Abstract
Background
Transcatheter closure of patent foramen ovale (PFO) has been demonstrated to be superior to medical therapy in stroke prevention in selected patients. Beyond traditional permanent metallic devices, NobleStitch EL, a suture-based system, has been developed as a potential alternative.
Case summary
A 50-year-old man underwent transcatheter closure of PFO with mild interatrial septal bulging and tunnel-like morphology with a NobleStitch device. A transthoracic echocardiography performed immediately after PFO closure showed residual shunt (RS), which persisted unchanged at staged controls, due to the inability of the delivery system to capture both the septum primum and the septum secundum. A second procedure was performed with the implantation of a Figulla Flex II 27/30 mm device, with no RS detectable at control echocardiography.
Discussion
The NobleStitch device is interesting in its concept, but several pitfalls may be encountered during its deployment. Opposite to permanent metallic devices, RSs after the procedure are not expected to decrease over time and should be managed with a different approach.
Keywords: Patent foramen ovale, PFO occluder, Metallic device, Suture-based PFO closure, Complications, Residual shunt, Case report
Learning points
The NobleStitch device, a suture-based tool for patent foramen ovale (PFO) closure, interesting in its concept, has a very limited experience reported in literature with good clinical and safety outcomes.
A residual shunt (RS) after successful PFO closure with a metallic device may be observed in a minority of cases. Usually, the RS grade decreases over time thanks to device endothelialization. A persistent RS has to be taken into account for cryptogenic stroke prevention management.
Residual shunt detected after NobleStitch use has to be considered a suture-failure, not to be monitored over time but requiring a different management.
Introduction
Transcatheter closure of patent foramen ovale (PFO) has been demonstrated to be superior to medical therapy in stroke prevention in selected patients.1,2 Beyond traditional permanent metallic devices,NobleStitch EL, a suture-based system, has been developed as an alternative approach limiting the burden of exogenous materialreleased at the cardiac level.3 However, despite promising, only few data are available detailing the effectiveness and the safety of the NobleStitch system.
In particular, given its peculiar mechanism based on the apposition of septum primum and septum secundum through a polypropylene suture, concerns persist in patients treated with NobleStitch regarding the management of residual shunt (RS), which are frequently detected following PFO closure, but which tend to decrease and mostly disappear over time with traditional permanent metallic devices due to endothelization.4
Timeline
| November 2018 | Minor stroke |
|---|---|
| January 2019 | Transoesophageal echocardiography detecting a tunnel-like patent foramen ovale (PFO) and planned PFO closure |
| January 2019 | Patient underwent suture-based NobleStitch PFO closure at another centre. Transthoracic echocardiography performed after procedure showed significant residual right-to-left (R-L) shunt |
| February and April 2019 | Transthoracic echocardiography confirming severe residual R-L shunt |
| September 2019 | Transthoracic echocardiography and bubble test showing unchanged severe R-L shunt |
| October 2019 | Patient asked for a second opinion consultation at our institute |
| November 2019 | Patient underwent PFO closure with a permanent metallic device implantation without residual intracardiac shunt |
Case presentation
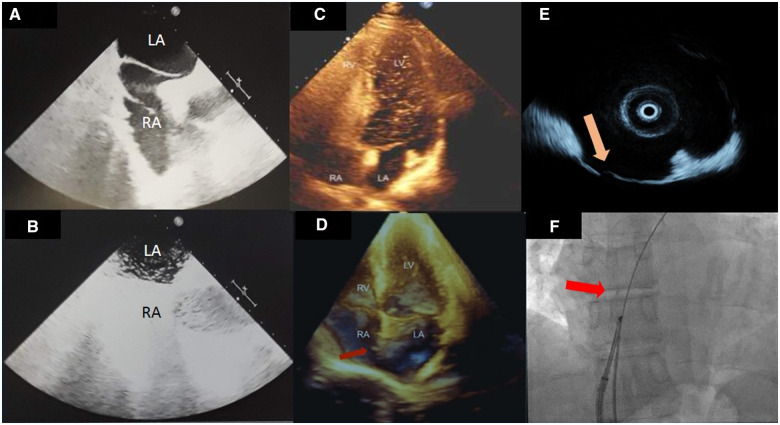
A 50-year-old man, with a known MTHFR homozygous genetic mutation and recent minor stroke underwent percutaneous PFO closure at another institute. After transoesophageal echo showing mild interatrial septal bulging with a tunnel-like PFO, clinicians planned a suture-based NobleStitch EL (Kardia, Italy) closure procedure (Figure 1A and B).
Figure 1.
(A and B) Transoesophageal echocardiography (A) showing a tunnel-like patent foramen ovale and bubble test (B) showing significant right–left shunt during Valsalva manoeuvre; (C) echocardiography with bubble test confirming the persistent patency of the foramen ovale 9 months following the closure attempt with NobleStitch; (D) 3D transthoracic echocardiography: the red arrow points to the Kwiknot device; (E) intracardiac echocardiography documenting the patency of the foramen ovale (yellow arrow); (F) basal fluoroscopy; a 0.035″ wire is seen crossing the fossa ovale from the right to the left atrium; the red arrow points at the Kwiknot device.
This unique device consists of two dedicated suture delivery catheters to capture and suture the septum secundum and the septum primum using a polypropylene suture. A third element, the KwiKnot™ catheter (HeartStitch, Inc.), is advanced over the septum secundum and septum primum sutures to approximate both septa, achieving closure by securing the stitch and trimming the excess suture material. Transthoracic echocardiography (TTE) performed after PFO closure showed significant residual right-to-left (R-L) shunt, unchanged in severity at 1- and 3-months of follow-up. The treating physician suggested for a TTE 6 months later, leaving the patient on single antiplatelet therapy (aspirin 100 mg/daily).
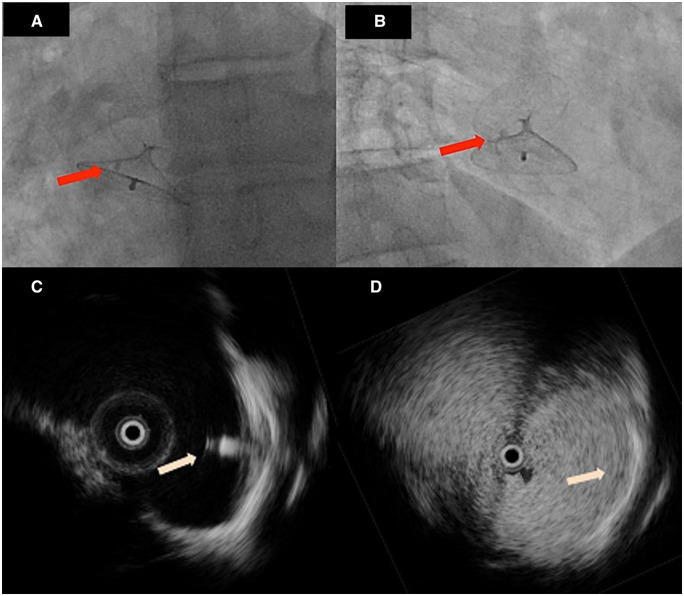
At 9 months of follow-up, after evidence of persistent severe R-L shunt at contrast-TTE (Figure 1C and Supplementary material online, Video S1), the patient, still on aspirin alone treatment, asked for a second opinion consultation. On consultation, patient was in good clinical conditions and physical examination was unremarkable. The consultant physician asked for a 3D echo (Supplementary material online, Video S2) showing interatrial septal bulging together with the persistence in place of one of the polypropylene sutures attached to the septum primum, together with the Kwiknot (Figure 1D). The patient was not allergic to nitinol and without contraindications to permanent metallic device implantation. Therefore, a device-based PFO closure was planned by bilateral femoral vein approach under intracardiac echo guidance (ICE; 9 F, 9-MHz Ultra ICE; EP Technologies, Boston Scientific Corporation, San Jose, CA, USA). ICE confirmed PFO patency (Figure 1E and F, Supplementary material online, Videos S3 and S4) and a 27–30 mm Figulla Flex II (Occlutech GmbH, Jena, Germany) occluder device deployment was performed (Figure 2A and B) with zero R-L shunt at intracardiac contrast injection (Figure 2C and D). At postoperative TTE, there was no evidence of device malposition or thrombosis. The patient was discharged on dual antiplatelet therapy (aspirin 100 mg/daily; clopidogrel 75 mg/daily for 3 months). At 6-month follow-up, patient was asymptomatic and contrast TTE showed device in place without RS.
Figure 2.
(A and B) Fluoroscopy images showing the deployed Occlutech Figulla Flex II 27/30 mm device; the red arrow points at the Kwiknot device, which can be seen ‘trapped’ between the two discs of the newly deployed device; (C) intracardiac echocardiographic image showing the correct positioning of the Occlutech Figulla Flex II 27/30 mm device (pink arrow); (D) bubble test during intracardiac echocardiographic monitoring, confirming the absence of residual shunt.
Discussion/conclusion
The NobleStitch device, despite interesting in its concept, has a very limited experience reported in literature with good clinical and safety outcomes.5 However, previous observations4 on septal tears after NobleStitch as well as residual septal defects and residual R-L shunts open few observations on the device full applicability in a consecutive cohort of patients. Residual R-L shunts may be detected following PFO closure, but they tend to decrease and mostly disappear over time after traditional permanent metallic devices implantation.6 Conversely, a residual R-L after a suture-based PFO closure, has to be classified as a procedure-failure with inherent controversial dilemmas on RS management. A transoesophageal echocardiography examination in these patients may be useful to study the mechanisms of residual shunting and differential diagnosis between a previously undetected atrial septal fenestration and an asymmetrical positioning of the suture causing a iatrogenic RS, A significant RS has been described in 10% of Noblestitch treated patients. Therapeutic options include pharmacological therapy with antiplatelet or oral anticoagulant drugs, percutaneous closure with a second device, or even surgical repair.7
We believe that a rigorous selection of the PFO anatomy avoiding long-tunnel-like and atrial septal aneurysms may play a key role in the procedural and device success.
Lead author biography

Daniela Trabattoni, MD; Position Chief, Invasive Cardiology Unit; Director, Women Heart Center, she founded in 2017, focused on cardiovascular prevention in women—Centro Cardiologico Monzino, IRCCS—Milan. Teaching clinical cardiology at the postgraduate cardiology school, University of Milan. She is elected committee member of the National Superior Institute of Health (ISS) for cardiovascular devices evaluation in 2011 and member of the ISS committee for a ministerial consensus document on gender-based medicine. She is reviewer and editorial member of national and international cardiology journals. She authored and co-authored 534 papers (130 peer-review manuscripts, 400 abstracts, and 4 chapters book) on national and international journals, ESC and ACC fellow.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
Supplementary Material
Contributor Information
Daniela Trabattoni, Centro Cardiologico Monzino, IRCCS, Via Carlo Parea 4, Milan, Italy.
Sebastiano Gili, Centro Cardiologico Monzino, IRCCS, Via Carlo Parea 4, Milan, Italy.
Giovanni Teruzzi, Centro Cardiologico Monzino, IRCCS, Via Carlo Parea 4, Milan, Italy.
Gloria Tamborini, Centro Cardiologico Monzino, IRCCS, Via Carlo Parea 4, Milan, Italy.
References
- 1. Ahmad Y, Howard JP, Arnold A, Shin MS, Cook C, Petraco R. et al. Patent foramen ovale closure vs. medical therapy for cryptogenic stroke: a meta-analysis of randomized controlled trials. Eur Heart J 2018;39:1638–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pristipino C, Sievert H, D'Ascenzo F, Mas J-L, Meier B, Scacciatella P. et al. ; European Association of Percutaneous Cardiovascular Interventions (EAPCI); European Stroke Organisation (ESO); European Heart Rhythm Association (EHRA); European Association for Cardiovascular Imaging (EACVI); Association for European Paediatric and Congenital Cardiology (AEPC); ESC Working group on GUCH; ESC Working group on Thrombosis; European Haematological Society (EHA). European position paper on the management of patients with patent foramen ovale. General approach and left circulation thromboembolism. EuroIntervention 2019;14:1389–1402. [DOI] [PubMed] [Google Scholar]
- 3. Ruiz CE, Kipshidze N, Chiam PT, Gogorishvili I.. Feasibility of patent foramen ovale closure with no-device left behind: first-in-man percutaneous suture closure. Catheter Cardiovasc Interv 2008;71:921–926. [DOI] [PubMed] [Google Scholar]
- 4. Baldetti L, Ferri LA, Ancona M, Bellini B, Visco E, Melillo F. et al. Interatrial septal tear after patent foramen ovale closure with the NobleStitch device. JACC Cardiovasc Interv 2019;12:e139–e140. [DOI] [PubMed] [Google Scholar]
- 5. Gaspardone A, De Marco F, Sgueglia GA, De Santis A, Iamele M, D'Ascoli E. et al. Novel percutaneous suture-mediated patent foramen ovale closure technique: early results of the NobleStitch EL Italian Registry. Eurointervention 2018;14:e272–e279. [DOI] [PubMed] [Google Scholar]
- 6. Trabattoni D, Gaspardone A, Sgueglia GA, Fabbiocchi F, Gioffrè G, Montorsi P. et al. AMPLATZER versus Figulla occluder for transcatheter patent foramen ovale closure. EuroIntervention 2017;12:2092–2099. [DOI] [PubMed] [Google Scholar]
- 7. Diaz T, Cubeddu RJ, Rengifo-Moreno PA, Cruz-Gonzalez I, Solis-Martin J, Buonanno FS. et al. Management of residual shunts after initial percutaneous patent foramen ovale closure: a single center experience with immediate and long-term follow-up. Catheter Cardiovasc Interv 2010;76:145–150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.