Abstract
Background
Plastic bronchitis (PB) and protein-losing enteropathy (PLE) are devastating complications after Fontan palliation that lead to uncontrolled loss of protein-rich lymphatic fluid into extra lymphatic compartments. Decompression of the thoracic duct is a new treatment option that effectively restores lymphatic system integrity by redirecting lymphatic flow into the low-pressure levels of the common atrium.
Case summary
We report a patient with severe failing Fontan circulation where surgical thoracic duct decompression leads to resolution of PLE and PB symptoms but worsening hypoxaemia that could be managed with banding of internal jugular vein.
Discussion
Thoracic duct decompression in patients with failing Fontan circulation can be a simple and effective treatment for PLE and PB. Hypoxaemia may occur but can be managed with banding of internal jugular vein.
Keywords: Fontan-operation, Protein-losing enteropathy, Plastic bronchitis, Thoracic duct decompression, Internal jugular vein banding, Hypoxaemia, Case report
Learning points
Thoracic duct decompression in severe failing Fontan circulation can be a highly effective simple and save treatment option for protein-losing enteropathy and plastic bronchitis.
Profound cyanosis may develop after thoracic duct decompression but can be managed with banding of the internal jugular vein.
Introduction
Plastic bronchitis (PB) and protein-losing enteropathy (PLE) in single ventricle type congenital heart disease are devastating complications after surgical palliation. Pathophysiologic processes are still poorly understood. An increased central venous pressure, a hallmark of Fontan haemodynamic, seems to be the critical feature. As a consequence, lymph overproduction and congestion give rise to the formation of lymphatic fistulas and loss of protein-rich lymphatic fluid into extra lymphatic compartments.1,2
Current therapeutic strategies focus on interventional closure of such fistulas without considering and changing underlying pathophysiologic mechanism.3–5
A new approach, the decompression of the thoracic duct by creating a surgical anastomosis between the innominate vein and the atrial appendage overcomes this limitation as lymphatic flow is redirected into the low-pressure common atrium.6–8 However, as a consequence of creation of an iatrogenic right to left shunt profound cyanosis can be a challenge. We report a case where thoracic duct decompression in a patient with failing Fontan circulation almost normalized protein levels but lead to profound hypoxaemia that could be managed with banding of internal jugular vein.
Timeline
| Age | Events |
|---|---|
| Past medical history: hypoplastic left heart syndrome, Norwood-OP (7days), Glenn-OP (3 months), extracardiac fenestrated Fontan-OP (3 years), closure of fenestration (3.5 years) | |
| 6 years | Diagnosis of protein-losing enteropathy (PLE) (total protein 3.6 mg/dL), IgG 86 mg/dL, leucopoenia (1.6 G/L), elevated random stool α-1 antitrypsin level |
| Diagnosis of cytomegalovirus enterocolitis, treatment with ganciclovir improved hypoproteinaemia | |
| 7 years | Recurrence of PLE (hypoproteinaemia) |
| Initial treatment with budesonide transiently increased protein levels but had to be removed due to side effects | |
| 8 years | Diagnosis of plastic bronchitis (PB) (dyspnoea, hypoxaemia, and coughing, removal of large branching casts during bronchoscopy) |
| 8 years 1 month | Lymphatic imaging via the lymph nodes/patient was listed for heart transplantation |
| 8 years 7 months | Thoracic duct decompression |
| Protein levels increased from 34 mg/dL preoperatively up to 72 mg/dL during follow-up but oxygen saturation dropped after the operation from 92–96% to 76–80% | |
| 8 years 11 months | Banding of internal jugular vein |
| After banding peripheral oxygen saturation (SpO2) was on average 85% in room air. Protein levels did not decrease | |
| Follow-up 6 months after banding | No signs of PLE or PB. Fatigue improved. SpO2: 82%, total protein 70.4 mg/dL |
Case presentation
A boy with a history of hypoplastic left heart syndrome who underwent the Norwood-, Glenn-, and an extracardiac fenestrated Fontan-operation at our facility presented at the age of 6 years with signs of PLE. Fenestration had been closed 3 years before after a trial occlusion demonstrated no reduction in cardiac output and no elevation of central venous pressure. He had hypoproteinaemia (total protein 3.6 mg/dL), hypogammaglobulinaemia (immunoglobulin G 86 mg/dL), leucopoenia (1.6 G/L), and elevated random stool α-1-antitrypsin level. During workup cytomegalovirus enterocolitis was diagnosed and hypoproteinaemia resolved after treatment with ganciclovir.
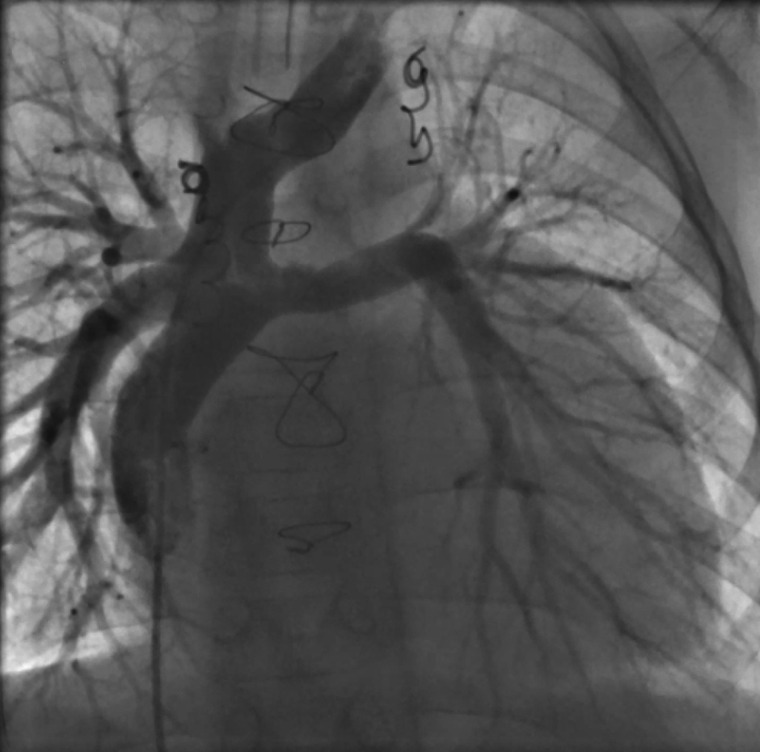
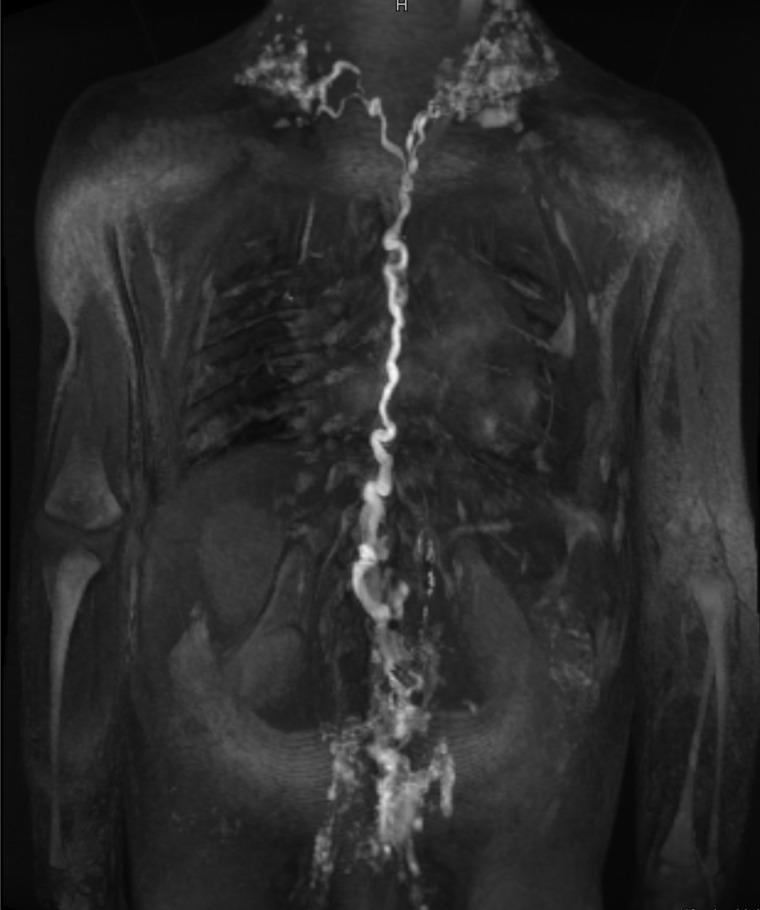
However, 1 year later hypoproteinaemia recurred and initial treatment with budesonide transiently increased protein levels but had to be removed due to side effects. When the patient was admitted to our hospital with dyspnoea, hypoxaemia, and coughing at the age of 8 years, bronchoscopy was initiated and large branching casts were removed from both sides of the lung indicating PB. Cardiac catheterization at that time showed a mean central venous pressure of 12 mmHg and no evidence of obstruction to pulmonary artery flow or pulmonary vein stenosis and a mildly reduced right ventricular systolic function. Aortopulmonary collaterals were excluded (Figure 1). Re-opening of the fenestration was considered at that time, but abandoned because parents disapproved the procedure and there was no obvious obstruction of pulmonary flow nor elevated central venous pressures. Lymphatic imaging via the lymph nodes demonstrated extensive lymph malformations that could not be treated with lymphatic intervention (Figure 2).
Figure 1.
Fluoroscopy of the Fontan pathway demonstrating unobstructed pulmonary flow.
Figure 2.
Dynamic contrast magnetic resonance lymphangiography in a patient with severe failing Fontan circulation. Note the tortuous and dilated thoracic duct.
Because of the severity of both conditions the patient was listed for cardiac transplantation. As bridging to transplant he underwent thoracic duct decompression as previously described by Hraška6 (Figure 3).
Figure 3.
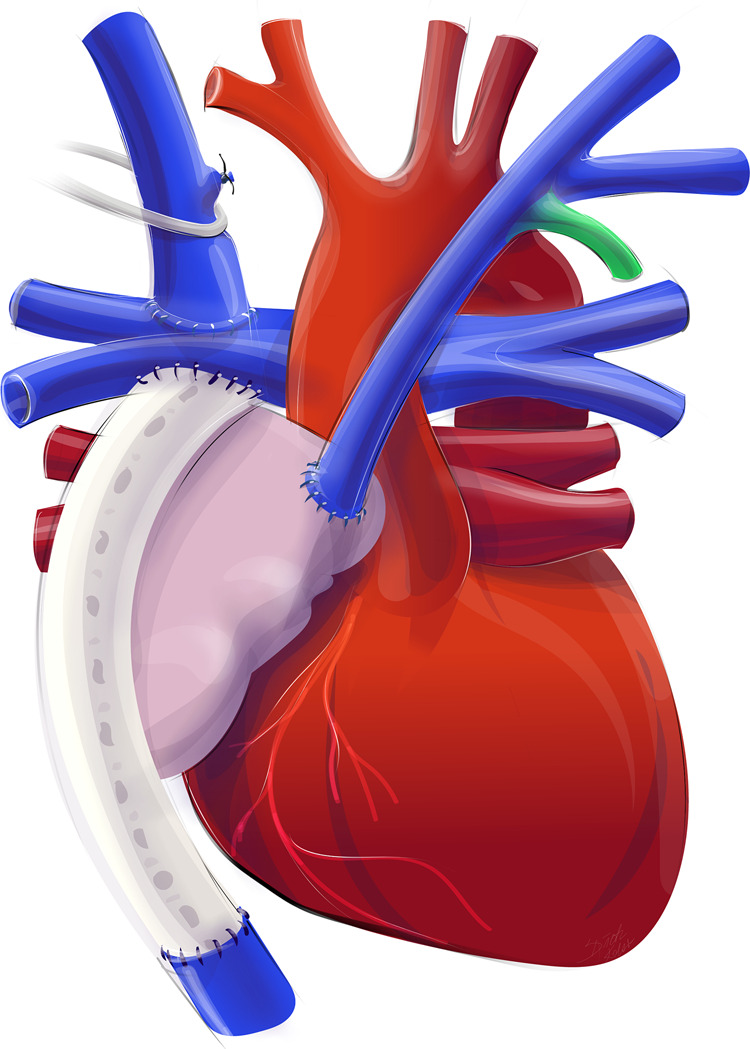
Creation of a surgical anastomosis between the innominate vein and the right atrial appendage.
Procedure: A median sternotomy was performed. Right atrium, Fontan-tunnel, aortopulmonary truncus as well as the superior vena cava and the innominate vein were dissected. The innominate vein was mobilized up to the left venous angle, the left jugular vein and the left subclavian vein were dissected exclusively on the anterior surface. All residual venous branches which could be reached and were not ligated during Glenn-procedure were eliminated. Then, the right atrial appendage was mobilized. ECMO was used for venous drainage, the innominate vein and the aorta were cannulated. The innominate vein was crossclamped and transected at the junction with the right jugular vein. The proximal stump was oversewn. The apex of the right atrial appendage was clamped and incised. Muscle bundles and trabecula were resected and an end-end anastomosis with the innominate vein was performed.
Surgery had immediate and dramatic effects on hypoproteinaemia (Figure 4).
Figure 4.
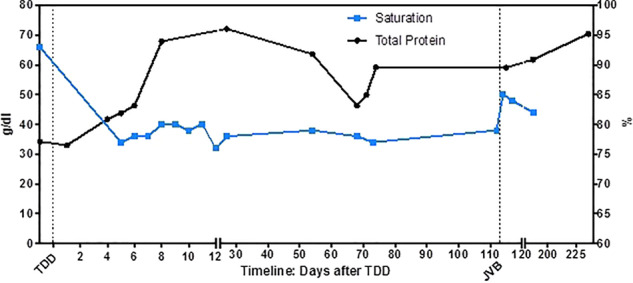
Total protein and oxygen saturation profile after thoracic duct decompression and jugular vein banding in a patient with severe failing Fontan circulation. JVB: jugular vein banding; TDD: thoracic duct decompression.
Total protein levels increased from 34 mg/dL preoperatively up to 68 mg/dL on the 8 post-operative day and normalized (72 mg/dL) during follow-up.
However, arterial oxygen saturation measured by pulse oximetry dropped after the operation from 92–96% to 76–80% (Figure 3). During the next 3 months the parents reported frequent saturations below 75% (home-measurement) and the patient complained of waxing fatigue. To improve hypoxaemia decision was made to decrease right to left shunt by banding the internal jugular vein.
Procedure: The patient was placed in a supine position, the head slightly extended and turned to the right. The incision was performed along the anterior border of the sternocleidomastoid muscle, which was then retracted laterally. The vessels of the carotid triangle were exposed and the internal jugular vein was dissected free, especially in proximal direction. A banding, made of a 7 mm polytetrafluoroethylene prosthesis, was placed around the vein. The banding was than tightened until saturation increased just below 90% (Figure 5).
Figure 5.
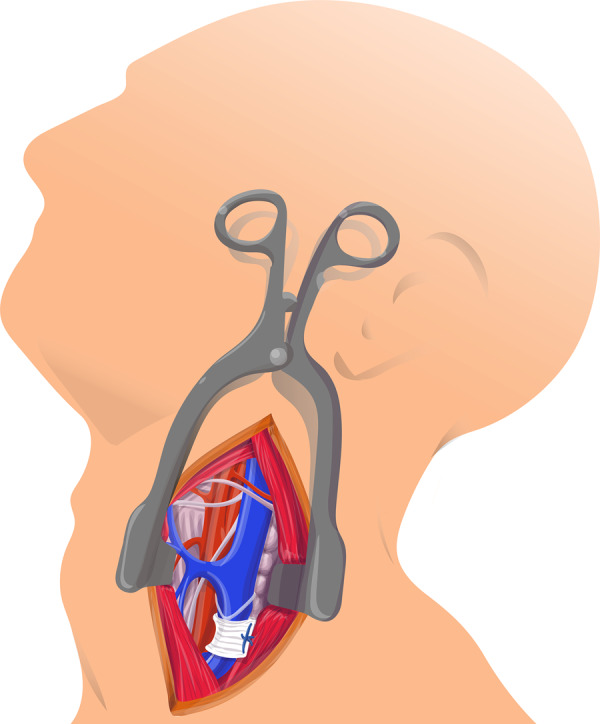
Banding of internal jugular vein.
After banding the peripheral oxygen saturation was on average 85% in room air. Protein levels did not decrease and fatigue improved. Since thoracic duct decompression the patient had no further signs of PLE nor did he expectorate any casts and cardiac transplantation could be abandoned.
Discussion
Decompression of the thoracic duct either by creating a surgical anastomosis or by a transcatheter approach has previously been reported to be an effective treatment of severe lymphatic failure in a small number of patients. It is based on the concept of diverting blood from the innominate vein to the lower pressure systemic atrium and thereby reversing the deleteriously effects of venous congestion on lymphatic system integrity.6–8
Unlike selective lymphatic embolization of abnormal lymphatic fistulas, it modifies the pathophysiologic impetus and hence may have the potential to initiate long-standing improvement. In our patient symptoms and laboratory values improved dramatically so that he could be taken off transplant list.
Profound hypoxaemia as a consequence of a new right to left shunt was a severe accompanying effect with a negative impact on quality of life. Banding of jugular vein allowed us to adjust venous flow and to achieve satisfactory oxygen saturation. The procedure itself was simple and safe. It had no effect on total protein levels and symptomatic and laboratory improvement persisted 6 months after the procedure.
Lead author biography
Dr Christoph Bauer graduated from Medical University of Graz, Austria in 2010. He completed his residency in Pediatric and Adolescent Medicine at the Kepler University Hospital. Currently, he is following his residency in Pediatric Cardiology at the Kepler University Hospital. His main research theme is Fontan failure in single ventricle patients.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Supplementary Material
Acknowledgements
We thank Dr Andreas Tulzer and Mr Dietmar Kolar for helping to create the figures for this report.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
Contributor Information
Christoph Bauer, Department of Pediatric Cardiology, Johannes Kepler University Linz, Krankenhausstrasse 26-30, 4020 Linz, Austria.
Roland Mair, Department of Pediatric Cardiac Surgery, Johannes Kepler University Linz, Krankenhausstrasse 26-30, 4020 Linz, Austria; Johannes Kepler University Linz, Altenbergerstrasse 68, 4040 Linz, Austria.
Rudolf Mair, Department of Pediatric Cardiac Surgery, Johannes Kepler University Linz, Krankenhausstrasse 26-30, 4020 Linz, Austria; Johannes Kepler University Linz, Altenbergerstrasse 68, 4040 Linz, Austria.
Gerald Tulzer, Department of Pediatric Cardiology, Johannes Kepler University Linz, Krankenhausstrasse 26-30, 4020 Linz, Austria.
References
- 1. Rychik J. The relentless effects of the Fontan paradox. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2016;19:37–43. [DOI] [PubMed] [Google Scholar]
- 2. Rychik J, Atz AM, Celermajer DS, Deal BJ, Gatzoulis MA, Gewillig MH. et al. ; On behalf of the American Heart Association Council on Cardiovascular Disease in the Young and Council on Cardiovascular and Stroke Nursing. Evaluation and management of the child and adult with Fontan circulation: a scientific statement from the American Heart Association. Circulation 2019;140:e234–e284. [DOI] [PubMed] [Google Scholar]
- 3. Itkin M, Piccoli DA, Nadolski G, Rychik J, DeWitt A, Pinto E. et al. Protein-losing enteropathy in patients with congenital heart disease. J Am Coll Cardiol 2017;69:2929–2937. [DOI] [PubMed] [Google Scholar]
- 4. Dori Y, Keller MS, Rome JJ, Gillespie MJ, Glatz AC, Dodds K. et al. Percutaneous lymphatic embolization of abnormal pulmonary lymphatic flow as treatment of plastic bronchitis in patients with congenital heart disease. Circulation 2016;133:1160–1170. [DOI] [PubMed] [Google Scholar]
- 5. Maleux G, Storme E, Cools B, Heying R, Boshoff D, Louw JJ. et al. Percutaneous embolization of lymphatic fistulae as treatment for protein-losing enteropathy and plastic bronchitis in patients with failing Fontan circulation. Catheter Cardiovasc Interv 2019;94:996–1002. [DOI] [PubMed] [Google Scholar]
- 6. Hraška V. Decompression of thoracic duct: new approach for the treatment of failing Fontan. Ann Thorac Surg 2013;96:709–711. [DOI] [PubMed] [Google Scholar]
- 7. Smith CL, Hoffman TM, Dori Y, Rome JJ.. Decompression of the thoracic duct: a novel transcatheter approach. Catheter Cardiovasc Interv 2020;95:E56–E61. [DOI] [PubMed] [Google Scholar]
- 8. António M, Gordo A, Pereira C, Pinto F, Fragata I, Fragata J.. Thoracic duct decompression for protein-losing enteropathy in failing Fontan circulation. Ann Thorac Surg 2016;101:2370–2373. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.