Highlights
-
•
A high platelet-to-lymphocyte ratio (PLR; >124) before curative surgical resection was found to be associated with reduced disease-free survival (DFS) in non-metastatic colon cancer patients.
-
•
A high PLR was predominant among patients with circulating tumor microemboli (CTM).
-
•
This prospective study revealed a mechanism of interaction between circulating tumor cells (CTCs) and other blood components, and implicated platelets in CTC survival.
-
•
The present findings support the use of blood biomarkers to better identify and follow high-risk colon cancer patients.
Keywords: Circulating tumor cells, Circulating tumor microemboli, Colon adenocarcinoma, Platelet-to-lymphocyte ratio, Liquid biopsy
Abbreviations: ACC, accuracy; AUC, area under the curve; CEA, carcinoembryonic antigen; CRC, colorectal cancer; CTC, circulating tumor cell; CTM, circulating tumor microemboli; DFS, disease-free survival; ISET, isolation by size of tumor cells; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; ROC, receiver operating characteristic; SE, sensitivity; SP, specificity; pS, pathological stage
Abstract
Colorectal cancer is a common and often deadly cancer. Circulating tumor cells (CTCs) have been implicated as a potentially valuable prognosis factor. The detection of circulating tumor microemboli (CTM) and of simple blood component parameters that reflect inflammatory status, such as the platelet-to-lymphocyte ratio (PLR) and neutrophil-to-lymphocyte ratio (NLR), may provide information about tumor progression. The aim of this study was to explore the importance of CTCs, CTM, PLR, and NLR prospectively in non-metastatic colon cancer progression. CTCs were enriched using ISETⓇ (Isolation by SizE of Tumor cells) and identified by immunocytochemical exclusion of leukocytes. We evaluated CTCs and blood cell parameters in a cohort of 69 stage I–III colon cancer patients (52.2% men; median age, 61 years; age range, 19–87 years) at a baseline timepoint prior to resection surgery. The median of CTC levels at baseline was 20 cells/8 mL (0–94) and higher levels were associated with CTM presence (p = 0.02). CTM were found in 18 (26.1%) patients. Of 18 stage I patients, 33.3% had CTM and of 51 stages II or III patients, 13.7% had CTM (p = 0.08). Patients with a high PLR (>124) were mostly (75.6%) diagnosed with high-risk stages II/III cancer (stages I/low-risk II, 24.4%; p = 0.014). All 8 patients that had disease recurrence during follow-up had a high PLR (p = 0.02 vs. low PLR). NLR was not significantly associated with disease stage or recurrence. The present results indicate that CTCs and PLR analyses may be clinically useful for colon cancer management and risk stratification.
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer worldwide. In 2018, CRC accounted for 10.2% (1.4 million) of all neoplasms diagnosed in both genders and was the second leading cause of death, representing 9.2% of all registered cancer deaths worldwide [1]. Surgical resection is the only curative treatment modality for localized colon cancer. The goal of surgical resection of primary colon cancer is complete removal of the malignant growth together with the major associated vascular pedicles and the lymphatic drainage basin for the affected colonic segment [2].
Pathological evaluation of surgical specimens is performed to determined pathological stage (pS). Follow-up monitoring is recommended for patients with pS I and II (low-risk) disease. For patients with colon cancer with pS II or III (high-risk) disease, adjuvant treatment is indicated with 5-fluorouracil alone or in combination with other chemotherapeutic agents, such as oxaliplatin (e.g. FOLFOX therapy) [3]. Although 5-year survival rates for patients with stage I–III colon cancer range are high (71–90%), some patients experience local or distant recurrences [4]. Thus, there is a need for biomarkers that can be used to identify which patients with non-metastatic tumors are likely to be responsive or resistant to therapies.
Circulating tumor cells (CTCs) and CTC clusters, known as circulating tumor microemboli (CTM), are found in peripheral blood and play a key role in cancer recurrence and progression [5,6]. Although CTCs carry robust information about the tumor and have been studied extensively in recent years, their biology is not well understood [7,8]. CTCs are extremely scarce compared to normal blood cells, with only 1–100 CTCs being present among millions of leukocytes and billions of erythrocytes [9,10]. Other blood components can be reliable biomarkers of epithelial-to-mesenchymal cell transition and CTC colonization of distant sites as well as mediators of tumor cell crosstalk in the blood that protects them from death [11].
Given that inflammation is a hallmark of cancer, it is sensible to investigate the influence and importance of immune system elements on colon cancer prognosis. Readily accessible blood element data, such as absolute neutrophil, lymphocyte, and platelet counts, may be useful for prognostic evaluations. The aim of the present study was to examine the hypothesis that simple blood element parameters related to systemic inflammatory status, namely the platelet-to-lymphocyte ratio (PLR) and the neutrophil-to-lymphocyte ratio (NLR), may be useful for prognosis determination in colorectal cancer [12], [13], [14], [15]. We thus analyzed blood components to explore the importance of CTCs, CTM, PLR, and NLR in non-metastatic colon cancer progression.
Materials and methods
Patient recruitment
A single-center prospective longitudinal study was conducted at A.C. Camargo Cancer Center in São Paulo, Brazil, and approved by its Institutional Review Board and Ethics Committee (code: 2141/15B). The patient inclusion criteria were: age > 18 years; undergoing resection surgery for colon cancer; diagnosis confirmed by pathology; ECOG (Eastern Cooperative Oncology Group) Performance Status between 0 and 2; and written consent. The exclusion criteria were: a surgical procedure in the past week; therapy in the past 3 weeks; prior history of another carcinoma in the past 2 years. Patients with non-metastatic colon tumors were recruited at their anesthesiologist visits, and had preoperative blood draws from July 2016 to October 2019. Cases were coded to preserve patient confidentiality. Information about clinical characteristic was collected retrospectively from medical record reviews. Blood from healthy individuals (Fig. 1E) was used as negative control, and blood from healthy individuals with HCT8 cell spikes (Fig. 1F) was used as positive controls.
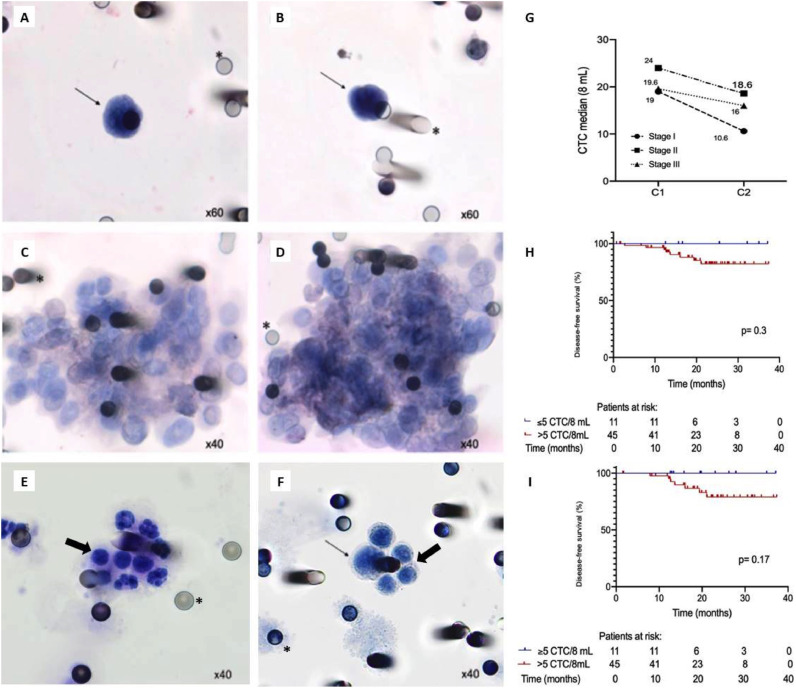
Fig. 1.
Circulating tumor cells (CTCs) isolated from non-metastatic colon cancer. Photomicrographs of CD45-immunonegative CTCs (A and B) and circulating tumor microemboli (CTM) (C and D) isolated from patient blood samples. Leukocytes isolated from a healthy individual (E) and cells from a healthy blood spiked with HCT-8 cell line (F). Thin arrows identify isolated CTCs, thick arrows indicate leukocytes, and asterisks show locations of 8-µm pores in polycarbonate membranes. (G) Median CTC counts (8 mL samples) by pathological stage (pS) at baseline (C1) and follow-up (C2). (H) Disease-free survival (DFS) in relation to CTC counts at C1 (≤5 vs. >5 CTCs/8 mL; p = 0.3). (I) DFS in relation to CTC counts at C2 (≤5 vs. >5 CTCs/8 mL; p = 0.17).
Pathological staging was conducted according to the American Joint Committee on Cancer's tumor-node-metastasis staging for colorectal cancer guidelines (7th edition) [16]. Baseline collections (C1) were performed preoperatively, Follow-up collection (C2) times differed among patients according to pS: 1–2 years for pS I; 6–9 months for low-risk pS II; and prior to commencing adjuvant chemotherapy for high-risk pS stage II and pS III (Fig. S1). Approximately 8 mL of blood was collected at each visit, and only those individuals who signed the informed consent form were enrolled in the study.
CTC isolation and identification
For each patient, 8-mL blood samples were collected in ethylenediamine tetraacetic acid tubes and maintained under gentle homogenization for up to 4 h at room temperature until being selectively filtered with ISETⓇ (Isolation by SizE of Tumor Cells; Rarecells, France), according to the manufacturer's instructions. ISET-filters are composed of 10 spots with 8-µm-diameter pores, retaining intact CTCs with diameters > 8 µm, which become negative pressure-bound to polycarbonate membranes. Following the ISET procedure, the membranes were stored at −20 °C.
CTCs were identified by light microscopy revealing negative immunocytochemical reaction to leukocyte common antigen with a hematoxylin counterstain [17]. In addition, CTCs were defined morphologically: diameter >16 µm, high nuclear-cytoplasmic ratio (≥0.8), and a hyperchromatic and irregular nucleus [18], (CD45, 1:100 CSB-PA010546; CusaBio, Wuhan, China).
Blood element counts
Laboratory data, including total neutrophil, lymphocyte, platelet counts and carcinoembryonic antigen (CEA) assays were obtained at C1 in a clinical analysis laboratory at A.C. Camargo Cancer Center. The data were used to analyze the prognostic value of the NLR and PLR. A NLR in the range of 0.78–3.53 was considered normal [19] and a PLR > 124 was considered high accordingly to the optimal cut-off value given by ROC (Receiver Operating Characteristic) curve. Preoperative infection status was determined by medical record review.
Statistical analysis
Patient characteristics were reported as absolute and relative frequencies for qualitative variables, and by median, minimum, and maximum values for quantitative variables. Data distributions were determined with Mann-Whitney U (2 variables) or Kruskall-Wallis (≥3 variables) tests. A sensitivity and specificity analysis were performed using the ROC curve to determine the optimal cut-off value for PLR at C1, and for CTCs at the two moments analyzed (C1 and C2), in order to better separate patients that had or not disease relapse. Kaplan-Meier survival curves were generated and then compared by log-rank testing. Disease-free survival (DFS) was assessed from C1 to the date of the first disease recurrence, as determined by imaging and/or biopsy. Patients who did not have a recurrence were censored on the date of their last hospital visit. The level of significance was 5% for all tests. All statistics were performed in SPSS version 24.0 (IBM, Armonk, NY), with a 5% significance criterion. Figures were constructed in GraphPad Prism version 8.2.1 (GraphPad Software, Inc., San Diego, CA).
Results
Patients
The demographic, clinical, and pathological characteristics of the study cohort of patients who underwent curative colon cancer resection (N = 69) are summarized in Table 1. Briefly, all tumors were classified histologically as adenocarcinomas, of which 85% (57/69) were moderately differentiated. The primary tumor site was in the distal colon in about two-thirds of the cases. A majority of patients (56.5%) were life-long non-smokers.
Table 1.
Demographic, clinical, and pathological characteristics of patients with non-metastatic colon cancer.
| Variable (N) | N | % |
|---|---|---|
| Total number of patients | 69 | 100 |
| Median age (range) at recruitment, years (69) | 61 (19–87) | |
| Gender, no. males/no. females (69) | 36/33 | 52.2/47.8 |
| Histological grade (67) | ||
| Mucinous adenocarcinoma | 5 | 7.5 |
| Well-differentiated adenocarcinoma | 4 | 6 |
| Moderately-differentiated adenocarcinoma | 57 | 85 |
| Poorly-differentiated adenocarcinoma | 1 | 1.5 |
| Primary tumor site (69) | ||
| Proximal colon | 23 | 33.3 |
| Distal colon | 46 | 66.7 |
| KRAS mutation status (54) | ||
| Wild-type | 35 | 64.8 |
| Mutant | 19 | 35.2 |
| NRAS mutation status (54) | ||
| Wild-type | 49 | 90.7 |
| Mutant | 5 | 9.3 |
| BRAF mutation status (47) | ||
| Wild-type | 47 | 100 |
| Mutant | 0 | 0 |
| MMR status (64) | ||
| Proficient | 57 | 89.1 |
| Deficient | 7 | 10.9 |
| Tumor size (69) | ||
| 1 | 4 | 5.8 |
| 2 | 20 | 29 |
| 3 | 37 | 53.6 |
| 4a/4b | 8 | 11.6 |
| Lymph node status (69) | ||
| N0 | 33 | 47.8 |
| N+ | 36 | 52.2 |
| pS (69) | ||
| I | 18 | 26.1 |
| IIA/IIB | 15 | 21.7 |
| IIIA/IIIB/IIIC | 36 | 52.2 |
| Therapeutic strategy (69) | ||
| Adjuvant chemotherapy | 40 | 58 |
| Follow-up | 29 | 42 |
| Median CEA at baseline, C1 (available for 65/69) | 2.5 (0.1–158) | |
| Median CEA at follow-up, C2 (available for 37/56) | 1.6 (0.6–33) | |
| Median CTC (8 mL) at baseline, C1 (available for 69/69) | 20 (0–94) | |
| Median CTC (8 mL) at follow-up, C2 (available for 56/56) | 13 (0–69) | |
| CTM at baseline, C1 (available for 69/69) | ||
| Presence | 13 | 18.8 |
| Absence | 56 | 81.2 |
| CTM at follow-up, C2 (available for 56/56) | ||
| Presence | 8 | 14.3 |
| Absence | 48 | 85.7 |
| Tumor-node-metastasis staging according to 2017 UICC guidelines. | ||
Both KRAS and NRAS mutational status (for both genes, codons 12, 13, 61, 117, 146 were evaluated) were available in 54/69 (78.3%) of the medical records, of which 19/54 (35.2%) were indicated to be KRAS mutant and 5/54 (9.3%) were indicated to be NRAS mutant. BRAF V600E was analyzed in 47/69 (68.1%) of cases, and all of them were considered as wild-type. Mismatch repair proficiency status was available in 64/69 (92.8%) of cases and only 10.1% (7 cases) had MMR-deficient tumors. None of the molecular analyses mentioned above had association with prognosis, or other biomarkers tested.
Of the 40 patients (58%) that received adjuvant chemotherapy, 6 (15%) had pS II disease and 34 (85%) had pS III disease. Among the pS II patients, about two-thirds (67%) received an adjuvant capecitabine regimen, whereas among the pS III patients, about four-fifths (79.5%) received adjuvant FOLFOX (5-fluorouracil plus leucovorin and oxaliplatin) or CAPOX (capecitabine plus oxaliplatin) regimen. The median follow-up time was 22.1 months (95% CI, 19–25.2 months). During patient follow-up, 8/69 patients (11.6%) had a cancer relapse, with a mean recurrence time of 33.7 months [95% confidence interval (CI), 31.3–36 months). Median DFS was not reached. None of patients progressed to death.
CTC and CTM evaluation
As shown in Fig. 1A and 1B, CTCs were identified by cytopathological analysis and CD45 immunonegativity (i.e. leucocyte exclusion). All morphologically characterized CTCs were CD45 immunonegative. Blood from 20 healthy individuals was used as a negative control, with a similar distribution when classified by age and sex, and none of them had CTCs (Fig. 1E). We analyzed CTCs from all 69 patients at C1 and from 56 patients at C2, due loss of follow-up (7 patients) and drop-out the study (6 patients). The detection rate was 94.2% at C1 and 94.6% at C2, and the median number of CTCs/8 mL was 20 (0–94) at C1 and 13.3 (0–69) at C2. Although the detection rate remained high at C2, a non-significant trend of a drop in CTC counts following tumor resection surgery was evident overall as well as within each pS group (Fig. 1G). For pS I, II, and III, we observed median CTC counts (in 8 mL) of 19, 24, and 19.6 at C1 and median CTC counts (in 8 mL) of 10.6, 18.6, and 16, respectively.
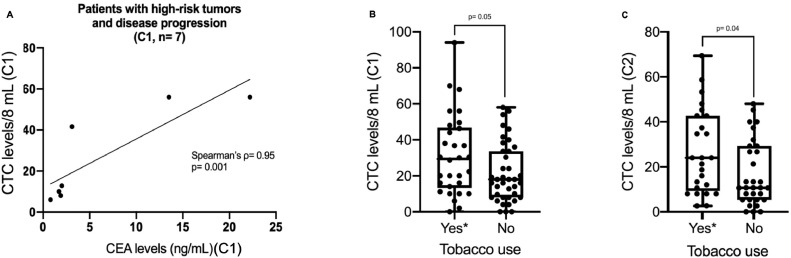
Based on ROC curve analyses (Figs. S2B and S2C), a cut-off of 5 CTCs/8 mL was chosen for both C1 (area under the curve [AUC]: 0.488, sensitivity [SE]: 100%, specificity [SP]: 11%, accuracy [ACC]: 21.7%) and C2 (AUC: 0.532, SE: 100%, SP: 18%, ACC: 25%). Patients with at or below the cut-off at C1 (p = 0.3; Fig. 1H) and at C2 (p = 0.17; Fig. 1I) both showed a tendency toward a higher DFS than those above the cut-off (DFS not calculated at either evaluation point). Among patients with high-risk tumors (submitted to adjuvant chemotherapy) who experienced disease recurrence, CTC counts correlated very significantly with CEA levels (Spearman's ρ= 0.95; p = 0.001; Fig. 2A).
Fig. 2.
CTC count comparisons across study subgroups. (A) Correlations of CTC counts with CEA at C1 in patients who had disease relapse and were treated with adjuvant chemotherapy (biomarkers data available for 7/7 cases at C1; Spearman's ρ = 0.95; p = 0.001). (B) Median CTC counts at C1 in smokers/former smokers versus in non-smokers (p = 0.05; Mann-Whitney U). (C) Median CTC counts at C2 in smokers/former smokers versus in non-smokers (p = 0.04; Mann-Whitney U). *Smokers/former smokers were grouped in the x-axis “yes” classification.
Interestingly, as shown in Fig. 2B and 2C, smokers/ex-smokers had significantly greater median CTC counts than non-smokers at both C1 (28.8 vs. 17.6 CTCs/8 mL; p = 0.05) and C2 (24 vs. 10.4 CTCs/8 mL; p = 0.04). CTM were found in 13/69 patients (18.8%) at C1 and in 8/56 patients (14.3%) at C2 (Figs. 1C and 1D). Compared to patients without CTM, patients with CTM had significantly higher distributions of CTCs at both C1 (p = 0.02) and C2 (p = 0.01). None of the morphologically characterized CTCs presented CD45 expression, nor did the healthy donors present CTCs on their membranes.
Blood element analyses
According to their medical records, none of the patients exhibited preoperative signs of infection. NLR was evaluated in 65/69 cases, and a median NLR value of 2.6 (range, 1.0–14.8) was obtained. NLRs were within the normal range in a majority of patients and NLRs were not associated with any analyzed patient characteristics, including DFS.
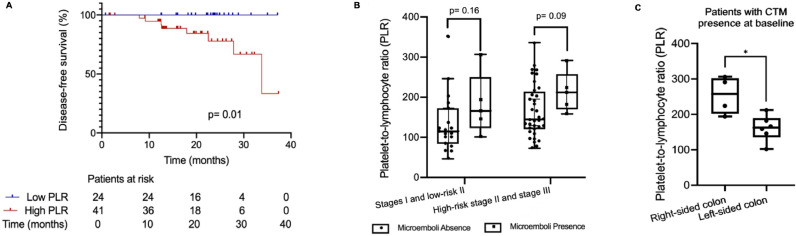
Based on ROC curve analyses (Fig. S2A), an optimal cut-off of 124 for PLR was chosen (AUC: 0.655, SE: 100%, SP: 42%, ACC: 49.2%). Patients with a high PLR (above 124) at C1 were more likely to have high-risk pS II or III disease (75.6%) than pS I or low-risk pS II disease (24.4%; p = 0.014). In addition, 90% of patients with CTM at C1 were in the high-PLR group (p = 0.11 vs. low PLR). All 8 patients who suffered disease recurrence had a high PLR (p = 0.02 vs. low PLR). Accordingly, analyzing the whole cohort, the high-PLR group had a shorter DFS than the low-PLR group (DFS time not calculated; p = 0.019; Fig. 3A). No correlation was found between the levels of PLR and CTC at baseline (p = 0.48), or at follow-up (p = 0.96). The amount of CTM as also the number of CTCs into the clusters were not correlated with the PLR values.
Fig. 3.
Platelet-to-lymphocyte ratios (PLRs) and circulating tumor microemboli (CTM) in non-metastatic colon cancer. (A) Comparison of DFS between patients with low PLRs (<124) versus those with high PLRs (≥124) at C1 (p = 0.01). (B) Distribution of PLR levels among patients with versus without CTM, by pS (stages I and low-risk II: p = 0.16; high-risk stage II and stage III). (C) Among patients with CTM (N = 10), PLRs were higher in patients with right-sided colon cancer (p = 0.01 vs. left-sided).
Analyzing patients according to disease stage, we found that among pS I patients, 80% of the patients with CTM at C1 had a high PLR (p = 0.11 vs. low PLR). Meanwhile, among patients with pS II or III disease, 100% of the patients with CTM at C1 had a high PLR (p = 0.31 vs. without CTM). Patients with pS I or low-risk pS II disease and no evident CTM had a median PLR of 115. Patients who were diagnosed with the same disease stage but with CTM had a median PLR of 166 (p = 0.16 vs. no CTM). Among patients with high-risk stage II/III disease, the median PLR was 212 among those with CTM but only 145 among those without CTM (p = 0.09; Fig. 3B). Among patients with CTM, the PLR was significantly greater in patients with right-sided tumors (p = 0.019 vs. left-sided; Fig. 3C).
Discussion
Because immune system homeostasis plays a central role in disease responses, immune deregulation can enable the development of several diseases [20,21], especially cancer [22,23]. However, immune system involvement with tumor components in blood has been only recently explored [11,24,25]. In this study, we obtained data suggesting that a greater value for the immune parameter PLR may indicate a worse prognosis, particularly when evaluated together with CTCs, and clinical characteristics. The presently observed tendency for a higher PLR distribution in patients with CTM, even in early stages of colon cancer, supports the hypothesis that there may be signaling between CTCs and platelets. Furthermore, we found that patients with a high PLR (>124) had a significantly lower DFS than those with a low PLR, demonstrating an interaction between platelet ratio and cancer recurrence. These findings are consistent with the findings of a prior meta-analysis of 26 studies (13,964 tumors of various types) showing that a high PLR is predictive of worse OS overall (hazard ratio, 1.60; 95% CI, 1.35–1.90) as well as for colorectal cancer specifically (hazard ratio,1.65; 95% CI, 1.33–2.05) [26]. This meta-analysis [23] and other studies [14,15,27] presented a dissimilarity in relation to the cut-off value of PLR, and most of them calculate and consider as “high PLR” according to the cohort that is being studied, results that show an agreement with our data. Concerning the NLR results obtained here, it was noticed a discrepancy when compared to the literature [12,28,29]. Probably, our unfavorable results in relation to NLR can be justified by the small sample size, heterogeneity of resectable colon cancer stages, and short follow-up time. Hence, accordant results could be obtained in a larger cohort study.
Using the ISET method, we obtained a high rate of CTC detection at both C1 (94.2%) and C2 (94.6%). High CTC detection rates (83% for colorectal cancer and 89% for colon cancer specifically) have also been reported with a MetacellⓇ filtration protocol [30]. The present study demonstrated, for the first time to our knowledge, significantly higher CTC counts in smokers/former smokers than in non-smokers at baseline and follow-up evaluation points.
The presently determined ROC curve-based cut-off separating patients with versus without cancer recurrence (5 CTCs/8 mL for C1 and C2), albeit without a statistically significant difference, was similar to the cut-off reported recently by Baek et al. [31] using another methodology (5 CTCs/7.5 mL). They did not obtain a significant difference in progression-free survival or overall survival between groups divided by this cut-off (p = 0.33). Using the CmX platform (a biomimetic lipid-coated microfluidics system for isolating viable CTCs/CTM), Tsai et al. [32] demonstrated that distant metastasis development in non-metastatic patients was likely in patients with ≥5 CTCs/2 mL than in patients with a lesser CTC presence.
Because we used an antigen-independent cell-isolation method, the CTCs that we isolated, even with all the cytopathological criteria of malignancy, may have heterogeneous capacities and biological mechanisms depending on their clone of origin in the primary tumor. Such heterogeneity may help to explain the high numbers of CTCs found in pS I. In addition, CTC presence can precede metastasis development by months, or even years. Using ISET to analyze the peripheral blood of 168 patients, Ilie et al. detected CTCs in 5/168 patients (3%) with chronic obstructive pulmonary disease, but without a lung cancer diagnosis, and then found that these 5 patients developed pulmonary nodules 1–4 years later, making early resection of these nodules possible and thus providing a long-term cure [33].
There remains a need to identify a set of blood markers that can be used to determine whether tumor resection surgery has been curative or not and thus to guide chemotherapy treatment planning, including monitoring of treatment effects on CTC levels. Available antigen-based CTC detection methods do not yield consistent results and have a lower CTC detection range than antigen-independent methods. Krebs et al. [18] evaluated 40 lung cancer patients with two technologies: CellSearchⓇ (Menarini Silicon Biosystem), which detects two epithelial markers (cytokeratin and EpCAM expression), and ISET. CTCs were found in 9/40 patients (23%) with CellSearch and found in 32/40 patients (80%) with ISET[18], suggesting that the former methodology is prone to false negative results [34].
In conclusion, in this study, we showed that it is feasible to explore the implications of CTCs and CTM in patients with non-metastatic colon cancer with the easy-to-use free-labeling ISET method. Additionally, the present results are suggestive of a possible interaction of CTCs with platelets. The parameters obtained with this method may be clinically useful for improving follow-up monitoring of patients at high risk of cancer or cancer recurrence. Certainly, this is a pilot study that includes patients with different stages of colon cancer and needs to be validated in larger cohorts.
Declaration of Competing Interest
The authors have declared no conflict of interest.
Acknowledgments
Funding
This work was supported by a grant from São Paulo Research Foundation (FAPESP; Grant: 2016/18786–9) and from Labour Public Ministry (TAC-MP-PAJ 000968.2012.10.000/0); EAA had a PhD fellowship from FAPESP (2015/16952–6); ACB is a PhD student with a fellowship from FAPESP (Grant: 2019/18100–8); JGRT had a M.Sc. fellowship from the Brazilian National Council for Scientific and Technological Development (CNPq; Grant: 190144/2017–3).
Data availability statement
Data underlying the findings of this study are available from the corresponding author upon reasonable request.
Author contributions
Conceptualization, LTDC; Data curation, LTDC; Formal analysis, EAA; Funding acquisition, MST; Investigation, EAA, VSS, ACB, JGRT, RMT, SAJ and LTDC; Methodology, EAA, ACB, JGRT; Project administration, EAA and LTDC; Resources, VAG and BECK; Supervision, LTDC; Writing – original draft, EAA; Writing – review & editing, VSS and LTDC.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2020.100932.
Appendix. Supplementary materials
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Vogel J.D., Eskicioglu C., Weiser M.R., Feingold D.L., Steele S.R. The American society of colon and rectal surgeons clinical practice guidelines for the treatment of colon cancer. Dis. Colon Rectum. 2017;60:999–1017. doi: 10.1097/DCR.0000000000000926. [DOI] [PubMed] [Google Scholar]
- 3.André T., Boni C., Navarro M., Tabernero J., Hickish T., Topham C. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J. Clin. Oncol. 2009;27:3109–3116. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 4.American Cancer Society. Colorectal Cancer 2020.
- 5.Eslami-S Z., Cortés-Hernández L.E., Alix-Panabières C. Circulating tumor cells: moving forward into clinical applications. Precis. Cancer Med. 2020;3:4. doi: 10.21037/pcm.2019.11.07. 4. [DOI] [Google Scholar]
- 6.Aceto N. Bring along your friends: homotypic and heterotypic circulating tumor cell clustering to accelerate metastasis. Biomed. J. 2020;43:18–23. doi: 10.1016/j.bj.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joosse S.A., Gorges T.M., Pantel K. Biology, detection, and clinical implications of circulating tumor cells. EMBO Mol. Med. 2015;7:1–11. doi: 10.15252/emmm.201303698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mamdouhi T., Twomey J.D., McSweeney K.M., Zhang B. Fugitives on the run: circulating tumor cells (CTCs) in metastatic diseases. Cancer Metastasis Rev. 2019;38:297–305. doi: 10.1007/s10555-019-09795-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Toom E.E., Verdone J.E., Gorin M.A., Pienta K.J. Technical challenges in the isolation and analysis of circulating tumor cells. Oncotarget. 2016;7:62754–62766. doi: 10.18632/oncotarget.11191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong B., Zu Y. Detecting circulating tumor cells: current challenges and new trends. Theranostics. 2013;3:377–394. doi: 10.7150/thno.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heeke S., Mograbi B., Alix-Panabières C., Hofman P. Never travel alone: the crosstalk of circulating tumor cells and the blood microenvironment. Cells. 2019:8. doi: 10.3390/cells8070714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dimitriou N., Felekouras E., Karavokyros I., Alexandrou A., Pikoulis E., Griniatsos J. Neutrophils to lymphocytes ratio as a useful prognosticator for stage II colorectal cancer patients. BMC Cancer. 2018;18:1202. doi: 10.1186/s12885-018-5042-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Z., Zhao R., Cui Y., Zhou Y., Wu X. The dynamic change of neutrophil to lymphocyte ratio can predict clinical outcome in stage I-III colon cancer. Sci. Rep. 2018:8. doi: 10.1038/s41598-018-27896-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato R., Oikawa M., Kakita T., Okada T., Abe T., Yazawa T. The prognostic value of the prognostic nutritional index and inflammation-based markers in obstructive colorectal cancer. Surg. Today. 2020;50:1272–1281. doi: 10.1007/s00595-020-02007-5. [DOI] [PubMed] [Google Scholar]
- 15.Guo D., Li X., Xie A., Cao Q., Zhang J., Zhang F. Differences in oncological outcomes and inflammatory biomarkers between right-sided and left-sided stage I-III colorectal adenocarcinoma. J. Clin. Lab. Anal. 2020;34:e23132. doi: 10.1002/jcla.23132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edge S.B., Compton C.C. The American joint committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 17.Abdallah E.A., Braun A.C., Flores B.C.T.C.P., Senda L., Urvanegia A.C., Calsavara V. The potential clinical implications of circulating tumor cells and circulating tumor microemboli in gastric cancer. Oncologist. 2019 doi: 10.1634/theoncologist.2018-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krebs M.G., Hou J.-.M., Sloane R., Lancashire L., Priest L., Nonaka D. Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches. J. Thorac. Oncol. 2012;7:306–315. doi: 10.1097/JTO.0b013e31823c5c16. [DOI] [PubMed] [Google Scholar]
- 19.Forget P., Khalifa C., Defour J.-.P., Latinne D., Van Pel M.-.C., De Kock M. What is the normal value of the neutrophil-to-lymphocyte ratio? BMC Res. Notes. 2017;10:12. doi: 10.1186/s13104-016-2335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Godbout J.P., Glaser R. Stress-induced immune dysregulation: implications for wound healing, infectious disease and cancer. J. Neuroimmune Pharmacol. 2006;1:421–427. doi: 10.1007/s11481-006-9036-0. [DOI] [PubMed] [Google Scholar]
- 21.Chovatiya R., Medzhitov R. Stress, inflammation, and defense of homeostasis. Mol. Cell. 2014;54:281–288. doi: 10.1016/j.molcel.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Visser K.E., Eichten A., Coussens L.M. Paradoxical roles of the immune system during cancer development. Nat. Rev. Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez H., Hagerling C., Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. 2018;32:1267–1284. doi: 10.1101/gad.314617.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szczerba B.M., Castro-Giner F., Vetter M., Krol I., Gkountela S., Landin J. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature. 2019;566:553–557. doi: 10.1038/s41586-019-0915-y. [DOI] [PubMed] [Google Scholar]
- 25.Guo B., Oliver T.G. Partners in crime: neutrophil–CTC collusion in metastasis. Trends Immunol. 2019;40:556–559. doi: 10.1016/j.it.2019.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou X., Du Y., Huang Z., Xu J., Qiu T., Wang J. Prognostic value of PLR in various cancers: a meta-analysis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0101119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu C., Gao P., Yang Y., Chen X., Wang L., Yu D. Prognostic evaluation of platelet to lymphocyte ratio in patients with colorectal cancer. Oncotarget. 2017;8:86287–86295. doi: 10.18632/oncotarget.21141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He W.-.Z., Hu W.-.M., Kong P.-.F., Yang L., Yang Y.-.Z., Xie Q.-.K. Systemic neutrophil lymphocyte ratio and mismatch repair status in colorectal cancer patients: correlation and prognostic value. J. Cancer. 2018;9:3093–3100. doi: 10.7150/jca.26669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ozdemir Y., Akin M.L., Sucullu I., Balta A.Z., Yucel E. Pretreatment neutrophil/lymphocyte ratio as a prognostic aid in colorectal cancer. Asian Pac. J. Cancer Prev. 2014;15:2647–2650. doi: 10.7314/apjcp.2014.15.6.2647. [DOI] [PubMed] [Google Scholar]
- 30.Eliasova P., Pinkas M., Kolostova K., Gurlich R., Bobek V. Circulating tumor cells in different stages of colorectal cancer. Folia Histochem. Cytobiol. 2017;55:1–5. doi: 10.5603/FHC.a2017.0005. [DOI] [PubMed] [Google Scholar]
- 31.Baek D.H., Kim G.H., Song G.A., Han I.S., Park E.Y., Kim H.S. Clinical potential of circulating tumor cells in colorectal cancer: a prospective study. Clin. Transl. Gastroenterol. 2019;10:e00055. doi: 10.14309/ctg.0000000000000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsai W.-.S., Chen J.-.S., Shao H.-.J., Wu J.-.C., Lai J.-.M., Lu S.-.H. Circulating tumor cell count correlates with colorectal neoplasm progression and is a prognostic marker for distant metastasis in non-metastatic patients. Sci. Rep. 2016;6:24517. doi: 10.1038/srep24517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ilie M., Hofman V., Long-Mira E., Selva E., Vignaud J.-.M., Padovani B. “Sentinel” circulating tumor cells allow early diagnosis of lung cancer in patients with chronic obstructive pulmonary disease. PLoS One. 2014;9 doi: 10.1371/journal.pone.0111597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raimondi C., Gradilone A., Naso G., Cortesi E., Gazzaniga P. Clinical utility of circulating tumor cell counting through CellSearch(Ⓡ): the dilemma of a concept suspended in Limbo. Onco Targets Ther. 2014;7:619–625. doi: 10.2147/OTT.S46200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data underlying the findings of this study are available from the corresponding author upon reasonable request.