Abstract
Persistent small-cell lymphocytosis in dogs with a concurrent mediastinal mass has been associated with both thymoma and small-cell lymphoma. In thymomas, neoplastic thymic epithelial cells induce overproduction and release of polyclonal lymphocytes, whereas thymic lymphoma results in thymic effacement by a clonal expansion of neoplastic lymphocytes and subsequent leukemic phase of lymphoma. Flow cytometry has been used to differentiate these 2 entities by immunophenotyping mediastinal mass aspirates. It has been reported that cases with mediastinal masses in which ≥ 10% of the associated small-cell lymphocytes were double positive for CD4 and CD8 were thymomas, whereas masses associated with < 10% were suggestive of lymphoma. We report a unique case of thymoma-associated lymphocytosis lacking the classic CD4+CD8+ immunophenotype. Our findings suggest that there may be more diversity in the thymoma-associated lymphocyte immunophenotype than has been identified previously; immunophenotyping alone might not be sufficient to differentiate thymic small-cell lymphoma from thymoma-associated lymphocytosis. In dogs with mediastinal masses and peripheral lymphocytosis, employing a variety of testing modalities to avoid misdiagnosis is prudent. These modalities include cytologic and/or histologic evaluation, immunophenotyping, and clonality assessment.
Keywords: dogs, flow cytometry, immunophenotype, lymphocytosis, PCR for antigen receptor gene rearrangement, thymoma
The presence of a mediastinal mass and persistent peripheral small-cell lymphocytosis has been associated with both thymoma and lymphoma.8,22 These circulating small lymphocytes have cytomorphologic features that overlap between polyclonal (reactive) and clonal (neoplastic) populations.1,2 In thymoma, an aberrant microenvironment is produced by the neoplastic cortical or medullary thymic epithelial cells (TECs) resulting in overproduction and release of non-neoplastic lymphocytes. In thymic lymphoma–associated small-cell lymphocytosis, a clonal expansion of neoplastic lymphocytes effaces and replaces the TECs, dendritic cells, and thymocytes normally found within the thymus, and spill over into the blood, resulting in a secondary leukemic phase of lymphoma.4,16
Flow cytometry (FC) can be used as part of the diagnostic workup of mediastinal masses composed primarily of small lymphocytes to assess immunophenotype and differentiate thymoma from lymphoma.14 Lymphocytes associated with canine thymoma were reported to be double positive (DP) for CD4 and CD8 in ≥ 10% of cells, whereas < 10% DP cells were determined to be diagnostic for lymphoma.14 Herein, we report a unique case of thymoma associated with lymphocytes lacking a DP immunophenotype.
A 9-y-old, spayed female Labrador Retriever was presented to Kansas State University’s Veterinary Health Center (Manhattan, KS) with a 6-wk history of exercise intolerance, dyspnea, and cough. The referring veterinarian’s bloodwork (VetScan HM5 v.2.2; Abaxis) demonstrated hypercalcemia and persistent moderate lymphocytosis (Table 1) with negative rickettsial indirect fluorescent antibody results for Ehrlichia canis, Borrelia burgdorferi, and Rickettsia rickettsii. Physical examination was unremarkable except for a thin body condition score (2 of 5) and muffled heart sounds.
Table 1.
Selected hematology and serum biochemistry results from a dog with thymoma-associated lymphocytosis and presumed paraneoplastic hypercalcemia.
| Analyte | Unit | Referring veterinarian |
KSVDL |
||||
|---|---|---|---|---|---|---|---|
| Day 0 | Day 59 | RI | Day 73 | Day 85 | RI | ||
| Hematology | |||||||
| HCT | L/L | 0.45 | 0.47 | 0.37–0.55 | 0.40 | 0.38 | 0.41–0.59 |
| WBC | ×109/L | 20.6* | 16.4 | 8.0–17.0 | 16.5 | 13.8 | 4.3–13.6 |
| Lymph | ×109/L | 9.8* | 8.8* | 1.0–4.8 | 10.1 | 5.9 | 0.8–4.3 |
| Basophil | ×109/L | 0.01 | 0.03 | 0.0–0.4 | 0.3 | 0 | 0.0–0.1 |
| Biochemistry | |||||||
| Albumin | g/L | 40 | – | 25–44 | 40 | 40 | 32–42 |
| Ca | mmol/L | 3.12 | – | 2.20–2.94 | 14.2 | 15.4 | 9.5–11.2 |
| Free Ca | mmol/L | – | – | – | 1.62 | 1.57 | No RI |
Ca = calcium; HCT = hematocrit; KSVDL = Kansas State Veterinary Diagnostic Laboratory (Manhattan, KS); Lymph = lymphocyte; RI = reference interval; WBC = white blood cell. Dash (–) indicates no results available. * Analyte flagged by analyzer and should be interpreted as minimum concentration.
Complete blood count via automated analyzer (ADVIA 2120i; Siemens) and manual differential count demonstrated mild leukocytosis with moderate lymphocytosis and mild basophilia (Table 1). Blood smear evaluation demonstrated predominantly small lymphocytes (Fig. 1) compatible with either a reactive or a neoplastic process. Measurement of free (ionized) calcium (Critical Care Express; Nova Bio) supported the moderate hypercalcemia previously identified on serum biochemistry (Table 1). Given that persistent hypercalcemia can be paraneoplastic,6 and basophilia has been associated with T-cell lymphoma in both dogs13 and cats,3 neoplastic lymphocytosis was of concern.
Figure 1.
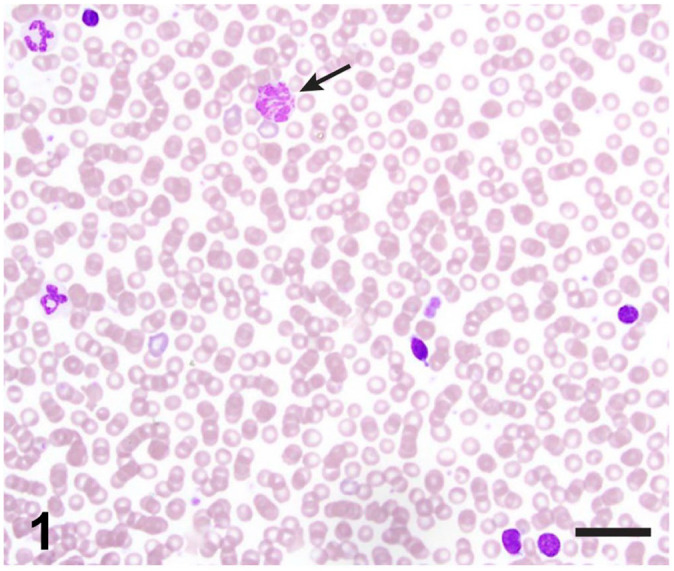
Representative photomicrograph of the peripheral blood smear demonstrating moderate lymphocytosis and mild basophilia. Lymphocytes are small (nuclei smaller than the diameter of a neutrophil) with mature condensed chromatin and scant basophilic cytoplasm. A basophil is indicated by the arrow. Modified Wright stain. Bar = 25 µm.
Radiographs and computed tomography (Suppl. Fig. 1) demonstrated a large, lobulated, opaque soft tissue mass with extensive vascular involvement occupying most of the cranial mediastinum with displacement of adjacent structures. Ultrasound-guided, fine-needle aspirate cytology samples of the mass were composed of a heterogeneous population of lymphocytes, predominantly small lymphocytes with lower numbers of medium and large lymphocytes (Fig. 2). Polygonal to slightly spindled cells arranged in variably sized clusters were also observed, but in much lower numbers (Fig. 2, arrows). Individual, well-granulated mast cells were frequently observed in association with clusters of epithelial cells (Fig. 2, asterisks). Based on these findings, the cytologic interpretation of the cranial mediastinal mass was thymoma.
Figure 2.
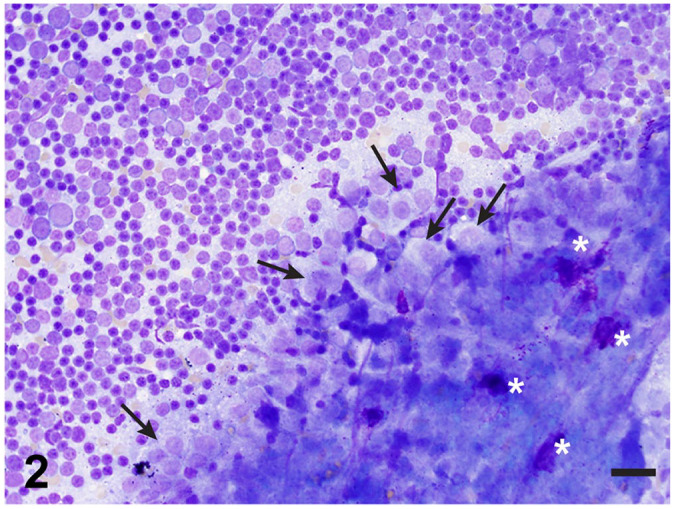
Mediastinal mass aspirate composed of a heterogeneous population of predominantly small lymphocytes with variably sized clusters of polygonal to slightly fusiform epithelial cells (arrows) and occasional scattered mast cells (asterisks).
Modified Wright stain. Bar = 25 µm.
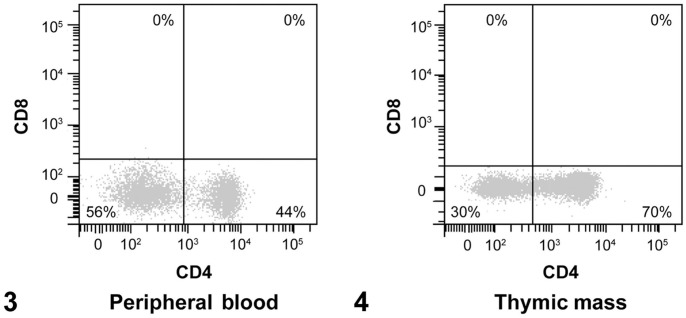
In order to reconcile the peripheral blood small-cell lymphocytosis and the mediastinal mass aspirate cytology, FC immunophenotyping was performed (Table 2). Immunophenotyping of the mediastinal mass and peripheral blood was accomplished via 2-parameter density plot development by gating on lymphocytes based on forward scatter (FSC) and side scatter (SSC) properties, followed by selection of events expressing the pan-leukocyte marker CD45 (Suppl. Figs. 2, 3). T lymphocytes within the CD45+ population were identified by expression of CD5 (Suppl. Figs. 2, 3) and T lymphocyte subset markers CD4 and CD8 (Figs. 3, 4). These findings demonstrated 30% and 56% CD5+CD4−CD8− T cells within the mediastinal mass and peripheral blood, respectively, with the remaining T lymphocytes being CD4+. Less than 1% of all T cells in both locations were DP T cells, which has previously been suggested as a feature of small-cell lymphoma in mediastinal mass aspirates.14 CD21, CD11b, CD14, and CD34 were detected in ≤ 2% of events (Suppl. Figs. 2, 3).
Table 2.
Antibody panel used for flow cytometric immunophenotyping of peripheral blood and mediastinal mass samples from a dog with thymoma-associated lymphocytosis.
| Antigen | Fluorophore | Clone | Manufacturer |
|---|---|---|---|
| CD34 | PE | 1H6 | eBioscience (Thermo Fisher Scientific) |
| CD45 | FITC | YKIX716.13 | eBioscience (Thermo Fisher Scientific) |
| CD5 | PerCP-eFluor 710 | YKIX322.3 | eBioscience (Thermo Fisher Scientific) |
| CD4 | Pacific Blue | YKIX302.9 | Bio-Rad |
| CD8 | AlexaFluor 700 | YCATE55.9 | Bio-Rad |
| CD21 | AlexaFluor 647 | CA2.1D6 | Bio-Rad |
| CD14 | APC-H7 | M5E2 | BD Biosciences |
| CD11b | PE-Dazzle 594 | M1/70 | Bio-Legend |
APC = allophycocyanin; CD = cluster of differentiation; FITC = fluorescein isothiocyanate; PE = phycoerythrin; PerCP = peridinin–chlorophyll–protein.
Figures 3, 4.
Flow cytometric immunophenotyping of peripheral blood (3) and thymic mass (4). Dot plots with subdivided T cells by detection of CD4 and CD8. The lower right quadrant contains CD4+ cells, lower left quadrant CD4−CD8− (double-negative) cells, upper left quadrant CD8+ cells, and the upper right quadrant CD4+CD8+ (double-positive) cells.
In order to rule out lymphoma, PCR for antigen receptor gene rearrangement (PARR) was performed at the Clinical Immunology Laboratory of Colorado State University (Fort Collins, CO) on both aspirated material from the mediastinal mass and peripheral blood. Amplicons of the T-cell receptor (TCR) gene were interpreted to reflect a population of polyclonal T cells.
Collectively, these findings are consistent with a diagnosis of thymoma with CD4 and CD8 double-negative (DN) lymphocytosis. Given the extensive vascular involvement of this mass, surgical resection was not feasible. The owner declined radiation therapy. The patient returned home and was reported to be deceased 4 mo later. A postmortem examination was not performed.
In dogs, data in the literature are limited regarding FC immunophenotyping of thymomas (2 case reports and 1 study with 6 cases) and thymoma-associated lymphocytosis (2 case reports).5,8,14,20 Of the 9 cases of thymoma (including our case herein) with immunophenotype reported, 7 of 9 (78%) had ≥ 10% DP T cells. The 3 cases of thymoma-associated lymphocytosis (including our case herein) all reported a significant population (> 28% in all cases) of DN circulating T cells and < 10% DP T cells.5,8 Hence, immunophenotyping alone may not be sufficient to differentiate thymic small-cell lymphoma from thymoma. Such findings suggest that there may be more diversity in thymoma-associated lymphocyte immunophenotype than has been identified previously.
The thymus—a 2-lobed organ located in the cranial mediastinum—is composed of thymic stromal cells (dendritic and epithelial cells) within a central medulla, and peripheral cortex surrounded by a thin capsule.7 The main purpose of the thymus is to produce functional, self-tolerant T cells while eliminating autoreactive T cells.15,19 From our understanding of thymocyte maturation in people, TECs release hormones such as thymopoietin, thymulin, thymosin-α1, and thymosin-β4, which affect T-cell antigen expression.4,22 In a healthy thymus, the initial hematopoietic precursor cells released from the bone marrow are attracted to the thymic corticomedullary junction by TEC chemokines such as thymotaxin.4,10,19 These precursor cells express CD34 but not CD4 or CD8, and have no TCR development.10,11,15,19,24 Once they enter the thymus, CD34 expression is lost and the cells become lymphoid progenitor cells. As they move into the DN compartment of the thymus, CD5 expression is acquired and they are considered DN thymocytes. These DN thymocytes normally comprise only 1–5% of developing T cells.8,23
Within the TEC-initiated microenvironment, thymocytes undergo selection resulting in either γδ or αβ TCR gene rearrangement.10,19 Those cells that do not successfully rearrange TCR genes are denied survival signals and undergo apoptosis. As maturation proceeds, αβ-TCR progenitor cells express both CD4 and CD8, making a population of DP cells8,10,19,24; 80–90% of developing thymic T cells are DP in dogs and cats.8,14,23 These thymocytes then move back to the corticomedullary region and undergo additional positive selection.10,23 The αβ-TCR binding to either MHC class I or class II antigen on the TEC, for upregulation of CD8 and CD4, respectively, results in continued survival signals for these cells.10,19,23,24 These thymocytes are now single-positive (SP) mature T cells. These SP cells move into the medullary compartment and undergo negative selection through interactions with TECs and antigen-presenting dendritic cells. Those T cells that bind too strongly to presented self-antigen are destroyed, thus eliminating autoreactive cells.10,19,24 Through this complex selection process, only 2% of thymocytes successfully emigrate into the peripheral circulation.
In a thymoma, altered neoplastic TEC hormone, cytokine, and chemokine production and release result in modified stroma–thymocyte interactions as well as thymic architectural and cell composition changes.16 These changes result in abnormal development of intratumoral T cells.16 As a result, thymomas have been associated with a variety of paraneoplastic syndromes, including autoimmune diseases (e.g., myasthenia gravis),16 hypercalcemia of malignancy secondary to parathyroid hormone–related peptide (PTH-rp) production,12 and peripheral T-cell lymphocytosis5,8 and basophilia.8,20 Indeed, 2 features of our case, hypercalcemia and basophilia, were suspected to be paraneoplastic in nature. Although PTH-rp concentration was not measured in our case, there was no evidence of other disorders associated with hypercalcemia, such as acute renal failure, hypoadrenocorticism, or granulomatous disease. The mechanism underlying thymoma-associated basophilia in dogs8,20 is unknown. In people, thymus stromal lymphopoietin (TSLP) has been demonstrated to influence basophil hematopoiesis in bone marrow and can elicit an expansion of this cell population, resulting in peripheral basophilia.21 Thus, neoplastic TECs overproducing TSLP is a plausible, but not proven, mechanism for thymoma-associated basophilia.
Given that neoplastic TECs alter the microenvironment in the thymus, one could speculate that an overabundance of any of the developing T-cell phenotypes could be seen. As such, it is important to consider γδ-TCR T cells as the DN lymphocyte population present in our case. One case report confirmed thymoma-associated lymphocytosis of γδ T-cell origin by FC immunophenotyping of peripheral blood and bone marrow samples, but not the mediastinal mass.8 An unstained blood smear from our case was submitted to the University of California–Davis Leukocyte Antigen Biology Laboratory for γδ- and αβ-TCR immunocytochemistry, but no evidence of positive immunoreactivity was noted for either TCR type. Therefore, we are unable to confirm that the DN population of lymphocytes responsible for the peripheral lymphocytosis are γδ T cells in our case.
The mechanism of thymocyte release into circulation with thymoma is unknown; however, several mechanisms have been proposed.4,5,8,25 The combination of overproduction and release of non-neoplastic lymphocytes by TECs as well as vascular structure violation could cause lymphocytes to “spill” into the circulation.4,5,8,22,25 Deranged bone marrow or lymphoid organ signals could induce abnormal TEC signals resulting in T-cell proliferation and disordered release.4,5,8,25 Altered cell-surface adhesion molecule interactions on lymphocytes and endothelial cells could impact T-cell migration from the thymus to the circulation and/or from the circulation into other lymphoid tissues.4,5,8 Last, lymphocytes within the thymus could be neoplastic resulting in lymphoma with a leukemic phase in peripheral blood. In our case, the peripheral polyclonal lymphocyte population had an identical phenotype to the expanded polyclonal lymphocytes present in the thymoma. This, coupled with the extensive vascular involvement also present, suggests that vascular structure violation with polyclonal lymphocytes spilling into the circulation is a feasible explanation for the lymphocytosis that we noted.
A limitation of our report is lack of histologic confirmation of the cytologic diagnosis of thymoma. However, 2 previous studies demonstrated substantial agreement between fine-needle aspirate cytology interpretation and final histologic diagnosis in thymomas.17,18 The cytologic features of thymoma in veterinary species are quite consistent with thymoma in people,18 and in humans, a cytologic diagnosis of thymoma is considered accurate when both the lymphoid and epithelial compartments are observed,9 as in our case. Although histologic confirmation would have been desirable, the cytologic interpretation in conjunction with the absence of a clonal lymphocyte population is strong evidence for the diagnosis of thymoma in our case.
Our case demonstrates that 1) immunophenotyping a thymic mass and peripheral blood lymphocytosis may result in a greater percentage of CD5+ DN polyclonal lymphocytes than expected with underlying thymoma; and 2) lack of a DP lymphocyte phenotype does not exclude thymoma. Hence, in dogs with mediastinal masses and peripheral lymphocytosis, one should employ a variety of testing modalities to avoid misdiagnosis as well as providing valuable prognostic and treatment information. These modalities include cytologic and/or histologic evaluation, FC for immunophenotyping, assessment of clonality, and immunophenotyping via immunocytochemistry or FC for γδ- and αβ-TCR, when available.1,2 To further characterize the immunophenotypic diversity within thymoma-associated lymphocytes and their circulating cohorts, more fully characterized thymoma cases are needed.
Supplemental Material
Supplemental material, Supplemental_material for CD4 and CD8 double-negative immunophenotype of thymoma-associated lymphocytes in a dog by Yvonne M. Wikander, Kaori Knights, Calli Coffee, William Vernau, David S. Biller, Mary Lynn Higginbotham and Nora L. Springer in Journal of Veterinary Diagnostic Investigation
Acknowledgments
We thank Mal Hoover, certified medical illustrator, for her assistance in figure preparation.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Nora L. Springer  https://orcid.org/0000-0003-4543-7313
https://orcid.org/0000-0003-4543-7313
Supplementary material: Supplementary material for this article is available online.
Contributor Information
Yvonne M. Wikander, Department of Diagnostic Medicine/Pathobiology, Kansas State Veterinary Diagnostic Laboratory
Kaori Knights, Department of Diagnostic Medicine/Pathobiology, Kansas State Veterinary Diagnostic Laboratory.
Calli Coffee, Department of Clinical Sciences, Veterinary Health Center.
William Vernau, College of Veterinary Medicine, Kansas State University, Manhattan, KS; Department of Pathology, Microbiology & Immunology, University of California–Davis, Davis, CA.
David S. Biller, Department of Clinical Sciences, Veterinary Health Center
Mary Lynn Higginbotham, Department of Clinical Sciences, Veterinary Health Center.
Nora L. Springer, Department of Diagnostic Medicine/Pathobiology, Kansas State Veterinary Diagnostic Laboratory.
References
- 1. Avery AC, Avery PR. Determining the significance of persistent lymphocytosis. Vet Clin North Am Small Anim Pract 2007;37:267–282. [DOI] [PubMed] [Google Scholar]
- 2. Avery PR, Avery AC. Molecular methods to distinguish reactive and neoplastic lymphocyte expansions and their importance in transitional neoplastic states. Vet Clin Pathol 2004; 33:196–207. [DOI] [PubMed] [Google Scholar]
- 3. Balan M, et al. Marked paraneoplastic basophilia accompanying eosinophilia in a cat with alimentary T-cell lymphoma. JFMS Open Rep 2017;3:2055116917730180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barton AD. T-cell lymphocytosis associated with lymphocyte-rich thymoma. Cancer 1997;80:1409–1417. [DOI] [PubMed] [Google Scholar]
- 5. Batlivala TP, et al. Paraneoplastic T cell lymphocytosis associated with a thymoma in a dog. J Small Anim Pract 2010; 51:491–494. [DOI] [PubMed] [Google Scholar]
- 6. Bergman PJ. Paraneoplastic hypercalcemia. Top Companion Anim Med 2012;27:156–158. [DOI] [PubMed] [Google Scholar]
- 7. Boehm T. Thymus development and function. Curr Opin Immunol 2008;20:178–184. [DOI] [PubMed] [Google Scholar]
- 8. Burton AG, et al. Thymoma-associated lymphocytosis in a dog. Vet Clin Pathol 2014;43:584–588. [DOI] [PubMed] [Google Scholar]
- 9. Chhieng DC, et al. Cytology of thymomas, emphasis on morphology and correlation with histologic subtypes. Cancer 2000;90:24–32. [PubMed] [Google Scholar]
- 10. Ciofani M, Zúñiga-Pflücker JC. The thymus as an inductive site for T lymphopoiesis. Annu Rev Cell Dev Biol 2007;23:463–493. [DOI] [PubMed] [Google Scholar]
- 11. Comazzi S, Gelain ME. Use of flow cytometric immunophenotyping to refine the cytological diagnosis of canine lymphoma. Vet J 2011;188:149–155. [DOI] [PubMed] [Google Scholar]
- 12. Foley P, et al. Serum parathyroid hormone-related protein concentration in a dog with thymoma and persistent hypercalcemia. Can Vet J 2000;41:867–870. [PMC free article] [PubMed] [Google Scholar]
- 13. Gupta A, et al. What is your diagnosis? Abdominal fluid from a dog. Vet Clin Pathol 2013;42:113–114. [DOI] [PubMed] [Google Scholar]
- 14. Lana S, et al. Diagnosis of mediastinal masses in dogs by flow cytometry. J Vet Intern Med 2006;20:1161–1165. [DOI] [PubMed] [Google Scholar]
- 15. Marx A, et al. The autoimmune regulator AIRE in thymoma biology: autoimmunity and beyond. J Thorac Oncol 2010;5(10 Suppl 4):S266–S272. [DOI] [PubMed] [Google Scholar]
- 16. Muller-Hermelink HK, et al. Characterization of the human thymic microenvironment: lymphoepithelial interaction in normal thymus and thymoma. Arch Histol Cytol 1997;60:9–28. [DOI] [PubMed] [Google Scholar]
- 17. Pintore L, et al. Cytological and histological correlation in diagnosing feline and canine mediastinal masses. J Small Anim Pract 2014;55:28–32. [DOI] [PubMed] [Google Scholar]
- 18. Rae CA, et al. A comparison between the cytological and histological characteristics in thirteen canine and feline thymomas. Can Vet J 1989;30:497–500. [PMC free article] [PubMed] [Google Scholar]
- 19. Shah DK, Zúñiga-Pflücker JC. An overview of the intrathymic intricacies of T cell development. J Immunol 2014;192:4017–4023. [DOI] [PubMed] [Google Scholar]
- 20. Simpson RN, et al. Massive thymoma with medullary differentiation in a dog. Vet Pathol 1992;29:416–419. [DOI] [PubMed] [Google Scholar]
- 21. Siracusa MC, et al. TSLP promotes IL-3-independent basophil hematopoiesis and type 2 inflammation. Nature 2011;477:229–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smith GP, et al. T-cell lymphocytosis associated with invasive thymomas. Am J Clin Pathol 1994;102:447–453. [DOI] [PubMed] [Google Scholar]
- 23. von Boehmer H. The developmental biology, function and specificity of lymphocyte subsets. Annu Rev Immunol 1988;6:309–326. [DOI] [PubMed] [Google Scholar]
- 24. Weksler B, Lu B. Alterations of the immune system in thymic malignancies. J Thorac Oncol 2014;9(9 Suppl 2):S137–S142. [DOI] [PubMed] [Google Scholar]
- 25. Zhao L, et al. Bone metastasis of malignant thymomas associated with peripheral T-cell lymphocytosis. BMC Surg 2016; 16:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_material for CD4 and CD8 double-negative immunophenotype of thymoma-associated lymphocytes in a dog by Yvonne M. Wikander, Kaori Knights, Calli Coffee, William Vernau, David S. Biller, Mary Lynn Higginbotham and Nora L. Springer in Journal of Veterinary Diagnostic Investigation