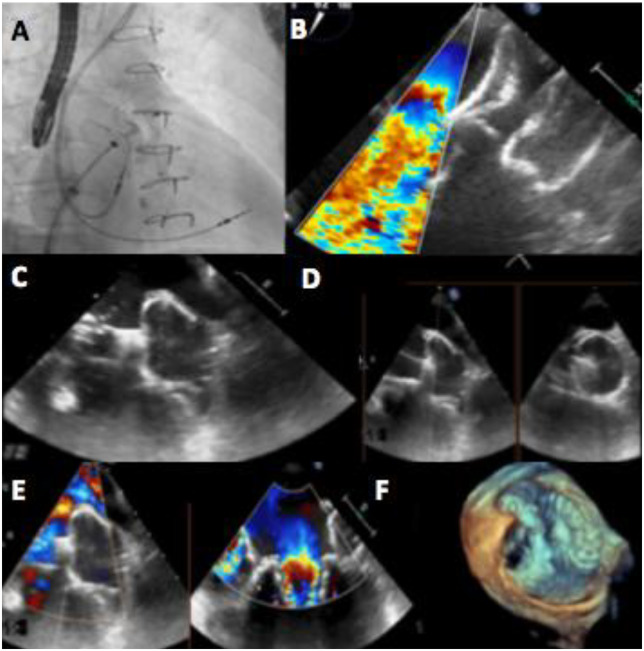
Percutaneous left atrial appendage (LAA) occlusion in patients with history of mitral valve surgery represent a challenge for interventional cardiologists, due to large-sized LAA and to the presence of prosthetic material. Patients with previous mitral valve surgery represent a scant population among the patients treated with percutaneous LAA occlusion and there is no evidence to date to support the use of a specific device in this particular clinical setting.
A 77-year-old woman with permanent atrial fibrillation (AF) and previous mitral regurgitation treated by surgical valve replacement with a biological prosthesis was referred for LAA occlusion due to multiple intracranial bleeding while on anticoagulant therapy. Preprocedural imaging with both transoesophageal echocardiography (TOE) and computed tomography scanning was performed, showing an LAA landing zone diameter of 31 mm requiring a 34 mm Amplatzer Amulet occluder (St. Jude, Golden Valley, MN, USA) implantation. Although the fluoroscopic guidance demonstrated proper positioning of the device (Panel A), TOE revealed a clear interference between the disc and the mitral prosthesis leaflets (Panel B). After several attempts, given the impossibility to avoid the conflict between the device and the valve, a second LAA occlusion procedure with a 35 mm Watchman FLX device (Boston Scientific, Plymouth, MN, USA) was performed. After the deployment of the device into the LAA, both fluoroscopic and TOE (Panel C) guidance showed a correct positioning and the ‘tug test’ was carried out to confirm the stability before final device release (Panel D). Transoesophageal echocardiography revealed the absence of interference between the device and the mitral prosthesis, with no residual peri-device shunt (Panels E and F). The device was released and an optimal final result was achieved.
Patients with bioprosthetic mitral valve or those previously treated with mitral valve repair represent a scant population among the patients treated with percutaneous LAA occlusion. In adjunction, for these patients, percutaneous LAA occlusion may present with particular procedural challenges, especially when using double-disk type devices, and little is known about this very specific population. These patients may more frequently present with enlarged LAA, as well as with a reduced distance between the ostium of the LAA and the neighbouring anatomical structures (mitral annular plane and left upper pulmonary vein). The Amulet device has been successfully used to occlude large LAAs, but the concomitant presence of a large LAA orifice in patients with previous mitral surgery might be considered as a predictor of interaction between the mitral valve and the device, especially when using the Amulet occluder: the larger the LAA size, the bigger the disc, the higher the risk of conflict with mitral valve. The Watchman FLX device is a new generation LAA closure device with a self-expanding nitinol frame structure with fixation anchors and a permeable polyester fabric cover facing the left atrium. It is characterized by a complete intra-LAA position. This feature, together with its round shape and the ability to fully recapture and reposition, makes the Watchman FLX device a very appealing option for treating patients iwth AF, previous mitral valve surgery and large-size LAA with absolute or relative contraindications for oral anticoagulation.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Supplementary Material
Contributor Information
Antonio Martellini, Structural Interventional Cardiology Division, Cardio-Toraco-Vascular Department, Careggi University Hospital, Largo Brambilla 3, 50141 Florence, Italy.
Francesco Meucci, Structural Interventional Cardiology Division, Cardio-Toraco-Vascular Department, Careggi University Hospital, Largo Brambilla 3, 50141 Florence, Italy.
Alessio Mattesini, Structural Interventional Cardiology Division, Cardio-Toraco-Vascular Department, Careggi University Hospital, Largo Brambilla 3, 50141 Florence, Italy.
Francesca Ristalli, Structural Interventional Cardiology Division, Cardio-Toraco-Vascular Department, Careggi University Hospital, Largo Brambilla 3, 50141 Florence, Italy.
Miroslava Stolcova, Structural Interventional Cardiology Division, Cardio-Toraco-Vascular Department, Careggi University Hospital, Largo Brambilla 3, 50141 Florence, Italy.
Carlo Di Mario, Structural Interventional Cardiology Division, Cardio-Toraco-Vascular Department, Careggi University Hospital, Largo Brambilla 3, 50141 Florence, Italy.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.