Abstract
Background
Selexipag is an oral selective prostacyclin IP receptor agonist approved in patients with low- and intermediate-risk pulmonary hypertension (PH); evidence in patients at high risk is lacking.
Case summary
A 42-year-old woman with worsening dyspnoea (World Health Organization functional class III–IV) and suspected PH at echocardiographic examination was evaluated in our Pulmonary Hypertension Centre. Right heart catheterization showed pre-capillary PH with reduced cardiac index and increased pulmonary vascular resistance. High-resolution computed tomography excluded parenchymal lung disease and ventilation/perfusion (V/Q) lung scan was negative for mismatched perfusion defects so the conclusive diagnosis was high-risk idiopathic pulmonary arterial hypertension (PAH). The patient refused an initial combination therapy including a parenteral prostacyclin analogue (PCA) in accordance with the ESC/ERS guidelines, so an off-label triple oral combination therapy including a phosphodiesterase-5 inhibitor, an endothelin receptor antagonist, and selexipag was started. At 3- and 6-month follow-up we found a clinical and haemodynamic improvement, so the patient was reclassified as low risk. Her clinical condition is currently stable.
Discussion
Despite the benefit of parenteral PCAs in high-risk PAH, low adherence to treatment may be explained by adverse side effects related to the intravenous route of administration. Given the potential effect seen in our patient, upfront triple oral combination therapy in PAH high-risk patients should be further evaluated in a controlled clinical trial.
Keywords: Pulmonary arterial hypertension, Upfront triple oral combination therapy, Selexipag, Case report
Learning points
Pulmonary arterial hypertension (PAH) is a severe disease with a poor prognosis. In high-risk patients an upfront combination therapy including intravenous prostacyclin analogues (PCAs) is recommended.
Considering the potential efficacy, an upfront initial triple oral combination therapy should be further evaluated in a controlled clinical trial, especially in high-risk PAH patients who are not candidates for parenteral administration of PCAs.
Introduction
Pulmonary arterial hypertension (PAH) is a rare and progressive disease with a poor prognosis. Three distinct signalling pathways can be addressed by specific drugs: the endothelin, the nitric oxide, and the prostacyclin pathway. Current guidelines recommend combination therapies targeting these pathways based on mortality-risk assessment. Upfront oral combination therapy is approved in low- to intermediate-risk patients, whereas initial combination therapy including parenteral prostanoids is recommended in the high risk. Selexipag is an oral selective IP prostacyclin receptor agonist approved in low and intermediate risk, but evidence in patients at high risk is lacking.1 We report a successful case of upfront triple oral combination therapy including selexipag in a young woman with high-risk idiopathic PAH (IPAH).
Timeline
| Day 0 |
|
| Days 1–2 |
|
| Days 4–6 |
|
| Day 7 |
|
| Day 10 |
|
| 3-Month follow-up |
|
| 6-Month follow-up |
|
Case presentation
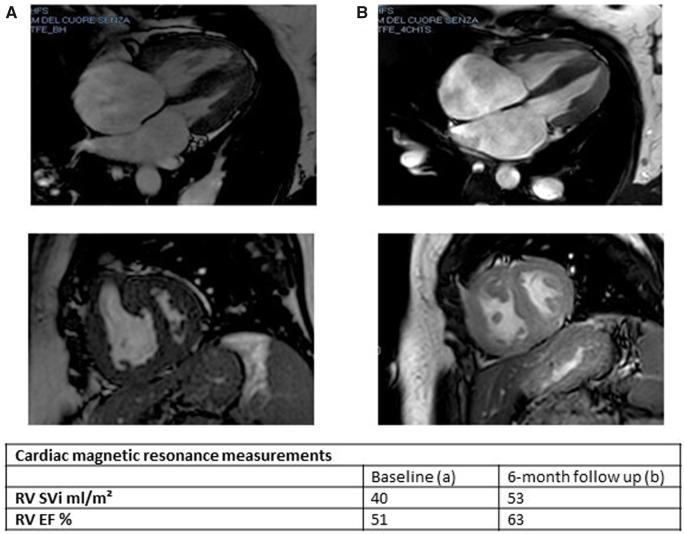
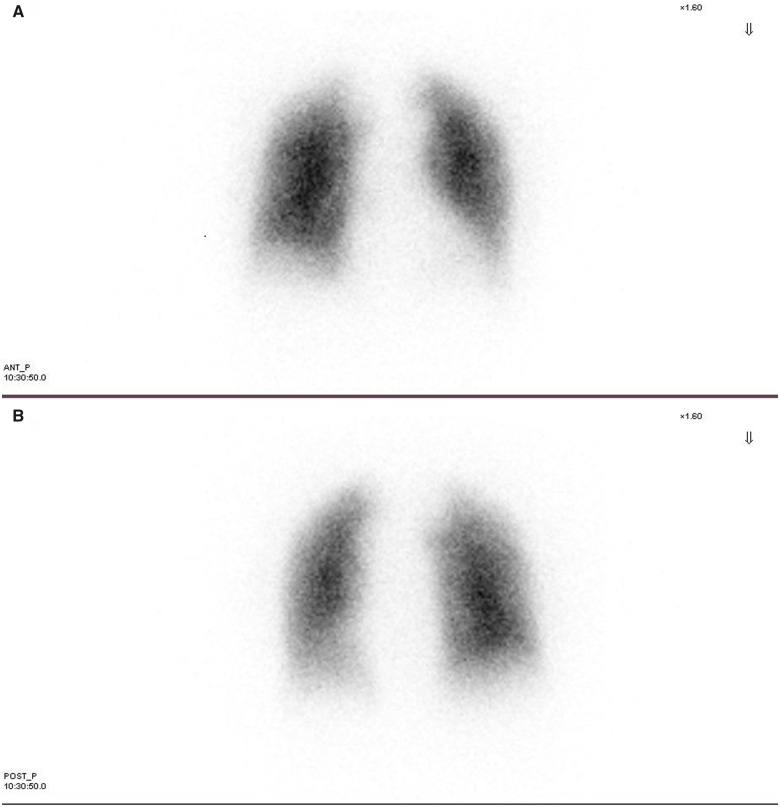
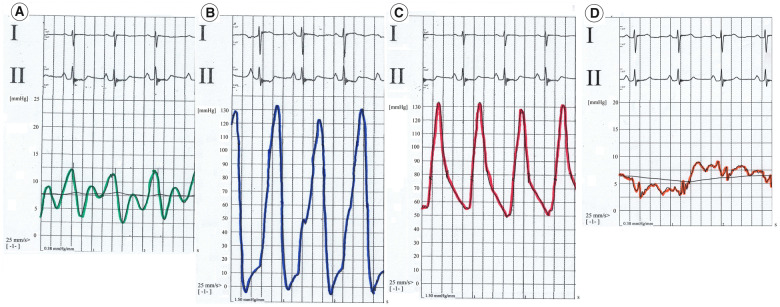
A 42-year-old woman with progressive exertional dyspnoea and World Health Organization functional class (WHO FC) III–IV and a recent episode of haemoptysis was referred to our Pulmonary Hypertension Centre. She had no cardiovascular risk factors and family medical history was not available because the patient was adopted at the age of 12. She had no history of human immunodeficiency virus infection, no exposure to drugs or toxins involved in the development of PAH, no history of chronic liver disease, deep vein thrombosis, or connective tissue disease. At our first physical examination, the patient did not have elevated jugular venous pressure, hepatomegaly, ascites, or peripheral oedema; but the auscultation revealed an accentuated pulmonary component of the second heart sound. The electrocardiogram showed sinus rhythm and right bundle branch block. Transthoracic echocardiography confirmed the suspicion of pulmonary hypertension (PH): systolic pulmonary arterial pressure was estimated to be 80 mmHg, the pulmonary artery trunk was dilated and the right ventricular (RV) outflow tract acceleration time was shortened. No pericardial effusion was detected. Six minutes walking test (6MWT) showed impairment in functional capacity (220 m). Cardiac magnetic resonance (CMR) imaging showed a hypertrophic, normal-volume right ventricle with mild depressed global systolic function (RV ejection fraction 51%) and no evidence of congenital heart disease. The left ventricle ejection fraction was normal with flattening of the interventricular septum (Figure 1A). High-resolution computed tomography excluded parenchymal lung disease and perfusion lung scan was negative for mismatched perfusion defects, ruling out the suspect of chronic thromboembolic PH (Figure 2). Although the patient had never been complaining of angina coronary angiography was performed to exclude left main stem coronary artery compression by the dilated pulmonary artery trunk. The exam did not demonstrate any coronary artery abnormality. Finally, right heart catheterization (RHC) confirmed pre-capillary PH with severe reduction of cardiac index and a remarkable increase of pulmonary vascular resistance (Figure 3 and Table 1). The patient was non-responder to acute vasoreactivity testing performed by inhaled nitric oxide and a conclusive diagnosis of IPAH was made. After a comprehensive clinical assessment, based on ESC/ERS table risk stratification, the patient was classified as high risk (Table 2) and an initial combination therapy including a parenteral prostacyclin analogue (PCA) was proposed.1 However, the patient refused PCA for personal concerns about the potential side effects. Consequently, oral therapy including low-dose diuretics (furosemide 50 mg once daily) and specific drugs acting on the three separate signalling pathway involved in PAH were used. So a phosphodiesterase-5 inhibitor (Sildenafil 20 mg three times daily), an endothelin receptor antagonist (Macitentan 10 mg once daily), and selexipag were started under strict medical supervision. Selexipag was started at dosage of 200 μg twice daily and titrated up to 1200 μg twice daily over 30 days. The therapy was well tolerated without hypotension. At 3- and 6-month follow-up on the same medication we found a significant clinical and haemodynamic improvement as confirmed by a comprehensive revaluation including echocardiography, CMR (Figure 1B), brain natriuretic peptide, 6MWT, and RHC (Table 1). So the patient was reclassified as low risk (Table 2). After 6 months clinical conditions were still stable on the same medical regimen, referral for lung transplantation is under evaluation.
Figure 1.
(A) Cardiac magnetic resonance at baseline. (B) Cardiac magnetic resonance at 6-month follow-up. RV EF, right ventricle ejection fraction; RV SVi, right ventricle stroke volume index.
Figure 2.
Perfusion lung scan, no perfusion defect was detected. (A) Anterior view. (B) Posterior view.
Figure 3.
Basal right heart catheterization pressure tracings. (A) Right atrium. (B) Right ventricle. (C) Pulmonary artery. (D) Pulmonary artery wedge pressure.
Table 1.
Haemodynamic evaluation at baseline, 3- and 6-month follow-up
| Baseline | 3-Month follow-up | 6-Month follow-up | |
|---|---|---|---|
| RAP (mmHg) | 7 | 6 | 6 |
| mPAP (mmHg) | 81 | 58 | 60 |
| PAWP (mmHg) | 8 | 9 | 7 |
| CI (L/min/mq) | 1.9 | 2.8 | 2.9 |
| PVR (Wood Unit) | 20 | 9.7 | 10.5 |
CI, cardiac index; mPAP, mean pulmonary arterial pressure; PAWP, pulmonary artery wedge pressure; PVR, pulmonary vascular resistance; RAP, right atrial pressure.
Table 2.
Risk assessment at baseline, 3- and 6-month follow-up according to ESC pulmonary arterial hypertension guidelines risk assessment
| Baseline | 3-Month follow-up | 6-Month follow-up | |
|---|---|---|---|
| Clinical sign of right heart failure | No | No | No |
| Progression of symptoms | Rapid | No | No |
| Syncope | No | No | No |
| WHO FC | III–IV | II | I |
| 6MWD (m) | 220 | 480 | 490 |
| BNP (ng/L) | 330 | 187 | 25 |
| Right atrium area (cmq) | 27 | 25 | 19 |
| Pericardial effusion | No | No | No |
| RAP (mmHg) | 7 | 6 | 6 |
| CI (L/min/mq) | 1.9 | 2.8 | 2.9 |
| SvO2 (%) | 65 | 66 | 66 |
Green, low-risk determinants; red, high-risk determinants; yellow, intermediate-risk determinants.
BNP, brain natriuretic peptide; CI, cardiac index; 6MWD, 6 minutes walking distance; RAP, right atrial pressure; SvO2, mixed venous oxygen saturation; WHO FC, World Health Organization functional class.
Discussion
Intravenous prostacyclin is the cornerstone of medical therapy in high-risk patients with PAH.2,3 Prostacyclin is an endogenous prostanoid that induces vasodilatation, inhibits platelet aggregation and cell proliferation. Prostacyclin is noticeably down-regulated in PAH.4 Despite the benefit of intravenous PCAs, many patients die without receiving this treatment, probably due to the high rate of adverse side effects.5 Long-term intravenous drug administration is associated with a not negligible risk of severe complications, ranging from 15% to 20% and including catheter occlusion, central venous thrombosis, line infections, and catheter-related sepsis.6,7
Selexipag is an orally available IP prostacyclin receptor agonist. Following absorption, it is hydrolyzed to an active metabolite, which has a 7.9-h half-life, allowing for twice-daily dosing. The density of prostacyclin receptors varies largely in humans, so the dose required for each patient needs to be adjusted, being the highest tolerated dose the goal of titration.8,9
The GRIPHON study was the pivotal randomized, event-driven, controlled trial evaluating the safety and efficacy of selexipag in PAH patients. In GRIPHON, selexipag reduced the risk of experiencing the primary mortality/morbidity endpoint event of 40% compared with placebo. Among the entire cohort of patients recruited, 376 received a background double combination therapy with a phosphodiesterase-5 inhibitor and an endothelin receptor antagonist before randomization.10 In a recent post hoc analysis of GRIPHON, the efficacy of selexipag as a third agent was evaluated in this subgroup, demonstrating the incremental benefit of selexipag compared to placebo, consistent with the results observed for the overall population.11 Also, the ongoing phase III TRITON trial could confirm these results.12 However, due to the low rate of WHO FC IV patients enrolled, GRIPHON cannot provide evidence about the efficacy of selexipag in the high-risk patients, so the medication has been approved only in low- or intermediate-risk status.
In the high-risk patients, selexipag efficacy was reported for the transition from parenteral to oral therapy in stable WHO FC I/II patients on parenteral prostanoids. The switching therapy to selexipag was well tolerated without any complication or impairment of clinical status.13
Our patient rejected the parenteral administration of PCAs, so we started an upfront triple oral combination therapy including selexipag, achieving significant clinical improvement. This case report may provide an insight into therapeutic options for patients at high risk who are not suitable for parenteral prostanoid administration. Nevertheless, further investigation is needed to clarify the role of an initial triple oral combination therapy in high-risk patients and gain knowledge about the ideal clinical setting where this strategy could be better applied.
Lead author biography

Serena Rossi is an interventional cardiologist working in SS. Annunziata Hospital in Chieti, Italy. After her medical degree, obtained in 2011, she gained expertise in coronary intervention, right and left heart catheterization and in the management of PH patients.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
Supplementary Material
Contributor Information
Serena Rossi, Cardiology Department, Interventional Cardiology Unit, SS. Annunziata Hospital, Via dei Vestini, 66100 Chieti, Italy.
Carla Pietrangelo, Department of Medical, Oral and Biotechnological Sciences, University “Gabriele d’Annunzio”, Via dei Vestini, 66100 Chieti, Italy.
Sante Donato Pierdomenico, Department of Medical, Oral and Biotechnological Sciences, University “Gabriele d’Annunzio”, Via dei Vestini, 66100 Chieti, Italy.
Livio Giuliani, Cardiology Department, Interventional Cardiology Unit, SS. Annunziata Hospital, Via dei Vestini, 66100 Chieti, Italy.
References
- 1. Galiè N, Humbert M, Vachiery J-L, Gibbs S, Lang I, Torbicki A. et al. ; ESC Scientific Document Group. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2016;37:67–119. [DOI] [PubMed] [Google Scholar]
- 2. Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB. et al. ; Primary Pulmonary Hypertension Study Group. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med 1996;334:296–302. [DOI] [PubMed] [Google Scholar]
- 3. Lang IM, Gaine SP.. Recent advances in targeting the prostacyclin pathway in pulmonary arterial hypertension. Eur Respir Rev 2015;24:630–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Badesch DB, McLaughlin VV, Delcroix M, Vizza CD, Olschewski H, Sitbon O. et al. Prostanoid therapy for pulmonary arterial hypertension. J Am Coll Cardiol 2004;16:56S–61S. [DOI] [PubMed] [Google Scholar]
- 5. Farber HW, Miller DP, Meltzer LA, McGoon MD.. Treatment of patients with pulmonary arterial hypertension at the time of death or deterioration to functional class IV: insights from the REVEAL Registry. J Heart Lung Transplant 2013;32:1114–1122. [DOI] [PubMed] [Google Scholar]
- 6. Barnes H, Yeoh HL, Fothergill T, Burns A, Humbert M, Williams T.. Prostacyclin for pulmonary arterial hypertension. Cochrane Database Syst Rev 2019;5:CD012785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Farber HW, Gin-Sing W.. Practical considerations for therapies targeting the prostacyclin pathway. Eur Respir Rev 2016;25:418–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sorensen LM, Wehland M, Kruger M, Simonsen U, Nassef MZ, Infanger M. et al. A special focus on selexipag—treatment of pulmonary arterial hypertension. Curr Pharm Des 2017;23:5191–5199. [DOI] [PubMed] [Google Scholar]
- 9. Simonneau G, Torbicki A, Hoeper MM, Delcroix M, Karlócai K, Galiè N. et al. Selexipag, an oral, selective prostacyclin receptor agonist for the treatment of pulmonary arterial hypertension. Eur Respir J 2012;40:874–880. [DOI] [PubMed] [Google Scholar]
- 10. Sitbon O, Channick R, Chin KM, Frey A, Gaine S, Galiè N. et al. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med 2015;373:2522–2533. [DOI] [PubMed] [Google Scholar]
- 11. Coghlan JG, Channick R, Chin K, Di Scala L, Galiè N, Ghofrani HA. et al. Targeting the prostacyclin pathway with selexipag in patients with pulmonary arterial hypertension receiving double combination therapy: insights from the randomized controlled GRIPHON study. Am J Cardiovasc Drugs 2018;18:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The efficacy and safety of initial triple versus initial dual oral combination therapy in patients with newly diagnosed pulmonary arterial hypertension (TRITON). ClinicalTrials.gov. 2017: NCT02558231.
- 13. Holthaus N, Prins K, Rose L, Prisco S, Pritzker M, Thenappan T.. Transition from parental prostacyclin to selexipag: a case series of five pulmonary arterial hypertension patients. Pulm Circ 2019;9:2045894019862167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.