Abstract
Fetal bovine serum (FBS) used in cell culture may be contaminated with adventitious agents, which can affect the production of biologicals and the results of clinical laboratory tests. We carried out a retrospective study to determine the incidence of adventitious agent contamination of Argentinean irradiated FBS dating from 2015 to 2019. We analyzed FBS batches for mycoplasma and adventitious viruses (bovine pestiviruses, bovine adenovirus, bluetongue virus, bovine parainfluenza virus 3, rabies virus, bovine parvovirus, bovine herpesvirus 1, bovine respiratory syncytial virus, and reovirus). Cell passages followed by direct immunofluorescence were carried out to check viability of the mentioned adventitious agents. Also, molecular detection of mycoplasma and pestiviruses was performed on the FBS samples. The presence of neutralizing antibodies against pestiviruses was determined. Molecular analyses indicated that frequencies of mycoplasma and pestiviruses in FBS were 14% and 84%, respectively. All of the batches were seronegative for pestiviral antibodies. After cell passages, all FBS samples were negative for hemadsorbent agents and by immunofluorescence for all of the viral species analyzed; PCR assays were negative for mycoplasma and pestiviruses. Our results demonstrate that, of all adventitious agents tested, local FBS batches only had traces of mycoplasma and pestiviruses; gamma irradiation was effective in inactivating them.
Keywords: biological products, contamination, fetal bovine serum, mycoplasma, pestiviruses
Introduction
Fetal bovine serum (FBS) is a universal growth supplement for cell and tissue culture media used in research, biotechnology, and pharmaceutical manufacturing in the production of viral vaccines, recombinant proteins, and biotherapeutics. FBS contains essential components for cell proliferation and maintenance, such as hormones, growth factors, vitamins, minerals, transport proteins, and trace elements.11 It is obtained from the blood drawn from bovine fetuses via a closed system of collection at slaughterhouses.
Pregnant cows arriving at slaughterhouses represent ~ 23% of the total female cattle slaughter, although this percentage varies according to the specific time and country.15,16 Approximately 800,000 L of FBS are produced annually worldwide, from 2,000,000 bovine fetuses, with production increasing annually.5 Argentina is one of the most important producers of FBS in South America, generating 7,000–8,000 L/y. The most important clients are local and foreign biopharmaceutical industries, vaccine producers, and to a lesser extent, research and diagnostic laboratories.
Cows can be infected with pathogens during pregnancy and can pass them through the placenta to fetuses. Hence, FBS may be contaminated with pathogens that may become adventitious agents during the manufacturing of biological products.6 Unlike microbial contamination (fungi, yeast, and common bacteria), mycoplasma and noncytopathic (ncp) viruses cannot be detected easily. Mycoplasmas and ncp viruses may cause silent infections in cell cultures, impacting research and diagnostic results. In addition, the contamination of cells and biological products could have potentially dramatic epidemiologic consequences, through the accidental introduction of pathogens into susceptible human or animal populations.12
Mycoplasmas are in the Mycoplasmataceae family of the Mollicutes class. Given their small size and membrane flexibility, mycoplasmas are pleomorphic and can alter their size and shape in response to environmental conditions. They can even penetrate sterilization-grade filters.27 Mycoplasmas are a very real concern for research laboratories because they are very common cell culture contaminants. Because these agents affect the cells’ overall behavior, contamination can result in false interpretation of experimental results and undermine the validity of the resulting data. Moreover, it may be very difficult to remove mycoplasmas from contaminated cells.17
Pestiviruses belong to the Flaviviridae family and are a group of enveloped, positive-sense, single-stranded RNA viruses. The pestivirus genus includes, among other viruses, bovine viral diarrhea virus 1 and 2 (BVDV-1, -2; Pestivirus A, B) and a putative atypical pestivirus, the HoBi-like virus. The bovine pestiviruses (BVDV-1, BVDV-2, and HoBi-like virus) are very common contaminants of biological materials such as FBS and cell cultures.3,25 BVDV-1 and -2 are some of the most common pestiviruses, and they are endemic almost worldwide.7,23 Given that 90% of BVDV strains are ncp, when cell lines are accidentally contaminated, it is impossible to detect BVDV under the optical microscope.21,22,28,31
One way to reduce the risk of contamination during the manufacturing process of a biological product derived from cell culture is to treat animal-derived materials, such as FBS, to inactivate possible contaminants. Gamma irradiation is one of the most widely used and effective methods for the inactivation of viruses and mycoplasmas in animal sera. Regulations from the United States (USDA 9 CFR 113.46-53) and the European Union (EMA/CHMP/BWP/457920/2012) require that all FBS, regardless of country of origin, be tested and/or treated (by heat or gamma irradiation) to assure its lack of mycoplasma and adventitious viruses, which are the following: bovine pestiviruses, rabies virus (RBV; Rabies lyssavirus), reovirus, bovine herpesvirus 1 (BoHV-1; Bovine alphaherpesvirus 1), bovine parainfluenza virus 3 (BPIV-3; Bovine respirovirus 3), bovine adenovirus (BoAdV; Bovine atadenovirus), bovine parvovirus (Ungulate bocaparvovirus 1), bluetongue virus (BTV), and bovine respiratory syncytial virus (BRSV; Bovine orthopneumovirus). However, some companies still market non-irradiated FBS.20
The Adventitious Virus Laboratory from the National Institute of Technological Agriculture (INTA) in Argentina offers specialized services for detecting contaminating adventitious agents in biopharmaceutical and biotherapeutic products for human and veterinary use. We carried out a retrospective study to evaluate contamination with mycoplasma and adventitious viruses in Argentinean batches of irradiated FBS.
Materials and methods
Samples
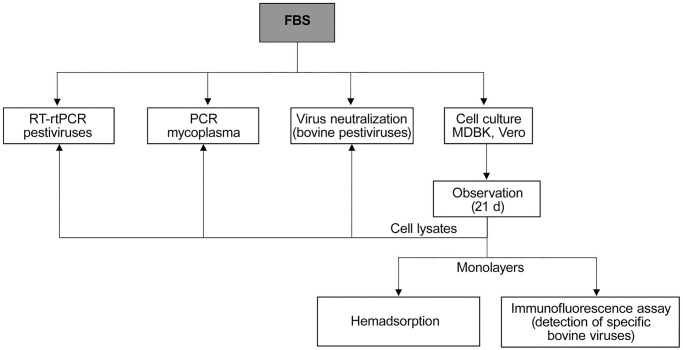
We evaluated 124 samples of irradiated FBS batches, belonging to the 5 largest FBS local manufacturers (A–E), by volume of production. Batches were produced and remitted to the laboratory between 2015 and 2019. In all cases, the dynamic gamma irradiation procedure was carried out in a local company at 25 kilograys prior to remission. The number of fetuses per batch was not communicated by the producers. Molecular and culture methods were used to analyze the samples (Fig. 1).
Figure 1.
Schematic representation of adventitious agent analysis of irradiated Argentinean fetal bovine serum batches.
Molecular analyses
Molecular detection of mycoplasma and bovine pestiviruses was performed in all of the FBS batches in 2 stages: in raw samples, and in the lysate of cultured cells after cell passages.
Mycoplasma detection: DNA extraction and PCR
DNA was obtained from raw FBS samples and from cell lysates (High Pure DNA isolation kit; Roche), following the manufacturer’s instructions. GPO3 and MGSO primers were used to amplify a 280-bp DNA fragment, complementary to the 16S rRNA region of mycoplasma, according to a previous report.13 Lysates of baby hamster kidney (BHK) cells contaminated with mycoplasma and minimal essential medium were used as positive and negative controls, respectively. PCR reactions were carried out in a total reaction volume of 25 µL containing 500 nM of each primer, 5× GoTaq reaction buffer (Promega), 25 mM of dNTPs mix (PBL), 0.25 U of Taq DNA polymerase (Promega), and 5 µL of extracted DNA. The PCR conditions were as follows: initial denaturing at 94°C for 5 min followed by 35 cycles of denaturing at 94°C for 1 min, primer annealing at 55°C for 1 min, and elongation at 72°C for 2 min. The final elongation step was extended to 2 min at 72°C. The presence of the amplified product was revealed by electrophoresis in 2% agarose gel, containing an intercalating agent (GelRed; Biotium), and visualized under ultraviolet light. The limit of detection of the PCR was determined previously to be 8 copies per reaction (data not shown).
Pestivirus detection: RNA extraction and real-time PCR
Viral RNA was extracted from raw FBS samples, cell lysates, and controls (High Pure viral RNA kit; Roche) according to the manufacturer’s recommendations. Untreated FBS, free from pestiviral antibodies (Abs) and RNA, were used as negative controls; freedom was determined by virus neutralization (VN) and real-time PCR (rtPCR) methods described below. BVDV-1 Singer strain stocks were diluted in pestivirus-negative FBS to a final titer of 102 tissue culture infective doses (TCID)/mL to be used as positive controls. Reverse transcriptions (RTs) were carried out in a final reaction volume of 25 μL using 500 ng of RNA. The reaction mixture consisted of 0.5 μg of random hexanucleotide primers (Biodynamics), 200 U of M-MLV (Moloney murine leukemia virus; Promega), 20 U of RNAsin ribonuclease inhibitor (Promega), 5× RT buffer (Promega), and 25 mM of dNTPs mix (PBL). The thermal condition was 60 min at 42°C, and then the reactions were heated to 95°C for 5 min in order to inactivate the enzyme.
A SYBR Green rtPCR was performed (StepOne cycler, Thermo Fisher Scientific; Fast-essential Green master mix, Roche) according to the manufacturers’ protocols. The reactions were carried out in a 12-µL volume, and ROX (5-carboxy-X-rhodamine; Invitrogen) was used as reference dye.
The primers used in the rtPCR are known as “189-389”14 and were used at a final concentration of 470 nM. These primers target a partial sequence of the 5′ untranslated region of BVDV, from position 190 to 390 of the NADL reference strain (GenBank accession M31182). PCR conditions were as follows: initial denaturing step at 95°C for 10 min, followed by 45 cycles of 95°C for 20 s, 60°C for 20 s, and 72°C for 20 s. Melting curve analysis was used to evaluate the results; specificity of the amplification was confirmed by agarose gel electrophoresis. This assay has previously been demonstrated to detect BVDV-1, BVDV-2, and HoBi-like virus, and the linear range of the rtPCR is 108–101 complementary DNA copies/µL, with 99% efficiency (unpublished data).
Virus neutralization
VN assays were performed to detect the presence of neutralizing Abs (nAbs) against pestiviruses in all of the raw FBS samples (Fig. 1), as described previously.18,19 Briefly, 100 TCID50 of cytopathic BVDV-1 (Singer strain), BVDV-2 (VS253 strain), or HoBi-like virus (83/10), were co-incubated with dilutions of inactivated serum samples (1/4–1/64) for 1 h at 37°C. Then, the mixture was added to plates with 3 × 104 Madin–Darby bovine kidney (MDBK) cells/well. Plates were incubated for 72 h at 37°C under 5% CO2. Reciprocals of the end point nAb titers were calculated using the Reed and Muench method.24
Cell culture
Samples of FBS were inoculated in duplicate on MDBK and Vero cell lines, according to international recommendations (9.CFR section 113.53, USDA-APHIS-USA, https://www.govinfo.gov/app/details/CFR-2014-title9-vol1/CFR-2014-title9-vol1-sec113-53/summary; Fig. 1). After an adsorption period of 1 h at 37°C, maintenance culture medium was added, and the cell cultures were incubated at 37°C with 5% CO2. Monolayers were subcultured every 7 d by a freeze–thaw cycle, and 15% of the volume of the culture was used to inoculate the subculture. Cells were examined regularly throughout the 21-d maintenance period for evidence of cytopathic agents. On day 21, monolayers were tested by hemadsorption and immunofluorescence assays, and cell lysates were used for molecular detection of mycoplasma and pestiviruses. For this purpose, monolayers were washed 3 times with PBS to eliminate any trace of culture medium, and then cells were lysed with a small volume of PBS by a freeze–thaw cycle.
Hemadsorption and immunofluorescence assays
In order to detect the presence of hemadsorbing agents, monolayers were washed 3 times with PBS, and cells were then incubated with a suspension of washed guinea pig and chicken red blood cells according to standard procedures.
Inoculated cell cultures were also evaluated by direct immunofluorescence using a panel of specific polyclonal Abs conjugated with fluorescein (VMRD; Suppl. Table 1). Briefly, cells were fixed with cold acetone for 20 min at −20°C and air-dried. The cells were washed once with PBS and then the Abs were added and incubated for 30 min at 37°C. Cells were then washed 3 times with PBS, and the stained slides were mounted on a glass slide with mounting fluid. Observation was performed with a fluorescent-light microscope with 20× and 40× objectives. Cells infected with reference strains for each viral species were used as positive controls. Non-infected cells were used as negative controls in all cases.
The master cell banks used in our study were provided by the tissue culture facilities at the Virology Institute (INTA), and they were adventitious agents–free according to prior studies carried out in our laboratory. All of the reagents used for growth and maintenance of the indicator cells (serum, trypsin, and cell culture medium) were of commercial origin. All of them were controlled to rule out the presence of adventitious agents by the methods explained above (molecular analyses, VN, cell culture, and hemadsorption and immunofluorescence assays).
Results
By PCR assays, 14% and 84% of the raw FBS analyzed samples were positive for mycoplasma and bovine pestiviruses, respectively. Only 8% of FBS samples were positive in both PCR tests. In contrast, 12 FBS samples (10%) were negative for both PCRs (Table 1). There was no association between the PCR results and the FBS manufacturer (Table 2).
Table 1.
Molecular detection of mycoplasma and pestiviral genetic material in raw fetal bovine serum samples.
| Pestivirus | Mycoplasma | Total | |
|---|---|---|---|
| Negative | Positive | ||
| Positive | 76% (94) | 8% (10) | 84% (104) |
| Negative | 10% (12) | 6% (8) | 16% (20) |
| Total | 86% (106) | 14% (18) | 100% (124) |
Number of samples in parentheses.
Table 2.
Detection of mycoplasma and pestivirus genetic material in fetal bovine serum batches from different manufacturers (A–E) each year.
| Manufacturer | 2015 |
2016 |
2017 |
2018 |
2019 |
Total samples |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Myco. | Pest. | Myco. | Pest. | Myco. | Pest. | Myco. | Pest. | Myco. | Pest. | ||
| A | 4/13 | 11/13 | 3/15 | 12/15 | 2/9 | 9/9 | 2/24 | 20/24 | 2/21 | 19/21 | 82 |
| B | 0/2 | 1/2 | 0/1 | 0/1 | 1/3 | 2/3 | 1/8 | 7/8 | 0/5 | 5/5 | 19 |
| C | – | – | 2/4 | 2/4 | 0/2 | 1/2 | 0/8 | 6/8 | 0/4 | 4/4 | 18 |
| D | 0/1 | 1/1 | 0/1 | 1/1 | – | – | – | – | – | – | 2 |
| E | – | – | – | – | 0/1 | 1/1 | 0/2 | 2/2 | – | – | 3 |
| + samples/total samples | 4/16 | 13/16 | 5/21 | 15/21 | 3/15 | 13/15 | 3/42 | 35/42 | 2/30 | 28/30 | |
Myco. = mycoplasma; Pest. = pestivirus; – = years without samples.
None of the FBS samples analyzed contained nAbs against BVDV-1, BVDV-2, or HoBi-like virus. None of the FBS samples under study showed evidence of cytopathic effect on MDBK or Vero cells during the entire culture observation period. No growth of contaminating microorganisms, such as bacteria and fungi, was observed during the entire culture observation period. After 21 d of culture, cell lysates showed negative results when analyzed by PCR or RT-rtPCR for mycoplasma or bovine pestiviruses, respectively. No hemadsorbing activity was detected, and immunofluorescence assays were negative for all of the viruses analyzed in all FBS samples.
Discussion
Based on clinical, virologic, and epidemiologic evidence, the Argentinean cattle population is known to be free of some pathogens considered as exotic, such as Rift Valley fever virus (Rift Valley fever phlebovirus), bovine spongiform encephalopathy prion, and rinderpest virus (Rinderpest morbillivirus), making the contamination of the source material with these agents unlikely. In contrast, some bovine viral infections are endemic in our country (bovine pestiviruses, BoHV-1, BRSV). The Argentinean sanitary status for other viral agents remains unknown because clinical or serologic evidence has not been reported (BoAdV, bovine parvovirus). Regulations specify that the products used in the production of biopharmaceuticals must be checked for the presence of various contaminating agents, and constitute the basis for analysis in our laboratory to assess the safety of the material under test.
Mycoplasmas affect cattle heath in some Argentinean farms. These farms may be suppliers of the FBS manufacturers at the slaughterhouses, and hence the local FBS batches may contain mycoplasma DNA. Added to this problem, cell culture contamination with mycoplasma is frequent around the world. The most likely sources of cell culture contamination with mycoplasma appear to be laboratory personnel, FBS, and other infected cell cultures.9,17 Microbiologic culture is the gold standard test to detect every kind of mycoplasma contamination in cell cultures without considering the origin and species of mycoplasma. However, culture is time-consuming and may lead to false-negative results. Mycoplasma contamination can also be detected by PCR, which is a simple, sensitive, specific, reliable, efficient, and cost-effective technique. Most mycoplasma PCR tests amplify the highly conserved sequence of the 16S rDNA region, which is shared by a broad range of species, including Mycoplasma bovis, M. arginini, and Acholeplasma laidlawii, which are the most frequent varieties found in cattle.17 We found mycoplasma DNA in 14% of the FBS batches tested. Although there is not much information about the expected rate of contamination, some authors have reported 0–19% of FBS batches to be positive for mycoplasma.1,2,6,9,22
Our pestiviral RT-rtPCR results indicated that a high proportion (84%) of the FBS batches contained viral RNA. Our data are in accordance with previous studies; multiple authors have reported pestiviral contamination rates of 22–100% in commercial FBS batches.3,10,20,26,29,30 In most of these reports, the number of fetuses per batch was not available but, definitely, an increased pool size enhances the rate of contamination. Also, the sensitivity of the molecular method used has a great impact on the ability to detect viral RNA in FBS samples. Finally, some of these studies have been carried out with primers that do not detect RNA from HoBi-like virus, whereas the primers used in our research have proved to efficiently detect BVDV-1, BVDV-2, and HoBi-like virus.14
The molecular detection of genetic material of mycoplasma and pestiviruses in the FBS batches indicates that the source material was contaminated with those agents but does not imply infectivity of the material. Discrimination between infectious and non-infectious mycoplasma and pestiviruses is essential and can only be achieved by cell culture methods. In our study, these infectious agents were absent after cell culture and subsequent passages, supporting correct viral inactivation by FBS producers through the gamma irradiation process. This treatment may profoundly minimize the risk for infectious agents in FBS,26,29 but the absence of infectious pathogens cannot be fully guaranteed until the product is strictly controlled by cell culture assays. In our study, the gamma irradiation process proved to be 100% effective in canceling the infectivity of the adventitious agents analyzed. The other adventitious viruses (BoAdV, BTV, BPIV-3, RBV, bovine parvovirus, BoHV-1, BRSV, and reovirus) were also absent in all of the FBS batches. Our results reinforce the importance of using irradiated FBS as a supplement reagent in cell culture assays for elaboration of biological products, in order to minimize the risk of adventitious contaminations in cells, and therefore in the final products. The presence of pestiviral RNA in FBS batches is still a matter of concern, especially if the sera are used for the manufacture of pestiviral vaccines.
Gamma irradiation cannot eliminate the viral Abs present in FBS batches. Therefore, laboratories are encouraged to implement a stringent Ab-detection protocol if FBS is used for pestiviral detection tests.8 Some researchers have reported uneven levels of nAbs in FBS batches.3,4 The Argentinean FBS batches that we evaluated contained no traces of nAbs against BVDV-1, BVDV-2, or HoBi-like virus, therefore nAbs would not be the main concern when using these inputs.
Supplemental Material
Supplemental material, Supplemental_material for Analysis of irradiated Argentinean fetal bovine serum for adventitious agents by Andrea Pecora, Jorgelina Pérez López, Maximiliano J. Jordán, Lautaro N. Franco, Romina Politzki, Vanesa Ruiz and Irene Alvarez in Journal of Veterinary Diagnostic Investigation
Acknowledgments
We thank the personnel from the Tissue Culture Section at the National Institute of Agricultural Technology (INTA), Virology Institute, for their excellent technical assistance.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This work was financially supported by project FONCYT PICT 2015-1291, project INTA PNSA-1115052, and extra budgetary funds from specialized technical services of the Laboratorio de Virus Adventicios (INTA) administered by Fundación Argeninta. A. Pecora, V. Ruiz, and I. Alvarez were also supported by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET).
ORCID iD: Irene Alvarez  https://orcid.org/0000-0001-5194-670X
https://orcid.org/0000-0001-5194-670X
Supplementary material: Supplementary material for this article is available online.
Contributor Information
Andrea Pecora, National Institute of Agricultural Technology (INTA), Virology Institute, Hurlingham, Buenos Aires, Argentina; National Scientific and Technical Research Council (CONICET), Ciudad Autónoma de Buenos Aires, Argentina.
Jorgelina Pérez López, National Institute of Agricultural Technology (INTA), Virology Institute, Hurlingham, Buenos Aires, Argentina.
Maximiliano J. Jordán, National Institute of Agricultural Technology (INTA), Virology Institute, Hurlingham, Buenos Aires, Argentina
Lautaro N. Franco, National Institute of Agricultural Technology (INTA), Virology Institute, Hurlingham, Buenos Aires, Argentina
Romina Politzki, National Institute of Agricultural Technology (INTA), Virology Institute, Hurlingham, Buenos Aires, Argentina.
Vanesa Ruiz, National Institute of Agricultural Technology (INTA), Virology Institute, Hurlingham, Buenos Aires, Argentina; National Scientific and Technical Research Council (CONICET), Ciudad Autónoma de Buenos Aires, Argentina.
Irene Alvarez, National Institute of Agricultural Technology (INTA), Virology Institute, Hurlingham, Buenos Aires, Argentina; National Scientific and Technical Research Council (CONICET), Ciudad Autónoma de Buenos Aires, Argentina.
References
- 1. Angulo AF, et al. Acholeplasma vituli sp. nov., from bovine serum and cell cultures. Int J Syst Evol Microbiol 2000;50:1125–1131. [DOI] [PubMed] [Google Scholar]
- 2. Barile MF, Kerns J. Isolation of Mycoplasma arginini from commercial bovine sera and its implication in contaminated cell cultures. Proc Soc Exp Biol Med 1971;138:432–437. [DOI] [PubMed] [Google Scholar]
- 3. Bauermann FV, et al. Lack of evidence for the presence of emerging HoBi-like viruses in North American fetal bovine serum lots. J Vet Diagn Invest 2014;26:10–17. [DOI] [PubMed] [Google Scholar]
- 4. Bolin SR, Ridpath JF. Prevalence of bovine viral diarrhea virus genotypes and antibody against those viral genotypes in fetal bovine serum. J Vet Diagn Invest 1998;10:135–139. [DOI] [PubMed] [Google Scholar]
- 5. Brindley D, et al. Peak serum: implications of serum supply for cell therapy manufacturing. Regen Med 2012;7:7–13. [DOI] [PubMed] [Google Scholar]
- 6. Cordero Camacho CP, et al. Detection of seven viruses and Mycoplasma in fetal bovine serum by real time PCR. Rev Colom Cienc Pecua 2011;24:585–597. [Google Scholar]
- 7. Deng M, et al. Prevalence and genetic diversity of bovine viral diarrhea virus in dairy herds of China. Vet Microbiol 2020;242:108565. [DOI] [PubMed] [Google Scholar]
- 8. Dubovi EJ. Laboratory diagnosis of bovine viral diarrhea virus. Biologicals 2013;41:8–13. [DOI] [PubMed] [Google Scholar]
- 9. Dvorakova H, et al. Detection of mycoplasma contamination in cell cultures. Vet Med 2005;6:262–268. [Google Scholar]
- 10. Giammarioli M, et al. Genetic detection and characterization of emerging HoBi-like viruses in archival foetal bovine serum batches. Biologicals 2015;43:220–224. [DOI] [PubMed] [Google Scholar]
- 11. Gstraunthaler G, et al. A plea to reduce or replace fetal bovine serum in cell culture media. Cytotechnology 2013;65:791–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hawkes P. Fetal bovine serum: geographic origin and regulatory relevance of viral contamination. Bioresour Bioprocess 2015;2:1–5. [Google Scholar]
- 13. Van Kupperveld FJM, et al. Genus- and species-specific identification of mycoplasmas by 16S rRNA amplification. Appl Environ Microbiol 1993;59:933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Monteiro FL, et al. Detection of bovine pestiviruses in sera of beef calves by a RT-PCR based on a newly designed set of pan-bovine pestivirus primers. J Vet Diagn Invest 2019;31:255–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nielsen OB, Hawkes P. Fetal bovine serum and the slaughter of pregnant cows: animal welfare and ethics. Bioprocess J 2019;18:1–4. [Google Scholar]
- 16. Nielsen SS, et al. Slaughter of pregnant cattle in Denmark: prevalence, gestational age, and reasons. Animals 2019;9:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nikfarjam L, Farzaneh P. Prevention and detection of mycoplasma contamination in cell culture. Cell J 2012;13:203–212. [PMC free article] [PubMed] [Google Scholar]
- 18. Pecora A, et al. First finding of genetic and antigenic diversity in 1b-BVDV isolates from Argentina. Res Vet Sci 2014;96:204–212. [DOI] [PubMed] [Google Scholar]
- 19. Pecora A, et al. Serologic evidence of HoBi-like virus circulation in Argentinean water buffalo. J Vet Diagn Invest 2017;29:926–929. [DOI] [PubMed] [Google Scholar]
- 20. Pecora A, et al. Molecular characterization of pestiviruses in fetal bovine sera originating from Argentina: evidence of circulation of HoBi-like viruses. Front Vet Sci 2019;6:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peterhans E, et al. Cytopathic bovine viral diarrhea viruses (BVDV): emerging pestiviruses doomed to extinction. Vet Res 2010;41:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pinheiro de Oliveira TF, et al. Detection of contaminants in cell cultures, sera and trypsin. Biologicals 201;41:1–8. [DOI] [PubMed] [Google Scholar]
- 23. Qi L, et al. Neighbourhood contacts and trade movements drive the regional spread of bovine viral diarrhoea virus (BVDV). Vet Res 2019;50:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reed JL, Muench H. A simple method of estimating fifty percent endpoints. Am J Epidemiol 1938;27:493–497. [Google Scholar]
- 25. Studer E, et al. Detection and characterization of pestivirus contaminations in human live viral vaccines. Biologicals 2002;30:289–296. [DOI] [PubMed] [Google Scholar]
- 26. Toohey-Kurth K, et al. Metagenomic assessment of adventitious viruses in commercial bovine sera. Biologicals 2017;47:64–68. [DOI] [PubMed] [Google Scholar]
- 27. Uphoff CC, Drexler HG. Eradication of mycoplasma contaminations from cell cultures. Curr Protoc Mol Biol 2014;106:28.5.1–28.5.12. [DOI] [PubMed] [Google Scholar]
- 28. World Organisation for Animal Health (OIE). Bovine viral diarrhea. Chapter 2.4.8. In: Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. OIE, 2018:698–711. [Google Scholar]
- 29. Xia H, et al. Detection and identification of the atypical bovine pestiviruses in commercial foetal bovine serum batches. PLoS One 2011;6:2–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yanagi M, et al. Contamination of commercially available fetal bovine sera with bovine viral diarrhea virus genomes: implications for the study of hepatitis C virus in cell cultures. J Infect Dis 1996;174:1324–1327. [DOI] [PubMed] [Google Scholar]
- 31. Zabal O, et al. Contamination of bovine fetal serum with bovine viral diarrhea virus. Rev Argent Microbiol 2000;1:27–32. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_material for Analysis of irradiated Argentinean fetal bovine serum for adventitious agents by Andrea Pecora, Jorgelina Pérez López, Maximiliano J. Jordán, Lautaro N. Franco, Romina Politzki, Vanesa Ruiz and Irene Alvarez in Journal of Veterinary Diagnostic Investigation