211–1222. doi: 10.1101/gad.9.10.1211. [DOI] [PubMed] [Google Scholar]
- Tian Z., Li X., Li M., Wu W., Zhang M., Tang C., Li Z., Liu Y., Chen Z., Yang M. Crystal structures of REF6 and its complex with DNA reveal diverse recognition mechanisms. Cell Discov. 2020;6:17. doi: 10.1038/s41421-020-0150-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada Y., Fang J., Erdjument-Bromage H., Warren M.E., Borchers C.H., Tempst P., Zhang Y. Histone demethylation by a family of JmjC domain-containing proteins. Nature. 2006;439:811–816. doi: 10.1038/nature04433. [DOI] [PubMed] [Google Scholar]
- Wang X., Gao J., Gao S., Li Z., Kuai B., Ren G. REF6 promotes lateral root formation through de-repression of PIN1/3/7 genes. J. Integr. Plant Biol. 2018;61:383–387. doi: 10.1111/jipb.12726. [DOI] [PubMed] [Google Scholar]
- Wang X., Gao J., Gao S., Song Y., Yang Z., Kuai B. The H3K27me3 demethylase REF6 promotes leaf senescence through directly activating major senescence regulatory and functional genes in Arabidopsis. PLoS Genet. 2019;15:1–24. doi: 10.1371/journal.pgen.1008068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Ichihashi Y., Suzuki T., Shibata A., Shirasu K., Yamaguchi N., Ito T. Abscisic acid-dependent histone demethylation during post-germination growth arrest in Arabidopsis. Plant Cell Environ. 2019;42:2198–2214. doi: 10.1111/pce.13547. [DOI] [PubMed] [Google Scholar]
- Xiao J., Wagner D. Polycomb repression in the regulation of growth and development in Arabidopsis. Curr. Opin. Plant Biol. 2014;23:15–24. doi: 10.1016/j.pbi.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Yamaguchi N., Matsubara S., Yoshimizu K., Seki M., Hamada K., Kamitani M., Kurita Y., Inagaki S., Suzuki T., Gan E.-S. Removal of repressive histone marks creates epigenetic memory of recurring heat in Arabidopsis. bioRxiv. 2020:1–36. doi: 10.1101/2020.05.10.086611. [DOI] [Google Scholar]
- Yan W., Chen D., Smaczniak C., Engelhorn J., Liu H., Yang W., Graf A., Carles C.C., Zhou D.-X., Kaufmann K. Dynamic and spatial restriction of Polycomb activity by plant histone demethylases. Nat. Plants. 2018;4:681–689. doi: 10.1038/s41477-018-0219-5. [DOI] [PubMed] [Google Scholar]
- Yan Y., Shen L., Chen Y., Bao S., Thong Z., Yu H. A MYB-domain protein EFM mediates flowering responses to environmental cues in Arabidopsis. Dev. Cell. 2014;36:437–448. doi: 10.1016/j.devcel.2014.07.004. [DOI] [PubMed] [Google Scholar]
- Yang H., Howard M., Dean C. Physical coupling of activation and derepression activities to maintain an active transcriptional state at FLC. Proc. Natl. Acad. Sci. U S A. 2016;113:9369–9374. doi: 10.1073/pnas.1605733113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Y., Sawikowska A., Neumann M., Posé D., Capovilla G., Langenecker T., Neher R.A., Krajewski P., Schmid M. Temporal dynamics of gene expression and histone marks at the Arabidopsis shoot meristem during flowering. Nat. Commun. 2017;8:15120. doi: 10.1038/ncomms15120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Li L.L., Guo M., Chory J., Yin Y. Modulation of brassinosteroid-regulated gene expression by Jumonji domain-containing proteins ELF6 and REF6 in Arabidopsis. Proc. Natl. Acad. Sci. U. S. A. 2008;105:7618–7623. doi: 10.1073/pnas.0802254105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zander M., Willige B.C., He Y., Nguyen T.A., Langford A.E., Nehring R., Howell E., McGrath R., Bartlett A., Castanon R. Epigenetic silencing of a multifunctional plant stress regulator. Elife. 2019;8:e47835. doi: 10.7554/eLife.47835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Clarenz O., Cokus S., Bernatavichute Y.V., Pellegrini M., Goodrich J., Jacobsen S.E. Whole-genome analysis of histone H3 lysine 27 trimethylation in Arabidopsis. PLoS Biol. 2007;5:e129. doi: 10.1371/journal.pbio.0050129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S., Hu H., Ren H., Yang Z., Qiu Q., Qi W., Liu X., Chen X., Cui X., Li S. The Arabidopsis H3K27me3 demethylase JUMONJI 13 is a temperature and photoperiod dependent flowering repressor. Nat. Commun. 2019;10:1303. doi: 10.1038/s41467-019-09310-x. [DOI] [PMC free article] [PubMed] [Google Scholar]