Highlights
-
•
The immune factors that determine the pathological and therapeutic effects of preoperative chemotherapy in patients with breast cancer are associated with local and systemic immune responses in the presence of tumor-infiltrating lymphocytes, in collaboration with downregulation of immunosuppressive factors mediated by vascular endothelial growth factor (VEGF) and cytotoxic T lymphocyte antigen 4 (CTLA-4) in regulatory T cells (Tregs) in the tumor microenvironment.
-
•
Multivariate analysis showed that grade 2 and better therapeutic effects tended to be associated with higher natural killer cell levels after preoperative chemotherapy (odds ratio = 1.02; 95% confidence interval, 0.99–1.05; p = 0.07).
-
•
Therapy targeting VEGF and CTLA-4 in Tregs to overcome tumor-derived immunosuppression may enhance the pathological and therapeutic responses to preoperative chemotherapy in patients with breast cancer.
Keywords: Breast cancer, Preoperative chemotherapy, Pathological and therapeutic effects, Immune factor, Tumor microenvironment
Abstract
Immune activation plays an important role in achieving the pathological and therapeutic effects of preoperative chemotherapy in patients with breast cancer. We evaluated how the immune response contributes to various therapeutic effects. This study was conducted on 43 patients with stages II–IV breast cancer who received preoperative chemotherapy followed by surgery. Peripheral natural killer (pNK) cell activity and the neutrophil-lymphocyte ratio, lymphocyte-monocyte ratio, and platelet-lymphocyte ratio (PLR) were assessed before and after chemotherapy. Tumor-infiltrating lymphocytes (TILs) and levels of 14 tumor microenvironmental factors, analyzed by next-generation sequencing, were assessed in formalin-fixed, paraffin-embedded sections of preoperative biopsy samples and surgical specimens. Univariate analysis showed that grade 2 (G2) and better therapeutic effects were significantly associated with human epidermal growth factor receptor 2 (HER-2)-positive cancer, lower PLRs, and higher NK cell and interleukin-6 levels after chemotherapy. The disappearance of axillary lymph-node metastasis was significantly associated with HER-2-positive cancer; increased pNK cell activity and lower PLRs and vascular endothelial growth factor (VEGF) levels after chemotherapy; and increased cytotoxic T lymphocyte antigen 4 (CTLA-4) levels in regulatory T cells (Tregs) and ≥5% TILs before chemotherapy. Multivariate analysis showed that G2 and better therapeutic effects tended to be associated with higher NK cell levels after chemotherapy (odds ratio = 1.02; 95% confidence interval, 0.99–1.05; P = 0.07). The activation of local and systemic immune responses by downregulation of immunosuppressive factors, such as VEGF and CTLA-4 in Tregs, had variable pathological and therapeutic effects after preoperative chemotherapy in patients with breast cancer.
Introduction
Preoperative chemotherapy has led to a paradigm shift in breast cancer treatment because the response to treatment predicts the long-term outcome based on the tumor subtype [1]. Pathological complete response (pCR) is a predictive marker for the therapeutic effect of neoadjuvant chemotherapy (NAC) on human epidermal growth factor receptor 2 (HER-2)-positive and triple-negative (TN) breast cancers, but not on luminal HER-2-negative breast cancer [2,3], and it is not a surrogate marker for long-term outcome [4]. The lack of association between pCR and long-term outcome is due to the heterogeneity of tumor characteristics, the role of pCR, and the effect of endocrine therapy among breast cancer subtypes [4]. pCR refers to the eradication of all tumor cells from the primary site and metastatic axillary lymph nodes after NAC, which is confirmed by the pathological evaluation of surgical specimens. However, circulating tumor DNA has been detected at the molecular level in peripheral blood cells by digital polymerase chain reaction in patients with breast cancer who had achieved pCR [5,6], indicating that residual tumor cells are still present in patients who have achieved pCR by pathological evaluation. In such cases, the residual tumor cells at the molecular level may reflect the long-term outcome of breast cancer patients achieving pCR, and certain immune responses elicited by NAC may affect the long-term outcome.
Tumor-infiltrating lymphocytes (TILs), including T lymphocytes and natural killer (NK) cells, have important and varied influences on the pathological and therapeutic effects of NAC in patients with various subtypes of breast cancer [7]. Compared with lower levels, higher levels of TILs before NAC are associated with higher pCR rates for TN, HER-2-positive, and luminal HER-2-negative breast cancers; they are associated with better prognoses for the former two cancer subtypes, but worse prognoses for the latter [7]. These findings imply that the amount of residual tumor per se after NAC is not a poor prognostic factor for luminal HER-2-negative breast cancer, and that endocrine therapy has a large effect. Although breast tumor shrinkage caused by NAC requires a local immune response with TILs at the primary site, the activation of both systemic and local immune responses contributes to the pathological and therapeutic effects of NAC [8]. Furthermore, tumor microenvironmental factors (TMEFs) play important roles in determining which immune responses are activated in patients with breast cancer during NAC [9], but the mechanisms of these actions remain unclear. In this study, we explored the roles of immune factors associated with the pathological and therapeutic effects of preoperative chemotherapy in patients with breast cancer.
Methods
Study design
Patients with stages II–IV breast cancer who received preoperative chemotherapy between 2013 and 2018 at the Hiroshima Mark Clinic, Hiroshima, Japan were enrolled in this cohort study. Blood samples were taken before and after chemotherapy for the analysis of peripheral natural killer (pNK) cell activity and determination of neutrophil–lymphocyte ratios (NLRs), lymphocyte–monocyte ratios (LMRs), and platelet–lymphocyte ratios (PLRs). TILs and TMEFs were examined comparatively in pre-chemotherapy vacuum-assisted biopsy (VAB) samples and post-chemotherapy surgical resection specimens.
Patient eligibility
Patient eligibility criteria were: diagnosis of stage II–IV breast cancer and receipt of preoperative chemotherapy; age < 70 years; female sex (menopausal status was not a criterion); ECOG (Eastern Cooperative Oncology Group) performance status = 0; white blood cell count ≥ 3000/mm3; platelet count ≥ 100,000/mm3; and normal bilirubin, aspartate aminotransferase/alanine aminotransferase, alkaline phosphatase, and creatine concentrations. Patients with active cardiac disease, pregnancy, and/or histories of breast cancer treatment or anthracycline treatment for any malignancy, and those receiving concurrent sex hormone treatment, were excluded. Patients with bilateral breast cancer and stage IV disease with multiple lung and/or liver metastases were also excluded. Disease stage was determined according to the tumor-node-metastasis (TNM) classification scheme recommended by the Union for International Cancer Control [10]. Axillary lymph-node metastasis (Ax+) was confirmed by fine-needle aspiration biopsy. For stage IV cases, locoregional treatment was considered to be palliative surgery to effectively downstage primary breast cancer.
Assessment of the pathological and therapeutic effects of preoperative chemotherapy
The pathological and therapeutic effects of preoperative chemotherapy were assessed according to the histopathological criteria of the Japanese Breast Cancer Society [11,12]. Pathological responses in intramammary lesions were graded as follows: grade 0 (G0), negligible or no change in cancer cells; grade 1a (G1a), mild changes in cancer cells or marked changes in less than one-third of cancer cells; grade 1b (G1b), marked changes in more than one-third but less than two-thirds of cancer cells; grade 2a (G2a), marked changes in more than two-thirds of cancer cells; grade 2b (G2b), disappearance of almost all cancer cells; and grade 3 (G3), apparent disappearance of all cancer cells. Therapeutic grades were determined according to the involvement of breast ducts and/or axillary lymph nodes. The disappearance of lymph-node metastasis was noted when it occurred. The disappearance of cancer cells from all breast tissues and axillary lymph nodes was considered to be a complete response (ypT0N0) because the axillary status has been suggested to be a better prognostic factor than the primary tumor response to NAC [4,13]. The disappearance of Ax+ was confirmed by the pathological analysis of surgically- dissected axillary lymph nodes.
Measurement of pNK cell activity and lymphocyte ratios
pNK cell activity in peripheral blood samples collected before and 3–4 weeks after chemotherapy was measured by the chromium release assay, which was conducted by SRL, Inc. (Tokyo, Japan) as described previously [14,15]. In brief, pNK cells were assayed for cytotoxic activity against 51Cr-labeled target (K562) cells. After the isolation of lymphocytes, effector and target cells were adjusted to a ratio of 20:1, plated, and incubated at 37 °C for 3.5 h under CO2. The cells were then centrifuged, and supernatants were collected and counted in a gamma counter. pNK cell activity was calculated as follows: pNK cell activity = experimental group release – background release/maximal release – background release. The NLR, LMR, and PLR were measured using peripheral blood samples taken before and 3–4 weeks after chemotherapy, before surgical treatment.
Evaluation of TILs
TILs in VAB sections obtained before chemotherapy and in surgical specimens obtained after chemotherapy were assessed by hematoxylin and eosin staining according to the recommendations of an international TIL working group [16]. In brief, TILs in the stromal compartment were counted, and the area of stromal tissue was used as the denominator to determine the percentage of stromal TILs. The stromal area was within the border of each invasive carcinoma. All mononuclear cells, including lymphocytes and plasma cells, were scored, except for the polymorphonuclear leukocytes that were present throughout the sections (4–5 μm; magnification, 200–400 ×). A pathologist determined the average number of TILs in each tumor area.
Assessment of TMEFs
TMEFs in formalin-fixed, paraffin-embedded sections collected from preoperative VAB samples and surgical specimens were analyzed by next-generation sequencing for CD4, CD8, NK, forkhead box protein P3 (FOXP3), cytotoxic T lymphocyte antigen 4 (CTLA-4), programmed cell death 1, programmed cell death ligand 1, interleukin (IL) 2, IL-6, IL-12, interferon γ, IL-10, transforming growth factor β, and vascular endothelial growth factor (VEGF) transcripts as described previously [14,15]. In brief, after the removal of paraffin using a deparaffinization solution, the samples were incubated in an optimized lysis buffer and heated briefly to partially reverse the formalin crosslinking of the released nucleic acids. Complementary DNAs (cDNAs) were synthesized using reverse transcriptase and random primers. The cDNA libraries were cleaned, combined, quantified, denatured, and diluted in hybridization buffer to create pooled libraries. Final TMEF expression levels were normalized to glyceraldehyde 3-phosphate dehydrogenase expression. TMEF levels were reported as medians and ranges of target genes from several immune-response amplicon sequences in the TME.
Statistical analysis
All data were analyzed using Statcel 4 software (OMS Publishing Inc., Tokyo, Japan). Continuous and independent variables were analyzed using the Mann–Whitney test. Categorical variables were analyzed using the chi-squared, Fisher's exact, and Wilcoxon signed-rank sum tests. Univariate and multivariate analyses were performed to evaluate associations between clinicopathological factors, pNK cell activity, and NLRs, LMRs, PLRs, TILs, and TMEF levels. For the multivariate analysis, a logistic regression model was used to determine statistical relationships between each dependent variable and the independent variables. Odds ratios (ORs) were reported with 95% confidence intervals (CIs). P values < 0.05 were considered to be significant.
Results
Patient characteristics and pathological effects
This study included 43 patients (median age, 50.0 years; range, 27–69 years) with stage II (n = 21), stage III (n = 17), or stage IV (n = 5) breast cancer. The clinical characteristics of the five stage-IV cases were T2N1M1 (bone), T3N3M1 (distant lymph node), T4bN1M1 (bone), T2N3M1 (bone), and T2N3M1 (lung/bone). The tumor subtypes were luminal (n = 27), HER-2-positive (n = 12), and TN (n = 4). The histological types were invasive ductal carcinoma (n = 38), which was not otherwise specified, and other (n = 5). The treatment regimens included taxanes, 5-fluorouracil (F), epirubicin (E), cyclophosphamide (C), and/or trastuzumab (Tz). Fourteen patients were treated with taxanes + FEC (+Tz in two cases), 20 patients were treated with FEC + taxanes (+Tz in nine cases), eight patients were treated with EC + taxanes (+Tz in one case), and one patient was treated with dose-dense EC + taxanes. Tz was used for HER-2-positive cases. Taxanes were given as nanoparticle albumin-bound paclitaxel, docetaxel, or paclitaxel. According to histopathological criteria, the pathological and therapeutic responses were G1a in 12 patients, G1b in 13 patients, G2a in seven patients, G2b in five patients, and G3 (complete) in six patients. The pCR rate was 13.9%.
Infiltration of TILs
The percentages of TILs in the VAB samples and surgical specimens ranged from 1% to 40%, but most samples contained ≤10% TILs. When the cutoff for TILs was set at ≥5%, pre-chemotherapy biopsy samples from 18 of 43 patients were positive. After NAC, TILs were present in samples from 12 of 43 patients, and the percentage of TILs had increased after chemotherapy in six of these cases.
Associations between pathological and therapeutic effects and clinicopathological factors, TILs, NLRs, LMRs, PLRs, and TMEFs
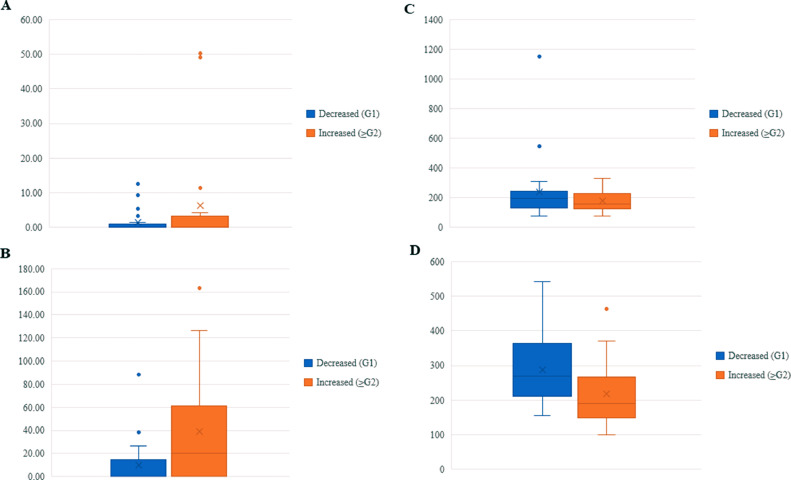
Univariate analysis showed that G2 and better pathological and therapeutic effects were significantly associated with HER-2 positivity (P < 0.01), Tz treatment (P < 0.01), and increased NK (P = 0.04) and IL-6 (P = 0.02) levels after chemotherapy. Changes in pNK cell activity after chemotherapy were not associated with any pathological or therapeutic effect. PLRs were significantly higher in the G1 effect (P <0.01) and significantly lower in the G2 and better therapeutic effects than in the G1 effect after chemotherapy (P = 0.02; Table 1). Box plot analyses of NK cell levels and PLRs are shown in Fig. 1.
Table 1.
Univariate associations of pathological responses with clinicopathological factors, TILs, and TMEFs in 43 patients with breast cancer who received preoperative chemotherapy.
| Variable | Pathological response |
P | ||
|---|---|---|---|---|
| Decreased (G1, n = 24) | Increased (≥G2, n = 19) | |||
| Median age, years (range) | 49 (27–69) | 52 (30–68) | NSa | |
| Stage, n | II | 14 | 7 | NSa |
| III | 6 | 11 | ||
| IV | 4 | 1 | ||
| Subtype, n | Luminal | 21 | 6 | <0.01b |
| HER-2-positive | 1 | 11 | ||
| TN | 2 | 2 | ||
| Treatment regimen, n | Taxanes/FEC | 11 (Tz: 1) | 3 (Tz: 1) | NSb |
| FEC/taxanes | 9 | 11 (Tz: 9) | ||
| EC/taxanes | 4 | 4 (Tz: 1) | ||
| ddEC/taxanes | 0 | 1 | ||
| Tz, n | Tz– | 23 | 8 | <0.01b |
| Tz+ | 1 | 11 | ||
| Nuclear grade, n | 1 | 2 | 0 | NSa |
| 2 | 4 | 3 | ||
| 3 | 18 | 16 | ||
| Ki-67 positivity, n | <15% | 6 | 2 | NSa |
| 15–35% | 6 | 4 | ||
| >35% | 12 | 13 | ||
| TILs, n (Pre-chemo) | <5% | 16 | 9 | NSb |
| ≥5% | 8 | 10 | ||
| TILs, n (Post-chemo) | <5% | 18 | 13 | NSb |
| ≥5% | 6 | 6 | ||
| Increase in TILs, n (%) | 3 (12.5%) | 3 (15.7%) | NSc | |
| Median levels (range) | 27.5 (12–49) | 28.0 (6–62) | NSa | |
| Pre-chemo pNK cells | 32.5 (8–61) | 34.0 (3–58) | NSa | |
| Post-chemo pNK cells P | NSd | NSd | ||
| Pre-chemo PLR | 196.28 (75–1151.66) | 159.74 (78.01–329.55) | NSa | |
| Post-chemo PLR P | 268.59 (166.84–541.25) <0.01d | 189.44 (106.08–462.50) NSd | 0.02a | |
| Median TMEF levels (range) | ||||
| Pre-chemo NK cells | 0 (0–12.50) | 0.04 (0–50.26) | NSa | |
| Post-chemo NK cells P | 0.38 (0–88.51) 0.02d | 20.23 (0–163.40) <0.01d | 0.04a | |
| Pre-chemo IL-6 | 0.44 (0–49.37) | 0.18 (0–42.92) | NSa | |
| Post-chemo IL-6 | 1.20 (0–412.72) | 7.08 (0–2710.88) | NSa | |
| P | NSd | 0.02d | ||
TIL tumor-infiltrating lymphocyte, TMEF tumor microenvironmental factor, G1 grade 1, G2 grade 2, NS not significant, HER-2 human epidermal growth factor receptor 2, TN triple negative, FEC 5-fluorouracil/epirubicin/cyclophosphamide, Tz trastuzumab, EC epirubicin/cyclophosphamide, ddEC dose-dense epirubicin/cyclophosphamide, pNK peripheral natural killer, PLR platelet-lymphocyte ratio, NK natural killer, IL-6 interleukin 6.
Mann–Whitney test.
chi-squared test.
Fisher's exact test.
Wilcoxon signed-rank sum test.
Fig. 1.
Box plot analysis between pathological responses in primary lesion and NK cell levels and PLRs before and after preoperative chemotherapy in 43 patients with breast cancer who received preoperative chemotherapy. A, B: NK cell levels between G1 and G2 and better therapeutic effects before and after preoperative chemotherapy (pre-chemo, NS; post-chemo, P = 0.04). A: Before; B: After; Vertical axis: Relative expression of NK levels normalized to glyceraldehyde 3-phosphate dehydrogenase expression. C, D: PLRs between G1 and G2 and better therapeutic effects before and after preoperative chemotherapy (pre-chemo, NS; post-chemo, P = 0.02). C: Before; D: After; Vertical axis: Platelet to lymphocyte ratio. NK natural killer, PLRs platelet-lymphocyte ratios, G grade, NS not significant.
Associations between the disappearance of Ax+ and clinicopathological factors, TILs, NLR, LMR, PLR, and TMEFs
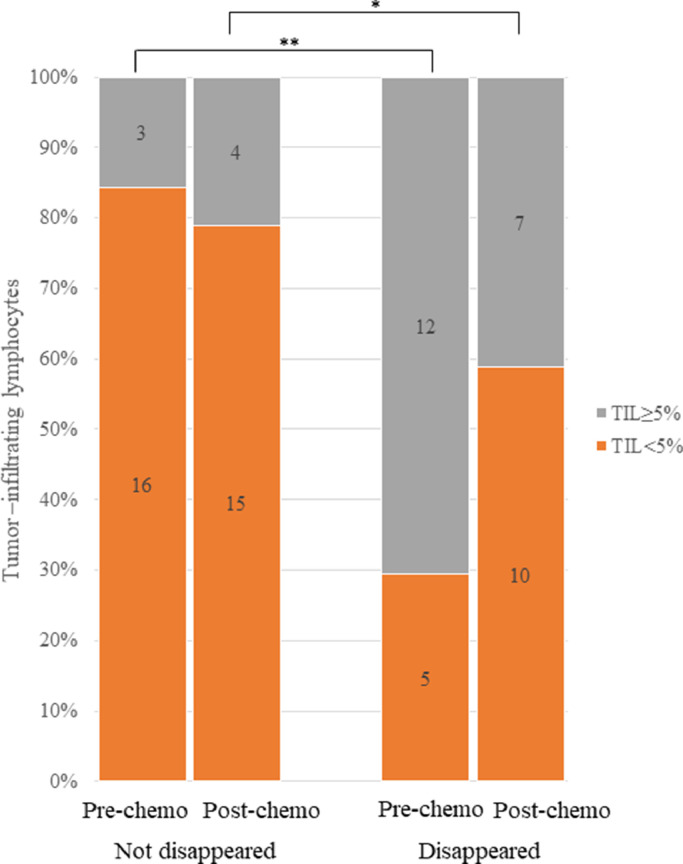
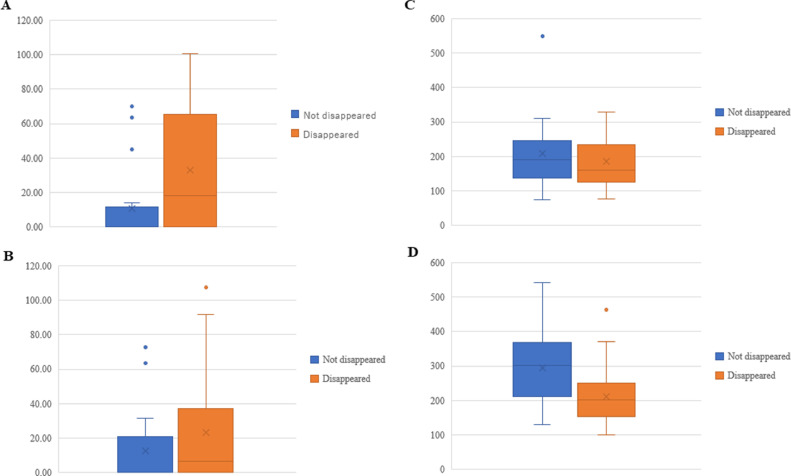
Univariate analyses showed that the disappearance of Ax+ was significantly associated with stage III disease (vs. stages II and IV; P = 0.02), the HER-2-positive and TN subtypes (P < 0.01), Tz treatment (P = 0.04), ≥5% TILs before chemotherapy (P < 0.01), increased pNK cell activity after chemotherapy (P = 0.03), decreased VEGF level after chemotherapy (P = 0.02), and higher CTLA-4 levels in regulatory T cells (Tregs) before chemotherapy (P = 0.02; due to decreases in CTLA-4 levels in Tregs after chemotherapy). PLRs were significantly higher in the patients for whom Ax+ had not disappeared (P <0.01) and significantly lower in those for whom Ax+ disappeared than in those for whom Ax+ had not disappeared after chemotherapy (P = 0.01; Table 2). Distribution in levels of TILs in disappearance of Ax+ before and after chemotherapy is shown in Fig. 2. Box plot analyses of CTLA-4 in Tregs and PLRs are shown in Fig. 3.
Table 2.
Univariate associations of Ax+ disappearance with clinicopathological factors, TILs, and TMEFs in 36 patients with breast cancer who received preoperative chemotherapy.
| Variable | Ax+ |
P | ||
|---|---|---|---|---|
| Not disappeared (n = 19) | Disappeared (n = 17) | |||
| Median age, years (range) | 49 (27–69) | 51 (35–67) | NSa | |
| Stage, n | II | 13 | 4 | 0.02a |
| III | 5 | 12 | ||
| IV | 1 | 1 | ||
| Subtype, n | Luminal | 16 | 6 | <0.01b |
| HER-2-positive | 3 | 8 | ||
| TN | 0 | 3 | ||
| Treatment regimen, n | Taxanes/FEC | 8 (Tz: 1) | 3 (Tz: 1) | NSb |
| FEC/taxanes | 7 (Tz: 2) | 10 (Tz: 6) | ||
| EC/taxanes | 4 | 4 (Tz: 1) | ||
| Tz, n | Tz– | 16 | 9 | 0.04b |
| Tz+ | 3 | 8 | ||
| Nuclear grade, n | 1 | 1 | 0 | NSa |
| 2 | 3 | 2 | ||
| 3 | 15 | 15 | ||
| Ki-67 positivity, n | <15% | 2 | 1 | NSa |
| 15–35% | 6 | 3 | ||
| >35% | 11 | 13 | ||
| TILs, n (Pre-chemo) | <5% | 16 | 5 | <0.01b |
| ≥5% | 3 | 12 | ||
| TILs, n (Post-chemo) | <5% | 15 | 10 | NSc |
| ≥5% | 4 | 7 | ||
| Increase in TILs, n (%) | 3 (15.7%) | 2 (11.7%) | NSc | |
| Median levels (range) | ||||
| Pre-chemo pNK cells | 30.0 (12–57) | 25.0 (6–62) | NSa | |
| Post-chemo pNK cells | 30.0 (8–60) | 41.0 (3–58) | NSa | |
| P | NSd | 0.03d | ||
| Pre-chemo PLR | 189.30 (100.00–548.23) | 160.21 (87.57–329.55) | NSa | |
| Post-chemo PLR | 301.72 (149.79–541.25) | 201.25 (106.08–462.50) | 0.01a | |
| P | <0.01d | NSd | ||
| Median TMEF levels (range) | ||||
| Pre-chemo CTLA-4 | 0.15 (0–69.91) | 18.40 (0–100.34) | 0.02a | |
| Post-chemo CTLA-4 | 0.27 (0–72.83) | 6.76 (0–107.46) | NSa | |
| P | NSd | NSd | ||
| Pre-chemo VEGF | 701.90 (0–4080.10) | 1022.62 (2.08–4005.85) | NSa | |
| Post-chemo VEGF | 508.07 (0–2740.96) | 452.67 (4.24–2555.05) | NSa | |
| p | NSd | 0.02d | ||
Ax+ axillary lymph node metastasis, TIL tumor-infiltrating lymphocyte, TMEF tumor microenvironmental factor, NS not significant, HER-2 human epidermal growth factor receptor 2, TN triple negative, FEC 5-fluorouracil/epirubicin/cyclophosphamide, Tz trastuzumab, EC epirubicin/cyclophosphamide, pNK peripheral natural killer, PLR platelet-lymphocyte ratio, CTLA-4 cytotoxic T lymphocyte antigen 4, VEGF vascular endothelial growth factor.
Mann–Whitney test,.
chi-squared test,.
Fisher's exact test,.
Wilcoxon signed-rank sum test.
Fig. 2.
Association between levels of TILs and disappearance of Ax+ before and after chemotherapy in 36 patients with breast cancer who received preoperative chemotherapy. Preoperative distribution of levels of TILs was significantly higher in patients in whom Ax+ disappeared after chemotherapy than in those in whom Ax+ did not disappear (**, P < 0.01; *, NS). TILs tumor-infiltrating lymphocytes, Ax+ axillary lymph node metastasis, NS not significant.
Fig. 3.
Box plot analysis between disappearance of Ax+ and levels of CTLA-4 in Tregs and PLRs before and after preoperative chemotherapy in 43 patients with breast cancer who received preoperative chemotherapy. A, B: Levels of CTLA-4 in Tregs between disappeared and not disappeared Ax+ before and after preoperative chemotherapy (pre-chemo, P = 0.02; post-chemo, NS). A: Before; B: After; Vertical axis: Relative expression of CTLA-4 levels normalized to glyceraldehyde 3-phosphate dehydrogenase expression. C, D: PLRs between disappeared and not disappeared Ax+ before and after preoperative chemotherapy (pre-chemo, NS; post-chemo, P = 0.01). C: Before; D: After; Vertical axis: Platelet to lymphocyte ratio. Ax+ axillary lymph node metastasis, CTLA-4 cytotoxic T lymphocyte antigen 4, Tregs regulatory T cells, PLRs platelet-lymphocyte ratios, NS not significant.
Associations between changes in PLRs and clinicopathological factors, TILs, and TMEFs
Given that G2 and better pathological and therapeutic effects and Ax+ disappearance were associated significantly with lower PLRs after chemotherapy, the associations between PLR reduction and clinicopathological factors, TILs, and TMEFs were assessed. Univariate analyses showed that PLR reduction was significantly associated with increased CD8 levels (P = 0.04), but not with G2 and better pathological and therapeutic effects or the disappearance of Ax+ after preoperative chemotherapy (Table 3).
Table 3.
Univariate associations of the PLR with clinicopathological Factors, TILs, and TMEFs in 43 patients with breast cancer who received preoperative chemotherapy.
| Variable |
PLR |
P | |||
|---|---|---|---|---|---|
| Increased (n = 30) | Decreased (n = 13) | ||||
| Median age, years (range) | 49 (27–69) | 52 (35–67) | NSa | ||
| Stage, n | II | 15 | 6 | NSa | |
| III | 10 | 7 | |||
| IV | 5 | 0 | |||
| Subtype, n | Luminal | 20 | 7 | NSb | |
| HER-2-positive | 6 | 6 | |||
| TN | 4 | 0 | |||
| Treatment regimen, n | Taxanes/FEC | 10 (Tz: 1) | 4 (Tz: 4) | NSb | |
| FEC/taxanes | 12 (Tz: 4) | 8 (Tz: 5) | |||
| EC/taxanes | 7 (Tz: 1) | 1 | |||
| ddEC | 1 | 0 | |||
| Tz, n | Tz– | 24 | 7 | NSc | |
| Tz+ | 6 | 6 | |||
| Nuclear grade, n | 1 | 2 | 0 | NSa | |
| 2 | 6 | 1 | |||
| 3 | 22 | 12 | |||
| Ki-67 positivity, n | <15% | 6 | 2 | NSa | |
| 15–35% | 9 | 1 | |||
| >35% | 15 | 10 | |||
| TILs, n (Pre-chemo) | <5% | 19 | 6 | NSb | |
| ≥5% | 11 | 7 | |||
| TILs, n (Post-chemo) | <5% | 21 | 10 | NSb | |
| ≥5% | 9 | 3 | |||
| Pathological response, n G1 | 19 | 5 | NSa | ||
| ≥G2 | 11 | 8 | |||
| Ax+, n disappeared | 9 | 8 | NSb | ||
| Not disappeared | 15 | 4 | |||
| Median levels (range) | |||||
| Pre-chemo pNK cells | 29.5 (8–62) | 24.0 (6–40) | NSa | ||
| Post-chemo pNK cells | 35.0 (3–61) | 30.0 (11–51) | NSa | ||
| P | NSd | NSd | |||
| Pre-chemo PLR | 153.46 (75.00–310.00) | 229.69 (125.45–548.23) | 0.01a | ||
| Post-chemo PLR | 274.18 (149.79–541.25) | 166.84 (100.52–286.66) | <0.01a | ||
| Median TMEF level (range) | |||||
| Pre-chemo CD8 | 44.19 (0.16–2123.70) | 124.97 (0–423.76) | NSa | ||
| Post-chemo CD8 | 7.60 (0–1372.71) | 200.12 (0–937.65) | NSa | ||
| P | NSd | 0.04d | |||
PLR platelet-lymphocyte ratio, TIL tumor-infiltrating lymphocyte, TMEF tumor microenvironmental factor, NS not significant, HER-2 human epidermal growth factor receptor 2, TN triple negative, FEC 5-fluorouracil/epirubicin/cyclophosphamide, Tz trastuzumab, EC epirubicin/cyclophosphamide, ddEC dose-dense epirubicin/cyclophosphamide, G1 grade 1, G2 grade 2, Ax+ axillary lymph node metastasis, pNK peripheral natural killer.
Mann–Whitney test,.
chi-squared test.
Fisher's exact test,.
Wilcoxon signed-rank sum test.
Associations of G2 and better pathological and therapeutic effects and the disappearance of Ax+ with pre-chemotherapy TILs, PLR, pNK cell activity, and TMEFs
Based on the results of univariate analyses, multivariate analysis of the associations of G2, better pathological and therapeutic effects, and the disappearance of Ax+ with pre-chemotherapy TILs, PLR, pNK cell activity, and TMEFs was performed. G2 and better therapeutic effects tended to be associated with higher NK levels after chemotherapy (OR = 1.02; 95% CI, 0.99–1.05; P = 0.07; Table 4).
Table 4.
Multivariate associations of G2 and better therapeutic effects and Ax+ disappearance with clinicopathological factors, TILs, and TMEFs in 43 patients with breast cancer who received preoperative chemotherapy.
| Dependent Variable | Independent Variable | OR (95% CI) | P |
|---|---|---|---|
| G2 and better therapeutic effects | Post-chemo NK cell level | 1.02 (0.99–1.05) | 0.07 |
| Post-chemo PLR | 0.99 (0.98–1.00) | 0.22 | |
| Disappearance of Ax+ | Pre-chemo TILs | 1.02 (0.92–1.13) | 0.61 |
| Post-chemo PLR | 0.99 (0.98–1.00) | 0.10 | |
| Pre-chemo CTLA-4 level | 1.02 (0.98–1.05) | 0.21 |
G2 grade 2, Ax+ axillary lymph node metastasis, TIL tumor-infiltrating lymphocyte, TMEF tumor microenvironmental factor, OR odds ratio, CI confidence interval, NK natural killer, PLR platelet-lymphocyte ratio, CTLA-4 cytotoxic T lymphocyte antigen 4.
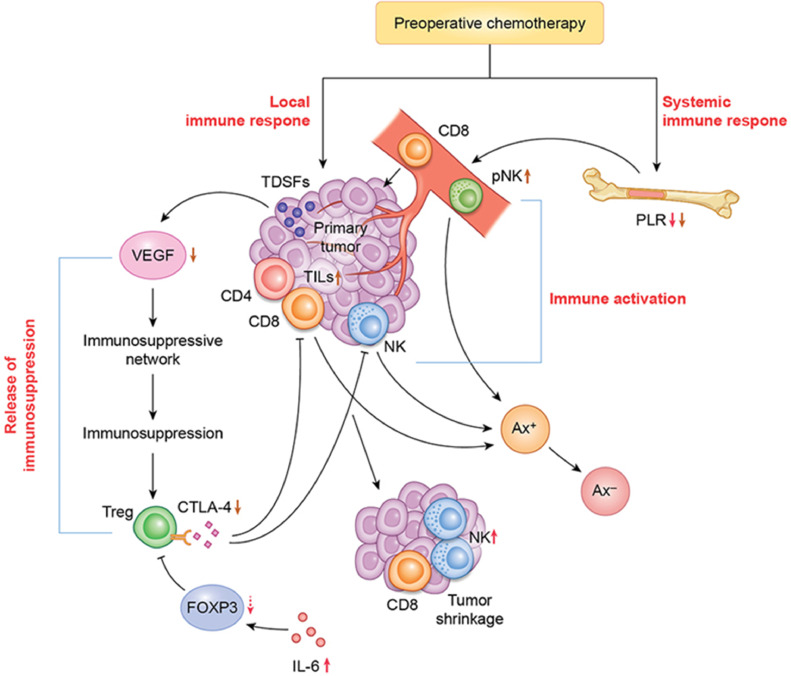
A model of the activation of local and systemic immune responses leading to pathological and therapeutic effects after preoperative chemotherapy in patients with breast cancer
In patients who responded to preoperative chemotherapy with anticancer agents, NK cell and IL-6 levels increased (local immune response) in the presence of TILs. In addition, the level of pNK cells derived from bone marrow increased, and the PLR decreased (systemic immune response) in these patients. These immune responses contributed to the shrinkage of primary tumors and eradication of metastatic tumor cells in axillary lymph nodes. Immunosuppressive conditions mediated by VEGF, a tumor-derived soluble factor, and CTLA-4 in Tregs in tumor microenvironments were overcome by preoperative chemotherapy in collaboration with these immune responses in responders (Fig. 4).
Fig. 4.
A proposed model of local and systemic activation of the immune response after preoperative chemotherapy in patients with breast cancer. In patients who responded to chemotherapy, NK cell and IL-6 levels increased, while the VEGF level and CTLA-4 level in Tregs decreased in the presence of TILs (local immune response). In addition, pNK cell activity increased, and PLRs decreased (systemic immune response) in these patients. These immune responses contributed to the eradication of breast cancer cells in primary tumors and metastatic axillary lymph nodes. Immunosuppressive states mediated by VEGF, a tumor-derived soluble factor, and CTLA-4 in Tregs in the tumor microenvironment were overcome after preoperative chemotherapy. NK natural killer, IL-6 interleukin 6, VEGF vascular endothelial growth factor CTLA-4 cytotoxic T lymphocyte antigen 4, Tregs regulatory T cells, TILs tumor-infiltrating lymphocytes, pNK peripheral NK, PLRs platelet-lymphocyte ratios.
Discussion
In this study, we showed that activation of the local and systemic immune responses by preoperative chemotherapy had different pathological and therapeutic effects in patients with breast cancer. Activation of the local immune response was induced by the presence of TILs before chemotherapy, and by increased NK cell levels and primary tumor shrinkage after chemotherapy. The levels of several immune cells, including NK and CD8 cells, were increased, and those of immunosuppressive factors, such as VEGF and CTLA-4 in Tregs, were decreased after chemotherapy. IL-6 is known to be responsible for immune activation and the enhancement of angiogenesis; increased IL-6 production may also be involved in the downregulation of FOXP3 expression in Tregs under inflammatory conditions [17]. Similarly, activation of the systemic immune response in collaboration with the local immune response, including via increased pNK cell activity and PLR reduction, contributed to primary tumor shrinkage and Ax+ disappearance. Importantly, in tumors with ≥5% TILs before chemotherapy, an increase in NK cells in the tumor microenvironment following chemotherapy was necessary to achieve G2, better pathological and therapeutic effects, and Ax+ disappearance. Thus, the coactivation of local and systemic immune responses is important for the improvement of pathological therapeutic responses after preoperative chemotherapy in patients with breast cancer.
Tumor heterogeneity can lead to different pathological and therapeutic responses, defined by local and systemic immune responses, after NAC. In this study, we found an increase in NK cells, associated with primary tumor shrinkage and G2 and better pathological and therapeutic responses, in patients with HER-2-positive breast cancer who were treated with Tz, due in part to the activation of NK cells by antibody-dependent cell-mediated cytotoxicity (ADCC) via FcγRIII upon Tz treatment [18]. In contrast, G2 and better pathological and therapeutic responses were not associated with the activation of pNK cells (systemic immune response), although the disappearance of Ax+ was associated with increased pNK cell activity after chemotherapy. These findings suggest that NK cells and pNK cell activity have different functional roles in primary tumor shrinkage and eradication of metastatic breast cancer cells in the axillary lymph nodes after preoperative chemotherapy.
As a limitation of interpretation of the immune factors responsible for achieving a therapeutic effect in this study, HER2-positive patients treated with Tz represent the major subgroup of patients, comprising 11 out of 19 patients (57.8%) who achieved G2 and better therapeutic effects. Because of the limited number of patients in the other groups, with only six out of 19 patients (31.5%) having luminal type and two out of 19 patients (10.5%) having TN type, there is a possibility that patients in the Tz-treated group could have a predominant effect on the overall study and may have caused bias in the interpretation of the results. Nevertheless, given that G2 and better pathological and therapeutic responses were observed in some patients with luminal HER-2-negative breast cancer after chemotherapy, the activation of local and systemic immune responses appears to be necessary for the achievement of pathological and therapeutic effects, regardless of Tz-induced ADCC.
Systemic immune parameters, including the NLR, LMR, and PLR, have been shown to be prognostic factors for patients with breast cancer [[19], [20], [21]]. Some recent studies have shown that a low pre-NAC NLR is a predictive factor for the pathological and therapeutic effects of NAC in patients with breast cancer [22,23], whereas other studies have not shown this effect [24,25]. Furthermore, low pre-NAC NLRs and PLRs have been shown to be predictive of pCR and better therapeutic and pathological responses after NAC in such patients [26,27]. Similarly, low pre-NAC PLRs have been associated with strong pathological and therapeutic responses after NAC in patients with breast cancer [28]. Low pre-NAC LMRs were also found to be predictive of pathological and therapeutic effects in patients with breast cancer after NAC [29]. Thus, the results are suggestive of roles of pre-NAC NLR, LMR, and PLR in prediction of the pathological and therapeutic effects of NAC; however, high pre-NAC levels of peripheral lymphocytes are not predictive of pathological and therapeutic responses after NAC. This lack of predictive power could be due to the inflammatory conditions that occur during tumor progression, based on the tumor stage and subtype, rather than to changes in lymphocyte levels between the periods before and after NAC.
In this study, differences in parameters before and after chemotherapy were assessed regardless of whether the systemic immune response was elicited by anticancer agents. We showed that lower PLRs associated with increases in CD8 levels, relative to platelets, were involved in G2, better therapeutic effects, and the disappearance of Ax+, in association with increased NK cell levels and decreased VEGF and CTLA-4 levels in Tregs (local immune responses), as well as increased pNK cell activity (systemic immune response) after chemotherapy. However, we do not know why relative increases in CD8+ peripheral lymphocytes relative to platelets, but not in neutrophils or monocytes, are good indicators of systemic immune response to therapy. Thus, increases in immune cells as local and systemic responses along with decreases in immunosuppressive factors play important roles in tumor shrinkage and the disappearance of Ax+ following tumor cell death induced by anticancer agents.
Although the results of this study are limited due to the small number of patients involved, the associations of immune responses with pathological and therapeutic outcomes were evaluated statistically, and the observed changes in parameters related to immunity demonstrate the important roles of local and systemic immune responses in patients with breast cancer who receive preoperative chemotherapy. In addition, patients with stage IV disease were included in this study to explore therapeutic immune factors inducing local and systemic immune responses after chemotherapy for a broad range of disease stages. A prospective randomized trial examining the effects of breast surgery in patients with stage IV disease showed that the survival rate following locoregional therapy was better than that following systemic therapy, overall and in patients with solitary bone metastases, but not in those with multiple lung and/or liver metastases [30].
Conclusions
Activation of the local and systemic immune responses by downregulation of immunosuppressive factors after chemotherapy in the presence of TILs in the primary tumor prior to chemotherapy is required to achieve G2, better pathological and therapeutic effects, and disappearance of Ax+ in patients with breast cancer. Therapy targeting VEGF and CTLA-4 in Tregs to overcome tumor-derived immunosuppression may enhance the pathological and therapeutic responses to preoperative chemotherapy in these patients.
CRediT authorship contribution statement
Ryungsa Kim: Conceptualization, Formal analysis, Investigation, Writing - original draft, Writing - review & editing. Ami Kawai: Conceptualization, Software, Formal analysis, Investigation, Data curation. Megumi Wakisaka: Resources, Data curation. Sayaka Sawada: Resources. Mika Shimoyama: Resources. Naomi Yasuda: Resources. Masayuki Hidaka: Conceptualization, Methodology, Formal analysis, Investigation. Yukitaka Morita: Conceptualization, Methodology, Formal analysis, Writing - review & editing. Shoichiro Ohtani: Resources, Data curation. Mitsuya Ito: Resources. Kensuke Kawasaki: Resources. Takanori Kin: Resources. Koji Arihiro: Resources, Data curation.
Declaration of Competing Interest
The authors have stated that they have no conflicts of interest.
Acknowledgments
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The datasets generated and analyzed in this study are available from the corresponding author upon reasonable request.
Ethics approval and informed consent
This study was performed in accordance with the Declaration of Helsinki. The Ethics Committee of Hiroshima Mark Clinic (July 1, 2012) approved the study with regard to the ethical, legal, and social implications. All samples were obtained with informed consent from the patients.
Acknowledgments
The authors would like to thank SRL, Inc. (Tokyo, Japan) for measuring pNK cell activity, and thank Elsevier scientific illustration services for creating Fig. 4. The authors also thank the patients and their families for their participation in this study.
References
- 1.von Minckwitz G., Untch M., Blohmer J.U. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol. 2014;30:1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 2.Hatzis C., Symmans W.F., Zhang Y. Relationship between complete pathologic response to neoadjuvant chemotherapy and survival in triple-negative breast cancer. Clin. Cancer Res. 2016;22:26–33. doi: 10.1158/1078-0432.CCR-14-3304. [DOI] [PubMed] [Google Scholar]
- 3.Broglio K.R., Quintana M., Foster M. Association of pathologic complete response to neoadjuvant therapy in her2-positive breast cancer with long-term outcomes: a meta-analysis. JAMA Oncol. 2016;2:751–760. doi: 10.1001/jamaoncol.2015.6113. [DOI] [PubMed] [Google Scholar]
- 4.Cortazar P., Zhang L., Untch M. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Murillas I., Schiavon G., Weigelt B. Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci. Transl. Med. 2015;7:302ra133. doi: 10.1126/scitranslmed.aab0021. [DOI] [PubMed] [Google Scholar]
- 6.B.R. McDonald, T. Contente-Cuomo, S.J. Sammut, et al., Personalized circulating tumor DNA analysis to detect residual disease after neoadjuvant therapy in breast cancer. Sci. Transl. Med. 11(2019) eaax7392. [DOI] [PMC free article] [PubMed]
- 7.Denkert C., von Minckwitz G., Darb-Esfahani S. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19:40–50. doi: 10.1016/S1470-2045(17)30904-X. [DOI] [PubMed] [Google Scholar]
- 8.Qian Y., Tao J., Li X. Peripheral inflammation/immune indicators of chemosensitivity and prognosis in breast cancer patients treated with neoadjuvant chemotherapy. Onco. Targets Ther. 2018;11:1423–1432. doi: 10.2147/OTT.S148496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.García-Martínez E., Gil G.L., Benito A.C. Tumor-infiltrating immune cell profiles and their change after neoadjuvant chemotherapy predict response and prognosis of breast cancer. Breast Cancer Res. 2014;16:488. doi: 10.1186/s13058-014-0488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brierley J.D., Gospodarowicz M.K., Wittekind C. 8th ed. WILEY-Blackwell; New Jersey: 2017. TNM Classification of Malignant Tumours. [Google Scholar]
- 11.Kurosumi M., Akashi-Tanaka S., Akiyama F. Committee for Production of histopathological criteria for assessment of therapeutic response of japanese breast cancer society. Histopathological criteria for assessment of therapeutic response in breast cancer (2007 version) Breast Cancer. 2008;15:5–7. doi: 10.1007/s12282-007-0016-x. [DOI] [PubMed] [Google Scholar]
- 12.Horii R., Akiyama F. Histological assessment of therapeutic response in breast cancer. Breast Cancer. 2016;23:540–545. doi: 10.1007/s12282-013-0499-6. [DOI] [PubMed] [Google Scholar]
- 13.Zhang G.C., Zhang Y.F., Xu F.P. Axillary lymph node status, adjusted for pathologic complete response in breast and axilla after neoadjuvant chemotherapy, predicts differential disease-free survival in breast cancer. Curr. Oncol. 2013;20:e180–e192. doi: 10.3747/co.20.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim R., Kawai A., Wakisaka M. A potential role for peripheral natural killer cell activity induced by preoperative chemotherapy in breast cancer patients. Cancer Immunol. Immunother. 2019;68:577–585. doi: 10.1007/s00262-019-02305-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim R., Kawai A., Wakisaka M. Immune correlates of the differing pathological and therapeutic effects of neoadjuvant chemotherapy in breast cancer. Eur. J. Surg. Oncol. 2020;46:77–84. doi: 10.1016/j.ejso.2019.09.146. [DOI] [PubMed] [Google Scholar]
- 16.Salgado R., Denkert C., Demaria S. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group. Ann. Oncol. 2014;26(2015):259–271. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao Y., Tang J., Chen W. Inflammation negatively regulates FOXP3 and regulatory T-cell function via DBC1. Proc. Natl. Acad. Sci. USA. 2015;112:E3246–E3254. doi: 10.1073/pnas.1421463112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clynes R.A., Towers T.L., Presta L.G., Ravetch J.V. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat. Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 19.Guo W., Lu X., Liu Q. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio for breast cancer patients: an updated meta-analysis of 17079 individuals. Cancer Med. 2019;8:4135–4148. doi: 10.1002/cam4.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeuchi H., Kawanaka H., Fukuyama S., Kubo N., Hiroshige S., Yano T. Comparison of the prognostic values of preoperative inflammation-based parameters in patients with breast cancer. PLoS One. 2017;12 doi: 10.1371/journal.pone.0177137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ni X.J., Zhang X.L., Ou-Yang G.W. An elevated peripheral blood lymphocyte-to-monocyte ratio predicts favorable response and prognosis in locally advanced breast cancer following neoadjuvant chemotherapy. PLoS One. 2014;9 doi: 10.1371/journal.pone.0111886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y., Chen K., Xiao X. Pretreatment neutrophil-to-lymphocyte ratio is correlated with response to neoadjuvant chemotherapy as an independent prognostic indicator in breast cancer patients: a retrospective study. BMC Cancer. 2016;16:320. doi: 10.1186/s12885-016-2352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chae S., Kang K.M., Kim H.J. Neutrophil-lymphocyte ratio predicts response to chemotherapy in triple-negative breast cancer. Curr. Oncol. 2018;25:e113–e119. doi: 10.3747/co.25.3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eryilmaz M.K., Mutlu H., Salim D.K., Musri F.Y., Tural D., Coskun H.S. The neutrophil to lymphocyte ratio has a high negative predictive value for pathologic complete response in locally advanced breast cancer patients receiving neoadjuvant chemotherapy. Asian Pac. J. Cancer Prev. 2014;15:7737–7740. doi: 10.7314/apjcp.2014.15.18.7737. [DOI] [PubMed] [Google Scholar]
- 25.Suppan C., Bjelic-Radisic V., La Garde M. Neutrophil/lymphocyte ratio has no predictive or prognostic value in breast cancer patients undergoing preoperative systemic therapy. BMC Cancer. 2015;15:1027. doi: 10.1186/s12885-015-2005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graziano V., Grassadonia A., Iezzi L. Combination of peripheral neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio is predictive of pathological complete response after neoadjuvant chemotherapy in breast cancer patients. Breast. 2019;44:33–38. doi: 10.1016/j.breast.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 27.Kim H.Y., Kim T.H., Yoon H.K., Lee A. The role of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in predicting neoadjuvant chemotherapy response in breast cancer. J. Breast Cancer. 2019;22:425–438. doi: 10.4048/jbc.2019.22.e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asano Y., Kashiwagi S., Onoda N. Platelet-lymphocyte ratio as a useful predictor of the therapeutic effect of neoadjuvant chemotherapy in breast cancer. PLoS One. 2016;11 doi: 10.1371/journal.pone.0153459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng Y., Chen R., Qu F. Low pretreatment lymphocyte/monocyte ratio is associated with the better efficacy of neoadjuvant chemotherapy in breast cancer patients. Cancer Biol. Ther. 2020;21:189–196. doi: 10.1080/15384047.2019.1680057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soran A., Ozmen V., Ozbas S. Randomized trial comparing resection of primary tumor with no surgery in stage IV breast cancer at presentation: protocol MF07-01. Ann. Surg. Oncol. 2018;25:3141–3149. doi: 10.1245/s10434-018-6494-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed in this study are available from the corresponding author upon reasonable request.