Abstract
Purpose
We report two patients who presented initially to ophthalmology clinics with symptoms and signs of orbital inflammation that led to a diagnosis of Erdheim-Chester Disease (ECD).
Observations
ECD is a rare form of non-Langerhans cell histiocytosis (LCH) which is characterised by multi-system organ involvement and poor prognosis with standard therapies. Both patients were positive for the BRAF V600E mutation on genetic testing and were treated with the BRAF inhibitors Vemurafenib and Dabrafenib respectively. These cases highlight the variable clinical presentation and course of ECD, the classical radiological and histopathological findings, and the high degree of clinical suspicion necessary to reach this diagnosis.
Conclusions and importance
The combination of xanthelasma and bilateral, diffuse intraconal orbital masses must suggest to the clinician the possibility of ECD; and consideration to arrange further investigation with a full body CT or FDG PET/CT scan should be given, even in the absence of wider systemic symptoms or signs. With the advent of targeted therapies such as BRAF inhibitors, it is of even more importance that a diagnosis of ECD is established in a timely manner in order to give these patients the best chance of reduced morbidity and increased survival.
Keywords: Histiocytosis, Erdheim-chester disease, Vemurafenib, BRAF, Orbital mass, Orbital inflammation
1. Introduction
Erdheim-Chester Disease (ECD) is a rare form of non-Langerhans cell histiocytosis (LCH) characterised by multi-system xanthogranulomotous infiltration by lipid-laden histiocytes.1 It was first described in two cases by the pathologists Jakob Erdheim and William Chester in 1930.2 Although there are now several hundred cases reported in the medical literature; there remains debate over the aetiology and pathogenesis of the disease. As of 2016, the World Health Organisation classified ECD as “a provisional entity under histiocytic and dendritic cell neoplasms, noting the association with B-rapidly accelerated fibrosarcoma gene (BRAF) mutations and the distinction from other members of the juvenile xanthogranuloma (JXG) family.3
Aside from the aetiological uncertainties, the complexity of ECD is amplified by a highly variable clinical presentation and course, ranging from an indolent focal disease to life-threatening multi-organ failure.4 The rarity of the disease coupled with the aforementioned characteristics means that many of the current treatments are based on anecdotal evidence from small studies or individual case reports and the overall prognosis remains poor in cases with multi-organ involvement.
ECD typically affects adults between the 5th and 7th decades with a relatively equal sex distribution. Histiocytic infiltration results in dense, progressive, recalcitrant fibrosclerosis of the bones, orbit and internal organs, including the mediastinal, pericardial, pleural, perinephric, and retroperitoneal spaces.5 From an ophthalmic perspective, orbital involvement in ECD is present in 27% of cases and is typically bilateral; often presenting as exophthalmos due to retro-orbital infiltration which is predominantly diffuse and intraconal.6
We report two cases which presented initially to ophthalmology clinics with symptoms and signs of orbital inflammation. These cases highlight the variable clinical presentation and course of ECD, the classical radiological and histopathological findings, and the high degree of clinical suspicion necessary to reach this diagnosis. Additionally, they demonstrate the disparity in treatment response, both to standard treatments and to the novel BRAF inhibitors which have shown promise in recent years.
2. Findings
2.1. Case 1
2.1.1. Initial presentation
Our first case is a 49-year-old patient who presented to the Ophthalmology department in Norfolk and Norwich University Hospital in March 2016 with chronic headaches and recurrent pain and swelling around his left eye over the last year. On initial presentation, the examination showed a 3mm left sided proptosis with restriction of eye movements, high intraocular pressure (25 mmHg) and marked swelling and erythema of the eyelid. There was minimal swelling of the right eyelid with no other positive findings. At this point there were no systemic symptoms and no xanthelasma, and reassuringly his vision at presentation was 6/6 in both eyes.
2.1.2. Investigations
Initial investigations showed a positive antinuclear antibody screen and bilateral intraconal masses on MRI imaging (Fig. 1A). Orbital biopsy showed CD3 positive T-cells and CD68 positive “foamy” macrophages and widespread collagenous fibrosis. The appearances at this point suggested an inflammatory process, but no clear cause. Despite a negative ANCA, the differential diagnosis at this point included granulomatosis with polyangiitis and the patient was started on high dose oral prednisolone (100mg od tapered down to 50mg over a few weeks). A lack of other symptoms and signs of wider systemic disease meant that histiocytic disorders such as ECD were not considered at this point in time.
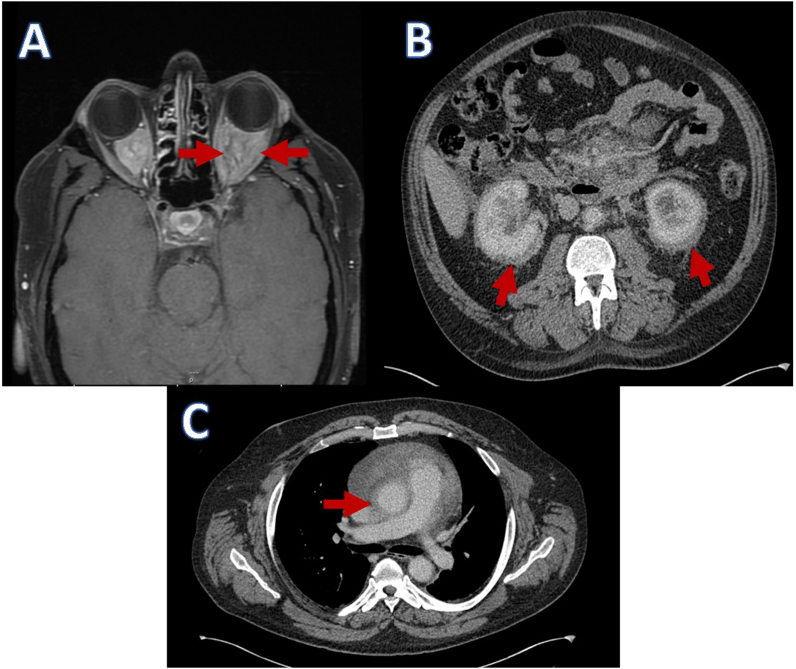
Fig. 1.
A: MRI demonstrating intraconal masses. B: CT abdomen demonstrating bilateral abnormal perirenal soft tissue and fat stranding. C: CT showing evidence of peri-aortitis.
Over the next seven months the patient had multiple courses of oral prednisolone due to episodes of orbital inflammation causing significant periocular pain. Trials with azathioprine and cyclophosphamide were both unable to control the symptoms sufficiently nor allow weaning of the steroids. Despite the symptoms of orbital inflammation, the vision remained at 6/6 in both eyes, with full colour vision and visual fields, but still some mild disc swelling clinically. A full body CT revealed no lymphadenopathy, but did demonstrate pericardial thickening and effusion, as well as abnormal soft tissue surrounding both kidneys and bilateral hydronephrosis with peri-nephric fat stranding (Fig. 1B and C). Further investigation with PET CT demonstrated Fluorodeoxyglucose (FDG) avid orbital masses (Fig. 2).
Fig. 2.
Axial CT, FDG PET and fused PETCT images showing FDG avid bilateral orbital masses.
In the context of orbital masses, vasculitis, and extensive pleural, peri-cardiac, renal, adrenal and mesenteric infiltration as well as the orbital biopsy reports; a diagnosis of ECD was made.
2.1.3. Treatment
Following the diagnosis, the symptoms continued to deteriorate with recurrent orbital inflammation and vision fluctuating between 6/6 and 6/24. Further immunosuppressive treatment with methotrexate and mycophenolate mofetil was initiated in combination with systemic steroids with little effect. Our patient in this case was identified as BRAF V600E mutation positive, and having failed to respond to four previous lines of therapy, in May 2017 (approximately 1 year after initial presentation) our patient was started on the BRAF inhibitor Vemurafenib 960mg orally (po) twice daily (bd).
Within one month of starting Vemurafenib the patient had complete cessation of episodes of orbital inflammation, and it was possible for the oral steroids to be weaned to 10mg once daily (od) without compromising vision. Repeat PET scans also showed improvement in FDG activity and no new lesions.
Over the course of the next year the dose of Vemurafenib was steadily reduced and systemic steroids were completely weaned by December 2017. As of December 2018, there was no clinical evidence of orbital inflammation, and PET scan continues to show stable appearances, in a metabolic complete remission, with no FDG activity in the intraconal masses or elsewhere. In June 2020 the patient remains clinically stable, with visual acuity at baseline, and maintained on Vemurafenib 240mg po od.
2.2. Case 2
2.2.1. Initial presentation
Our second case also presented to our outpatient department; but what followed was a distinctly more aggressive course of disease. This 70-year-old patient presented in February 2019 following an optician referral of suspected optic neuritis with a two month history of painless, gradual reduction of vision in both eyes. He was systemically well and no other symptoms were elicited on systemic enquiry.
On presentation the visual acuity [VA] was 6/15 in both eyes with a left relative afferent pupillary defect, bilaterally swollen optic discs and choroidal folds (Fig. 3, Fig. 4). Baseline investigations showed non-specifically raised ESR (60 mm/hr) and CRP (73 mg/L) with no space occupying lesion reported on CT scan. Initial consideration was given to idiopathic inflammatory syndrome and oral steroids (Prednisolone 60mg od) initiated.
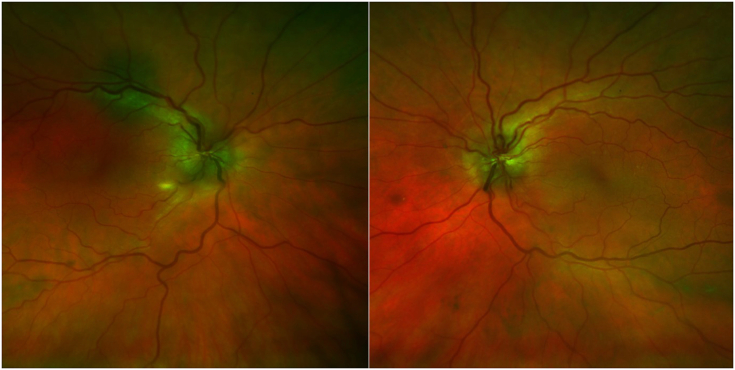
Fig. 3.
OPTOS fundus photos at initial presentation demonstrating bilateral optic disc swelling.
Fig. 4.
FFA images at initial presentation showing leaky optic discs.
2.2.2. Investigations
One month later the patient presented with further drop in vision of the left eye to 6/38 and xanthelasma was noted around both eyes (Fig. 5). Further blood tests including ANCA, TB, T. pallidium, Serum ACE, B12/Folate, and immunoglobulins were all unremarkable. An MRI was arranged which showed bilateral intraconal orbital masses that were diffuse and homogenous (Fig. 6).
Fig. 5.
External photograph demonstrating the presence of bilateral Xanthelasma.
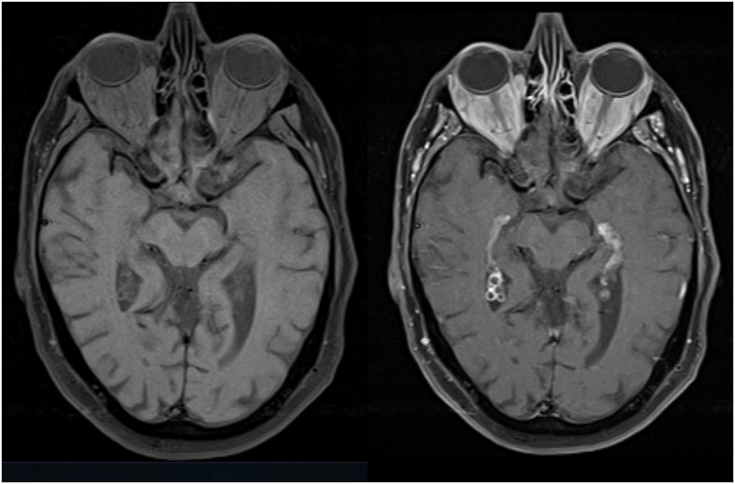
Fig. 6.
Initial MRI, Axial T1 slices shown here pre (left) and post (right) gadolinium enhancement demonstrating bilateral, diffuse, intraconal orbital masses.
At this point the differential diagnosis included: granulomatosis with polyangiitis, IgG4-related orbital inflammation, orbital lymphoma, and idiopathic orbital inflammatory disease. A referral was made to the rheumatology team who organised a full body CT, long bone x-rays and subsequent PET scan. These demonstrated aortitis, periaortitis, pleural involvement, osteosclerosis in the pelvic bones and tibia, and perinephric fat stranding on both kidneys (Fig. 7 A–D). Concurrently an urgent orbital biopsy was arranged (May 2019) which revealed adipose tissue infiltrated by foamy histiocytes that were CD68 positive including touton-type multinucleated cells and scattered lymphocytes. No IgG4 nor CD1a staining was noted. This constellation of radiological and histopathological findings led to a diagnosis of multi-system ECD in June 2019 with widespread skeletal involvement, retroperitoneal disease, proteinuria, gross dependent oedema (serum albumin 13 g/L; normal range 35–50 g/L) and peri-orbital disease. Having been previously independent his WHO performance status was 3.
Fig. 7.
A–B: CT scans with retroperitoneal inflammatory fat stranding involving the perirenal and periaortic spaces. C: Pre-treatment PET CT showing orbital uptake (red arrows), FDG avid bone lesions (yellow arrows), perineural and dural uptake (green arrows), periaortitis (purple arrow), peri renal disease and partial renal obstruction (blue arrows). D: post treatment PET CT showing complete metabolic response to treatment of all the previous findings except sclerotic bone infarcts in the distal femora, tibias and left calcaneum.
2.2.3. Treatment
Similar to case 1, our second patient tested BRAF positive and started treatment with interferon and steroids but continued to clinically deteriorate. In August 2019 he was commenced on the BRAF inhibitor Dabrafenib 150mg po bd, with immediate subjective improvement in his systemic symptoms, achieving a very good partial metabolic response on PET scan 2 months later. However, despite this treatment and high doses of systemic steroids the patient had already suffered a deterioration in his vision to no perception of light (NPL) and at his most recent follow up in June 2020, the patient remained NPL in both eyes, with improvement of his systemic symptoms, resolution of the dependent oedema, improvement in serum albumin (31 g/L) and improvement of his WHO performance status to 1.
3. Discussion
Diagnosis of ECD is often challenging because of its highly variable presentation and clinical course, multi-system involvement, and rarity of the disease. In terms of prognosis, morbidity, and mortality; CNS and cardiovascular involvement are the most significant.4 The most specific signs of ECD are the radiological and histological findings, but this means that a high degree of suspicion must be present in order to uncover them.
3.1. A diagnostic challenge
Given that orbital involvement and xanthelasma are present in 27% and 33% of cases respectively, and that exophthalmos is one of the most common presenting signs in patients with ECD; ophthalmologists are reasonably likely to be the first clinicians to encounter these cases.7 Although much rarer; it is important for ophthalmologists to note that ECD can also present with intraocular involvement such as choroidal infiltrates with associated central serous retinal detachment and choroidal neovascular membrane.8 Our first patient presented with a combination of neurological symptoms (headache) and orbital signs including intermittent periocular pain and swelling and unilateral exophthalmos. In contrast, the disease in our second patient manifested as painless, gradual reduction in vision in both eyes due to orbital infiltration; and additionally, through the presence of xanthelasma of the eyelids. Both cases, however, were similarly devoid of other signs or symptoms of wider systemic disease. MRI scans in both patients revealed bilateral, diffuse, intraconal masses which is the typical finding in ECD patients with orbital infiltration. However, in the absence of systemic disease, the differential diagnosis of these pseudotumoural lesions was centred on granulomatosis with polyangiitis, IgG4-related orbital inflammation, orbital lymphoma, and idiopathic orbital inflammatory disease. A full body CT scan in both cases revealed the cluster of radiographic findings consistent with ECD: long bone osteosclerosis, perinephric stranding, periaortic infiltration, pleural involvement and pericardial effusion.
One of the diagnostic criteria required for ECD is identification of xanthogranulomatous lesions characterised by CD68 (+)/CD1a (−) histiocytes, often with mixed inflammation and fibrosis on a tissue biopsy from an affected organ. However, there is considerable heterogeneity of histological appearances seen in ECD patients depending on the anatomical biopsy site, even in the same patient.9 In our first case, an orbital biopsy taken early in the disease course showed CD68 positive “foamy” macrophages and widespread collagenous fibrosis. The pathology report at this point (taken in the context of orbital masses, but with no systemic involvement identified clinically or radiographically) suggested an inflammatory process, but identified no clear cause. It wasn't until several months later when the radiographic findings were uncovered that a diagnosis of ECD was established. In contrast, the orbital biopsy in our second case also revealed adipose tissue infiltrated by foamy CD68 positive histiocytes; corroborating the already suspected diagnosis of ECD based on previous findings on imaging. Our experience in the first case demonstrates that a high degree of suspicion is necessary to establish a diagnosis of ECD – even when a biopsy demonstrates the typical histological findings.
3.2. A mixed response to modern treatment
The disease course in our first case was relatively indolent with episodes of pain associated with orbital inflammation intermittently affecting the patient for a year before initial presentation. Although there was mild unilateral proptosis present, there was no disturbance of visual acuity or visual field and this was maintained for approximately 18 months following the onset of symptoms. The episodes of painful orbital inflammation were controlled with oral steroids, but there was no improvement of the manifestations of systemic infiltration despite treatment with multiple immunosuppressive agents. Treatment with a novel BRAF inhibitor (Vemurafenib) was commenced approximately 2 years after onset of symptoms and resulted in reversal of visual loss and complete systemic improvement for this patient.
In contrast, the disease course in our second patient was much more aggressive, having presented with a two month history of painless reduction of vision in both eyes which deteriorated to NPL vision within six months. In this case the time frame from presentation to suspected radiographic diagnosis of ECD was 6 weeks, with histopathological confirmation following shortly thereafter. Despite treatment with oral steroids and a novel BRAF inhibitor (Dabrafenib) we were not able to prevent the patient losing his vision from the focal orbital infiltration. However, treatment with the BRAF inhibitor has led to significant improvements in the patient's systemic condition clinically and radiographically.
In recent years, molecular profiling has allowed the identification of mutations along the mitogen activated protein kinase-extracellular signal regulated kinase (MAPK-ERK) pathway which has altered our understanding of the biology of ECD.10 The BRAFV600E mutation is the most clinically relevant mutation in ECD and is present in approximately 57–75% of cases.11 As a result of these findings, ECD is now considered a clonal hematopoietic disorder marked by BRAFV600E and other MAPK pathway mutations, with clinical manifestations caused by neoplastic histiocytic infiltrates and uncontrolled inflammation.10 The discovery of this common BRAFV600E mutation (which is shared with hairy cell leukaemia and malignant melanoma) has led to the appropriation of targeted BRAF inhibitor therapies for use in ECD patients.
Treatment of BRAFV600E-mutant ECD patients with these drugs has been shown to be effective in multiple case reports and case series, particularly in patients who have progressed despite other therapies.10 A recent cohort study demonstrated prolonged efficacy of Vemurafenib with a confirmed objective response rate of 54.5%; progression-free survival of 86% and overall survival of 96% at 2 years.12 Dabrafenib, for which there are fewer reports in the literature in treating ECD, has also been used to good effect. In one case report it was used in combination with Trametinib (a MAPK inhibitor) to produce a progressive and sustained response in a patient with significant brain-stem disease and neurological symptoms.13 The authors of this case report chose Dabrafenib preferentially as it has shown improved overall survival without increased toxicity in clinical trials of melanoma treatment and improved CNS penetrance relative to Vemurafenib.13 There are currently no studies which have directly compared the efficacy of Vemurafenib and Dabrafenib in the treatment of ECD; but as demonstrated in our cases, both patients achieved a good clinical and radiological response to each of the drugs.
4. Conclusions
In summary we report two cases of BRAFV600E-mutant ECD which presented to our ophthalmology outpatient clinics over the course of the last three years. These cases highlight the variable clinical presentation and course of ECD, the classical radiological and histopathological findings, and the high degree of clinical suspicion necessary to reach this diagnosis. The combination of xanthelasma and bilateral, diffuse intraconal orbital masses must suggest to the clinician the possibility of ECD; and consideration to arrange further investigation with a full body CT or FDG PET/CT scan should be given, even in the absence of wider systemic symptoms or signs. With the advent of targeted therapies such as BRAF inhibitors, which have been shown to be highly efficacious, it is of even more importance that a diagnosis of ECD is established in a timely manner in order to give these patients the best chance of reduced morbidity and increased survival.
Patient consent
Written consent from both patients has been obtained to allow details of these cases to be published.
Funding
No funding or grant support.
Authorship
All authors attest that they meet the ICMJE criteria for Authorship.
CRediT authorship contribution statement
James Brodie: Conceptualization, Visualization, Writing - original draft, Writing - review & editing. Sean Zhou: Conceptualization, Writing - original draft, Writing - review & editing. Damodar Makkuni: Writing - review & editing. Clare Beadsmoore: Resources, Writing - review & editing. Chetan Mukhtyar: Writing - review & editing. Janak Saada: Writing - review & editing. Kristian M. Bowles: Writing - review & editing. Bijan Beigi: Writing - review & editing. Ben J.L. Burton: Supervision, Writing - review & editing.
Declaration of competing interest
The following authors have no financial disclosures (JB, SZ, DM, CB, CM, KMB, BB, BJLB).
Acknowledgements
None.
References
- 1.Diamond E.L., Dagna L., Hyman D.M. Consensus guidelines for the diagnosis and clinical management of Erdheim-Chester disease. Blood. 2014;124:483–492. doi: 10.1182/blood-2014-03-561381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chester W., Über Lipoid granulomatose Virchows Arch Pathol Anat. 1930;279:561–602. [Google Scholar]
- 3.Swerdlow S.H., Campo E., Pileri S.A. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazor R.D., Manevich-Mazor M., Shoenfeld Y. Erdheim-Chester Disease: a comprehensive review of the literature. Orphanet J Rare Dis. 2013;8:137. doi: 10.1186/1750-1172-8-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdelfattah A.M., Arnaout K., Tabbara I.A. Erdheim-chester disease: a comprehensive review. Anticancer Res. 2014;3262:3257–3261. [PubMed] [Google Scholar]
- 6.Sivak-Callcott J.A., Rootman J., Rasmussen S.L. Adult xanthogranulomatous disease of the orbit and ocular adnexa: new immunohistochemical findings and clinical review. Br J Ophthamol. 2006;90:602–608. doi: 10.1136/bjo.2005.085894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdellateef E.E., Abdelhai A.R., Gawish H. The first reported case of Erdheim-Chester Disease in Egypt with bilateral exophthalmos, loss of vision, and multi-organ involvement in a young woman. Am J Case Rep. 2016;17:360–370. doi: 10.12659/AJCR.897479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang L.C., Topping K.L., Gratzinger D. Orbital and chorioretinal manifestations of Erdheim-Chester disease treated with Vemurafenib. Am J Ophthalmol Case Rep. 2018;11:158–163. doi: 10.1016/j.ajoc.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozkaya N., Rosenblum M.K., Durham B.H. The histopathology of Erdheim-Chester Disease: a comprehensive review of a molecularly characterized cohort. Mod Pathol. 2018;31:581–597. doi: 10.1038/modpathol.2017.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haroun F., Millado K., Tabbara I. Erdheim-Chester Disease: comprehensive review of molecular profiling and therapeutic advances. Anticancer Res. 2017;37:2777–2783. doi: 10.21873/anticanres.11629. [DOI] [PubMed] [Google Scholar]
- 11.Haroche J., Papo M., Cohen-Aubart F. Erdheim-Chester disease (ECD), an inflammatory myeloid neoplasia. Presse Med. 2016;46:96–106. doi: 10.1016/j.lpm.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 12.Diamond E.L., Subbiah V., Lockhart A.C. Vemurafenib for BRAF V600-mutant erdheim-chester disease and langerhans cell histiocytosis: analysis of data from the histology-independent, phase 2, open-label VE-BASKET study. JAMA Oncol. 2018;4(3):384–388. doi: 10.1001/jamaoncol.2017.5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al Bayati A., Plate T., Al Bayati M. Dabrafenib and Trametinib treatment for erdheim-chester disease with brain stem involvement. Mayo Clin Proc Innov Qual Outcomes. 2018;2(3):303–308. doi: 10.1016/j.mayocpiqo.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]