Highlights
-
•
Histoplasmose is an unfrequented associated to esophageal carcinoma.
-
•
Pulmonary disease may interfere in the outcome after esophagectomy.
-
•
Vacuum treatment is a good option after anastomotic leakage.
Keywords: Esophageal cancer, Histoplasmosis, Endoscopic vacuum, Anastomotic leak, Case report
Abstract
Introduction
Esophagectomy is a challenging procedure associated with considerable morbidity. Previous pulmonary diseases, such as histoplasmosis fungal infection, may interfere in operative and postoperative outcomes after esophagectomy. Anastomotic leakage is one of the most feared complications after esophagectomy. However, new therapies developed such as vacuum procedure and esophageal prosthesis have been provenly beneficial.
Presentation of case
We present a case with squamous cell carcinoma of the mid esophagus portion on a young patient with a pulmonary histoplasmosis history. After a multidisciplinary board, the patient underwent transhiatal esophagectomy with gastric-pull up and cervical anastomosis due to pulmonary disease. The patient later developed an anastomotic leak with mediastinal abscess. We describe this complication's management via an endoscopic vacuum system, esophageal prosthesis, and exhibit a video illustrating the technique.
Discussion
We illustrate the management of esophageal cancer associated with previous pulmonary disease. Histoplasmosis may misunderstand the esophageal cancer staging, and it can contribute to anastomotic leakage occurrence. An endoscopic vacuum system is an excellent tool for treating esophagogastric anastomosis fistula after esophagectomy, even when the drainage is accumulated in the mediastinum. The esophageal prosthesis may be used after mediastinal abscess resolution.
Conclusion
Treatment of the association of esophageal cancer and histoplasmosis is feasible. However, care should be taken to avoid highly potential postoperative complications.
1. Introduction
Histoplasmosis is a fungal disease caused by a dimorphic fungus called Histoplasma capsulatum; contamination occurs via inhalation that can affect the patient systemically depending on the immunological status; the lung is the main affected organ [1]. Esophageal involvement is very rare and can manifest itself in two ways: Direct organ damage with the presence of ulcerations along the mucosal layer or extrinsic compression secondary to mediastinal lymph node enlargement [2,3]. Preexisting histoplasmosis is a challenge in the context of surgical treatment of esophageal cancer:
-
1.
Pulmonary sequelae can be confused with metastases [4];
-
2.
The resulting scar tissue may offer additional complexity such as adhesions and pulmonary perfusion to surgical procedure;
-
3.
Pulmonary previous infection might interfere in the incidence of surgical complications.
We describe here the management of a patient with pulmonary histoplasmosis and advanced esophageal cancer. We discuss this surgical approach including the management of a cervical anastomotic leak via a vacuum sponge. The following report was done in line with the SCARE criteria [5].
2. Case report
A 66-year-old male patient presented vomiting, dysphagia, and loss of 3 kg over a thirty-day period. He had a history of pulmonary histoplasmosis 46 years prior treated with Amphotericin B with a full clinical recovery. Other comorbidities include hypertension, glaucoma, and appendicitis at the age of 13 as well as smoking from 11 to 50 years of age 2 packs a day and ongoing use of cannabis.
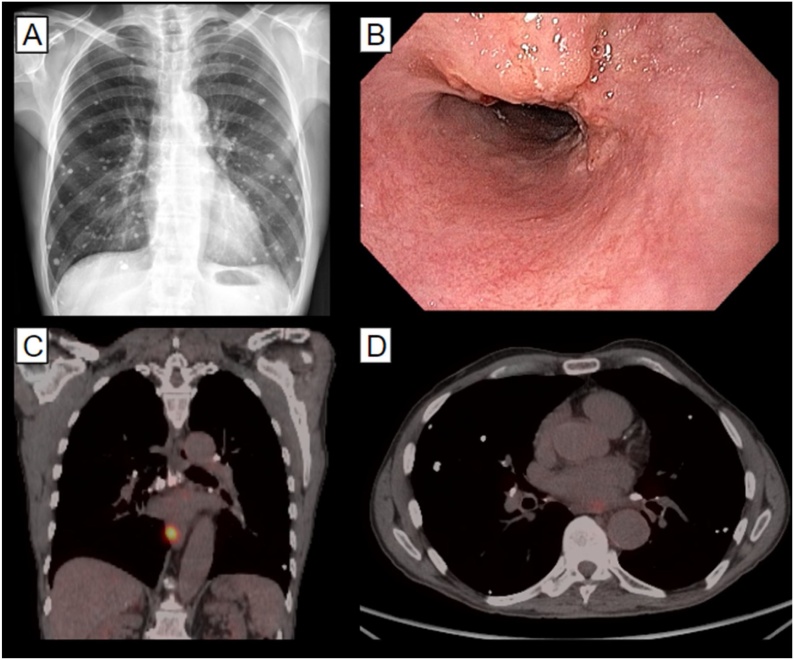
Upper digestive endoscopy revealed an ulcerative infiltrating lesion 32–35 cm from the incisors diagnosed through biopsy as a squamous cell carcinoma. This was staged as T2 N1 M0. Staging was done through positron emission tomography (PET-CT) and endoscopic ultrasound. The results showed an affected left para-esophageal lymph node. Chest imaging revealed many pulmonary nodules with signs of chronicity and calcification. These findings were attributed to the prior fungal infection; thus, they did not interfere in the staging process (Fig. 1).
Fig. 1.
Imaging studies and upper endoscopy on diagnosis. A: Chest x-ray showing multiple sequelary nodules; B: Upper endoscopy showing an ulcerated lesion; C: Positron-emission tomography (PET-CT) showing the primary lesion; D: PET-CT showing periesophageal lymph node enhancement.
The patient underwent chemo-radiotherapy with neoadjuvant intent receiving 4140 cGy in 23 fractions with one cycle of carboplatin plus paclitaxel. After discussion in a multidisciplinary board, it was decided to proceed with laparoscopic transhiatal esophagectomy with cervical anastomosis (McKeown procedure) because thoracoscopic access was deemed riskier due to pulmonary sequelae. Pathologic staging was ypTis, ypNo, with negative margins.
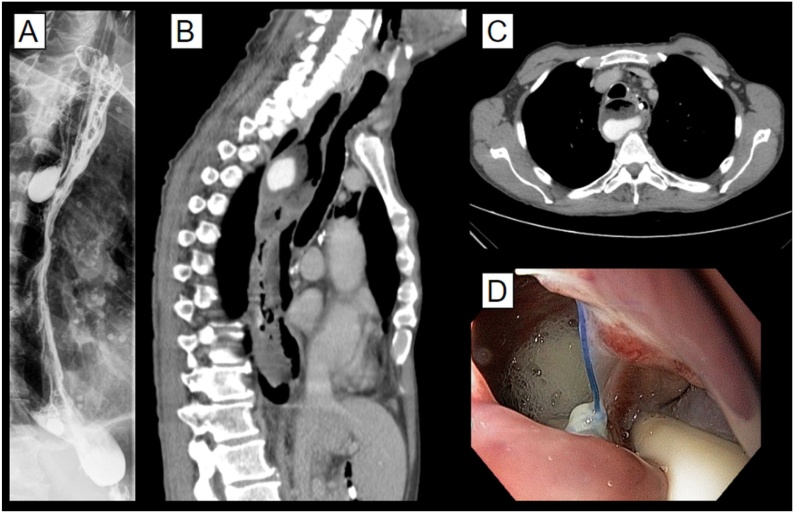
On the third postoperative day, the patient presented episodes of tachycardia, sweating, and elevated WBC count. Oral contrast X-ray and later CT scan were performed revealing a leak on the esophagogastric anastomosis and contiguous mediastinal fluid collection that was contemplated by the cervical drain (Fig. 2). Management was initially conservative with antibiotics, clinical support, and exclusive enteral tube feeding. Upon persistent mediastinal fluid collection, an endoscopic vacuum sponge system was installed, and an endoscopic jejunostomy was performed (see video).
Fig. 2.
Imaging studies at the time of the anastomotic leak. A: oral contrast chest X-ray showing contrast posterior to the gastric tube; B and C: Chest tomography shows similar findings; D: Upper endoscopy demonstrating a gap in the esophagogastric anastomosis, and the resulting mediastinal fluid collection.
The vacuum sponge was revised and exchanged on the 9th and on the 16th day showing progressive signs of cavity reduction and tissue granulation. On the 24th day, the vacuum system was removed, and a fully covered esophageal stent was placed. Oral contrast X-ray following stent placement revealed a small remaining leak and no fluid collection; thus, the patient was discharged from the hospital on exclusive enteral feeding after a stay of 53 days. An oral diet was reintroduced with no additional complications thirty days later when radiological studies and upper endoscopy showed no signs of anastomotic leakage. After two years of follow-up, the patient has a satisfactory oral intake, stable weight, and only mild symptoms with a self-reported good quality of life.
3. Discussion
Histoplasmosis is endemic to Brazil in all regions. It predominantly affects males in the fourth and fifth decade of life [1], and it may cause a pulmonary sequela. To the best of our knowledge, this is the first case report of esophageal cancer on a patient with a history of pulmonary histoplasmosis. As with other systemic fungal diseases, infection by histoplasma capsulatum may have a variety of manifestations—the most common includes multiple lung nodules [6], which must be differentiated from those of metastatic origin. These often present a challenge in this regard during staging [[7], [8], [9]].
Pulmonary histoplasmosis frequently courses with the formation of mediastinal granulomas, adhesions, and fibrosis. This feature determines a greater intraoperative challenge secondary to the obliteration of normal tissue planes [10] although there is no contraindication to surgical approaches in such cases; surgery under these conditions should be performed by an experienced team in centers with a large volume of cases. We consider thoracoscopic access to be an inherently riskier option and favor the transhiatal technique when applicable.
An anastomotic leak is a much-feared postoperative complication that is linked to high mortality and a prolonged hospital stay. Among the classically defined risk factors for leaks, there are many conditions that lead to microvascular damage and tissue hypoxia such as smoking, hypertension, diabetes, chronic kidney disease, and others [11]. In addition, an intraoperative drop in arterial pH below 7.25 can increase the risk of a leak [12]. Based on these principles, we propose that previous pulmonary infections and their sequelae may play a role in the development of this surgical complication as illustrated in this case.
A decreased blood supply to the anastomosed region is a key factor in the development of a leak. Specific surgical techniques can be used to prevent anastomotic leaks in high-risk patients such as microsurgical anastomosis of gastric tube vessels with the transverse cervical artery and vein. This showed promising results in a recent trial [13].
Once an esophageal anastomosis has developed a leak, the treatments available include conservative, surgical, and endoscopic approaches. Endoscopic treatment, endoluminal stenting, and vacuum therapy are among the most common strategies. A recent meta-analysis compared endoscopic techniques and vacuum therapy and demonstrated that vacuum with sponge provides a higher success rate for leak resolution with shorter hospital stays [14,15]. In the case evaluated here, sponge vacuum treatment played an essential role in the resolution of the complication.
As of today, no clear link between histoplasmosis and cancer has yet been established, but some studies hypothesize a possible association between esophageal candidiasis and squamous cell carcinoma [16] suggesting that fungal agents are a factor in oncogenesis although further research is needed on this topic.
4. Conclusion
Esophageal cancer is a complex disease with several challenges ranging from correct diagnosis and staging to management. In this context, a history of histoplasmosis presents an additional layer of difficulty surrounding the case. Knowledge of these issues as presented here may prove valuable to enhance patient care. The development of an anastomotic leak represents a severe complication and an endoscopic vacuum therapy is an effective treatment strategy as exemplified by the case presented.
Conflicts of interest
The authors declare no conflict of interest.
Funding
The authors received no specific funding for this work.
Ethical approval
Ethical approval exemption was given for this study.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
Flavio Roberto Takeda: Conceptualization, writing, formal analysis.
Rodrigo N Garcia: writing – review and editing.
Marcelo Simas de Lima: writing – review and editing radiological images.
Gustavo Gonçalves Yogolare: writing – review and editing.
Ulysses Ribeiro Junior: writing and Supervision.
Fauze Maluf Filho: review and supervision.
Rubens Antonio Aissar Sallum: review and supervision.
Ivan Cecconello: Supervision.
Registration of research studies
Not applicable.
Guarantor
Flávio Roberto Takeda.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Footnotes
Supplementary material related to this article can be found, in the online version, at https://doi.org/10.1016/j.ijscr.2020.10.107.
Contributor Information
Flavio Roberto Takeda, Email: flavio.takeda@hc.fm.usp.br.
Rodrigo Nicida Garcia, Email: Rodrigo.garcia@hc.fm.usp.br.
Marcelo Simas de Lima, Email: marcelotete@hotmail.com.
Gustavo Gonçalves Yogolare, Email: gustavoyogolare@gmail.com.
Ulysses Ribeiro Junior, Email: Ulysses.ribeiro@usp.br.
Fauze Maluf Filho, Email: fauze.maluf@terra.com.br.
Rubens Antonio Aissar Sallum, Email: rsallum007@gmail.com.
Ivan Cecconello, Email: icecconello@hotmail.com.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Almeida Marcosde Abreu. The occurrence of histoplasmosis in Brazil: a systematic review. Int. J. Infect. Dis. 2019;86:147–156. doi: 10.1016/j.ijid.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Marshall J.B., Singh R., Demmy T.L., Bickel J.T., Everett E.D. Mediastinal histoplasmosis presenting with esophageal involvement and dysphagia: case study. Dysphagia. 1995;10(1):53–58. doi: 10.1007/BF00261282. [DOI] [PubMed] [Google Scholar]
- 3.Finniss M., Lewis P., Myers J., Ibrahim L., Patel P. A case of gastrointestinal histoplasmosis with esophageal involvement. Clin. J. Gastroenterol. 2020;13(2):173–177. doi: 10.1007/s12328-019-01036-z. [DOI] [PubMed] [Google Scholar]
- 4.Dall Bello A.G., Severo C.B., Guazzelli L.S. Histoplasmosis mimicking primary lung cancer or pulmonary metastases. J. Bras. Pneumol. 2013;39:63–68. doi: 10.1590/S1806-37132013000100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agha R.A., Fowler A.J., Saetta A. A protocol for the development of reporting criteria for surgical case reports: the SCARE statement. Int. J. Surg. 2016;27:187–189. doi: 10.1016/j.ijsu.2016.01.094. [DOI] [PubMed] [Google Scholar]
- 6.PPTES Torres, Rabahi M.F., Moreira M.A.C., Santana P.R.P., Gomes A.C.P., Marchiori E. Tomographic assessment of thoracic fungal diseases: a pattern and signs approach. Radiol. Bras. 2018;51(5):313–321. doi: 10.1590/0100-3984.2017.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perko R., Messinger Y., Moertel C. Pseudometastasis secondary to histoplasmosis infection: false-positive PET/CT findings. Pediatr. Blood Cancer. 2010;54(4):621–623. doi: 10.1002/pbc.22363. [DOI] [PubMed] [Google Scholar]
- 8.Ye C., Zhang G., Wang J., Chai Y. Histoplasmosis presenting with solitary pulmonary nodule: two cases mimicking pulmonary metastases. Niger. J. Clin. Pract. 2015;18(2):304–306. doi: 10.4103/1119-3077.151075. [DOI] [PubMed] [Google Scholar]
- 9.Dall Bello A.G., Severo C.B., Oliveira Fde M., Hallal Junior R.J., Hochhergger B., Severo L.C. Histoplasmosis presenting with multiple pulmonary nodules. A case mimicking radiological features of pulmonary metastasis. Rev. Inst. Med. Trop. Sao Paulo. 2013;55(3) doi: 10.1590/S0036-46652013000300013. S0036-46652013000300209. [DOI] [PubMed] [Google Scholar]
- 10.Hammoud Z.T., Rose A.S., Hage C.A., Knox K.S., Rieger K., Kesler K.A. Surgical management of pulmonary and mediastinal sequelae of histoplasmosis: a challenging spectrum. Ann. Thorac. Surg. 2009;88(2):399–403. doi: 10.1016/j.athoracsur.2009.04.041. [DOI] [PubMed] [Google Scholar]
- 11.Kassis E.S., Kosinski A.S., Ross P., Jr., Koppes K.E., Donahue J.M., Daniel V.C. Predictors of anastomotic leak after esophagectomy: an analysis of the society of thoracic surgeons general thoracic database. Ann. Thorac. Surg. 2013;96(6):1919–1926. doi: 10.1016/j.athoracsur.2013.07.119. [DOI] [PubMed] [Google Scholar]
- 12.Goense L., van Rossum P.S., Tromp M. Intraoperative and postoperative risk factors for anastomotic leakage and pneumonia after esophagectomy for cancer. Dis. Esophagus. 2017;30(1):1–10. doi: 10.1111/dote.12517. [DOI] [PubMed] [Google Scholar]
- 13.Takeda F.R., Tutihashi R., Tustumi F. Supercharged cervical anastomosis for esophagectomy and gastric pull-up [published online ahead of print, 2020 Jun 26] J. Thorac. Cardiovasc. Surg. 2020 doi: 10.1016/j.jtcvs.2020.06.021. S0022-5223(20)31737-2. [DOI] [PubMed] [Google Scholar]
- 14.Rausa E., Asti E., Aiolfi A., Bianco F., Bonitta G., Bonavina L. Comparison of endoscopic vacuum therapy versus endoscopic stenting for esophageal leaks: systematic review and meta-analysis. Dis. Esophagus. 2018;31(11) doi: 10.1093/dote/doy060. [DOI] [PubMed] [Google Scholar]
- 15.Chevallay M., Jung M., Chon S.H., Takeda F.R., Akiyama J., Mönig S. Esophageal cancer surgery: review of complications and their management [published online ahead of print, 2020 Sep 15] Ann. N. Y. Acad. Sci. 2020 doi: 10.1111/nyas.14492. [DOI] [PubMed] [Google Scholar]
- 16.Delsing C.E., Bleeker-Rovers C.P., van de Veerdonk F.L. Association of esophageal candidiasis and squamous cell carcinoma. Med. Mycol. Case Rep. 2012;1(1):5–8. doi: 10.1016/j.mmcr.2012.02.003. Published 2012 Mar 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.