Abstract
Background
Celiac disease is a digestive inflammatory syndrome with several complications. It is associated with coagulation and platelets abnormalities leading to thromboembolic events. Cerebral venous thrombosis is an exceptional localization of thrombosis in celiac disease and could be life-threatening.
Case summary
A 17-year-old female patient with history of celiac disease and not following a gluten-free diet, checked in to the emergency department for a sudden, 2-week-old, and deteriorating, onset of intense headache and muscle weakness. The cerebral computed tomography-scan showed bilateral fronto-parietal hypodensity with micro-bleeds. We investigated using a cerebral magnetic resonance imaging that revealed superior longitudinal sinus thrombosis and right transverse and sigmoid sinuses thrombosis, along with right haematoma and ischaemic areas. The patient was prescribed anticoagulation therapy. Follow-ups over a 2-year period confirmed a favourable outcome and a complete regression of symptoms.
Discussion
Evolution of celiac disease could be associated with several complications. Eighty-five percent of patients is potentially exposed to thromboembolic events due to the hypercoagulability state of the disease and different coagulation and fibrinolysis abnormalities (e.g. hyperhomocysteinaemia, protein C and S deficiencies, vitamin K and B deficiencies). Cerebral venous thrombosis is a rare thromboembolic localization. Anticoagulation is efficient in most cases though endovascular treatment might be required.
Keywords: Celiac disease complications, Cerebral venous thrombosis, Case report
Learning points
Thromboembolic events are common complications of celiac disease.
Cerebral venous thrombosis is an exceptional localization, but could be life-threatening.
Raise patient’s awareness of the importance of complying to a gluten-free diet, to avoid complications.
Introduction
Celiac disease is a digestive inflammatory syndrome caused by gluten intolerance. It affects 0.6–1.0% of the world population.1 It was first observed in infants; however recently, celiac disease is increasingly diagnosed in adults. The classical digestive symptoms are dominated by malabsorption syndrome, diarrhoea, and abdominal pain. But it can also have several extra-intestinal symptoms, such as amenorrhoea, dermatitis herpetiformis, and neuropsychological disorders.2
Different complications of celiac disease were previously described. Venous thrombosis is a common thromboembolic complication, with a 25% higher risk in patients with celiac disease compared with the general population.3 It is mostly located in the abdomen or lower limbs, but the cerebral localization was exceptionally described.
We report a rare case of cerebral venous thrombosis in a 17-year-old female patient suffering from celiac disease.
Timeline
| Time | Events |
|---|---|
| Childhood | The patient was diagnosed for celiac disease. |
| 2 weeks before the presentation | Sudden onset of muscular weakness with intense headache. |
| The day of presentation |
|
| The same day |
|
| 1 week after presentation | The patient left the hospital with regression of the motor deficit and relieve of the headache |
| 2 years of follow-up | Good evolution with progressive improvement and complete regression of the motor deficit |
Case presentation
A 17-year-old Caucasian female patient, with a history of celiac disease and not following a gluten-free diet, presented a sudden, 2-week-old, onset of intense headache and muscle weakness that was worsening progressively. There was no trauma or fall prior to the onset of her symptomatology. She was not taking hormonal contraception or any other medications and was not an active smoker.
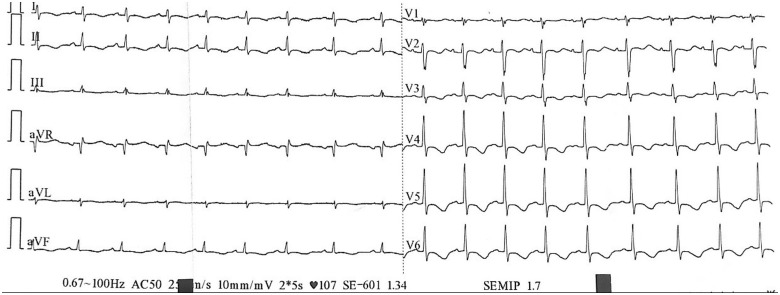
On clinical examination, the patient was conscious, dyspnoeic (Class III of NYHA classification), without any chest pain, she had a heart rate of 107 b.p.m., a blood pressure of 100/80 mmHg, normal heart sounds on auscultation. The neurological examination showed a tetraparesis (2/5 on the left arm and leg and 4/5 on the right limbs), there were no signs of sensory deficit. The left leg examination revealed swollen, indurated, painful, reddish leg, with a positive Homans sign, consistent with a deep venous thrombosis. The pulmonary and abdominal examinations were normal. An electrocardiogram was performed and showed a regular sinus rhythm with circumferential inverted T waves (Figure 1).
Figure 1.
Electrocardiogram.
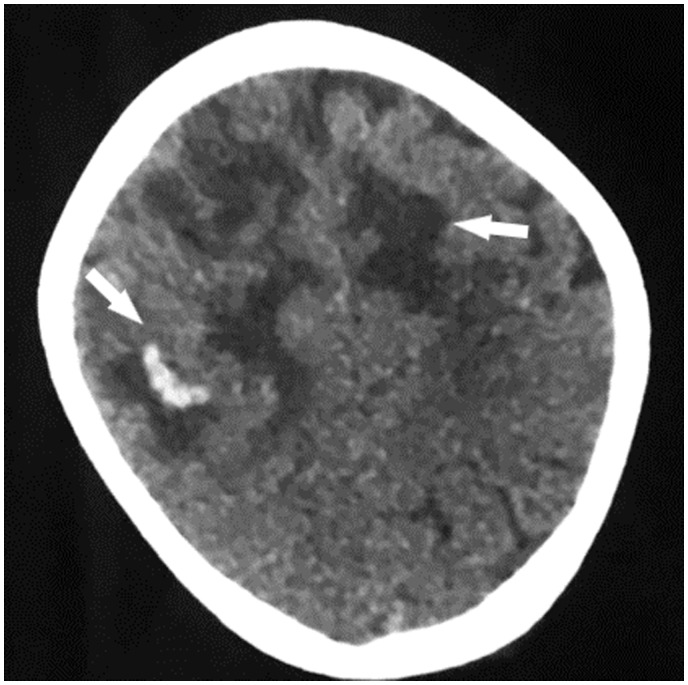
A venous Doppler was performed on the left leg and confirmed a deep venous thrombosis of the common and superficial femoral vein extending to the popliteal vein. The cerebral computed tomography scan showed a bilateral fronto-parietal hypodensity with micro-bleeds (Figure 2).
Figure 2.
Unenhanced computed tomography scan showing bilateral fronto-parietal hypodensity with bleeding spots.
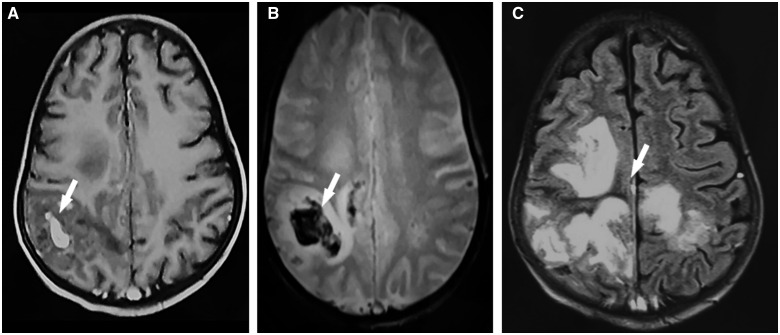
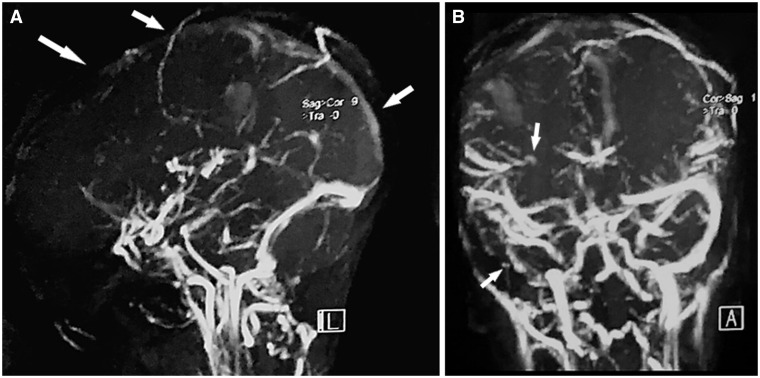
The magnetic resonance imaging displayed a cerebral venous thrombosis of the superior longitudinal sinus and the right transverse and sigmoid sinuses with parietal right haematomas, an important mass effect without brain herniation, with fronto-parietal meningeal haemorrhage (Figures 3 and 4).
Figure 3.
Magnetic resonance imaging: (A) T1-weighted and enhanced axial sequence showing bleeding as hyperintensity and (B) T2* weighted axial sequence; showing bleeding as hypo intensity. (C) FLAIR axial sequence showing hyperintensity of ischaemia. FLAIR, fluid-attenuated inversion recovery.
Figure 4.
Venous angio-magnetic resonance imaging: (A) Sagittal view showing superior longitudinal sinus thrombosis. (B) Frontal view showing right transverse and sigmoid sinuses thrombosis.
The transthoracic echocardiography (TTE) showed a normal left ventricle with a left ventricular ejection fraction of 60%, a mild tricuspid regurgitation with a pulmonary artery pressure estimated at 34 mmHg. Transthoracic echocardiography also revealed a small ostium secundum atrial septal defect of 8 mm with left to right shunt, without dilation or dysfunction of the right ventricle.
Routine laboratory tests revealed an inflammatory syndrome with elevated sedimentation rate at 21 mm (<20 mm), an anaemia with haemoglobin level at 9.3 g/dL (11.5–17.0 g/dL), an increased white blood cell count of 11 480/µL (4000–10 000), platelet count of 297 000 (150 000–460 000), C-reactive protein level of 9.8 mg/L (<5 mg/L), hypocalcaemia of 68.9 mg/L (86–103 mg/L), abnormal hepatic enzymes with elevated alanine aminotransferase (ALT) of 100 IU/L (<34) and aspartate aminotransferase (AST) of 110 IU/L (<31), and a normal gamma-glutamyltransferase (GT) level of 17 IU/L (<39).
Laboratory tests for hypercoagulability abnormalities were negative: protein C level was measured at 73% (70–140%), protein S at 70% (60–130%), homocysteine at 10 µmol/L (<20 µmol/L), vitamin B12 at 155 pg/mL (150–900 pg/mL), and normal values of anti-phospholipid antibodies.
Anticoagulation treatment (with low-molecular-weight heparin, followed by warfarin) was immediately initiated after diagnosis (target INR range of 2–3), with a progressive improvement and complete regression of the motor deficit over a 2-year clinical follow-up.
Discussion
Celiac disease is an auto-immune enteropathy triggered by gluten intolerance in genetically susceptible patients [specially predisposing human leukocyte antigen (HLA) genotypes].4
The pathology is characterized by a wide spectrum of clinical presentations, an autoantibody response, and damage to the small intestinal-mucosa with variable degrees of severity. The diagnosis is frequently suspected when patients have digestive expression of the disease such as chronic diarrhoea, weight loss, abdominal pain, and bloating, which could be found in 40–50% of cases. The diagnosis is based on serologic testing, especially serum Ig A anti-tissue transglutaminase antibodies, then backed up by a biopsy of the small intestine highlighting a partial or total villous atrophy.
The evolution of the disease can be associated with multiple complications such as infertility (and/or recurrent abortions), osteoporosis, ulcerative jejunoileitis, thromboembolic complications or cancers. Enteropathy-associated T-cell lymphoma and jejunal adenocarcinoma are rare complications.5 The risk of venous thrombosis in presence of celiac disease is 1, 27 times higher than the general population.6 It is frequently located in abdominal veins, specifically splanchnic vein thrombosis, and less frequently in lower limbs. The cerebral localization is unusual, and cerebral venous thrombosis represents 1% of all strokes.7
Thromboembolic events are due to quantitative and qualitative abnormalities of platelets, coagulation, or fibrinolysis mechanisms.2 The pathogenesis of this association is not well defined. But risk factors for hypercoagulability conditions are present in 85% of celiac disease patients with venous thrombosis, such as thrombophilia, oral contraception, pregnancy, hyperhomocysteinaemia, protein C or S deficiencies, Vitamin K malabsorption, neoplasms, or genetic mutation (methylene tetrahydrofolic acid reductase MTHFR mutations/JAK 2 mutation).8
Homocysteine is an intermediate metabolite of the methionine synthesis pathway. Folate and B vitamins (especially B6 and B12) are essential co-factors of homocysteine metabolism.9 Deficiencies in B vitamins are commonly identified in celiac disease and induce hyperhomocysteinaemia, which is an independent risk factor for arterial and venous thrombosis. It is present in 20% of celiac disease cases.10
With regards to our patient, her blood tests were normal and we could not deduce any specific aetiology to the thromboembolic complication. Nevertheless, she was not on a gluten-free diet, which leads us to believe that intestinal damages induced by gluten proteins could be a potential aetiology. However, as a limitation of our study, further genetic investigations searching for the cerebral venous thrombosis’s cause were not feasible.
Furthermore, celiac disease found to be part of a multiple auto-immune syndrome (MAS) in several cases.11 MAS is a condition that is defined by the presence of three or more concomitant auto-immune diseases in a single patient. The pathophysiology of this association is not well described but could expose patients to a higher risk of developing thrombosis. This condition is another possible explanation for the thromboembolic complications in our patient, but no concomitant auto-immune disease was diagnosed in this case.
Treatment of celiac disease requires a gluten-free diet and correction of different deficiencies (vitamin K supplementation, vitamin B 12 supplementation, etc). No gluten consumption is the ideal treatment but daily gluten restriction is difficult. According to Catassi et al.,12 the lowest gluten threshold to avoid intestinal mucosa lesion is fixed at 10–50 mg/day. With a good gluten-free diet, healing of the intestinal damage typically occurs within 6–24 months. In our case, although being diagnosed for celiac disease since childhood, our patient did not respect the gluten-free diet.
For cerebral venous thrombosis, anticoagulation or thrombolytic therapy is efficient in 90% of cases.13 Endovascular treatment is a good alternative in patients presenting a resistance or a threatening end result despite a good anticoagulation. Some researchers support the benefits and safety of systemic anticoagulation despite the presence of intracranial haemorrhage.14 Concerning our patient, we immediately started anticoagulation therapy, with a good outcome and without any bleeding complications.
To the best of our knowledge, there are no clear guidelines for cerebral venous thrombosis in patients with celiac disease, nor for anticoagulation therapy and its duration. In our case report, the anticoagulation therapy was completed in 6 months without any adverse effects.
Anticoagulation treatment (with low-molecular-weight heparin) as thromboembolic prophylaxis should be considered depending on the circumstances, especially when celiac disease is associated with other auto-immune disorders.15
Conclusions
Celiac disease is an auto-immune enteropathy that is associated with several coagulation and platelet aggregation abnormalities inducing thromboembolic events. Cerebral venous thrombosis is a rare complication that could be life threatening. Patients should be aware of the importance of a gluten-free diet. Thromboembolic prophylaxis could be discussed on a case by case basis, although guidelines and expert consensus are yet to be agreed upon this matter.
Lead author biography

Dr Maha Bouziane started her cardiology residency at the Cardiology Department of Ibn Rochd University Hospital of Morocco in 2016. She then completed her training as a fellow at the heart valve disease clinic of the Cardiology Department of the University Hospital of Liege in Belgium in 2018. Her areas of interest include non-invasive cardiovascular imaging, echocardiography, and valvular heart disease.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Funding
The authors received no specific funding for this work.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
Supplementary Material
Contributor Information
Maha Bouziane, Cardiology Department, P37, Ibn Rochd University Hospital, 1, Rue des Hôpitaux, Quartier des Hôpitaux, Casablanca, Morocco.
Salim Arous, Cardiology Department, P37, Ibn Rochd University Hospital, 1, Rue des Hôpitaux, Quartier des Hôpitaux, Casablanca, Morocco.
Rachida Habbal, Cardiology Department, P37, Ibn Rochd University Hospital, 1, Rue des Hôpitaux, Quartier des Hôpitaux, Casablanca, Morocco.
References
- 1. Biagi F, Klersy C, Balduzzi D, Corazza GR.. Are we not over-estimating the prevalence of celiac disease in the general population? Ann Med 2010;42:557–561. [DOI] [PubMed] [Google Scholar]
- 2. Halfdanarson TR, Litzow MR, Murray J.. Hematologic manifestations of coeliac disease. Blood 2007;109:412–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ungprasert P, Wijarnpreecha K, Tanratana P.. Risk of venous thromboembolism in patients with celiac disease: a systematic review and meta-analysis. J Gastroenterol Hepatol 2016;31:1240–1245. [DOI] [PubMed] [Google Scholar]
- 4. Mustalahti K, Catassi C, Reunanen A, Fabiani E, Heier M, McMillan S. et al. The prevalence of celiac disease in Europe: results of a centralized, international mass screening project. Ann Med 2010;42:587–595. [DOI] [PubMed] [Google Scholar]
- 5. Sharaiha RZ, Lebwohl B, Reimers L, Bhagat G, Green PH, Neugut AI.. Increasing incidence of enteropathy-associated T-cell lymphoma in the United States, 1973-2008. Cancer 2012;118:3786–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ludvigsson JF, Welander A, Lassila R, Ekbom A, Montgomery SM.. Risk of thromboembolism in 14,000 individuals with coeliac disease. Br J Haematol 2007;139:121–127. [DOI] [PubMed] [Google Scholar]
- 7. Beyrouti R, Mansour M, Kacem A, Derbali H, Mrissa R.. Recurrent cerebral venous thrombosis revealing celiac disease: an exceptional case report. Acta Neurol Belg 2017;117:341–343. [DOI] [PubMed] [Google Scholar]
- 8. Lerner A, Blank M.. Hypercoagulability in celiac disease an update. Autoimmun Rev 2014;13:1138–1141. [DOI] [PubMed] [Google Scholar]
- 9. McCully KS. Homocysteine, vitamins, and vascular disease prevention. Am J Clin Nutr 2007;86:1563S–1568S. [DOI] [PubMed] [Google Scholar]
- 10. Grover PJ, Jayaram R, Madder H.. Management of cerebral venous thrombosis in patients with Law-Hamilton syndrome and coeliac disease, epilepsy and cerebral calcification syndrome. Br J Neurosurg 2010;24:684–685. [DOI] [PubMed] [Google Scholar]
- 11. Harpreet S, Deepak J, Kiran B.. Multiple autoimmune syndrome with celiac disease. Reumatologia 2016;6:326–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Catassi C, Fabiani E, Iacono G, D’Agate C, Francavilla R, Biagi F. et al. Prospective, double-blind, placebo-controlled trial to establish a safe gluten threshold for patients with celiac disease. Am J Clin Nutr 2007;85:160–166. [DOI] [PubMed] [Google Scholar]
- 13. Ferro JM, Canhão P, Stam J, Bousser M-G, Barinagarrementeria F; ISCVT Investigators. Prognosis of cerebral vein and dural sinus thrombosis: results of the international study on cerebral vein and dural sinus thrombosis (ISCVT). Stroke 2004;35:664–670. [DOI] [PubMed] [Google Scholar]
- 14. Medel R, Monteith SJ, Crowley RW, Dumont AS.. A review of therapeutic strategies for the management of cerebral venous sinus thrombosis. Neurosurg Focus 2009;27:E6. [DOI] [PubMed] [Google Scholar]
- 15. Jorge O, Jorge A, Camus G.. Celiac disease associated with antiphospholipid syndrome. Rev Esp Enferm Dig 2008;100:102–103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.