Abstract
Loggerhead (Caretta caretta; Cc) and green sea (Chelonia mydas; Cm) turtles admitted to rehabilitation facilities may require blood transfusions for supportive treatment of disorders resulting in life-threatening anemia, but, considering the unique erythrocyte chemistry of sea turtles, standardized donor red blood cell (RBC) storage protocols have not been established. Prolonged cold storage and the effects of various anticoagulant-preservative solutions have been associated with increased RBC osmotic fragility across a broad range of species. Increased RBC fragility in stored RBC products has been associated with acute transfusion reactions. The osmotic fragility test is used to measure erythrocyte resistance to hemolysis while being exposed to a series of dilutions of a saline solution. We obtained baseline measurements for osmotic fragility in healthy Cc and Cm. Osmotic fragility testing was performed on samples from 10 Cc to 10 Cm. Fifty percent (50%) RBC hemolysis was identified at a mean NaCl concentration of 0.38% in both species. Results of our study will help guide future studies evaluating optimal storage solutions for sea turtle blood products.
Keywords: erythrocyte, osmotic fragility, red blood cell, sea turtles, transfusion
According to the National Oceanic and Atmospheric Administration, loggerhead (Caretta caretta; Cc) and green sea (Chelonia mydas; Cm) turtles are both listed as either endangered or threatened at various worldwide locations under the endangered species act (https://www.fisheries.noaa.gov/species/loggerhead-turtle, https://www.fisheries.noaa.gov/species/green-turtle). Both of these species are frequently admitted to rehabilitation facilities in which blood transfusions may become necessary as part of supportive care in cases of chronic emaciation or after surgical removal of external fibropapillomatosis-associated lesions.17 The availability of stored whole blood can be useful in situations when appropriate sea turtle donors of the same species are not available at a rehabilitation facility.
For both storage and transport of whole blood from donor turtles, it is essential to understand optimal storage conditions for each species. However, little is known with respect to selecting optimal storage solutions for the preservation of donor sea turtle red blood cells (RBCs) prior to transfusion to a recipient turtle. Current pre-transfusion RBC storage recommendations have been extrapolated from studies conducted in humans, domestic mammals (e.g., dogs, cats, and horses), one American alligator study, and one anticoagulant short-term storage study in sea turtles.9,12,20,23
Studies evaluating preservation media for RBC storage typically evaluate RBCs stored in various commercial storage media (e.g., acid–citrate–dextrose, citrate–phosphate–dextrose, and citrate–phosphate–dextrose–adenine) over the course of 4–6 wk. It is well documented in the literature that damage occurs to RBCs during the course of storage.8 Cold storage of donor RBCs is notably associated with an increase in RBC osmotic fragility, RBC hemolysis, leakage of intracellular enzymes and electrolytes, and decreased ATP and 2,3-diphosphoglycerate synthesis.1,4,6 Moreover, prolonged cold storage and the effects of various anticoagulant-preservative solutions have been associated with increased RBC osmotic fragility across a broad range of species.3,7,11,19 Increased RBC fragility in stored RBC products has also been associated with acute transfusion reactions.16 In addition, hypercoagulability5 and endothelial damage18 are just a few of the potential adverse reactions associated with the transfusion of storage-damaged human RBCs.
When evaluating storage solutions, RBC osmotic fragility is utilized as a measure of RBC susceptibility to hemolysis, making knowledge of species-specific osmotic fragility critical to the development of proper storage protocols.4,14 To date, studies evaluating the osmotic fragility of Cc and Cm have not been reported. Our objective was to obtain baseline measurements for osmotic fragility in Cc and Cm. We tested the hypothesis that the osmotic fragilities of both Cc and Cm RBCs are similar.
For determination of osmotic fragility, whole blood was collected from 10 Cc to 10 Cm at The Turtle Hospital, Marathon, FL. Samples were collected in accordance with Florida Fish and Wildlife Conservation Commission Marine Turtle Permit 16-021B; the study was approved by the University of Florida Institutional Animal Care and Use Committee (Protocol 201406823). All study animals were deemed clinically healthy based on physical examination and absence of any clinicopathologic abnormalities based on complete blood count and serum biochemistry profile. Housing at The Turtle Hospital consists of large pools and tanks filled with water sourced from the nearby bay. Tanks are outdoors and open to the public during business hours. All turtles sampled were recovered patients at The Sea Turtle Hospital and either awaiting release or permanent residents at the hospital. Samples were collected from October 2016 through January 2017. Turtles were manually caught from their tanks and restrained for sampling. Based on notch-to-notch and notch-to-tip measurements, all turtles were adult, and of undetermined sex. From each turtle, after surgical cleaning of the venipuncture site, a venous blood sample (1.5 mL) was collected into a 3-mL lithium heparin blood collection tube (Vacutainer; Fisher Scientific) from either the right or left dorsal cervical sinus using a 21-ga, 5-cm needle and a 3-mL syringe.
Insulated samples were shipped overnight on wet ice to the University of California–Davis School of Veterinary Medicine, Veterinary Blood Bank at the UC Davis William R. Pritchard Veterinary Medical Teaching Hospital for analysis. Samples were maintained at 4–6°C until evaluation, which was within 36 h of collection. Upon arrival, grossly visible hemolysis was absent in all specimens, and an aliquot of the sample was spun down and the supernatant evaluated (Plasma/Low Hb system; HemoCue America) for the presence of hemolysis.
Osmotic fragility was determined as described previously, with minor modifications.2 A range of NaCl solutions (0.0, 0.15, 0.20, 0.25, 0.30, 0.35, 0.40, 0.45, 0.50, 0.55, 0.60, 0.65, 0.70, and 0.90%) was used to produce a gradient such that the range of NaCl concentrations could be observed from hemolysis initiation through complete hemolysis. Complete hemolysis was confirmed via microscopic examination of the fluid. The series of solutions contained a 0.0% NaCl solution (distilled water) to ensure complete lysis, and a 0.9% NaCl solution that did not produce any cell lysing. In brief, 100 µL of well-mixed anticoagulated whole blood was added to 5 mL of increasing concentrations of NaCl (0.00–0.90%), mixed, incubated for 1 h at room temperature, and then centrifuged for 5 min at 2,500 × g (Sorvall T1 centrifuge; Thermo Scientific). The optical density (OD) at 540 nm of 500 μL of the supernatant was measured by spectrophotometry (Ultrospec 3300 Pro spectrophotometer; Amersham Biosciences). ODs were measured once on each blood sample for each NaCl dilution.
Hemolysis was considered 0% in 0.9% NaCl and 100% in 0.0% NaCl. The percent hemolysis in each sample was calculated with the use of the following equation: % hemolysis = (OD sample)/(OD in 0% saline) × 100%.10 A sigmoid fragility curve was drawn by plotting % hemolysis on the y-axis and NaCl concentration on the x-axis (Figs. 1, 2). Mean NaCl concentrations at which 50% hemolysis occurred were identified as the point where the osmotic fragility curve intersected the point of 50% lysis. In both species, 50% hemolysis occurred at 0.38% NaCl concentration (Figs. 1, 2).
Figure 1.
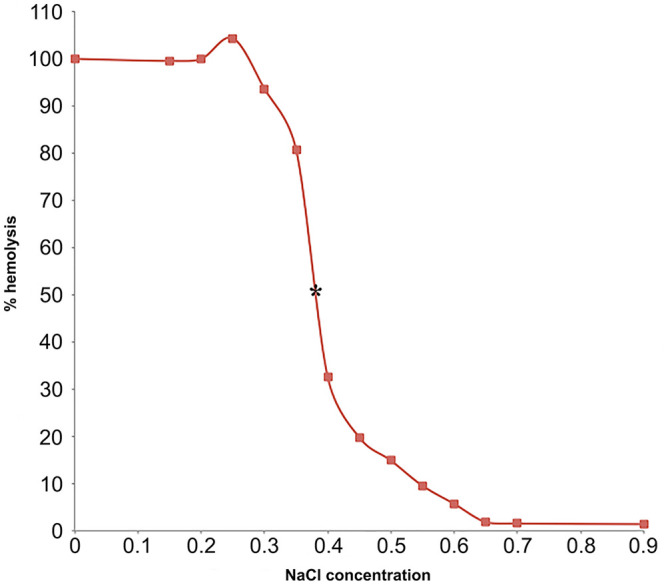
Osmotic fragility curve of loggerhead sea turtle (Caretta caretta) red blood cells; 50% hemolysis occurs at 0.38% [NaCl] marked with asterisk (*).
Figure 2.
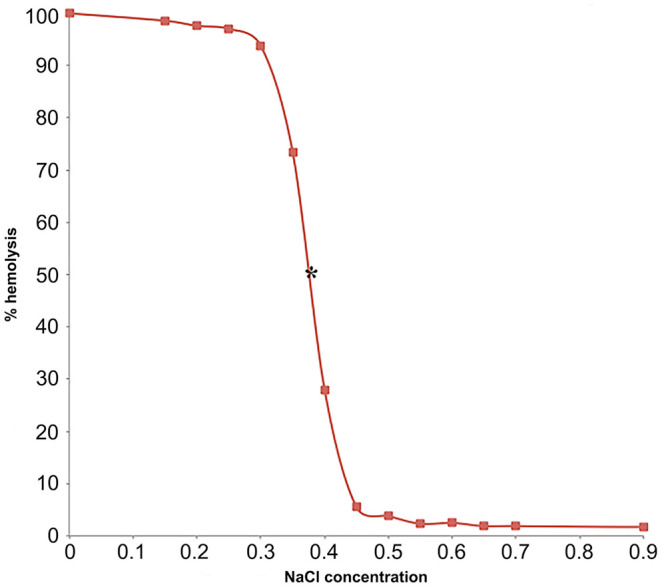
Osmotic fragility curve of green sea turtle (Chelonia mydas) red blood cells; 50% hemolysis occurs at 0.38% [NaCl] marked with asterisk (*).
Statistical evaluation of all data points was performed with commercial software (Analyse-it). All data were analyzed using a Student t-test with p ≤ 0.05 considered significant; data were normally distributed. There was no statistically significant difference in the osmotic fragility measurements between Cc and Cm (p = 0.074). No outliers were found in the data.
Our study provides important and novel information regarding the erythrocyte osmotic fragility of Cc and Cm, laying the foundation for future evaluations of commercial storage media for preservation of sea turtle RBC products. Generally, it has been noted that nucleated erythrocytes are more resistant to osmotic stress than non-nucleated erythrocytes.2,22 In our study, and in both species, 50% hemolysis occurred at 0.38% NaCl concentration. By comparison, 50% hemolysis occurred at 0.44% NaCl concentration in healthy dogs in one study,15 and at 0.45–0.57% NaCl in healthy cats in another study.21 A comparative study on osmotic fragilities found that amphibians were the most resistant to osmotic stress followed by reptiles, birds, and then mammals.2 Compared to the mammals examined in the study, all of the ectothermic vertebrates examined had RBCs that were more osmotically resistant.2 Proposed physiologic mechanisms for this difference included size of RBCs, the presence of nuclei in RBCs, body temperature, and osmoregulation. Among reptiles, there was no significant difference in fragilities for aquatic or terrestrial species.2
Reptile erythrocytes are thought to be more resistant to lysis because their ectothermic nature requires their erythrocytes to be exposed to a wider range of body temperatures. Furthermore, ectothermic erythrocytes have longer lifespans than endothermic erythrocytes. Therefore, they would need to be more osmotically resistant to account for their long survival time. Finally, nucleated erythrocytes are more osmotically resistant than non-nucleated, and larger erythrocytes are more resistant than small (i.e., lower NaCl concentrations at which 50% hemolysis occurred).2
There are a few options for anticoagulants used in the processing of reptilian blood. To date, heparin is used most commonly and was the anticoagulant in our study. And, given that K2-EDTA can cause erythrolysis in chelonians,13 we used heparin to avoid erythrocyte lysis associated with other anticoagulants.
There are limitations in our study beyond small sample size. For one, an explanation of the increase in percent hemolysis above 100% may be the result of residual binding of hemoglobin to erythrocyte membranes.22,24 Such binding would falsely lower the OD in samples in which it occurred, which might affect the osmotic fragility curve and calculated 50% RBC lysis. Only sampling turtles in rehabilitation settings limits data to those in very controlled environments in terms of water quality and temperature. Furthermore, all of these turtles sampled are therefore nonbreeding. Variations in osmotic fragility are likely in those turtles that are in more natural habitats, given increased diversity in age, sex, breeding status, and environment. Future work could be performed examining sea turtles in different environments—such as sampling those migrating versus those nesting because water temperature difference and seasonal variation may play a role in sea turtle erythrocyte osmotic fragility.
Acknowledgments
We thank Dr. Dori Borjesson, Naomi Walker, and the staff of the UC Davis School of Veterinary Medicine Blood Bank and Hematology Lab; Samantha Clark and Ashley Isaac at Loggerhead Marinelife Center; and Caitlin Greene, Richie Moretti, and rehabilitation technicians and staff at The Turtle Hospital.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This work was supported in part by funding from the UC Davis School of Veterinary Medicine Students Training in Advanced Research (STAR) Program
Contributor Information
Rebecca Radisic, Department of Pathology, Microbiology, and Immunology, School of Veterinary Medicine, University of California–Davis, Davis, CA.
Sean D. Owens, Department of Pathology, Microbiology, and Immunology, School of Veterinary Medicine, University of California–Davis, Davis, CA.
Charles A. Manire, Veterinary Services, Juno Loggerhead Marinelife Center, Juno Beach, FL
Nicole Montgomery, Veterinary Services, Juno Loggerhead Marinelife Center, Juno Beach, FL.
Doug Mader, The Turtle Hospital, Marathon, FL.
Bette Zirkelbach, The Turtle Hospital, Marathon, FL.
Nicole I. Stacy, Department of Comparative, Diagnostic, and Population Medicine, College of Veterinary Medicine, University of Florida, Gainesville, FL
References
- 1. Acker JP, et al. A quality monitoring program for red blood cell components: in vitro quality indicators before and after implementation of semiautomated processing. Transfusion 2014;54:2534–2543. [DOI] [PubMed] [Google Scholar]
- 2. Aldrich K, et al. Comparison of erythrocyte osmotic fragility among amphibians, reptiles, birds and mammals. Trans Kansas Acad Sci 2006;109:149–158. [Google Scholar]
- 3. Almizraq R, et al. Storage of red blood cells affects membrane composition, microvesiculation, and in vitro quality. Transfusion 2013;53:2258–2267. [DOI] [PubMed] [Google Scholar]
- 4. Alshalani A, et al. Impact of blood manufacturing and donor characteristics on membrane water permeability and in vitro quality parameters during hypothermic storage of red blood cells. Cryobiology 2018;80:30–37. [DOI] [PubMed] [Google Scholar]
- 5. Aung HH, et al. Procoagulant role of microparticles in routine storage of packed red blood cells: potential risk for prothrombotic post-transfusion complications. Pathology 2017;49:62–69. [DOI] [PubMed] [Google Scholar]
- 6. Barshtein G, et al. Storage-induced damage to red blood cell mechanical properties can be only partially reversed by rejuvenation. Transfus Med Hemother 2014;41:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beutler E, et al. The osmotic fragility of erythrocytes after prolonged liquid storage and after reinfusion. Blood 1982;59:1141–1147. [PubMed] [Google Scholar]
- 8. Blasi B, et al. Red blood cell storage and cell morphology. Transfus Med 2012;22:90–96. [DOI] [PubMed] [Google Scholar]
- 9. Emerson J, et al. Preservation of red blood cells in the American alligator (Alligator mississippiensis) over time and in two different anticoagulants. J Herpetol Med Surg 2014;24:82–94. [Google Scholar]
- 10. Ezell GH, et al. The osmotic fragility of some fish erythrocytes in hypotonic saline. Comp Biochem Physiol 1969;28:409–415. [DOI] [PubMed] [Google Scholar]
- 11. Igbokwe NA. A review of the factors that influence erythrocyte osmotic fragility. Sokoto J Vet Sci 2018;16:1–23. [Google Scholar]
- 12. Mudge MC, et al. Comparison of 4 blood storage methods in a protocol for equine pre-operative autologous donation. Vet Surg 2004;33:475–486. [DOI] [PubMed] [Google Scholar]
- 13. Muro J, et al. Effects of lithium heparin and tripotassium EDTA on hematologic values of Hermann’s tortoises (Testudo hermanni). J Zoo Wildl Med 1998;29:40–44. [PubMed] [Google Scholar]
- 14. Oyewale JO. Changes in osmotic fragility of nucleated erythrocytes resulting from blood storage. Zentralbl Veterinarmed A 1994;41:475–479. [DOI] [PubMed] [Google Scholar]
- 15. Paes G, et al. The use of the rapid osmotic fragility test as an additional test to diagnose canine immune-mediated haemolytic anaemia. Acta Vet Scand 2013;55:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Patterson J, et al. In vitro lysis and acute transfusion reactions with hemolysis caused by inappropriate storage of canine red blood cell products. J Vet Intern Med 2011;25:927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Phillips B, et al. Evaluation of three anticoagulants used for short-term storage of loggerhead sea turtle (Caretta caretta) whole blood. J Herpetol Med Surg 2017;27:97–103. [Google Scholar]
- 18. Rifkind JM, et al. The pathophysiology of extracellular hemoglobin associated with enhanced oxidative reactions. Front Physiol 2014;5:500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sarkar M, et al. The effect of anti-coagulants on the osmotic fragility of erythrocytes in the yak (Poephagus grunniens). Vet J 1999;157:91–93. [DOI] [PubMed] [Google Scholar]
- 20. Stacy NI, et al. Chronic debilitation in stranded loggerhead sea turtles (Caretta caretta) in the southeastern United States: morphometrics and clinicopathological findings. PLoS One 2018;13:e0200355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tritschler C, et al. Increased erythrocytic osmotic fragility in anemic domestic shorthair and purebred cats. J Feline Med Surg 2016;18:462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Viscor G, et al. Method of determining the osmotic fragility curves of erythrocytes in birds. Lab Anim 1982;16:48–50. [DOI] [PubMed] [Google Scholar]
- 23. Wardrop KJ. Selection of anticoagulant-preservatives for canine and feline blood storage. Vet Clin North Am Small Anim Pract 1995;25:1263–1276. [DOI] [PubMed] [Google Scholar]
- 24. Welbourn EM, et al. The mechanism of formation, structure and physiological relevance of covalent hemoglobin attachment to the erythrocyte membrane. Free Radic Biol Med 2017;103:95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]


