Abstract
Childhood anaemia and stunting are major public health concerns in Ghana. Using the 2014 Ghana Demographic and Health Survey, we evaluated whether childhood anaemia (Haemoglobin concentration < 110 g/L) and stunting (height-for-age z score < −2) co-occur beyond what is expected in Ghana, and employed spatial analysis techniques to determine if their co-occurrence is spatially correlated. There was no statistically significant difference between the observed and expected frequency of co-occurrence. Among 24–35 month and 36–59-month-old children, belonging to a high wealth household compared to low wealth household was associated with lower odds of the co-occurrence of childhood anaemia and stunting (OR, 95% CI: 0.3[0.1, 0.8] and 0.2[0.1, 0.5], respectively). Children aged 6–23 months with caregivers who had formerly been in union compared to their counterparts with caregivers who have never been in union had higher odds of co-occurrence of anaemia and stunting (5.1, [1.1, 24.3]). Overall, households with high wealth and having a mother with secondary or more education were associated with lower odds of the co-occurrence of childhood anaemia and stunting (OR, 95% CI: 0.4[0.2, 0.8] and 0.5[0.3, 0.9], respectively). There was substantial spatial clustering of co-occurrence, particularly in the northern region of the country. Interventions purposed to improve linear growth and anaemia must identify the specific factors or context which contribute to childhood anaemia and stunting.
Keywords: Anaemia, Stunting, Malnutrition, Co-occurrence, Spatial distribution, Ghana
Highlights
-
•
Childhood anaemia and stunting are serious public health issues in Ghana.
-
•
There is an association between household wealth and mother's education, and the co-occurrence of anaemia and stunting.
-
•
Co-occurrence of anaemia and stunting is spatially distributed.
-
•
These findings suggest cause specific approaches to addressing anaemia and stunting.
Introduction
The burden of childhood anaemia and stunting (height-for-age Z-score < -2 SD) continues to be a widespread public health concern, particularly in low- and middle-income countries (Gosdin et al., 2018). A report from the World Health Organization (WHO) indicated that an estimated 273.2 million children age 6–59 months were anaemic in the year 2011, with the sub-Saharan African region having the highest proportion of cases (WHO, 2011). The aetiology of anaemia is complex. However, the causes can broadly be grouped into nutrition-related diseases or genetic disorders (Hotez et al., 2004; Jourdan et al., 2018; Menendez et al., 2000). Deficiencies in iron, vitamin B12, and folate are the main causes of nutritional anaemia whereas anaemia triggered by infections or genetic disorder can be chronic and hereditary or an acute response to a disease state (WHO, 2016). Globally, although anaemia is most commonly attributable to iron deficiency (Kassebaum et al., 2014), there are significant variations with respect to the causes of anaemia among different population groups and different areas due to specific prevailing conditions (De Benoist et al., 2008). Critical area-specific, non-iron deficiency causes of anaemia includes infections caused by soil-transmitted helminthiasis (particularly in low socioeconomic regions) and malaria (Haldar & Mohandas, 2009; Jourdan et al., 2018). Succinctly, these infections cause inflammation which triggers the release of proinflammatory cytokines altering iron metabolism. The alteration of the iron metabolism leads to iron been sequestered within cells of the liver and spleen and intestinal enterocytes and reduction in the production and life span of red blood cells (Hoffman et al., 2013; Weiss & Goodnough, 2005). Increasingly hemoglobinopathies have also been recognized as a significant contributors of anaemia among different populations, particularly those of Sub-Saharan Africa and Asia (Weatherall, 2008). Hemoglobinopathies are a group of inherited or genetical acquired blood disorder causing abnormal formation of hemoglobin or a decrease in its production.
Globally, iron-deficiency anaemia is the single largest cause of years lived with disability in children and adolescents (Leung et al., 2011). A meta-analysis involving 12,000 children from six African countries showed that for 1 g/dL increase in Hb, the risk of death was reduced by 24% (Scott et al., 2014). Beside significantly contributing to increasing under-five mortality, anaemia in children leads to delayed motor development, reduced mental capacity and poor educational attainment (Walter, 1994). Although there is generally a downward trend for prevalence of anaemia in Ghana, anaemia is observed to increase with increase in age, particularly after exclusive breastfeeding has ended. Currently, in Ghana, about two-thirds (66%) of children age 6–59 months are anaemic (GDHS, 2014).
Childhood stunting is considered a major indicator for assessing children's well-being (de Onis & Branca, 2016). Globally, approximately 159 million children under 5 years are stunted with over 90% of them living in low- and middle-income countries (Adair et al., 2013). The consequence of stunting includes poor growth, poor cognition, low educational performance and suboptimal function later in life, including adverse effects such as restricted uterine blood flow and growth of the uterus, placenta and foetus during pregnancy (Dewey & Begum, 2011). Stunting is considered as a major indicator for post-2015 development agenda. Thus, its reduction was an important target among the six global nutrition targets for 2025 at the World Health Assembly in 2012 (De Onis et al., 2013). The first 1000 days of life is usually the period in which stunting occurs. Although with adequate nutrition and health there could be catch growth, a child who becomes stunted within this period may not reach his/her normal growth trajectory (Fink & Rockers, 2014). Stunting, particularly in developing countries, has been shown to be linked to household members' earnings (Galasso et al., 2016). For example, a centimetre increase in height was found to be associated with a 2% increase in hourly earnings among male workers in Mexico (Vogl, 2014). Similarly, 1% difference in height was associated with a 2.3% increase in hourly earnings in Indonesia (LaFave & Thomas, 2017).According to the last Multiple Indicator Cluster Survey (MICS4), 23% of children under five years in Ghana were stunted (GSS, 2011). Though Ghana has experienced a decline in stunting over the years (28% in 2008 to 19% in 2014) (Ghana Statistical Service et al., 2015), the present prevalence is still considered unacceptable. More so, given the absolute numbers of children that are stunted as a result of population growth, the incidence of stunting needs attention (Ghana Statistical Service, 2019). An estimated GH'4.6 billion (or US$2.6 billion), equivalent to 6.4% of Ghana's Gross Domestic Product in 2012 was lost due to conditions associated with stunting and other forms of malnutrition (NDPC, 2016).
Conceptual frameworks for malnutrition, anaemia and stunting as seen in the frequently cited UNICEF, World Bank Group and WHO frameworks respectively, share similar underlining drivers for stunting and anaemia (UNICEF, 1990; WHO, 2006; World Bank, 2006). Given the scarce resources available to address country-level nutrition concerns in children, identifying shared predictors of anaemia and stunting could lead to targeted interventions that will simultaneously mitigate these two public health concerns. This notwithstanding, results from studies that compare the frequency of co-occurrence of anaemia and stunting and their expected frequencies are inconsistent. The observed frequency of co-occurrence was reported not to be significantly greater than their expected frequencies in Latin American countries. This suggest that, these conditions should be treated independently of each other (Albalak et al., 2000; Castejon et al., 2004). A more recent study in Peru found the frequencies of co-occurrence to be similar to frequencies expected by chance (Gosdin et al., 2018). Analysis of data from 46 low- and middle-income countries showed that though there is a wide range for the prevalence of co-occurrence of anaemia and stunting in these countries, overall the condition is highly prevalent (Duc Tran et al., 2018). Few studies have however explored predictors of co-occurrence and compared its prevalence with the expected estimates and whether or not co-occurrence was spatially distributed.
The specific aims of this study were: (1) to examine if anaemia and stunting co-occur beyond what is expected in Ghana, and (2) to determine the predictors of co-occurrence of anaemia and stunting among children 6–59 months and, (3) to assess the level of spatial distribution of co-occurrence of anaemia and stunting. The third objective is against the backdrop that child malnutrition might be spatially distributed due to particular cultural and environmental factors, like population density and disease distribution, food availability and production. There is a paucity of studies that have examined the determinants of the co-occurrence of anaemia and stunting, and even fewer studies have explored the underlying spatial factors. This is critical in ensuring that nutrition intervention programmes give the needed attention to identify hotspots or cold spots of co-occurrence to reduce child malnutrition.
Methods
Study design
We used data from the most recent (2014) national representative Ghana Demographic and Health Survey (GDHS). The GDHS, like other demographic and health surveys (DHS), are cross-sectional studies conducted periodically in low- and middle-income countries to monitor and evaluate population, health and nutrition programs (Zuehlke, 2013). Households for the GDHS surveys are sampled using a two-staged sampling procedure. Firstly, enumeration areas (clusters) were selected with probability proportion-to-size (PPS) methodology followed by an equal probability systematic selection of households from each sampled cluster. All children in sampled households who were under 5 years of age were eligible for the survey. Women of childbearing age or caregivers were interviewed face-to-face using a structured questionnaire.
Data collection
The survey employed standardized data collection procedures. Using structured questionnaires, data were collected through one-on-one in-person interviews to capture characteristics of sampled households, mothers/caregivers and children.
Anthropometric measurement of children's weight and height were taken using lightweight electronic SECA scales (Seca, Hamburg, Germany) and measuring boards (Shorr Production), respectively. Children's nutritional status was calculated based on the WHO Multicenter Growth Reference Study Standards (WHO, 2006). Children with a height/length-for-age z score less than −2 were classified as stunted. Using a drop of capillary blood, a HemoCue (Hemocue, Inc., Brea, CA) was used to determine the children's haemoglobin concentration.
Main dependent variable
Co-occurrence was determined by identifying children who had any kind of anaemia (i.e. Hb concentration <110 g/L) concurrently with having a height-for-age z score less than −2.
Independent variables
Model covariates were selected based on the existing UNICEF conceptual framework for malnutrition and scholarship on the determinants of childhood anaemia status and stunting (Ijarotimi, 2013; Tengco et al., 2008; UNICEF, 2008). The predictors included household wealth, household size, sanitation status, source of drinking water, whether or not child had taken iron tablets in previous 7 days, child fever and diarrhoea in the previous 2 weeks, child's feeding practices (specifically dietary diversity) were explored.
Analysis
The data analysis was conducted using the Stata statistical software package version 14.2 (2017; StataCorp, College Station, TX, USA). The survey design required the use of the “svyset” command to identify sampling weights, cluster, and strata variables. This accounted for the non-proportional allocation of the samples to different regions of the country and ensured the results generated are representative at the national and domain levels (GSS, 2015). Anova and Pearson's chi-squared test statistics were also calculated as well as test for differences in means and proportions respectively among households with children within the ages of 6–23 months, 24–35 months and 36–59 months. By default, using the svy: tabulate in STATA displays Pearson χ2 statistic with the Rao and Scott second-order correction (Rao and Scott, 1981, 1984).
The expected frequency of co-occurrence of anaemia and stunting was computed as the product of the prevalence of stunting and anaemia. The squared of the difference between the observed and expected frequencies of co-occurrence divided by the expected was the chi-squared statistic with one degree of freedom.
Our dependent variable predicted a dichotomous situation (i.e., 1 = children who are anaemic and stunted at the same time; 0 = children who are not anaemic and stunted at the same time). Considering that the outcome variable was binary, a logistic regression technique was used (Pohlmann & Leitner, 2003). The F-statistics of models run were used to determine the goodness of fit of the models at a 5% significance level.
ArcMap in ArcGIS was used to map the cluster distribution of co-occurrence of anaemia and stunting. The dependent variable was explored in Open Geoda using a number of descriptive and exploratory tools including a histogram, a box plot, and a cartogram. The presence of spatial clustering of co-occurrence was determined using the local spatial autocorrelation (LISA) statistic and the univariate Moran's I statistic. Due to survey respondents' confidentiality, points in clusters in the DHS dataset were randomly displaced within certain limit. Although this has the potential of introducing measurement error and covariate misspecification, the current spatial analysis was within the clusters, which were not displaced outside the districts within which they exist.
Ethical approval
The 2014 GDHS data sets are in the public domain and full access is provided to researchers upon justifying the reason for use. The 2014 GDHS survey protocol including biomarker collection, was reviewed and approved by the Ghana Health Service Ethical Review Committee and the Institutional Review Board of ICF International. Written informed consent was obtained from all participants who were interviewed for the survey.
Results
Table 1 presents the demographic information and characteristics of households and caregivers/mothers by children's age groups. A little over half (55%) of the sample were from rural areas. Nearly two-thirds (65%) and about 84% of the households used improved sanitation and water facilities, respectively. The majority of the households were headed by males with a quarter (25%) being headed by females. About 81% of the households had mosquito nets. Less than one-third (27%) of the caregivers had no formal education. About two in five households (43%) fall within the low wealth scale. Significantly more children between 6 and 23 months were anaemic compared to their counterparts in other age groups. There was no difference between the children's age categories with respect to stunting and the co-occurrence of stunting and anaemia. Significantly more caregivers of children who were in the 36–59 months age group had ever been married compared to their counterparts with children that were between 6-23 months and 24–34 months. There was no significant difference among the age groups with regards to the proportion of caregivers who had no education, primary, secondary and tertiary education.
Table 1.
Household, women and children characteristics by age-group of children.
| All | 6–23 months | 24–35 months | 36–59 months | P-value | |
|---|---|---|---|---|---|
| Household | |||||
| Urban | 46.3 | 46.7 | 48.0 | 45.2 | 0.649 |
| Rural |
53.6 |
53.3 |
52.0 |
54.8 |
|
| Male-headed household |
75.0 |
75.9 |
74.9 |
74.2 |
0.773 |
| Household wealth1 | |||||
| Low | 42.9 | 43.3 | 39.7 | 44.2 | 0.522 |
| Medium | 19.9 | 19.5 | 19.7 | 20.4 | |
| High | 37.2 | 37.2 | 40.6 | 35.4 | |
| Household use of mosquito bed net | |||||
| No child | 29.5 | 27.1 | 30.6 | 31.0 | 0.882 |
| All children | 45.2 | 48.1 | 43.6 | 43.5 | |
| Some children | 5.8 | 5.3 | 5.5 | 6.4 | |
| No net in house |
19.5 |
19.5 |
20.2 |
19.1 |
|
| Household with improved water source |
84.3 |
86.2 |
82.1 |
83.8 |
0.118 |
| Household with improved sanitation |
64.6 |
64.1 |
67.9 |
65.6 |
0.458 |
| Caregiver/mother | |||||
| Marital status | |||||
| Never in union | 6.1 | 7.6 | 5.7 | 4.9 | 0.021 |
| Currently in union | 87.8 | 88.4 | 88.5 | 87.0 | |
| Ever in union | 6.1 | 3.9 | 5.8 | 8.1 | |
| Parity (mean ± Standard deviation) | 3.6 ± 2.1 | 3.4 ± 2.1 | 3.3 ± 1.9 | 3.9 ± 2.1 | <0.001 |
| Educational level | |||||
| None | 28.7 | 28.1 | 25.5 | 30.8 | 0.259 |
| Primary | 19.6 | 18.8 | 19.3 | 20.5 | |
| Secondary and higher | 51.7 | 53.1 | 55.1 | 48.7 | |
| Children | |||||
| Male child |
53.1 |
50.5 |
55.5 |
54.1 |
0.210 |
| Child had fever (previous 2wks) |
14.1 |
16.1 |
17.1 |
14.1 |
0.389 |
| Had diarrhoea (previous 2wks) | 12.5 | 16.0 | 17.0 | 7.0 | <0.001 |
| Child has taken vitamin A | 62.0 | 69.4 | 61.0 | 55.8 | <0.001 |
| Breast feeding status | |||||
| Still breastfeeding | 31.8 | 80.0 | 8.8 | 1.4 | <0.001 |
| Ever breastfeed | 67.1 | 19.5 | 90.4 | 96.9 | |
| Never breastfeed | 1..7 | 0.5 | 0.8 | 1.7 | |
| Dietary diversity score ≥ 4 |
12.5 |
25.5 |
1.3 |
6.9 |
<0.001 |
| Nutritional status | |||||
| Anaemic | 66.8 | 77.4 | 65.9 | 58.2 | <0.001 |
| Stunted | 14.5 | 12.6 | 13.9 | 16.6 | 0.224 |
| Co-occurrence (Anaemic + Stunted) | 11.8 | 10.6 | 11.2 | 13.3 | 0.454 |
More younger children (6–23 months) had diarrhoea within the past two weeks prior to the survey compared to the 35–59 months old children. Also, more of the youngest age group had received vitamin A capsules over the past six months than children of the other age groups.
Observed vs. the expected chance of co-occurrence of anaemia and stunting
Table 2 shows the observed and expected prevalence of co-occurrence of anaemia and stunting. Overall, 66.8% and 13.3% of the children were anaemic and stunted, respectively. About 12% of the children were observed to have both anaemia and stunting concurrently whereas the expected co-occurrence of both conditions was about 9%. There was no statistically significant difference between the observed and expected frequency of co-occurrence for the pooled sample and for all sub-groups of children.
Table 2.
Observed co-occurrence of anaemia and stunting versus the expected prevalence of co-occurrence.
| Pooled | Age groups |
|||
|---|---|---|---|---|
| 6–23 months | 24–35 months | 36–59 months | ||
| Anaemia | 66.8 | 77.4 | 66.0 | 58.2 |
| Stunting | 13.3 | 12.6 | 13.9 | 16.6 |
| Observed co-occurrence | 11.8 | 10.6 | 11.2 | 13.3 |
| Expected co-occurrence | 8.9 | 9.7 | 9.2 | 9.6 |
| X2 | 0.98 | 0.07 | 0.46 | 1.36 |
| P-value | 0.32 | 0.79 | 0.50 | 0.24 |
Predictors of co-occurrence of anaemia and stunting
Table 3 presents the predictors of co-occurrence of anaemia and stunting by age categories. The F-statistics of all the models show that the set of covariates significantly predict co-occurrence of anaemia and stunting. For children within 6–23 months age brackets, having a caregiver/mother who had ever been in union compared to those who had never been in union had a higher odds of being anaemic and stunted concurrently. However, having a caregiver with secondary school education compared to children of caregivers with no formal education lower the odds of being anaemic and stunted concurrently, i.e. caregivers' secondary school education is protective against the co-occurrence of anaemia and stunting in children. No such effect of caregiver's marital status and formal education on the con-occurrence of anaemia and stunting were observed for children within 36–59 months; belonging to a high wealth household was protective against the co-occurrence of anaemia and stunting for 36–59 months old children. The pooled data showed that, children belonging to high wealth households compared with their counterparts in low wealth households had significantly lower odds of being anaemic and stunted concurrently. Specifically, compared with households in low wealth tertile, children in households with high wealth are 0.4 times as likely (lower odds) to simultaneously have anaemia and stunting, and this is statistically significant at 1% level. Similarly, children of caregivers with secondary and higher education compared to no education had significantly lower odds of being anaemic and stunted concurrently. For example, setting no formal education as the base, children of caregivers with higher education are 0.1 times as likely to have anaemia and stunting concurrently, and this is statistically significant at 5% level. Among children between the ages of 6–23 months, having a caregiver/mother who has ever being in a union, compared to those who have not, had a significantly higher odds to being anaemic and stunted concurrently. Children's sex, health status (experience of fever and diarrhoea), vitamin A supplement intake, breastfeeding status and dietary intake did not predict the co-occurrence of anaemia and stunting.
Table 3.
Predictors of co-occurrence of anaemia and stunting by age group.
| Variables | 6–23 months |
24–35 |
36–59 |
Pooled (ALL) |
|---|---|---|---|---|
| AOR [CI 95%] | AOR [CI 95%] | AOR [CI 95%] | AOR [CI 95%] | |
| Household | ||||
| Location (ref = rural) | ||||
| Urban | 0.8 [0.4, 1.9] | 1.0 [0.4, 2.3] | 0.9 [0.4, 1.9] | 1.0 [0.6, 1.5] |
| Sex of household head (ref = female) | ||||
| Male | 0.8 [0.4, 1.4] | 1.0 [0.4, 2.8] | 1.0 [0.4, 2.3] | 0.9 [0.6, 1.4] |
| Wealth (tertile) (ref = low) | ||||
| Middle | 1.1 [0.5, 2.3] | 0.3 [0.1, 0.9] ** | 0.6 [0.3, 1.1] * | 0.7 [0.5, 1.0] |
| High | 1.3 [0.5, 3.8] | 0.3 [0.1, 0.8]** | 0.2 [0.1, 0.5] *** | 0.4 [0.2, 0.8] *** |
| Water source (ref = unimproved) | ||||
| Improved | 0.8 [0.4, 1.8] | 1.5 [0.7, 3.1] | 0.9 [(0.5, 1.7] | 1.0 [0.6, 1.6] |
| Sanitation(ref = unimproved) | ||||
| Improved | 1.5 [0.8, 2.7] | 0.7 [0.4, 1.3] | 1.0 [0.6, 1.7] | 1.0 [0.7, 1.5] |
| Sleep under mosquito bed net (ref = no) | ||||
| All children | 1.0 [0.5, 1.7] | 0.5 [0.2, 1.4] | 1.1 [0.6, 1.8] | 0.9 [0.6, 1.3] |
| Some children | 2.1 [0.8, 5.3] | 1.0 [0.3, 3.3] | 2.0 [0.8, 4.8] | 1.6 [0.8, 3.2] |
| No net in household | 0.9 [0.4, 2.0] | 0.4 [0.1, 1.7] | 1.2 [0.6, 2.4] | 0.9 [0.5, 1.5] |
| Caregiver/mother | ||||
| Marital status (ref = never in union) | ||||
| Currently in union | 1.9 [0.5, 7.9] | 0.9 [0.3, 3.3] | 1.0 [0.3, 4.0] | 1.6 [0.7, 3.3] |
| EverFormerly in union | 5.1 [1.1, 24.3] ** | 0.5 [0.1, 3.7] | 1.3 [0.4, 4.2] | 2.1 [0.9, 5.0] |
| Parity (number of children born) | 1.1 [1.0, 1.3] | 1.1 [0.9, 1.2] | 0.9 [0.8, 1.0] | 1.0 [0.9, 1.1] |
| Educational level (ref = no education) | ||||
| Primary | 0.6 [0.3, 1.2] | 1.5 [0.7, 3.3] | 0.6 [0.3, 1.1] | 0.7 [0.5, 1.1] |
| Secondary and higher | 0.3 [0.2, 0.6] *** | 0.6 [0.2, 1.7] | 0.7 [0.3, 1.6] | 0.5 [0.3, 0.9] ** |
| Children | ||||
| Age (ref: 6–23 months) | ||||
| 24–35 months | 0.8 [0.5, 1.5] | |||
| 36–59 months | 0.9 [0.5, 1.6] | |||
| Sex (ref = female) | ||||
| Male | 1.3 [0.8, 2.1] | 0.8 [0.4, 1.4] | 1.1 [0.7, 1.8] | 1.1 [0.8, 1.4] |
| Had fever (previous 2weeks) (ref = no) | ||||
| Yes | 1.1 [0.5, 2.1] | 0.7 [0.3, 1.6] | 1.2 [0.6, 2.4] | 1.0 [0.6, 1.4] |
| Had diarrhoea (previous 2weeks) (ref = no) | ||||
| Yes | 0.9 [0.5, 1.7] | 1.0 [0.4, 2.4] | 0.5 [0.2, 1.3] | 0.8 [0.5, 1.2] |
| Taken vitamin A supplement -in pervious 6 months (ref = no) | ||||
| Yes | 1.2 [0.7, 2.2] | 2.0 [1.0, 4.1] | 0.6 [0.3, 1.0] | 0.9 [0.6, 1.2] |
| Breastfeeding status (ref = still breastfeeding) | ||||
| Ever breastfed, not currently breastfeed | 1.7 [0.9, 2.9] | 1.2 [0.5, 2.6] | 0.4 [0.1, 3.2] | 1.4 [0.9, 2.4] |
| Never breastfed | 56.3 [2.8–129.2] *** | 0.1 [0.0, 1.6] | 1.2 [0.3, 4.7] | |
| Minimum dietary diversity score (ref = 4) | ||||
| ≥4 |
0.6 [0.3, 1.2] |
2.4 [0.3, 22.4] |
1.5 [0.6, 3.6] |
0.9 [0.5, 1.6] |
|
Observations |
842 |
548 |
972 |
2365 |
| F-Statistics |
F (20, 311) = 2.25 P-value = 0.00 |
F (21, 262) = 3.33 P-value = 0.00 |
F (21, 318) = 3.73 P-value = 0.00 |
F (23, 375) = 3.27 P-value = 0.00 |
Values represents odds ratios and 95% confident intervals1Wealth status of the household determined using an asset-based index using principal components analysis to create standardized which were then categorized into quintiles.Children who were anaemic and stunted at the same time is considered to be having the two condition concurrently, thus the co-occurrence of anaemia and stunting. **P < 0.05 ***P < 0.001.
Spatial distribution of co-occurrence of anaemia and stunting
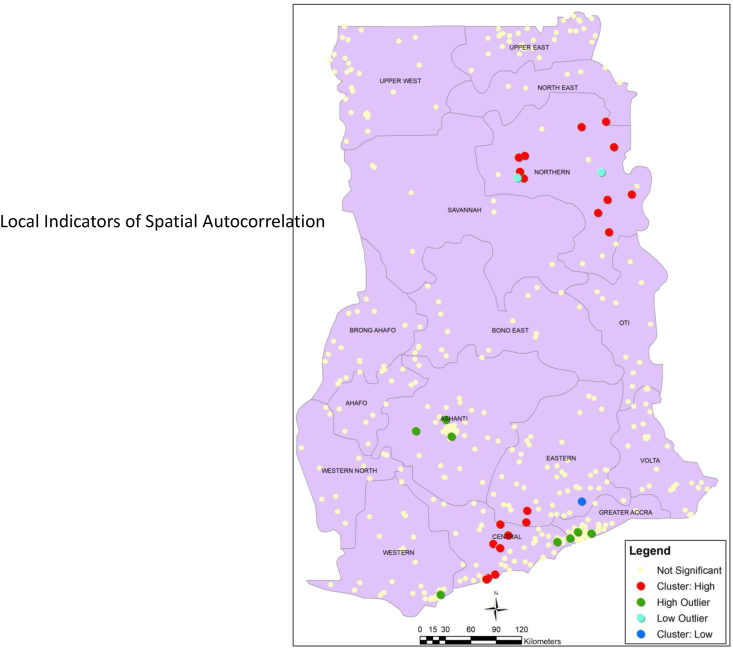
The geographic distribution (percentage) of co-occurrence is shown in Fig. 1. Using Local Indicators of Spatial Autocorrelation (LISA) statistics, we identified significant spatial variability on the distribution of co-occurrence in the district (Fig. 2). We identified statistically significant clusters of high prevalence of co-occurrence of stunting and anaemia (hotspots) in parts of the Northern and Greater Accra regions, and clusters of low prevalence (cold spots) in parts of the Greater Accra region (Fig. 3). The results show local spatial clustering in co-occurrence of anaemia and stunting. The univariate Moran's I statistics gave a value of 0.136 (P < 0.01).
Fig. 1.
Percentage of co-occurrence of anaemia and stunting across Metropolitan, Municipal and District Assemblies (MMDA's).
Fig. 2.
Local Indicators of Spatial Autocorrelation (LISA) significance plot showing spatial autocorrelation in co-occurrence of anaemia and children stunting among.
Fig. 3.
Getis-ord Gi map showing hot and cold spots at 90, 95 and 99% confidence in co-occurrence of anaemia and stunting among children.
Discussion
This study was carried out to determine if childhood anaemia and stunting co-occur beyond what is expected in Ghana and that their co-occurrence is spatially distributed. Similar to earlier studies (Albalak et al., 2000; Castejon et al., 2004; Gosdin et al., 2018), we found no significant difference between the observed and expected frequency of co-occurrence of stunting and anaemia in the pooled data as well as for the different children's age groups. The co-occurrence of stunting and anaemia (CAS) was, however, spatially clustered in selected regions of Ghana.
Belonging to a high wealth household and having a mother or caregiver who had secondary or more formal education was protective against co-occurrence of stunting and anaemia. Generally, belonging to lower economic strata household is associated with both childhood stunting and anaemia (Barros et al., 2010; Mal-EdNetwork Investigators, 2017). Mothers' educational status emerges as a predictor of CAS. This result is consistent with the effect of maternal education on childhood nutrition shown in studies of similar settings (Alemayehu et al., 2015; Herrador et al., 2014). Improved maternal education results in better feeding practices, better water and sanitation practices and general well-being that is protective against malnutrition (Imdad et al., 2011). Other studies have also reported that children of more educated mothers were more likely to be immunized than those of less educated mothers (Forshaw et al., 2017). The marital status of mothers, specifically ever being in union (i.e. separated, divorce and widowed) compared to being single women was a significant predictor of anaemia among the 6–23 months old children. Although some studies have demonstrated some association between parental marital status and other child's anaemia and other nutritional status such as their body mass index and (Schmeer, 2013; Yannakoulia et al., 2008), the exact mechanism through which the marital status of a mother affect children nutrition status is still evolving. Given the possible effect of marital status on caregiving practices and resource availability, further research is needed explore pathways through which family structures affects nutritional outcomes such as anaemia of children.
We found no sex difference with respect to the co-occurrence of anaemia and stunting. Other studies have shown that being a girl was negatively associated with anaemia and stunting (Foote et al., 2013; Sobrino et al., 2014). Another study has however suggested a higher risk of the co-occurrence of anaemia and stunting in boys than girls (Duc Tran et al., 2018). Thus, the reasons for some observed differences not fully explained in extant scholarship. Existing sex differences observed in child nutrition includes the unfavourable caregiving towards the girl child (Tumilowicz et al., 2015), the difference between male and females with respect gain of muscle mass which has an effect on needed iron (Antunes et al., 2012), and the fact the girls are breastfed longer than boys, noting that breastfeeding has an effect on children's growth (Mosha et al., 1998; Obermann-Borst et al., 2013).
The clustering of co-occurrence of anaemia and stunting suggests the condition to be a result of shared determinants within particular regions rather than occurring by chance. Adverse care environments, limited household and community resources and inadequate knowledge are some factors accounting for child malnutrition as stipulated in the UNIICEF framework (Black et al., 2008). The Northern region of Ghana reports higher incidence of malnutrition among children compared to the southern part of the country (Ghana Statistical Service et al., 2014). This has been attributed to the regions’ higher incidence of poverty and food insecurity. Earlier studies from low-and-middle income countries have shown poverty to be a major contributor to the burden of malnutrition in children (Khan & Mohanty, 2018). Although the relationship between malnutrition and poverty is very complex, our results confirmed that more deprived regions have higher prevalence of malnutrition.
The findings have important programmatic implications for health and particularly nutrition interventions in Ghana. For example, the hotspots for co-occurrence provide impetus to employ multiple strategies to effectively tackle and prevent the incidence of anaemia and stunting. Understanding the spatial distribution of this co-occurrence is not only critical for identifying regions to prioritize for intervention, but also important for identifying regional- and community-level determinants that are unique from household or individual behaviours (Jones et al., 2016). Thach et al. (2018) suggested that since childhood anaemia and stunting often cluster in low- and middle-income countries, these two conditions need to be addressed under a syndemic framework. For example, programs addressing childhood anaemia should work to improve iron supplementation as well as address household food security, a primary determinant of both anaemia and stunting (Duc Tran et al., 2018). Results from current study suggest the need to employ intervention that address place specific causes of anaemia and stunting while acknowledging that certain locations, particularly resource poor location may have higher prevalence of both and their co-occurrence.
Our study is not without limitations. Some of the limitations that should be mentioned include the absence of controlling for some important predictors of both anaemia and stunting such as food security in our model. Controlling for household wealth may mitigate the absence of a food security indicator, as wealth is known to predict both anaemia and stunting and has been interchanged for food security in some studies (Hong et al., 2006; Pasricha et al., 2010). Finally, cross-sectional data and an analysis such as this are weak in establishing causal inferences.
Conclusion
This study shows that there is a substantial percentage of children 6–59 months in Ghana who are both anaemic and stunted at the same time and also illustrates the spatial heterogeneity of their co-occurrence. This is a serious public health condition given that it is above ten percent. Co-occurrence of stunting and Anaemia (CAS) may result in an elevated risk of childhood mortality in areas where these conditions cluster. This study suggests directing funds and the implementation of specific child health interventions in the geographical hotspots of the co-occurrence of anaemia and stunting. These current results could help generate etiologic hypotheses of co-occurrence and identify spatial anomalies in co-occurrence incidence in vulnerable regions in Ghana. The finding supports employing integrated approaches to children's nutrition challenges in Ghana.
Ethics
The 2014 GDHS data sets are in the public domain and full access is provided to researchers upon justifying the reason for use. The 2014 GDHS survey protocol including biomarker collection, was reviewed and approved by the Ghana Health Service Ethical Review Committee and the Institutional Review Board of ICF International. Written informed consent was obtained from all participants who were interviewed for the survey. Participated in the survey.
All values represent weighted percentages 1Wealth status of the household determined using an assets-based index using principal components analysis to create standardized which were then categorized into quintiles. Children with haemoglobin <110 g/L are considered anaemic. Children with height-for-age z score less than −2 are considered stunted. A child who is anaemic and stunted at the same time is considered to be having the two condition concurrently, thus the co-occurrence of anaemia and stunting. Marital status: Never in union (never married or cohabited with someone or has always been single), Currently in union (individuals that are currently married or cohabiting with a partner), Ever in union (individuals who have ever been married, cohabited but currently divorced, separated or widowed).).
CRediT authorship contribution statement
Aaron Kobina Christian: Formal analysis, Writing - original draft, designed the study, performed the main analyses, and wrote the first draft of the manuscript. Caesar Agula: Formal analysis, assisted with the statistical analyses and. Philip-Neri Jayson-Quashigah: Formal analysis, Writing - original draft, carried out the spatial statistical analyses and contributed to the writing of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We appreciate and will like to thank the Ghana Statistical Service (GSS) for providing us with the data for this study. We also thank ICF International for implementing the MEASURE DHS and making the data available for public use.
Contributor Information
Aaron Kobina Christian, Email: akchristian@ug.edu.gh.
Caesar Agula, Email: caesaragula@gmail.com.
Philip-Neri Jayson-Quashigah, Email: pnjquashigah@gmail.com.
References
- Adair L.S., Fall C.H., Osmond C., Stein A.D., Martorell R., Ramirez-Zea M., Sachdev H.S., Dahly D.L., Bas I., Norris S.A., Micklesfield L., Hallal P., Victora C.G. Associations of linear growth and relative weight gain during early life with adult health and human capital in countries of low and middle income: Findings from five birth cohort studies. Lancet. 2013;382:525–534. doi: 10.1016/S0140-6736(13)60103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albalak R., Ramakrishnan U., Stein A.D., Van der Haar F., Haber M.J, Schroeder D.…Martorell R. Co-occurrence of nutrition problems in Honduran children. Journal of Nutrition. 2000;130:2271–2273. doi: 10.1093/jn/130.9.2271. [DOI] [PubMed] [Google Scholar]
- Alemayehu M., Tinsae F., Haileslassie K., Nutrition O.S. U., 2015. Undernutrition status and associated factors in under-5 children, in Tigray, Northern Ethiopia. Nutrition. 2015;31:964–970. doi: 10.1016/j.nut.2015.01.013. [DOI] [PubMed] [Google Scholar]
- Antunes H., Santos C., Carvalho S., Costa-Pereira A. Male gender is an important clinical risk factor for iron deficiency in healthy infants. The ESP Journal. 2012;7:219–222. [Google Scholar]
- Barros F., Victora C.G., Scherpbier R., Gwatkin D. Socioeconomic inequities in the health and nutrition of children in low/middle income countries. Revista de Saúde Pública. 2010;44 doi: 10.1590/s0034-89102010000100001. [DOI] [PubMed] [Google Scholar]
- Black R., Allen L., Bhutta Z., Caulfield L. Maternal and child undernutrition: Global and regional exposures and health consequences. Lancet. 2008;371:243–260. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- Castejon H.V., Ortega P., Amaya D., Gomez D., Leal J., Castejon O.J. Co-existence of anemia, vitamins A deficiency and growth retardation among children 24-84 months old in Maracaibo. Venezuela. Nutr. Neurosci. 2004;7:113–119. doi: 10.1080/10284150410001704534. [DOI] [PubMed] [Google Scholar]
- De Benoist B., Cogswell M., Egli I., McLean E. 2008. Worldwide prevalence of anaemia 1993-2005; WHO Global Database of anaemia. [DOI] [PubMed] [Google Scholar]
- De Onis M., Dewey K.G., Borghi E., Onyango A.W., Blössner M., Daelmans B., Piwoz E., Branca F. The world health organization's global target for reducing childhood stunting by 2025: Rationale and proposed actions. Maternal and Child Nutrition. 2013;9:6–26. doi: 10.1111/mcn.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey K.G., Begum K. Long-term consequences of stunting in early life. Maternal and Child Nutrition. 2011;7:5–18. doi: 10.1111/j.1740-8709.2011.00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duc Tran T., Biggs B.-A., Holton S., Thi Minh Nguyen H., Hanieh S., Fisher J. Co-morbid anaemia and stunting among children of pre-school age in low-and middle-income countries: A syndemic. Public Health Nutrition. 2018;22:35–43. doi: 10.1017/S136898001800232X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink G., Rockers P.C. Childhood growth, schooling, and cognitive development: Further evidence from the Young Lives study. American Journal of Clinical Nutrition. 2014 doi: 10.3945/ajcn.113.080960. [DOI] [PubMed] [Google Scholar]
- Foote E.M., Sullivan K.M., Ruth L.J., Oremo J., Sadumah I., Williams T.N., Suchdev P.S. Determinants of anemia among preschool children in rural, western Kenya. The American Journal of Tropical Medicine and Hygiene. 2013;88:757–764. doi: 10.4269/ajtmh.12-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forshaw J., Gerver S.M., Gill M., Cooper E., Manikam L., Ward H. The global effect of maternal education on complete childhood vaccination: A systematic review and meta-analysis. BMC Infectious Diseases. 2017 doi: 10.1186/s12879-017-2890-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GDHS . Ghana Statistical Service; Accra, Ghana: 2014. 2014. Ghana demographic and health survey report. [Google Scholar]
- Ghana Statistical Service . 2019. Ghana multiple indicator cluster survey 2017/18, Ghana statistical Service. [DOI] [Google Scholar]
- Ghana Statistical Service, (GSS) GSS,GHS, and ICF International; Rockville, Maryland, USA: 2014. ICF-international, 2015. Ghana demographic and health survey. [Google Scholar]
- Gosdin L., Martorell R., Bartolini R.M., Mehta R., Srikantiah S., Young M.F. The co-occurrence of anaemia and stunting in young children. Maternal and Child Nutrition. 2018;14 doi: 10.1111/mcn.12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GSS . 2011. Multiple indicator cluster survey, 2011: Monitoring the situation of children, women, and men; with an enhanced malaria module and biomarker. [Google Scholar]
- Haldar K., Mohandas N. Malaria, erythrocytic infection, and anemia. Hematology Am. Soc. Hematol. Educ. Program. 2009 doi: 10.1182/asheducation-2009.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrador Z., Sordo L., Gadisa E., Moreno J., Nieto J., Benito A., Aseffa A., Cañavate C., Custodio E. Cross-sectional study of malnutrition and associated factors among school aged children in rural and urban settings of fogera and libo kemkem districts, Ethiopia. PloS One. 2014;9 doi: 10.1371/journal.pone.0105880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman R.E.B., Jr., Silberstein L., Heslop H. 2013. Hematology: Basic principles and practice. [Google Scholar]
- Hong R., Banta J.E., Betancourt J.A. Relationship between household wealth inequality and chronic childhood under-nutrition in Bangladesh. International Journal for Equity in Health. 2006;5:15. doi: 10.1186/1475-9276-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez P.J., Brooker S., Phil D., Bethony J.M., Bottazzi M.E., Loukas A., Xiao S. Hookworm infection. New England Journal of Medicine. 2004 doi: 10.1056/NEJMra032492. [DOI] [PubMed] [Google Scholar]
- Ijarotimi O.S. Determinants of childhood malnutrition and consequences in developing countries. Curr. Nutr. Rep. 2013 doi: 10.1007/s13668-013-0051-5. [DOI] [Google Scholar]
- Imdad A., Yakoob M., Bhutta Z.B. Impact of maternal education about complementary feeding and provision of complementary foods on child growth in developing countries. BMC Public. 2011;11(3):25. doi: 10.1186/1471-2458-11-S3-S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A.D., Acharya Y., Galway L.P. Urbanicity gradients are associated with the household-and individual-level double burden of malnutrition in sub-Saharan Africa. The Journal of Nutrition. 2016;146(6):1257–1267. doi: 10.3945/jn.115.226654. [DOI] [PubMed] [Google Scholar]
- Jourdan P.M., Lamberton P.H., Fenwick A., Addiss D.G. Soil-transmitted helminth infections. The Lancet. 2018;391(10117):252–265. doi: 10.1016/S0140-6736(17)31930-X. [DOI] [PubMed] [Google Scholar]
- Kassebaum N.J., Jasrasaria R., Naghavi M., Wulf S.K., Johns N., Lozano R., Regan M., Weatherall D., Chou D.P., Eisele T.P., Flaxman S.R., Pullan R.L., Brooker S.J., Murray C.J.L. A systematic analysis of global anemia burden from 1990 to 2010. Blood. 2014 doi: 10.1182/blood-2013-06-508325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan J., Mohanty S.K. Spatial heterogeneity and correlates of child malnutrition in districts of India. BMC Public Health. 2018;18 doi: 10.1186/s12889-018-5873-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFave D., Thomas D. Height and cognition at work: Labor market productivity in a low income setting. Economics & Human Biology. 2017;25:52–64. doi: 10.1016/j.ehb.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung C.W., Williams D.R., Villamor E. Very low food security predicts obesity predominantly in California Hispanic men and women. Public Health Nutrition. 2011;15:2228–2236. doi: 10.1017/S1368980012000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mal-Ed Network Investigators Childhood stunting in relation to the pre- and postnatal environment during the first 2 years of life: The MAL-ED longitudinal birth cohort study. PLoS Medicine. 2017 doi: 10.1371/journal.pmed.1002408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez C., Fleming A., Today P.A.-P. Elsevier; 2000. U., 2000. Malaria-related anaemia. [DOI] [PubMed] [Google Scholar]
- Mosha T.C., Laswai H.S., Dakiyo S.O.S. Breastfeeding, weaning practices and anthropometric status of children in Morogoro district, Tanzania. Ecology of Food and Nutrition. 1998;37(4) doi: 10.1080/03670244.1998.9991551. [DOI] [Google Scholar]
- NDPC . NATIONAL DEVELOPMENT PLANNING COMMISION (NDPC); 2016. Social and economic impact of child undernutrition on Ghana's long-term development. [Google Scholar]
- Obermann-Borst S.A., Eilers P.H.C., Tobi E.W., De Jong F.H., Slagboom P.E., Heijmans B.T., Steegers-Theunissen R.P.M. Duration of breastfeeding and gender are associated with methylation of the LEPTIN gene in very young children. Pediatric Research. 2013;74(3) doi: 10.1038/pr.2013.95. [DOI] [PubMed] [Google Scholar]
- de Onis M., Branca F. Childhood stunting: A global perspective. Maternal and Child Nutrition. 2016 doi: 10.1111/mcn.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasricha S.R., Black J., Muthayya S., Shet A., Bhat V., Nagaraj S.…Shet A.S. Determinants of anemia among young children in rural India. Pediatrics. 2010;126(1):e140–e149. doi: 10.1542/peds.2009-3108. [DOI] [PubMed] [Google Scholar]
- Rao J.N.K., Scott A.J. The analysis of categorical data from complex sample surveys: Chi-squared tests for goodness of fit and independence in two-way tables. Journal of the American Statistical Association. 1981;76:221–230. doi: 10.1080/01621459.1981.10477633. [DOI] [Google Scholar]
- Pohlmann J.T., Leitner D.W. A comparison of ordinary least squares and logistic regression 1. Ohio Journal of Science. 2003;103(5):118–125. [Google Scholar]
- Rao J., Scott A. On chi-squared tests for multiway contingency tables with cell proportions estimated from survey data. Annals of Statistics. 1984 [Google Scholar]
- Schmeer K.K. Family structure and child anemia in Mexico. Social Science & Medicine. 2013 doi: 10.1016/j.socscimed.2012.10.028. [DOI] [PubMed] [Google Scholar]
- Scott S.P., Chen-Edinboro L.P., Caulfield L.E., Murray-Kolb L.E. The impact of anemia on child mortality: An updated review. Nutrients. 2014;6:5915–5932. doi: 10.3390/nu6125915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrino M., Gutierrez C., Publica A.C., de S. U., 2014. Child malnutrition in children under 5 years of age in Peru: Trends and determinants/desnutricion infantil en menores de cinco anos en Peru: Tendencias y factores. Revista Panamericana de Salud Públic. 2014;35:104–113. [PubMed] [Google Scholar]
- Tengco L.W., Rayco-Solon P., Solon F.S., Solon J.A., Sarol J.N. Determinants of anemia among preschool children in the Philippines. Journal of the American College of Nutrition. 2008;27(2):229–243. doi: 10.1080/07315724.2008.10719695. [DOI] [PubMed] [Google Scholar]
- Tumilowicz A., Habicht J., Pelto G., Pelletier D. Gender perceptions predict sex differences in growth patterns of indigenous Guatemalan infants and young children. American Journal of Clinical Nutrition. 2015;102:1249–1258. doi: 10.3945/ajcn.114.100776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNICEF . 1990. United nations children fund (UNICEF) conceptual framework, adapted from united nations children's fund (UNICEF), strategy for improved nutrition of children and women in developing countries. [DOI] [Google Scholar]
- UNICEF . 2008. UNICEF conceptual framework for malnutrition. [Google Scholar]
- Vogl T.S. Height, skills, and labor market outcomes in Mexico. Journal of Development Economics. 2014;107:84–96. [Google Scholar]
- Walter T. 4 Effect of iron-deficiency anaemia on cognitive skills in infancy and childhood. Baillieres. Clin. Haematol. 1994;7:815–827. doi: 10.1016/s0950-3536(05)80126-x. [DOI] [PubMed] [Google Scholar]
- Weatherall D. Hemoglobinopathies worldwide: Present and future. Current Molecular Medicine. 2008;8:592–599. doi: 10.2174/156652408786241375. [DOI] [PubMed] [Google Scholar]
- Weiss G., Goodnough L.T. Anemia of chronic disease. New England Journal of Medicine. 2005;352:1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- WHO . 2006. WHO Multicentre Growth Reference Study Group 2006. WHO child growth standards. Length/height-for-age, weight-for-age, weight-for-length, weight-for. Geneva. [Google Scholar]
- WHO . 2011. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. [Google Scholar]
- WHO . 2016. World health statistics 2016: Monitoring health for the SDGs sustainable development goals. [Google Scholar]
- World Bank . 2006. Repositioning nutrition as central to development: A strategy for large-scale Action. [Google Scholar]
- Yannakoulia M., Papanikolaou K., Hatzopoulou I., Efstathiou E., Papoutsakis C., Dedoussis G.V. Association between family divorce and children's BMI and meal patterns: The GENDAI study. Obesity. 2008 doi: 10.1038/oby.2008.70. [DOI] [PubMed] [Google Scholar]
- Zuehlke E. 2013. The demographic and health surveys at 25 years and beyond. [Google Scholar]