Abstract
We review herein infections by Conidiobolus spp., Cryptococcus spp., and Aspergillus spp. in sheep and goats. Conidiobolus spp. are common causes of rhinitis in sheep and are less frequent in goats, in which Conidiobolus spp. also cause skin lesions. Cryptococcus spp. cause rhinitis, meningitis, encephalitis, and pneumonia in goats, and are rarely observed in sheep. Aspergillus spp. may cause rhinitis in goats, and pneumonia and mastitis in sheep and goats. Gross and microscopic lesions caused by these 3 fungal infections may be similar to each other. The diagnosis of these diseases must be based on gross and microscopic lesions, coupled with detection of the agent by immunohistochemical, molecular, and/or culture-based methods.
Keywords: Aspergillus spp., Conidiobolus spp., Cryptococcus spp., goats, sheep
Introduction
The frequency of fungal infections in humans has been increasing in several parts of the world since the 1990s.10,11,20,21,37,54,56,60,64 In veterinary medicine, fungal diseases tend to be underestimated and are often misdiagnosed, probably because of the difficulties in the ability of some laboratories to culture fungal agents. Approximately 1.5 million species of fungi are known to be responsible for the natural degradation of biomass of the planet, but few of these are pathogenic for animals.12 However, environmental and climate changes, usually of anthropogenic origin, are promoting the emergence of fungal pathogens in humans, domestic and wild animals, and plants.6,21,52 In some countries, such as Brazil, there has been a significant increase in the number of diagnoses of diseases caused by fungi in goats and sheep.7,10,22,47,54,59,69
We review herein the main features of conidiobolomycosis, cryptococcosis, and aspergillosis in sheep and goats. We searched electronic databases, including CAB Abstracts, Medline, PubMed, Web of Science, Scopus, and Google Scholar, for original research articles and reviews using the following key words: conidiobolomycosis, Conidiobolus, aspergillosis, Aspergillus, cryptococcosis, Cryptococcus, sheep mycosis, and goat mycosis. Additional publications were identified by checking the references included in the articles found in those searches. We selected these 3 diseases because they may occur with similar clinical and pathologic characteristics, and diagnosticians should be aware of their similarities to establish an accurate diagnosis.
Conidiobolomycosis
Etiology and epidemiology
The genus Conidiobolus (Entomophthorales, Ancylistaceae)29 inhabits tropical and subtropical regions, and can be found as a saprobe in decaying vegetation and soil.58 Conidiobolus coronatus, C. incongruous, and C. lamprauges cause conidiobolomycosis, which is characterized by granulomatous inflammation of the nose and facial subcutaneous tissue, in immunocompetent humans,58 dogs,27 horses,30 sheep,7,22,45,48,50,60,72 and goats (Pedroso P.M.O., unpublished data).
In sheep, conidiobolomycosis was first described in Australia in 1992; during a 3-mo period, 700 sheep from 52 farms died of this disease after a clinical course of 7–10 d.11 Ten years later, the disease was reported in a sheep in Trinidad and Tobago.45 In Brazil, conidiobolomycosis in sheep was misdiagnosed as enzootic ethmoidal tumor until 2007.60 Conidiobolomycosis has been reported in sheep in midwestern7,70 and northeastern9,44,50,53,55,60,61 Brazil, with a morbidity of up to 6% and a lethality of 100%. Sporadic cases have been also reported in southern Brazil22,48 and in Uruguay.57 Conidiobolomycosis is endemic in northeastern Brazil (annual rainfall of 1,000–1,600 mm, temperature of 19–36°C, and relative humidity of 40–80%).61 In this region, 25 sheep flocks with conidiobolomycosis were systematically monitored for 3 y; prevalence was up to 12.5% in individual farms, average annual incidence was 2.8%, and 91% of the cases occurred during the rainy and warm period of the year.61
High humidity and temperature promote the growth of Conidiobolus spp. in soil and decaying vegetation, which may explain the higher incidence of outbreaks during rainy summers,22,61 especially in sheep kept in flooded areas.7,48,53 However, in the semiarid Brazilian region of Paraíba, where the annual rainfall is 350–800 mm, the average relative humidity is 50%, and there is a long dry season, the disease has been observed frequently even during the dry season.1 In these cases, overcrowding of sheep around ponds during periods of drought has been suggested as an important risk factor for conidiobolomycosis.1 Also, decaying vegetation around ponds provides ideal conditions for the growth of saprobes, including Conidiobolus spp.1 These climate and environmental conditions in some Brazilian tropical wet and semiarid regions may explain why conidiobolomycosis has being reported mainly in Brazil7,9,44,50,53,55,60,61,70 and rarely in other countries.11,45,57
The habit of grazing close to the ground has been suggested as a cause of high susceptibility of sheep to conidiobolomycosis, given that this would increase the chances of inhalation of fungal conidia from the soil or decaying vegetation.61 It has also been speculated that the conidia may enter the nasal mucosa through wounds generated by thorny plants providing traumatic implantation of conidia present on the surface of the plants or from the surrounding environment.34
Conidiobolomycosis was reported in 2 adult goats in the state of Bahia, Brazil (Pedroso P.M.O., unpublished data). To our knowledge, no reports of the disease in goats have been published.
Clinical signs
Clinically, conidiobolomycosis has 2 forms in sheep: (1) rhinopharyngeal, the most common, which affects mainly the ethmoid region, and (2) rhinofacial, which is less common and affects the nasal vestibulum and the skin of the nose.60,70 Clinical signs of both forms of the disease include serous or mucous nasal discharge, fever, apathy, anorexia, weight loss, and marked respiratory distress.7,22,55,60,61,70 In the rhinopharyngeal form, the expansion of the inflammation to one of the eye orbits may result in unilateral exophthalmos, which is often associated with marked craniofacial asymmetry and ulcerative keratitis (Fig. 1A, 1B). In addition, when inflammation invades the frontal lobe of the brain, sheep typically develop neurologic signs, including obtundation, circling, abnormal head postures, and head pressing.7,60,61 In goats, clinical signs include serous nasal discharge, dyspnea, weight loss, and dermatitis in the limbs and ears. Neither craniofacial asymmetry nor exophthalmos have been described in goats (Pedroso P.M.O., unpublished data). The clinical course of the disease is ~ 3 mo in both sheep and goats. Despite the success of treatment of conidiobolomycosis in humans,20 horses,63 and cattle,66 treatment of affected sheep in Brazil has not been effective, probably because the disease is frequently diagnosed when the lesions are very advanced.61 No information on treatment of goats with conidiobolomycosis is available.
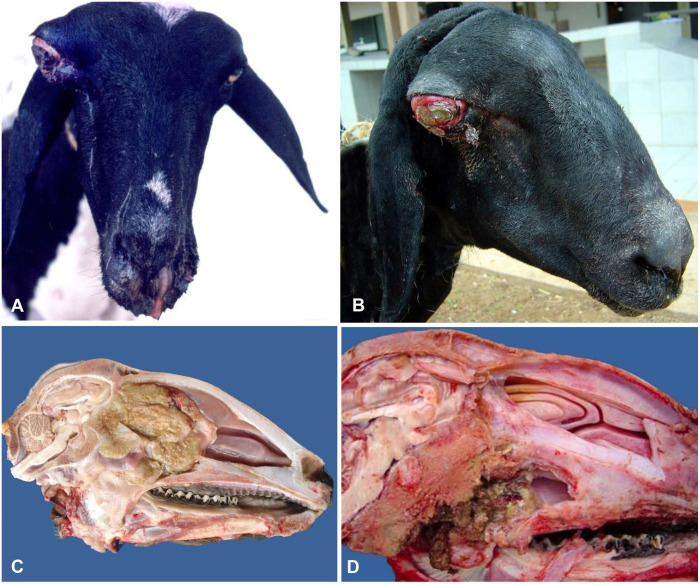
Figure 1.
Conidiobolomycosis in sheep. A. Nasal discharge and unilateral exophthalmos with marked craniofacial asymmetry and ulcerative keratitis. Published with permission of Vet Pathol.60 B. Unilateral exophthalmos with severe conjunctivitis and ulcerative keratitis. Reprinted with permission of Braz J Vet Res.61 C, D. Mid-sagittal section of heads showing a large yellow and firm mass in the caudal part of the nasal cavity (C) and pharynx (D).
Gross changes
In the ovine rhinopharyngeal form, the main gross lesions of conidiobolomycosis are localized in the ethmoid region, pharynx, and/or the nasal turbinates, and are characterized by focally extensive, yellow, firm irregular masses with occasional friable dark foci (Fig. 1C, 1D). Usually, the lesion extends to the orbit, cribriform plate, and nasal sinuses.7,22,55,60,70 In rare cases, the lesion may affect the rhinofacial region producing swelling of the nose, upper lip, and skin of the face, and ulceration of the hard palate. In these cases, a sagittal section of the head reveals a white-yellow mass that extends from the mucocutaneous junction of the nostrils to the mid-portion of the nasal cavity, and involves subcutaneous tissue, nasal vestibule, turbinates, and hard palate.70
Hematogenous spread of Conidiobolus spp. occurs occasionally. The lungs and retropharyngeal and parotid lymph nodes are the main organs involved, although other lymph nodes, liver, intestine, kidneys, and gallbladder can also be affected.53,60,70 The lymph nodes are enlarged, yellow and firm, with loss of corticomedullary definition.7,22,53,60,70 In other organs, the lesions consist of granulomas.7,22,53,55,60,70
In goats, the gross lesions are similar to those of the rhinofacial form of conidiobolomycosis in sheep. In addition, nodules are observed occasionally in the skin of the limbs, and in the internal and external region of the pinna (Pedroso P.M.O., unpublished data).
Microscopic findings
Histologically, the lesions are similar in sheep and goats, regardless of the organ affected, and consist of pyogranulomas, with eosinophilic central areas of necrosis with a small number of neutrophils and eosinophils, and surrounded by epithelioid macrophages, multinucleate giant cells, lymphocytes, and plasma cells. In hematoxylin and eosin (H&E)-stained sections, unstained hyphae, often surrounded by Splendore–Hoeppli reaction, can be seen in the central areas of necrosis (Fig. 2A). The hyphae are 5–30 µm diameter, irregularly shaped, thin-walled, laterally branched, rarely septate, and may have bulbous apical dilations.53,55,60,70 The hyphae stain well with Grocott methenamine silver (GMS; Fig. 2B) and other silver stains, but do not stain or stain weakly with periodic acid–Schiff (PAS).70 Occasionally, vasculitis and thrombi are associated with fungal vascular invasion. In the turbinates and cribriform plate, the bone undergoes osteoclastic resorption and replacement by fibrous tissue.52,55,60,70
Figure 2.
Conidiobolomycosis in goats. A. Granulomatous exudate in the nasal vestibulum with a necrotic center and non-stained hyphae. B. Grocott methenamine silver stain showing typical Conidiobolus spp. hyphae.
Cryptococcosis
Etiology and epidemiology
At least 322 species of the genus Cryptococcus (Tremellales, Agaricomycotina) have been described.31 However, only Cryptococcus neoformans (var. neoformans and var. grubii) and C. gattii have been described as causing disease in humans and domestic animals.40,59 Since 2014, a nomenclature change grouped the pathogenic species of Cryptococcus for humans into the C. neoformans species complex. This complex includes 7 species: C. neoformans (previously known as var. grubii), C. deneoformans (previously known as var. neoformans), C. gattii, C. bacillisporus, C. deuterogattii, C. tetragattii, and C. decagattii,31 with the last 5 species identified previously as C. gattii.31 For consistency and to reflect the evolving nature of fungal taxonomy, we have retained the nomenclature used in publications prior to these taxonomic revisions.
The pathogenic species of Cryptococcus are the only species of the order Tremellales able to grow well at temperatures > 30°C,51 and their capacity to grow at 37°C is one of their main virulence factors.37 The host’s inability to produce an effective cell-mediated immune response to Cryptococcus spp. has been attributed to the antiphagocytic and immunosuppressive action of the organism’s polysaccharide capsule, which prevents recognition of yeast by phagocytes and blocks migration of leukocytes to the site of fungal replication.15 Humans infected by strains with poorly developed capsules demonstrate an active cell-mediated immune response, which suggests a negative correlation between capsular production capacity and inflammatory response.47 Synthesis of melanin by Cryptococcus spp. also protects them from the damage caused by antioxidants produced by the host.8,37
Inhalation of blastospores has been described as the primary route of infection for C. neoformans and C. gattii in humans and animals.8,13 Thus, the respiratory system is primarily affected, but depending on the immune status of the patient and the virulence of the infecting strain, the infection may be disseminated to other organs, particularly the central nervous system.13,23,24,28,47,56
Infections by C. neoformans and C. gattii have different epidemiologic features. C. neoformans is ubiquitous in the environment, usually affects immunocompromised individuals, and is often isolated from the feces of clinically healthy birds, mainly pigeons, but has also been found in soil and in decaying vegetation.8,26,69 C. gattii may affect healthy individuals, mainly in subtropical and tropical regions.35,69 However, there have been reports of infection by this fungus in temperate regions, including Canada,38,64 Europe,71 and the northwestern United States.38 C. gattii has been isolated from several species of trees,26 including Eucalyptus camaldulensis and E. tereticornis.19 In Brazil, C. gattii has been isolated from trees of the genera Cassia, Ficus, Guettarda, Erythrina, and Licania.69
Only a few reports of cryptococcosis in sheep have been published.36,62 In Brazil, C. neoformans var. neoformans was isolated from pulmonary lesions of a clinically healthy sheep at slaughter.36 Also in Brazil, rhinitis caused by Cryptococcus sp. (species and subspecies not determined) was described in a 2-y-old Santa Inês sheep that developed a nasal granuloma 10 d after a traumatic head injury.62
In goats, cryptococcosis outbreaks occurred between 1990 and 1994 in 5 herds under extensive grazing conditions in Extremadura, Spain. All affected animals had severe subacute-to-chronic (2–6 wk clinical course) respiratory disease, and in 3 of the 5 affected herds, concomitant neurologic signs were observed. The percentage of affected animals was 2.5–12% with 100% lethality.3 It was suggested that the wide distribution of this pathogen in this temperate region was a result of extensive reforestation with Eucalyptus spp., especially E. camaldulensis,17 which is a natural habitat for C. gattii.3,17 Sporadic cases of cryptococcosis in goats have also been associated with granulomatous rhinitis, pneumonia, meningitis, and dermatitis of the head.14,39,65
In Brazil, cryptococcosis has been reported in goats affecting the lung58 (Pimentel L, 2012, Federal University of the Recôncavo Baiano, Bahia, Brazil; unpublished data) and brain (Pimentel L, 2012, unpublished data).
Clinical signs
Sheep and goats with nasal cryptococcosis may have swelling of the nasal region, with abundant granulation tissue and hemorrhagic exudate in the nostrils (Fig. 3A) and obstruction of the nostrils resulting in severe dyspnea, usually accompanied by purulent nasal discharge.62 Although these are the only clinical signs reported in sheep,36 goats may have severe respiratory disease, including cough, anorexia, fever, and severe weight loss.3,14,59 Neurologic signs may also be observed in goats.3,65 The infection is subacute-to-chronic, with a clinical course of 2–6 wk in goats and sheep.3,62
Figure 3.
Cryptococcosis in sheep and goats. A. Sheep with swollen right nasal region, and granulation tissue and hemorrhagic exudate in the right nostril. Reprinted with permission of Braz J Vet Pathol.62 B. Large yellow, gelatinous, cystic mass in the lung of a goat. C. Brain of a goat with bilateral and symmetrical cystic and gelatinous lesions in the corpus striatum. D. Lung of a sheep with round-to-crescentic, lightly stained or non-staining, 5–20 µm diameter yeast, with thin walls and surrounded by a clear, 5–10 μm wide halo. H&E. Photos C and D from Luciano Pimentel, Universidad Federal do Recôncavo Baiano, Brazil.
Gross findings
Grossly, cryptococcosis is characterized by white or yellow focal lesions, which are similar regardless of the organ or animal species affected (Fig. 3B, 3C). The main organs affected in goats are lungs, brain, nasal cavity, and the skin of the face.3,14,59,65 In sheep, gelatinous masses with polypoid nodules are reported only in the nasal cavities.62
Microscopic findings
Histologically, lesions of cryptococcosis vary from severe granulomatous inflammation with intralesional capsulated blastospores, to accumulations of numerous capsulated blastospores with few inflammatory cells, and tissue necrosis resulting from compression caused by the large masses of blastospores.28,47 The blastospores are spherical-to-oval, 5–10 µm diameter, encapsulated, with narrow-based budding. The polysaccharide capsule forms a clear halo around the blastospores, which gives the organism a characteristic aspect of “soap bubbles” in sections stained with H&E (Fig. 3D), PAS, and GMS.28 India ink staining demonstrates the encapsulated yeast cells in fluids and/or pus samples. The capsule can be stained with Mayer mucicarmine or alcian blue.47 In addition, because Cryptococcus spp. synthesize melanin, the blastospores may also be stained with Fontana–Masson38,47; this technique can be used to identify capsule-deficient strains, which may give false-negative results in sections stained with Mayer mucicarmine or alcian blue.47
Aspergillosis
Etiology and epidemiology
Fungi of the genus Aspergillus (Eurotiales, Trichocomaceae) are saprobes that are widely distributed in nature. Spread occurs via aerosols of spores present in soil, decaying vegetation, and occasionally animal tissues.5 There are ~ 250 species within the genus Aspergillus,16 but only a few of them are commonly associated with infection, mainly A. fumigatus, but A. niger, A. flavus, A. terreus, and A. nidulans are opportunistic pathogens of humans and/or animals.18,32,33,67 The hyphae of Aspergillus spp. are narrow (3–12 μm wide) and septate, with dichotomous branching and parallel borders. They stain with PAS and GMS and can also be seen in H&E-stained sections. Conidiophores, a specialized hyphal structure of some fungi that produce conidia (asexually produced spores borne externally to the cells), can be observed in highly oxygenated tissues, such as those of the respiratory system.28
Aspergillus spp. may cause infections in a variety of domestic animals. Disease produced by these fungi includes sinonasal and sino-orbital aspergillosis in cats and dogs, guttural pouch mycosis and keratitis in horses, and gastroenteritis, pneumonia, mastitis, and abortion in ruminants.18,32 Pneumonia produced by Aspergillus spp. has been described occasionally in sheep and goats, preferentially in young, feedlot-confined animals.25,41,42,46 The use of antimicrobials for prolonged periods, concomitant chronic diseases, and eating moldy feed are factors that have been implicated in pulmonary aspergillosis in ruminants.41 Mastitis produced by Aspergillus spp. has also been described in goats and sheep.33 In the semiarid region of Paraíba, Brazil, a case of cutaneous and nasal aspergillosis caused by A. niger was described in a previously healthy goat.10 Nasal aspergillosis has not been reported in sheep to date.
Clinical signs
Pulmonary aspergillosis in sheep and goats is characterized clinically by apathy, anorexia, cough, dyspnea, and nasal discharge.2,41 In goats, a nasal form of aspergillosis may occur, and is characterized by loss of condition associated with necrosis of the nasal mucosa and nasal turbinate bones, which may result in severe dyspnea. Cutaneous nodules, 0.3–3 cm diameter, occasionally ulcerated, may be observed in the dorsal nasal region and ears (Fig. 4A, 4B). Areas of depigmentation, like those observed in nasal aspergillosis in dogs, may be observed in the affected nostrils10 and have been attributed to the action of fungal toxins.49
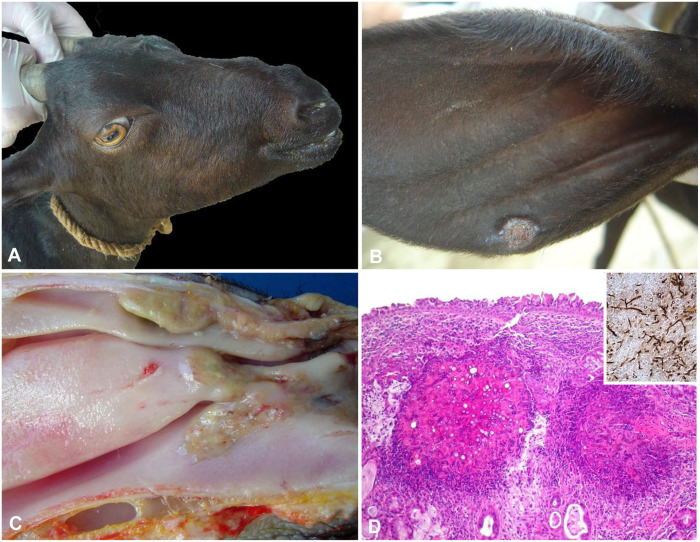
Figure 4.
Aspergillosis in goats. A. Nodular lesions in the nose. B. A nodular ulcerated lesion in the pinna. Reprinted with permission of J Comp Pathol.10 C. Ulcerated areas covered by yellow exudate in the nasal vestibule, nasal turbinates, nasal meatus, and nasal septum. D. Granulomatous exudate in the submucosa with necrotic foci containing numerous non-stained hyphae. Inset: hyphae stained by Grocott methenamine silver stain, H&E. Reprinted with permission of J Comp Pathol.10
Gross findings
Pulmonary aspergillosis in sheep and goats is characterized by granulomatous pneumonia.46 In the nasal cavity of goats, there are irregular and bilateral yellow masses than can affect the nostrils, nasal vestibule, nasal turbinates, nasal meatus, and nasal septum (Fig. 4C). Destruction of the turbinate bones, similar to the lesion in dogs with nasal aspergillosis,4,49 may also occur.10 Such lesions have also been attributed to the action of fungal toxins.67
Microscopic findings
The hallmark histologic lesions of aspergillosis in all organs affected are granulomas composed of a necrotic center containing hyphae with morphology compatible with Aspergillus spp. surrounded by neutrophils, macrophages, lymphocytes, and multinucleate giant cells (Fig. 4D).41
Diagnosis
A presumptive diagnosis of conidiobolomycosis, cryptococcosis, or aspergillosis can be based on macroscopic and histologic lesions. Confirmation, however, depends on detection of the agent by immunohistochemical, molecular, and/or culture-based methods, which should be associated with the presence of the agent within the histologic lesions. The use of pan-fungal PCR on animal tissues is an alternative diagnostic approach43; however, the generic nature of this PCR increases the potential for amplification of environmental DNA and should be treated with caution if used in isolation. Serologic detection has been used for the diagnosis of several fungal infections, mainly in humans.54,68 Fluorescent antibody and agglutination tests may be used to detect cryptococcal antibodies. Cryptococcal antigens may be detected in serum and/or cerebrospinal fluids by latex agglutination test, enzyme immunoassay, and lateral flow immunoassay.68 Immunodiffusion, complement fixation, and ELISA can be used to detect antibodies against Aspergillus spp.54
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Pedro M. O. Pedroso  https://orcid.org/0000-0001-9024-9149
https://orcid.org/0000-0001-9024-9149
Franklin Riet-Correa  https://orcid.org/0000-0001-5738-7785
https://orcid.org/0000-0001-5738-7785
Contributor Information
Priscila Maria Silva do Carmo, Hospital Veterinário, Centro de Saúde e Tecnologia Rural (CSTR), Universidade Federal de Campina Grande (UFCG), Bairro Santa Cecília, Patos, Brazil.
Francisco A. Uzal, California Animal Health and Food Safety Laboratory, University of California–Davis, San Bernardino, CA
Pedro M. O. Pedroso, Laboratório de Patologia Veterinária, Faculdade de Veterinária, Universidade Nacional de Brasília, Brasília, Brazil
Franklin Riet-Correa, Instituto Nacional de Investigación Agropecuaria (INIA), Plataforma de Investigación en Salud Animal, Estación Experimental INIA La Estanzuela, Colonia, Uruguay.
References
- 1. Aguiar GMN, et al. Aspectos epidemiológicos da conidiobolomicose em ovinos na região semiárida do nordeste do Brasil [Epidemiologic aspects of conidiobolomycosis in sheep in the northeastern Brazilian semiarid region]. Cienc Rural 2014;44:2210–2216. Portuguese. [Google Scholar]
- 2. Austwick PK, et al. Pulmonary aspergillosis in lambs. Vet Rec 1960;72:19–21. [Google Scholar]
- 3. Baró T, et al. First identification of autochthonous Cryptococcus neoformans var. gattii isolated from goats with predominantly severe pulmonary disease in Spain. J Clin Microbiol 1998; 36:458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Benitah N. Canine nasal aspergillosis. Clin Tech Small Anim Pract 2006;21:82–88. [DOI] [PubMed] [Google Scholar]
- 5. Bennett JW. An overview of the genus Aspergillus. In: Machida M, Gomi K, eds. Aspergillus Molecular Biology and Genomics. Caister Academic Press, 2010:1–17. [Google Scholar]
- 6. Black P, Nunn M. Impact of climate change and environmental changes on emerging and re-emerging animal disease and animal production. Paper Presented at the World Organisation for Animal Health 77th general session, May 2009, Paris, France https://www.standardsfacility.org/sites/default/files/OIE_Technical_Session_Climate_Change_Report.pdf [Google Scholar]
- 7. Boabaid FM, et al. Conidiobolomicose em ovinos no estado de Mato Grosso [Conidiobolomycosis in sheep in the state of Mato Grosso]. Pesq Vet Bras 2008;28:77–81. Portuguese. [Google Scholar]
- 8. Bovers M, et al. Diversity of the Cryptococcus neoformans-Cryptococcus gattii species complex. Rev Iberoam Micol 2008;25:S4–S12. [DOI] [PubMed] [Google Scholar]
- 9. Câmara ACL, et al. Rhinocerebral and rhinopharyngeal conidiobolomycosis in sheep. Cienc Rural 2011;41:862–868. [Google Scholar]
- 10. Carmo PMS, et al. Nasal and cutaneous aspergillosis in a goat. J Comp Pathol 2014;150:4–7. [DOI] [PubMed] [Google Scholar]
- 11. Carrigan MJ, et al. Ovine nasal zygomycosis caused by Conidiobolus incongruus. Aust Vet J 1992;69:237–240. [DOI] [PubMed] [Google Scholar]
- 12. Casadevall A. Fungal virulence, vertebrate endothermy, and dinosaur extinction: is there a connection? Fungal Genet Biol 2005;42:98–106. [DOI] [PubMed] [Google Scholar]
- 13. Castellá G, et al. Criptococosis y animales de compañía [Cryptococcosis and companion animals]. Rev Iberoam Micol 2008;25:S19–S24. Spanish. [DOI] [PubMed] [Google Scholar]
- 14. Chapman HM, et al. Cryptococcus neoformans infection in goats. Aust Vet J 1990;67:263–265. [DOI] [PubMed] [Google Scholar]
- 15. Chaturvedi V, Chaturvedi S. Cryptococcus gattii: a resurgent fungal pathogen. Trends Microbiol 2011;19:565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Desoubeaux G, Cray C. Animal models of aspergillosis. Comp Med 2018;68:109–123. [PMC free article] [PubMed] [Google Scholar]
- 17. Devesa JA. Vegetación y flora de Extremadura [Vegetation and fauna of Extremadura]. Ed. Universitas, 1995:402–403. Spanish. [Google Scholar]
- 18. Elad D, Segal E. Diagnostic aspects of veterinary and human aspergillosis. Front Microbiol 2018;9:1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ellis DH, Pfeiffer TJ. Natural habitat of Cryptococcus neoformans var. gattii. J Clin Microbiol 1990;28:1642–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Erker C, et al. Successful treatment of invasive Conidiobolus infection during therapy for acute lymphoblastic leukemia. J Pediatr Hematol Oncol 2018;40:446–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fisher M, et al. Emerging fungal threats to animal, plant and ecosystem health. Nature 2012;484:186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Furlan FH, et al. Conidiobolomicose causada por Conidiobolus lamprauges em ovinos no estado de Santa Catarina [Conidiobolomycosis caused by Conidiobolus lamprauges in sheep in the state of Santa Catarina]. Pesq Vet Bras 2010;30:529–532. Portuguese. [Google Scholar]
- 23. García ME, Blanco JL. Principales enfermedades fúngicas que afectan a los animales domésticos [Main fungal diseases affecting domestic animals]. Rev Iberoam Micol 2000;17:S2–S7. Spanish. [PubMed] [Google Scholar]
- 24. Gazzoni AF, et al. Histopathology, serology and cultures in the diagnosis of cryptococosis. Rev Inst Med Trop 2009;51:255–259. [DOI] [PubMed] [Google Scholar]
- 25. Gonzalez JF, et al. Pulmonary aspergillosis in young lambs. Rev Iberoam Micol 1993;10:98–99. [Google Scholar]
- 26. Granados DP, Castañeda E. Influence of climatic conditions on the isolation of members of the Cryptococcus neoformans species complex from trees in Colombia from 1992–2004. FEMS Yeast Res 2006;6:636–644. [DOI] [PubMed] [Google Scholar]
- 27. Grooters AM. Pythiosis, lagenidiosis, and zygomycosis in small animals. Vet Clin North Am Small Anim Pract 2003;33:695–720. [DOI] [PubMed] [Google Scholar]
- 28. Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev 2011;24:247–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gryganskyi AP, et al. Phylogenetic lineages in Entomophthoromycota. Persoonia 2013;30:94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Humber R, et al. Equine zygomycosis caused by C. lamprauges. J Clin Microbiol 1989;27:573–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Idnurm A, Lin X. Rising to the challenge of multiple Cryptococcus species and the diseases they cause. Fungal Genet Biol 2015;78:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jensen HE, et al. Gastrointestinal aspergillosis and zygomycosis of cattle. Vet Pathol 1994;31:28–36. [DOI] [PubMed] [Google Scholar]
- 33. Jensen HE, et al. Caprine mastitis due to aspergillosis and zygomycosis: a pathological and immunohistochemical study. J Comp Pathol 1996;114:183–191. [DOI] [PubMed] [Google Scholar]
- 34. Ketterer PJ, et al. Rhinocerebral and nasal zygomycosis in sheep caused by Conidiobolus incongruus. Aust Vet J 1992;69: 85–87. [DOI] [PubMed] [Google Scholar]
- 35. Leão CA, et al. Primary cutaneous cryptococcosis caused by Cryptococcus gattii in an immunocompetent host. Med Mycol 2011;49:352–355. [DOI] [PubMed] [Google Scholar]
- 36. Lemos LS, et al. Pulmonary cryptococcosis in slaughtered sheep: anatomopathology and culture. Vet Microbiol 2007; 125:350–354. [DOI] [PubMed] [Google Scholar]
- 37. Lester SL, et al. Cryptococcosis: update and emergence of Cryptococcus gatti Vet Clin Pathol 2011;40:4–17. [DOI] [PubMed] [Google Scholar]
- 38. MacDougall L, et al. Spread of Cryptococcus gattii in British Columbia, Canada, and detection in the Pacific Northwest, USA. Emerg Infect Dis 2007;13:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maestrale C, et al. Genetic and pathological characteristics of Cryptococcus gattii and Cryptococcus neoformans var. neoformans from meningoencephalitis in autochthonous goats and mouflons, Sardinia, Italy. Vet Microbiol 2015;177:409–413. [DOI] [PubMed] [Google Scholar]
- 40. Magalhães GM, et al. Cerebral cryptococcomas in a cow. J Comp Pathol 2012;147:106–110. [DOI] [PubMed] [Google Scholar]
- 41. Mahmoud MA, et al. Prevalence of some respiratory diseases among sheep and goats in Shalateen, Halaieb and Abu-Ramad areas. Beni-Suef Vet Med J 2005;15:196–202. [Google Scholar]
- 42. Mandal PC, Gupta PP. Sequential pathological studies in goats infected intratracheally with Aspergillus fumigatus. Mycopathologia 1993;121:77–81. [DOI] [PubMed] [Google Scholar]
- 43. Meason-Smith C, et al. Panfungal PCR for identification of fungal pathogens in formalin-fixed animal tissues. Vet Pathol 2017;54:640–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mendonça FS, et al. Conidiobolomycosis in sheep in the State of Pernambuco. Rev Bras Med Vet 2012;34:241–246. [Google Scholar]
- 45. Morris M, et al. Rhinocerebral zygomycosis in a sheep. Can Vet J 2001;42:227–228. [PMC free article] [PubMed] [Google Scholar]
- 46. Ohshima K, et al. Pathological studies on mucormycoses of the forestomach and abomasums in ruminants: a report on six cases complicated with candidiasis or pulmonary aspergillosis. Jpn J Vet Sci 1976;38:269–280. [DOI] [PubMed] [Google Scholar]
- 47. Pappalardo MCSM, Melhem MSC. Cryptococcosis: a review of the Brazilian experience for the disease. Rev Inst Med Trop Sao Paulo 2003;45:299–305. [DOI] [PubMed] [Google Scholar]
- 48. Pedroso PMC, et al. Rinite micótica rinofaríngea em um ovino Texel no Rio Grande do Sul [Rhinopharyngeal mycotic rhinitis in a Texel sheep]. Acta Sci Vet 2009;37:49–52. Portuguese. [Google Scholar]
- 49. Peeters D, Clercx C. Update on canine sinonasal aspergillosis. Vet Clin North Am Small Anim Pract 2007;37:901–916. [DOI] [PubMed] [Google Scholar]
- 50. Peixoto TC, et al. Surtos de conidiobolomicose ovina por Conidiobolus lamprauges no Estado da Bahia, Nordeste do Brasil [Outbreaks of conidiobolomycosis caused by Conidiobolus lamprauges in sheep in the state of Bahia, northeastern of Brazil]. Braz J Vet Med 2017;39:252–263. [Google Scholar]
- 51. Perfect JR. Cryptococcus neoformans: the yeast that likes it hot. FEMS Yeast Res 2006;6:463–468. [DOI] [PubMed] [Google Scholar]
- 52. Pinto J, et al. Climate change and animal diseases in South America. Rev Sci Tech 2008;27:599–613. [PubMed] [Google Scholar]
- 53. Portela RA, et al. Doenças da cavidade nasal em ruminantes no Brasil [Diseases of the nasal cavity in ruminants in Brazil]. Pesq Vet Bras 2010;30:844–854. Portuguese. [Google Scholar]
- 54. Richardson M, Page I. Role of serological tests in the diagnosis of mold infections. Curr Fungal Infect Rep 2018;12(3):127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Riet-Correa F, et al. Outbreaks of rhinofacial and rhinopharyngeal zygomycosis in sheep in Paraíba, northeastern Brazil. Pesq Vet Bras 2008;28:29–35. [Google Scholar]
- 56. Riet-Correa F, et al. Bovine cryptococcal meningoencephalitis. J Vet Diagn Invest 2011;23:1056–1060. [DOI] [PubMed] [Google Scholar]
- 57. Schild CO, et al. Conidiobolomicosis nasal en una oveja (Ovis aries) en Uruguay [Nasal conidiobolomycosis in a sheep (Ovis aries) in Uruguay]. Vet Montev 2015;52:25–30. Spanish. [Google Scholar]
- 58. Shaikh N, et al. Entomophthoramycosis: a neglected tropical mycosis. Clin Microbiol 2016;22:688–694. [DOI] [PubMed] [Google Scholar]
- 59. Silva EC, et al. Cryptococcus gattii molecular type VGII infection associated with lung disease in a goat. BMC Vet Res 2017;13:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Silva SMMS, et al. Conidiobolomycosis in sheep in Brazil. Vet Pathol 2007;44:314–319. [DOI] [PubMed] [Google Scholar]
- 61. Silva SMMS, et al. Epidemiologia e sinais clínicos da conidiobolomicose em ovinos no Estado do Piauí [Epidemiology and clinical signs of conidiobolomycosis in sheep in the state of Piauí]. Pesq Vet Bras 2007;27:184–190. Portuguese. [Google Scholar]
- 62. Silva STG, et al. Nasal cryptococcosis in a sheep in Brazilian semi-arid. Braz J Vet Pathol 2010;3:127–130. [Google Scholar]
- 63. Steiger RR, Williams MA. Granulomatous tracheitis caused by Conidiobolus coronatus in a horse. J Vet Intern Med 2000;14:311–314. [PubMed] [Google Scholar]
- 64. Stephen C, et al. Multispecies outbreak of cryptococcosis on southern Vancouver Island, British Columbia. Can Vet J 2002;43:792–794. [PMC free article] [PubMed] [Google Scholar]
- 65. Stilwell G, Pissarra H. Cryptococcal meningitis in a goat—a case report. BMC Vet Res 2014;10:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tadano T, et al. Entomoftoromicose (zigomicose) causada por Conidiobolus coronatus em Mato Grosso (Brasil): relato de caso [Entomophthoramycosis (zygomicosis) caused by Conidiobolus coronatus in Matto Grosso (Brazil): case report]. Rev Soc Bras Med Trop 2005;38:188–190. Portuguese. [DOI] [PubMed] [Google Scholar]
- 67. Tell LA. Aspergillosis in mammals and birds: impact on veterinary medicine. Med Mycol 2005;43:71–73. [DOI] [PubMed] [Google Scholar]
- 68. Tintelnot K, et al. Pitfalls in serological diagnosis of Cryptococcus gattii infections. Med Mycol 2015;53:874–879. [DOI] [PubMed] [Google Scholar]
- 69. Trilles L, et al. Regional pattern of the molecular types of Cryptococcus neoformans and Cryptococcus gattii in Brazil. Mem Inst Oswaldo Cruz 2008;103:455–462. [DOI] [PubMed] [Google Scholar]
- 70. Ubiali DG, et al. Pathology of nasal infection caused by Conidiobolus lamprauges and Pythium insidiosum in sheep. J Comp Pathol 2013;149:137–145. [DOI] [PubMed] [Google Scholar]
- 71. Viviani MA, et al. Molecular analysis of 311 Cryptococcus neoformans isolates from a 30-month ECMM survey of cryptococcosis in Europe. FEMS Yeast Res 2006;6:614–619. [DOI] [PubMed] [Google Scholar]
- 72. Weiblen C, et al. Aspectos epidemiológicos, clínicos e de diagnóstico da conidiobolomicose ovina no Brasil [Epidemiological, clinical and diagnostic aspects of sheep conidiobolomycosis in Brazil]. Cienc Rural Santa Maria 2016;46:839–846. [Google Scholar]