Abstract
Background
Vitamin K antagonists (VKAs) have been regarded as the therapy of choice for intracardiac thrombosis for decades based mostly on observational data. The advent of direct oral anticoagulants (DOACs) has displaced VKAs as the first-line therapy for multiple thrombotic disorders but not for intracardiac thrombosis. Although limited, there is growing evidence that DOACs are effective for intracardiac thrombosis and some data suggest that thrombus resolution might be superior to that with warfarin.
Case summary
Here, we present a series of six patients with left atrial appendage thrombi were treated with a venous thromboembolic dose of DOACs with resolution within 2–6 months with no reported complications.
Discussion
This case series adds to the accumulating evidence supporting the efficacy of DOACs in the treatment of intracardiac thrombi.
Keywords: Oral anticoagulants, Intracardiac thrombosis, DOAC, Case series
Learning points
Atrial arrhythmias are associated with high risk of intracardiac thrombus formation.
Venous thromboembolic therapeutic dose of rivaroxaban maybe effective in treatment of intra-atrial thrombosis in patients with atrial arrhythmias.
Introduction
Intracardiac thrombosis can be identified in any of the four cardiac chambers (including atrial appendages) of the heart. Most intracardiac thrombi are seen in the left atrial appendage (LAA) and left ventricle in the setting of atrial fibrillation (AF) and post-myocardial infarction.1 The development of intracardiac thrombi stems from Virchow’s triad: stasis, endothelial injury, and hypercoagulable state. Intracardiac thrombosis has multiple risk factors including AF, valvular disease, cardiac devices, heart failure with reduced ejection fraction (EF), dilated cardiomyopathy, acute myocardial infarction, and chronic left ventricular (LV) aneurysm with other systemic diseases such as amyloidosis, Behcet’s disease.2–4
The most fearful complication of an intracardiac thrombus is embolization; thrombi that are protruding, mobile or with central echolucency have a greater predisposition to embolize.5 Transthoracic echocardiography is the most used modality to diagnose intracardiac thrombosis. It has a sensitivity of 90–95% and a specificity 85–90% for the detection of LV thrombi.6 Transoesophageal echocardiography (TOE) has an even greater sensitivity and specificity by providing more detailed information but is considerably more invasive and not without associated risks. Intracardiac echocardiography maybe similarly useful in LAA assessment for thrombus presence.7 Contrast-enhanced computed tomography of the chest is emerging as well as helpful tool in intracardiac thrombus assessment.8
The standard of care for intracardiac thrombus is a treatment with a vitamin K antagonist (VKA), but the guidelines are largely based on observational studies.4 The use of VKA is limited by interactions with various foods, bleeding complications, and need for frequent monitoring; hence, non-VKAs have been used increasingly for the treatment of intracardiac thrombosis. Therefore, direct oral anticoagulants (DOACs) appear as a very attractive alternative. To date, no large randomized trials supporting the use of DOACs in the management of intracardiac thrombosis have been conducted; however, effectiveness of DOACs has been confirmed in a variety of thrombotic conditions.9
The aim of this article is to report a series of cases showing the resolution of intracardiac thrombi with the use of DOACs.
Timeline
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | |
|---|---|---|---|---|---|---|
| Time 0 | ||||||
| Indication for anticoagulation | Persistent atrial fibrillation | Paroxysmal atrial fibrillation | Newly diagnosed atrial fibrillation | Persistent atrial fibrillation | Atrial flutter | Persistent atrial fibrillation |
| Type of anticoagulation | Rivaroxaban | Rivaroxaban | None | Rivaroxaban | Rivaroxaban | Rivaroxaban |
| Dose of anticoagulation | 20 mg daily | 20 mg daily | 20 mg daily | 20 mg daily | 20 mg daily | |
| Time 1 | ||||||
| Presenting symptoms | Palpitation and dizziness | Palpitation and weakness | Palpitation | Palpitation | Palpitation and dizziness | Palpitation |
| Echocardiographic finding | Left atrial thrombus | Left atrial thrombus | Left atrial thrombus | Left atrial thrombus | Left atrial thrombus | Left atrial thrombus |
| Type of anticoagulation for Management | Rivaroxaban | Rivaroxaban | Rivaroxaban | Rivaroxaban | Rivaroxaban | Rivaroxaban |
| Dose of anticoagulation | 15 mg twice a day for 3 weeks then 20 mg daily | 15 mg twice a day for 3 weeks then 20 mg daily | 15 mg twice a day for 3 weeks then 20 mg daily | 15 mg twice a day for 3 weeks then 20 mg daily | 15 mg twice a day for 3 weeks then 20 mg daily | 15 mg twice a day for 3 weeks then 20 mg daily |
| Time 2 | ||||||
| Follow-up echocardiography | No thrombi | No thrombi | No thrombi | No thrombi | No thrombi | No thrombi |
| Follow-up time | 7 months | 2 months | 6 months | 5 months | 2 months | 2 months |
Case presentation
Patient 1
A 49-year-old African American female with a past medical history of hypertension, hypertrophic cardiomyopathy, hyperlipidaemia, obesity, atrial flutter with two ablation procedures, that presented for recurrent atrial fibrillation after electrical cardioversion. She was on rivaroxaban 20 mg daily for 12 months. The patient reported palpitations and dizziness. Her exercise tolerance decreased from 10 blocks to 2 blocks. A TOE in preparation for cardioversion revealed normal EF, moderately dilated left atrium (LA), and a density at the bottom of the appendage consistent with a loosely organized thrombus (Figure 1A).
Figure 1.
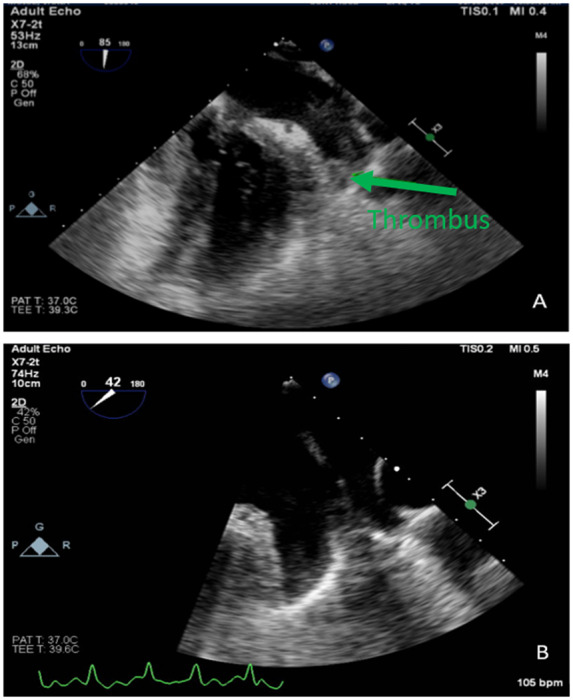
TOE images of left atrial appendage thrombus and subsequent thrombus resolution.
The patient was given rivaroxaban 15 mg twice a day for 3 weeks then 20 mg daily for intracardiac thrombus. On follow-up visit at 7 months, the patient reported compliance on medications. A repeat TOE was performed which revealed spontaneous echo contrast in the appendage with streaming and no thrombus seen (Figure 1B). She went for elective electrical cardioversion and converted to sinus rhythm.
Patient 2
A 63-year-old African American male with a past medical history of non-ischaemic cardiomyopathy, heart failure with reduced EF, hypertension, hyperlipidaemia, paroxysmal atrial fibrillation with two trials of electrical cardioversion, and a trial of chemical cardioversion with dofetilide and amiodarone came for follow-up due to symptomatic atrial fibrillation. He was on 20 mg rivaroxaban for anticoagulation for 4 months. The patient reported palpitations and weakness. He was sent for TOE in preparation for cardioversion which revealed an estimated EF of 25% with diffuse hypokinesia, moderately dilated LA, and a thrombus at the bottom of the appendage (Figure 2A).
Figure 2.
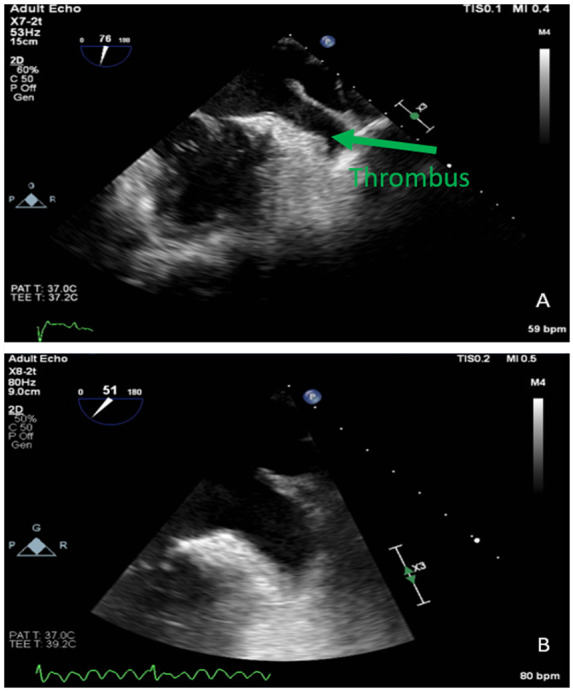
TOE images of left atrial appendage thrombus and subsequent thrombus resolution.
The patient was given rivaroxaban 15 mg twice a day for 3 weeks then 20 mg daily for intracardiac thrombus. On follow-up after 2 months, a repeat TOE which revealed markedly dilated LA with spontaneous echo contrast (‘smoke’) at the appendage without a thrombus (Figure 2B). He was converted to sinus rhythm with electrical cardioversion.
Patient 3
A 69-year-old African American female with a past medical history of hypertension presented with a newly diagnosed atrial fibrillation. The patient reported intermittent palpitations. She was sent for TOE in preparation for cardioversion which revealed normal EF, moderately dilated LA, and mobile oblong shaped mass at the distal portion of the appendage consistent with thrombus (Figure 3A).
Figure 3.
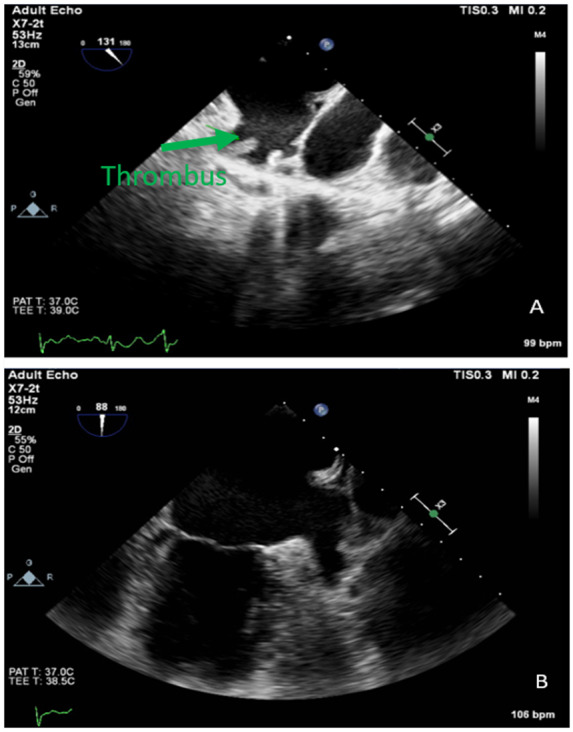
TOE images of left atrial appendage thrombus and subsequent thrombus resolution.
She was started on rivaroxaban 15 mg twice a day for 3 weeks then 20 mg daily for intracardiac thrombus. During follow-up after 6 months, she was sent for a repeat TOE which revealed spontaneous echo contrast in the appendage with streaming and no thrombus seen (Figure 3B). She went for elective electrical cardioversion and converted to sinus rhythm.
Patient 4
A 67-year-old African American female with a past medical history of Type II diabetes mellitus, hypertension, hyperlipidaemia, breast cancer after mastectomy, and atrial fibrillation was referred for atrial fibrillation ablation despite therapy with dofetilide. She was on 20 mg rivaroxaban daily for 3 months. The patient reported palpitations. She was sent for TOE in preparation for ablation which revealed a normal EF and a thrombus at the bottom of the appendage (Figure 4A).
Figure 4.
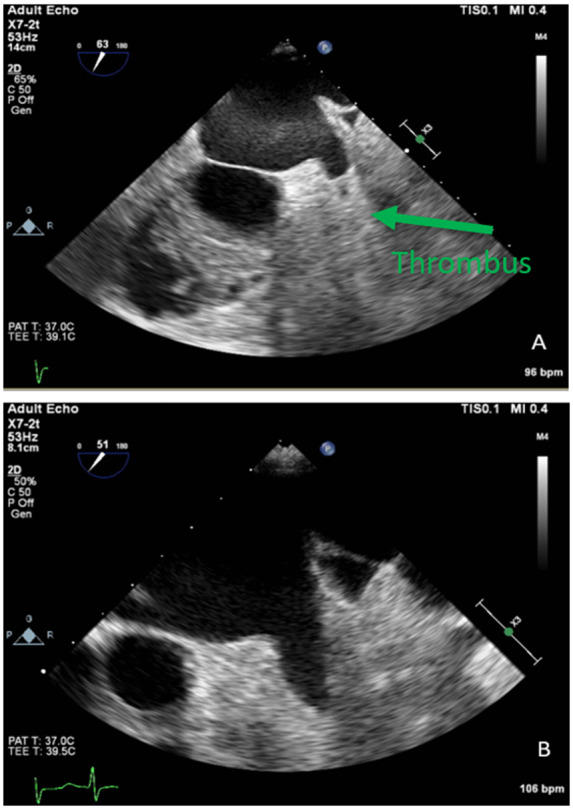
TOE images of left atrial appendage thrombus and subsequent thrombus resolution.
The patient was switched to rivaroxaban 15 mg twice a day for 3 weeks then 20 mg daily for intra-atrial thrombus. On follow-up after 5 months, she was sent for a repeat TOE which revealed mildly dilated LA with no thrombus identified (Figure 4B). She went for an elective ablation which was successful.
Patient 5
A 70-year-old African American male with a past medical history of hypertension, Type 2 diabetes mellitus, and atrial flutter was referred for ablation therapy. The patient is being treated with rivaroxaban 20 mg daily for stroke prophylaxis for the last 6 months. The patient reported intermittent palpitations and dizziness. He was sent for TOE which revealed normal EF, mildly dilated LA, and density in the tip of the appendage consistent with thrombus (Figure 5A).
Figure 5.
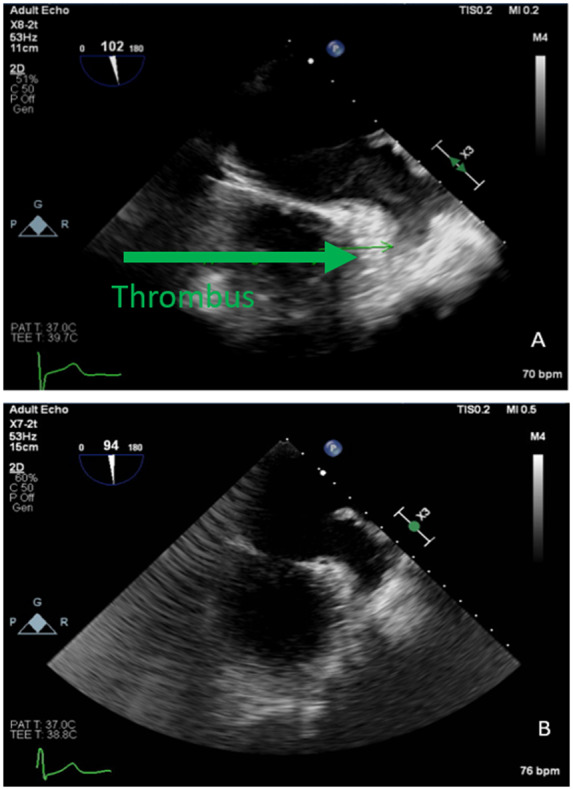
TOE images of left atrial appendage thrombus and subsequent thrombus resolution.
His medications were modified to rivaroxaban 15 mg twice a day for 3 weeks then 20 mg daily for intracardiac thrombus. Follow-up after 2 months, the patient reported compliance on medications. He was sent for a repeat TOE which revealed no thrombus (Figure 5B). He later underwent a successful ablation.
Patient 6
A 64-year-old African American female with a past medical history of chronic obstructive pulmonary disease on home oxygen, non-ischaemic cardiomyopathy, heart failure with mid-range EF, obstructive sleep apnoea, pulmonary arterial hypertension, Type 2 diabetes mellitus, hypertension, mitral valve replacement, hypothyroidism, and atrial flutter resistant to rate control presented after referral for atrial flutter ablation. She is being treated with rivaroxaban 20 mg daily for 12 months. The patient still reported palpitations. She underwent TOE which revealed an estimated EF of 40% with diffuse hypokinesia, markedly dilated LA, and a thrombus was identified at the LAA (Figure 6A).
Figure 6.
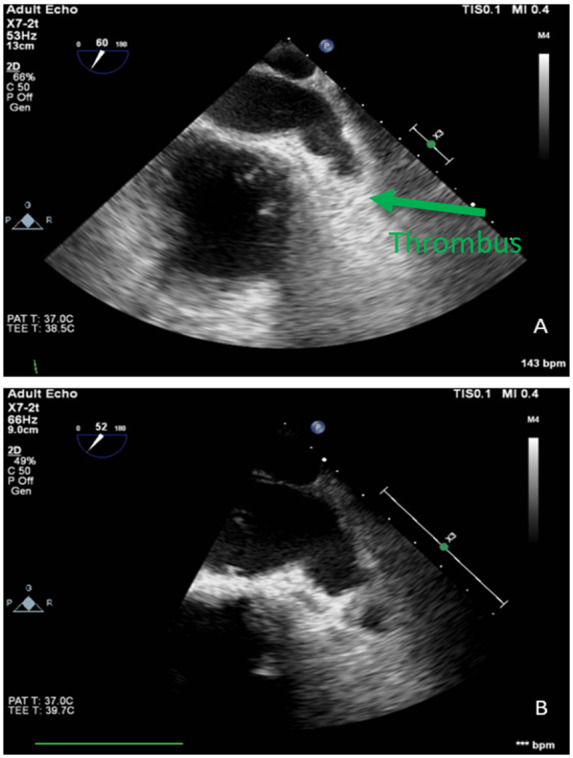
TOE images of left atrial appendage thrombus and subsequent thrombus resolution.
The patient was given rivaroxaban 15 mg twice a day for 3 weeks then 20 mg daily for intracardiac thrombus. On follow-up after 2 months, she was sent for a repeat TOE which revealed markedly dilated LA with spontaneous echo contrast (‘smoke’) at the appendage without a thrombus (Figure 6B). She then underwent atrial flutter ablation.
Discussion
The current guidelines recommend anticoagulation with VKA for the treatment of LV thrombus, with a target international normalized ratio (INR) of 2.0–3.0 for at least 3 months.10 Similarly, left atrial thrombus should be treated with VKA (INR 2–3) for at least 3 weeks.11 These recommendations are supported mainly by observational data. A meta-analysis of observational studies of patients with ST-elevation myocardial infarction and LV thrombus compared the risk of embolization and effects of treatment with VKA showing decrease in the risk of embolization and formation of mural thrombus.4 However, warfarin, considered the preferred treatment for intracardiac thrombosis, poses many challenges to both clinicians and patients because of its slow onset or action, bleeding complications, narrow therapeutic window requiring frequent monitoring, and interactions with various foods and drugs.12
These concerns have been largely mitigated with the use of DOACs. Direct oral anticoagulants possess several advantages over VKA. These include rapid onset, no need for laboratory monitoring, fixed dosing lesser risk of bleeding and no interaction with food.1 Direct oral anticoagulants are currently approved for the treatment of acute venous thromboembolism (VTE), prevention of recurrent VTE, prevention of stroke, and systemic embolism in non-valvular AF and VTE prophylaxis in major orthopaedic surgery.13 Despite the many advantages of DOACs, the lack of randomized trials has resulted in their limited use for the treatment and prevention of intracardiac thrombosis.14,15
Our cases are supported by multiple published articles providing evidence on the efficacy of DOACs in the treatment of intracardiac thrombosis. Shokr et al.16 demonstrated multiple cases revealing the resolution of LV thrombus with apixaban and rivaroxaban. Several case reports have been published on LV thrombus secondary to tachycardia-induced and hypertrophic cardiomyopathies that have been treated with DOACs.17,18 Hammerstingl et al.19 evaluated the resolution rates of LAA thrombi with DOACs in patients who failed VKA therapy. The absolute resolution rates of DOACs (61%) were significantly higher than VKA (8.1%). Hao et al.20 compared the efficacy and safety of dabigatran (fixed dose of 150 mg) with warfarin for the treatment of intracardiac thrombus in patients with non-valvular AF. Out of the 19 patients treated with dabigatran, 17 patients (89.5%) had complete thrombus resolution in 3 months. Furthermore, a collection of data from multiple case reports suggested that DOACs had a 90% complete thrombus resolution rate without causing any bleeding complications.1
Conclusion
This case series adds to the accumulating evidence supporting the efficacy of DOACs in the treatment of intracardiac thrombi. Further randomized trials are needed to provide solid evidence with that respect with one of them being apixaban vs. warfarin in patients with LV thrombus (ClinicalTrials.gov identifier: NCT03232398).
Lead author biography

Mohammed Al-Sadawi is an Internal medicine resident at SUNY Downstate Medical Center in Brooklyn, New York, USA. He is going to start cardiovascular medicine fellowship at SUNY Stony Brook.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patients in line with COPE guidance.
Conflict of interest: none declared.
Supplementary Material
Contributor Information
Mohammed Al-Sadawi, Department of Internal Medicine, SUNY Downstate, 450 Clarkson Ave Box 1199, Brooklyn, NY 11203, USA.
Jonathan Francois, Department of Internal Medicine, SUNY Downstate, 450 Clarkson Ave Box 1199, Brooklyn, NY 11203, USA.
Romy Rodriguez Ortega, Department of Internal Medicine, SUNY Downstate, 450 Clarkson Ave Box 1199, Brooklyn, NY 11203, USA.
Violeta Capric, Department of Internal Medicine, SUNY Downstate, 450 Clarkson Ave Box 1199, Brooklyn, NY 11203, USA.
Adam S Budzikowski, Division of Cardiovascular Medicine, Electrophysiology Section, Department of Medicine, SUNY Downstate, 450 Clarkson Ave Box 1199, Brooklyn, NY 11203, USA.
References
- 1. Ghaffarpasand E, Tehrani MD, Marszalek J, Chi G.. Non-vitamin K antagonist oral anticoagulants for the treatment of intracardiac thrombosis. J Thromb Thrombolysis 2018;46:332–338. [DOI] [PubMed] [Google Scholar]
- 2. Waller BF, Grider L, Rohr TM, McLaughlin T, Taliercio CP, Fetters J.. Intracardiac thrombi: frequency, location, etiology, and complications: a morphologic review—part I. Clin Cardiol 1995;18:477–479. [DOI] [PubMed] [Google Scholar]
- 3. Chesebro JH, Adams PC, Fuster V.. Antithrombotic therapy in patients with valvular heart disease and prosthetic heart valves. J Am Coll Cardiol 1986;8:41B–56B. [DOI] [PubMed] [Google Scholar]
- 4. Vaitkus PT, Barnathan ES.. Embolic potential, prevention and management of mural thrombus complicating anterior myocardial infarction: a meta-analysis. J Am Coll Cardiol 1993;22:1004–1009. [DOI] [PubMed] [Google Scholar]
- 5. Visser CA, Kan G, Meltzer RS, Dunning AJ, Roelandt J.. Embolic potential of left ventricular thrombus after myocardial infarction: a two-dimensional echocardiographic study of 119 patients. J Am Coll Cardiol 1985;5:1276–1280. [DOI] [PubMed] [Google Scholar]
- 6. Visser CA, Kan G, David GK, Lie KI, Durrer D.. Two dimensional echocardiography in the diagnosis of left ventricular thrombus. A prospective study of 67 patients with anatomic validation. Chest 1983;83:228–232. [DOI] [PubMed] [Google Scholar]
- 7. Baran J, Stec S, Pilichowska-Paszkiet E, Zaborska B, Sikora-Frąc M, Kryński T. et al. Intracardiac echocardiography for detection of thrombus in the left atrial appendage: comparison with transesophageal echocardiography in patients undergoing ablation for atrial fibrillation: the Action-Ice I Study. Circ Arrhythm Electrophysiol 2013;6:1074–1081. [DOI] [PubMed] [Google Scholar]
- 8. Kawaji T, Numamoto H, Yamagami S, Mabuchi R, Kitamura T, Enoki N. et al. Real-time surveillance of left atrial appendage thrombus during contrast computed tomography imaging for catheter ablation: THe Reliability of cOMputed tomography Beyond UltraSound in THROMBUS detection (THROMBUS) study. J Thromb Thrombolysis 2019;47:42–50. [DOI] [PubMed] [Google Scholar]
- 9. Jun M, Lix LM, Durand M, Dahl M, Paterson JM, Dormuth CR. et al. ; Canadian Network for Observational Drug Effect Studies (CNODES) Investigators. Comparative safety of direct oral anticoagulants and warfarin in venous thromboembolism: multicentre, population based, observational study. BMJ 2017;359:j4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kushner FG, Hand M, Smith SC Jr, King SB 3rd, Anderson JL, Antman EM. et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2009;54:2205–2241. [DOI] [PubMed] [Google Scholar]
- 11.European Heart Rhythm Association; European Association for Cardio-Thoracic SurgeryCamm AJ, Kirchhof P, Lip GYH, Schotten U, Savelieva I, Ernst S. et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010;31:2369–2429. [DOI] [PubMed] [Google Scholar]
- 12. Savelieva I, Camm AJ.. Practical considerations for using novel oral anticoagulants in patients with atrial fibrillation. Clin Cardiol 2014;37:32–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rao PSS, Burkart T.. Advances in oral anticoagulation therapy—what’s in the pipeline? Blood Rev 2017;31:205–211. [DOI] [PubMed] [Google Scholar]
- 14. Vandvik PO, Lincoff AM, Gore JM, Gutterman DD, Sonnenberg FA, Alonso-Coello P. et al. Primary and secondary prevention of cardiovascular disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e637S–ee68S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O’Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA. et al. ; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;127:529–555. [DOI] [PubMed] [Google Scholar]
- 16. Shokr M, Ahmed A, Abubakar H, Sayedahmad Z, Rashed A, Afonso L. et al. Use of direct oral anticoagulants in the treatment of left ventricular thrombi: a tertiary center experience and review of the literature. Clin Case Rep 2019;7:135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nakasuka K, Ito S, Noda T, Hasuo T, Sekimoto S, Ohmori H. et al. Resolution of left ventricular thrombus secondary to tachycardia-induced heart failure with rivaroxaban. Case Rep Med 2014;2014:814524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaya A, Hayiroglu MI, Keskin M, Tekkesin AI, Alper AT.. Resolution of left ventricular thrombus with apixaban in a patient with hypertrophic cardiomyopathy. Turk Kardiyol Dern Ars 2016;44:335–337. [DOI] [PubMed] [Google Scholar]
- 19. Hammerstingl C, Lambers M, Schueler R, Nickenig G.. Direct acting oral anticoagulants are more effective than vitamin-K-antagonists for the resolution of established left atrial thrombi in patients with atrial fibrillation. J Am Coll Cardiol 2015;65:A357. [Google Scholar]
- 20. Hao L, Zhong JQ, Zhang W, Rong B, Xie F, Wang JT. et al. Uninterrupted dabigatran versus warfarin in the treatment of intracardiac thrombus in patients with non-valvular atrial fibrillation. Int J Cardiol 2015;190:63–66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


