Abstract
Study Design:
Retrospective review
Objective:
To determine if adjuvant radiation therapy (RT) improves overall survival (OS) following surgical resection of chordomas.
Summary of Background Data:
The role of RT for the treatment of chordomas remains incompletely described. Previous studies have not found adjuvant RT to improve OS, but these studies did not group patients based on surgical margin status or radiation dose or modality. We used the National Cancer Database to investigate the role of RT in chordomas following surgical resection.
Methods:
Patients were stratified based on surgical margin status (positive vs negative). Utilizing the Kaplan Meier method, OS was compared between treatment modalities (surgical resection alone, therapeutic RT alone, and surgical resection plus therapeutic RT). OS was subsequently compared between patients treated with palliative dose (<40Gy), low dose (40– 65Gy), and high dose (>65Gy) RT. Similarly, OS was compared between advanced RT modalities including proton beam therapy (PBT) and intensity-modulated radiation therapy (IMRT), stereotactic radiosurgery (SRS), and external beam radiation therapy (EBRT). A multivariable model was used to determine adjusted variables predictive of mortality.
Results:
1,478 chordoma patients were identified; skull base (n=567), sacral (n=551), and mobile spine (n=360). Surgical resection and therapeutic adjuvant RT improved 5-year survival in patients with positive surgical margins (82% vs 71%, p=0·03). No clear survival benefit was observed with the addition of adjuvant RT in patients with negative surgical margins. High dose RT was associated with improved OS compared to palliative and low dose RT (p<0·001). Advanced RT techniques and SRS were associated with improved OS compared to EBRT. In the multivariate analysis high dose advanced RT (>65Gy) was superior to EBRT.
Conclusion:
Patients with positive surgical margins benefit from adjuvant RT. Optimal OS is associated with adjuvant RT administered with advanced techniques and cumulative dose >65 Gy.
Keywords: chordoma, radiation therapy, overall survival, surgical resection
Mini-Abstract:
The purpose of this study was to determine the role for radiation therapy (RT) in chordoma patients following surgical resection. A retrospective review of 1,478 chordoma patients was performed. Therapeutic RT improves survival in the setting of positive surgical margins.
INTRODUCTION:
Chordomas are low-grade neoplasms that arise from undifferentiated notochordal remnants along the axial skeleton.1 Historically, the low incidence of these tumors made it difficult to identify patient demographics, tumor characteristics, and therapeutic vulnerabilities. Specifically, the role for radiation therapy (RT) for chordoma patients remains incompletely described and practices are not uniform. The advent of tumor registries provided an opportunity to obtain epidemiologic, treatment, and survival data for chordomas on a national scale.
Despite adjuvant RT being commonly utilized, it has not been statistically shown to improve survival in previous database studies. The failure to demonstrate improved survival with RT was potentially related to the under-representation of contemporary radiation treatment modalities. Specifically, a large percentage of these patients received external beam radiation therapy (EBRT) with what would today be considered an inadequate radiation dose.
Previous database studies have investigated RT in a binary manner, patients either received or did not receive RT.2 However, the administration of RT for chordoma patients is not uniform and varies by the type of radiation, method of radiation delivery, radiation fraction size, and total radiation dose. In addition, these previous studies did not differentiate patients based on surgical margin status. To our knowledge, no adequately powered study has investigated the role for RT following incomplete R1/2 surgical resection.3
The primary objective of this study was to compare overall survival (OS) rates in chordoma patients with R1/2 surgical resections treated with or without therapeutic adjuvant RT. The secondary objective was to determine the impact of radiation dose and radiation modality on OS.
METHODS:
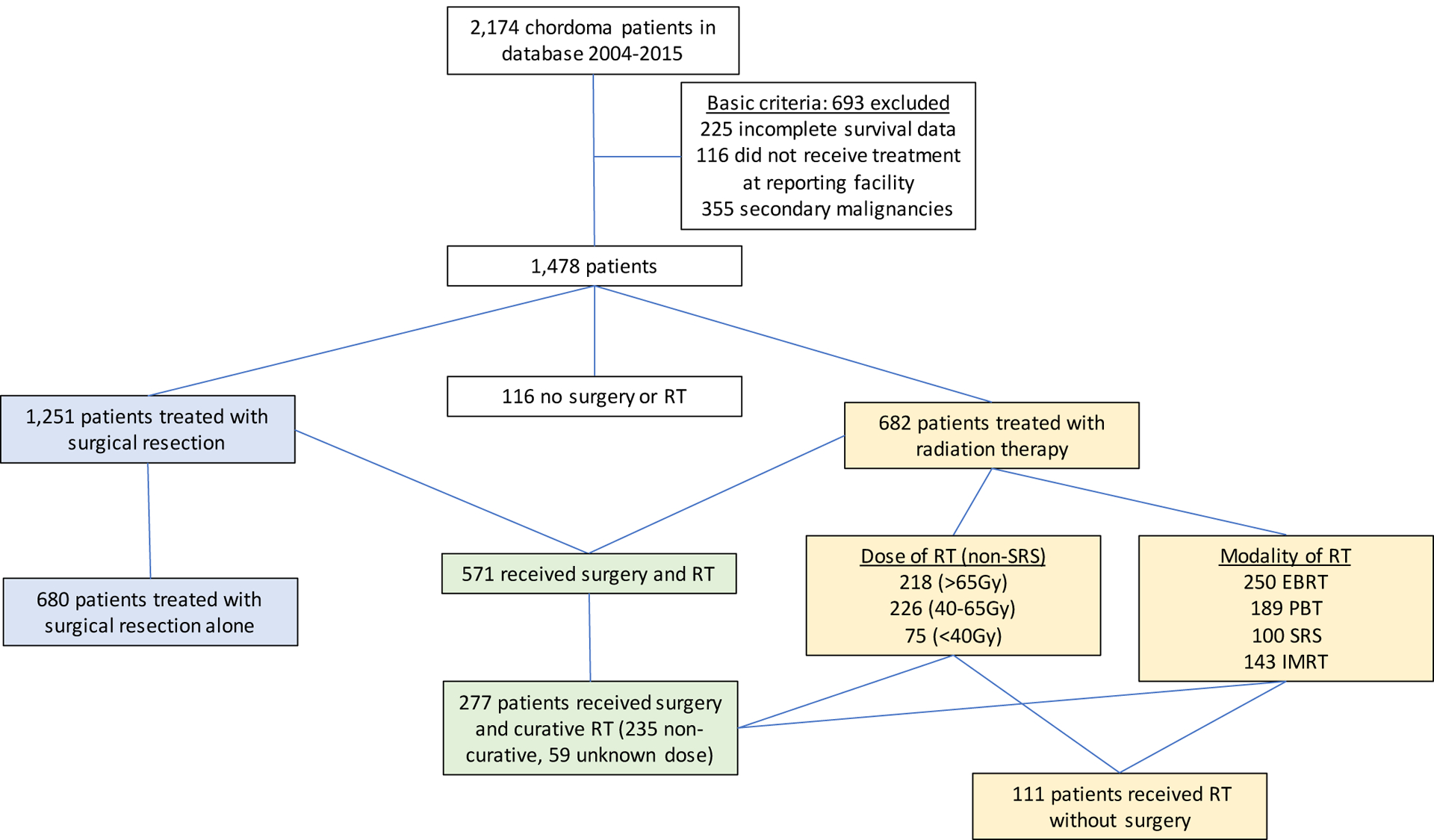
Chordoma patients treated between 2004 and 2015 were identified within the National Cancer Database (NCDB). The NCDB is the most complete tumor registry in the world capturing 70% of all new cancer diagnoses from over 1,500 Commission on Cancer-accredited institutions. The Participant User File was searched for patients with chordomas of the skull base, mobile spine, and sacrum. The patients were identified using the International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) topography codes C41.2 (Bones and Joints: Vertebral column). Patients were excluded because of incomplete survival data, if they did not receive any treatment for the chordoma at the reporting facility (NCDB Class of Case 00), and because of secondary malignancies (NCDB Sequence number >0) (Figure 1).
Figure 1:
Overall Survival by Treatment
Patient characteristics, tumor characteristics, and treatment characteristics were collected from the NCDB dataset. Conventional radiation modalities included EBRT and conformal techniques. Advanced radiation modalities included intensity modulated radiation therapy (IMRT), stereotactic radiosurgery (SRS) which included Gamma-Knife and LINAC techniques, and proton beam therapy (PBT).
The unadjusted OS rate between patients treated with surgery alone, surgery and RT, and RT alone were compared utilizing the Kaplan Meier (KM) method. The unadjusted OS rate was next compared between patients with positive and negative surgical margins treated with or without therapeutic RT. Therapeutic RT was defined as EBRT, PBT, or IMRT with total dose received >65Gy, or any patient who received SRS regardless of dose. Within the NCDB, patients coded as “no residual tumor” were considered to have negative surgical margins, and patients coded as “microscopic residual tumor,” “macroscopic residual tumor,” and “residual tumor NOS” were considered to have positive surgical margins. Because SRS utilizes a high radiation dose per day, but few treatment days, the biological effective dose of the total dose delivered cannot be directly compared to the total dose from standard radiation fraction sizes.
For the secondary study objective, patients not receiving SRS were divided into three cumulative dose RT groups; palliative dose (<40 Gy), low-dose (40–65 Gy), and high-dose (>65 Gy). They were also categorized by radiation modality, EBRT (including conformal techniques), SRS, IMRT, and PBT. An unadjusted survival analysis was performed to determine the OS rates between palliative dose, low dose, and high dose RT; and to determine the OS rate between conventional and advanced modalities.
Lastly, a multivariable regression model was used to adjust for baseline patient characteristics, tumor characteristics, and treatment variables to determine the adjusted hazard ratio when comparing treatment types.
Statistical Analysis
Comparisons across groups were performed using the chi-square test or Fisher’s exact test for categorical variables and the Wilcoxon rank-sum test for continuous variables.
The KM estimator with the log-rank test was used to assess survival across stratified groups of patients based on treatment type (surgery alone, surgery and RT, and RT alone), as well as to assess survival across radiation doses (<40 Gy, 40–65, >65 Gy) and between radiation modality groups (advanced, conventional, or SRS). The log-rank tests consider all follow-up available while the KM plots were truncated at year five for presentation. The fixed-point survival rates at year five were compared between groups using Greenwood’s KM variance formula. The alpha for multiple pairwise comparisons was adjusted using Tukey’s method.
A multivariable Cox proportional hazards (PH) model was used to adjust patient baseline, tumor, and treatment characteristics associated with increased mortality and possibly confounded with patient treatment across all available follow-up. We used the Kolmogorov-type supremum test, test of time interaction effects, and graphical evaluation of the hazard function over time to determine if the hazard function for each variable met the linearity and proportional hazards assumptions required of the Cox model. Multiple imputation was then used to estimate missing values in order to maximize the effective sample size and avoid spurious results for the regression model. Thirty imputed datasets were constructed in a two-step process, using a monotone Markov chain and then regression methods. Each of the 30 complete datasets was analyzed using the Cox PH model, with parameter estimates (coefficients and standard errors) from each imputation combined for inference. The hazard ratio (HR) with 95% confidence interval (CI) were reported for each covariate, as well as for contrasts of interest. A p-value < 0.05 indicates statistical significance, and statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS:
A total of 1,478 patients were identified. Skull base chordomas were the most frequently represented (n=567, 38·4%), followed by sacral chordomas (n=551, 37·3%) and mobile spine chordomas (n=360, 24·4%). Surgical resection was performed on 1,251 patients (84·6%). RT was utilized in 682 patients (46·1%). 571 patients (38·6%) were managed with surgical resection and RT, 524 patients received RT following surgery (92%), 37 patients received RT before surgery (6·5%), and it was unknown if RT was before or after surgery in 10 patients (1·7%). RT was more commonly utilized in patients with positive surgical margins following surgery compared to negative surgical margins (64·4% v. 32·1%, p<0·001) (Table 2). RT was used as monotherapy in 111 patients (7·5%). Lastly, 116 patients (7·8%) did not undergo any form of treatment and these patients were excluded from the KM and multivariable Cox PH models (Table 2).
Table 2:
Baseline patient and tumor characteristics by radiation modality and dose
| Radiation Modality | Radiation Dose1 | |||||
|---|---|---|---|---|---|---|
| Variable | Advanced (n=332) | Conventional (n=250) | SRS (n=100) | <40 Gy (n=75) | 40–65 Gy (n=226) | >65 Gy (n=218) |
| Age, years ***††† | 50 (20) | 56 (20) | 58 (18) | 57 (20) | 56 (19) | 49 (20) |
| Female sex | 141 (42.5%) | 99 (39.6%) | 42 (42.0%) | 36 (48.0%) | 93 (41.2%) | 87 (39.9%) |
| Race | ||||||
| Caucasian | 286 (86.1%) | 210 (84.0%) | 80 (80.0%) | 61 (81.3%) | 187 (82.7%) | 192 (88.1%) |
| Black | 16 (4.8%) | 15 (6.0%) | 10 (10.0%) | 6 (8.0%) | 15 (6.6%) | 8 (3.7%) |
| Asian | 20 (6.0%) | 13 (5.2%) | 7 (7.0%) | 4 (5.3%) | 13 (5.8%) | 14 (6.4%) |
| Other | 10 (3.0%) | 12 (4.8%) | 3 (3.0%) | 4 (5.3%) | 11 (4.9%) | 4 (1.8%) |
| Comorbidity Score ≥ 2 | 9 (2.7%) | 9 (3.6%) | 5 (5.0%) | 5 (6.7%) | 6 (2.7%) | 3 (1.4%) |
| Income above median† | n=325 | n=248 | n=99 | n=74 | n=222 | n=217 |
| 219 (67.4%) | 160 (64.5%) | 65 (65.7%) | 53 (71.6%) | 135 (60.3%) | 152 (71.0%) | |
| Insurance†† | n=331 | n=245 | n=99 | n=74 | n=222 | n=217 |
| Private | 197 (59.5%) | 129 (52.7%) | 49 (49.5%) | 38 (51.4%) | 107 (48.2%) | 141 (65.0%) |
| Government | 118 (35.6%) | 107 (43.7%) | 49 (49.5%) | 34 (45.9%) | 97 (43.7%) | 71 (32.7%) |
| Uninsured | 16 (4.8%) | 9 (3.7%) | 1 (1.0%) | 2 (2.7%) | 18 (8.1%) | 5 (2.3%) |
| Academic Facility *††† | n=236 | n=200 | n=80 | n=63 | n=180 | n=151 |
| 183 (77.5%) | 133 (66.5%) | 54 (67.5%) | 51 (81.0%) | 110 (61.1%) | 124 (82.1%) | |
| Tumor Size > 5 cm ***† | n=241 | n=196 | n=76 | n=55 | n=171 | n=167 |
| 97 (40.2%) | 106 (54.1%) | 17 (22.4%) | 32 (58.2%) | 88 (51.5%) | 65 (38.9%) | |
| Tumor Site ***††† | ||||||
| Skull | 168 (50.6%) | 70 (28.0%) | 56 (56.0%) | 19 (25.3%) | 80 (35.4%) | 113 (51.8%) |
| Spine | 96 (28.9%) | 75 (30.0%) | 29 (29.0%) | 31 (41.3%) | 60 (26.5%) | 61 (28.0%) |
| Pelvis | 68 (20.5%) | 105 (42.0%) | 15 (15.0%) | 25 (33.3%) | 86 (38.1%) | 44 (20.2%) |
| Metastases **† | n=320 | n=237 | n=94 | n=74 | n=214 | n=207 |
| 3 (0.9%) | 12 (5.1%) | 0 (0.0%) | 4 (5.4%) | 9 (4.2%) | 1 (0.5%) | |
| Surgery **†† | n=218 | n=178 | n=73 | n=56 | n=162 | n=138 |
| Positive Margins | 100 (45.9%) | 55 (30.9%) | 33 (45.2%) | 17 (30.4%) | 58 (35.8%) | 65 (47.1%) |
| Negative Margins | 84 (38.5%) | 66 (37.1%) | 20 (27.4%) | 17 (30.4%) | 60 (37.0%) | 52 (37.7%) |
| No Surgery | 34 (15.6%) | 57 (32.0%) | 20 (27.4%) | 22 (39.3%) | 44 (27.2%) | 21 (15.2%) |
| 1RT Dose *** | n=296 | n=223 | ||||
| <40 Gy | 20 (6.8%) | 55 (24.7%) | ||||
| 40–65 Gy | 117 (39.5%) | 109 (48.9%) | ||||
| >65 Gy | 159 (53.7%) | 59 (26.5%) | ||||
Note:
Dose groups only given if RT modality is Advanced or Conventional.
p<0.05,
p<0.01 and
p<0.001 for comparing modality groups.
p<0.05,
p<0.01 and
p<0.001 for comparing dose groups. Values given as mean (SD) or as count (percentage).
The 5-year survival rate for all chordoma patients was 76·1% (95%CI: 73–78%). The 5-year survival rates for skull base, mobile spine, and sacral chordomas were 84% (95% CI 80–87%), 70% (95% CI 65–76%), and 72% (95% CI 67–76%) respectively. For the entire cohort, surgical resection and therapeutic RT had the highest 5-year survival rate at 84·75% (95%CI: 79–89%) followed by surgical resection alone (79·7%; 95%CI:76–83%), and RT alone (61·9%; 95%CI: 42–77%), with the rate of survival significantly different only between RT only and surgery and RT (p=0·005) (Figure 2). Overall, a negative surgical margin following surgical resection was achieved in 64·5% of chordoma patients (79·2% sacral chordomas, 65·1% mobile spine chordomas, and 45·7% skull base chordomas) (Table 2). For patients with positive surgical margins, the 5-year survival rate was higher for patients treated with surgical resection and therapeutic RT (82·3%; 95%CI: 72–89%) compared to surgical resection alone (70·6; 95%CI: 60–79%) with a statistically different overall survival rate (p=0·047) (Figure 1). For patients with negative surgical margins the OS between patients treated with surgical resection and therapeutic adjuvant RT was similar to patients treated with surgical resection alone (p=0·889) with 5-year survival rates of 88.2% (95%CI: 75–95%) and 85.1% (95%CI: 80–89%) respectively (Figure 3). For patients receiving RT, the modality of therapy was available for 682 patients. Conventional external-beam radiation (EBRT) was utilized in 250 (36·7%) patients and advanced techniques were used in 432 (63·3%). The advanced techniques included PBT (n=189), IMRT (n=143), or SRS (n=100). The highest 5-year survival was seen within the advanced RT group (81·2%; 95%CI: 76–85%) followed by SRS (74·9%; 95%: 64–83%) and conventional (68·2; 95%CI: 61–75%) with survival rates for both advanced (p<0·001) and SRS (p=0·003) significantly higher than conventional RT (Figure 4). The survival rate for patients receiving PBT (p<0·001), SRS (p=0·006), or IMRT (p=0·011) were all statistically higher compared to EBRT, but not statistically different amongst themselves. The total dose of RT was analyzed for 599 patients. The dose was palliative (<40 Gy) in 75 patients, low dose (40–65 Gy) in 226 patients, and high dose (>65 Gy) in 218 patients. The high dose radiation group had significantly improved overall survival rates compared to the low dose radiation group (p<0·001) with 5-year survival rates of (85·1%; 95%CI: 79–90%) and (69·3%; 95%CI 61–76%), respectively (Figure 5).
Figure 2:
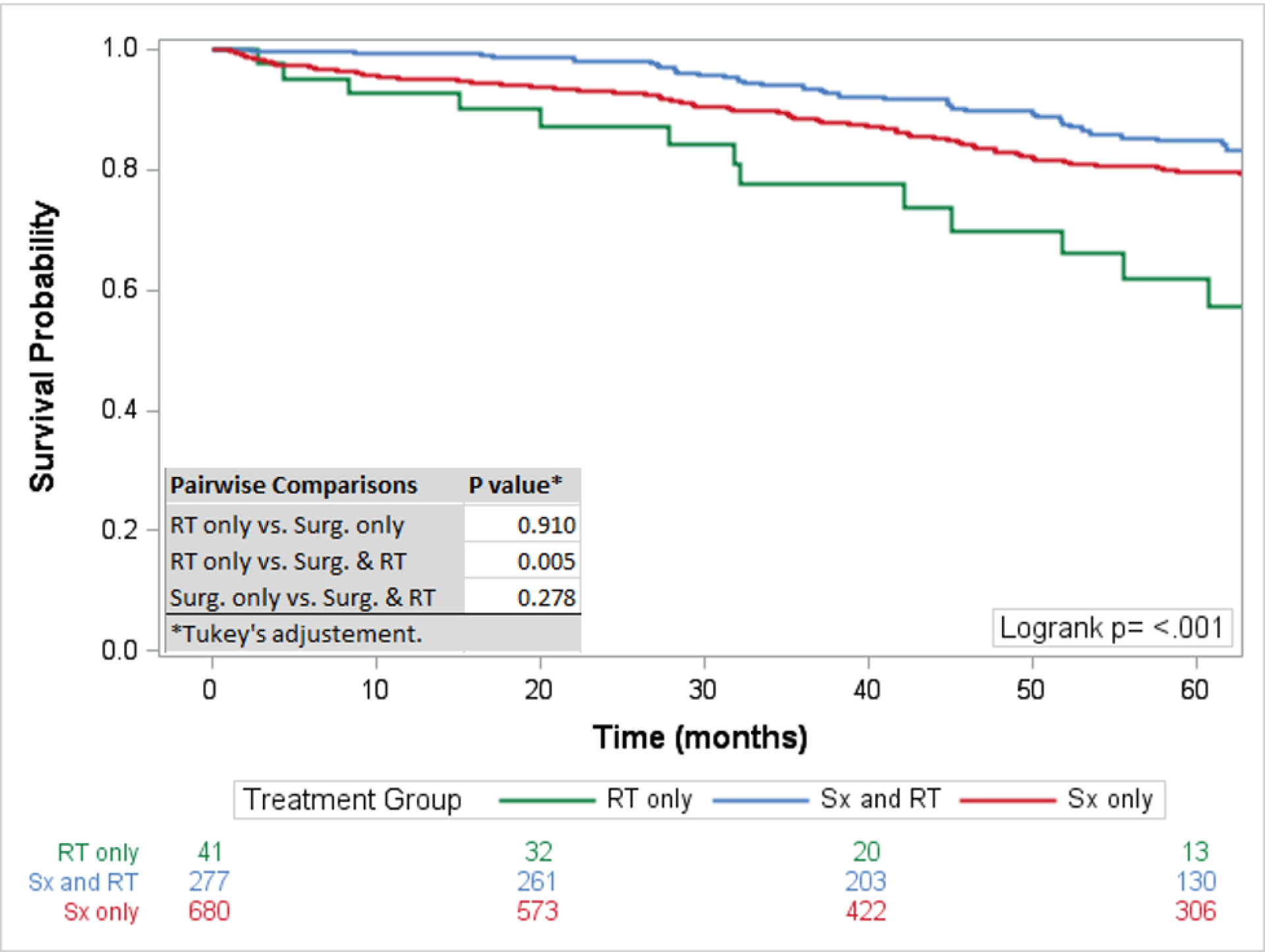
Overall Survival by Treatment: Survival analysis comparing overall survival by type of treatment. Statistically significant improved survival seen with surgical resection and radiation therapy compared to radiation therapy alone.
Figure 3:
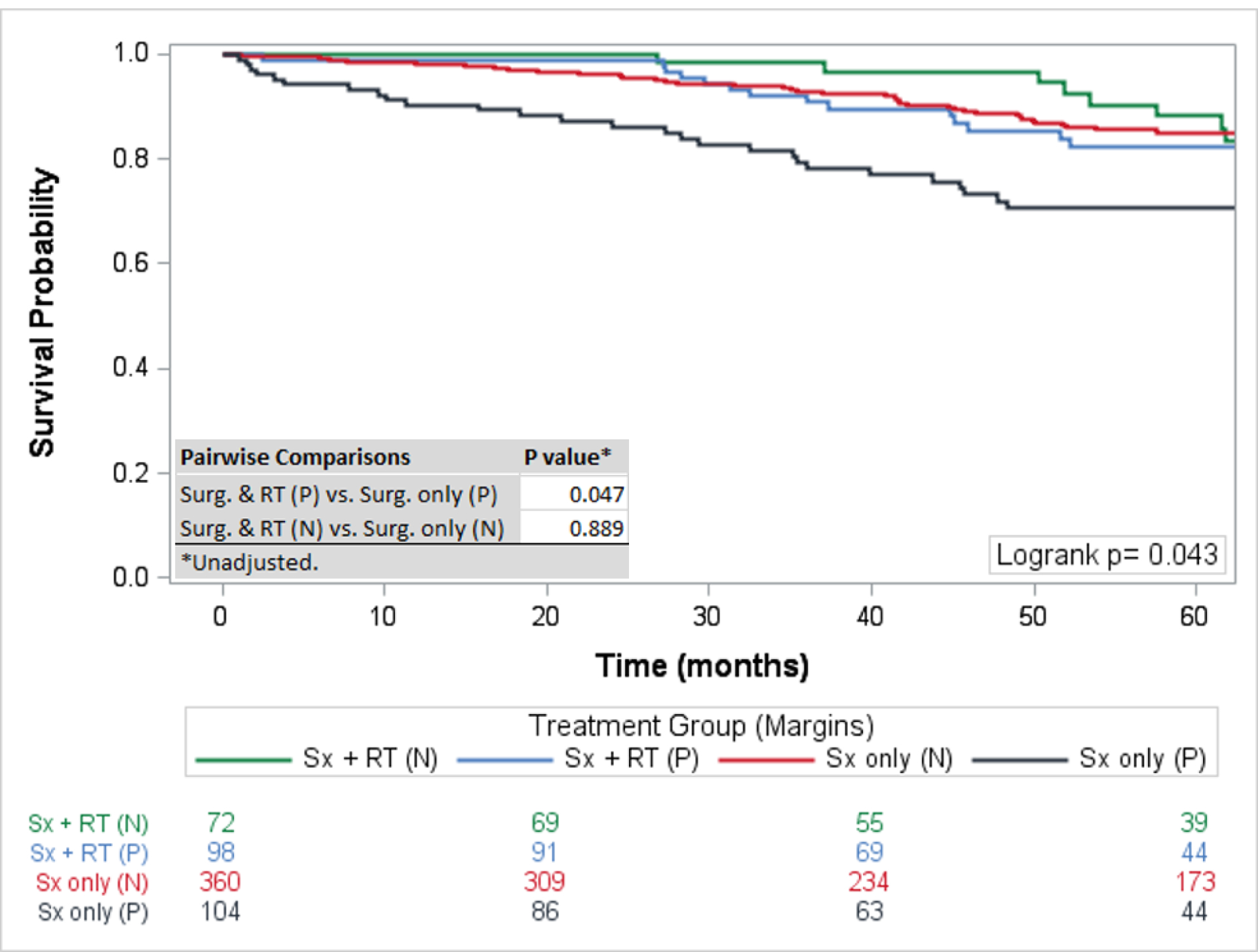
Overall Survival by Treatment and Margins: Statistically significant improved survival was observed for patient’s with positive surgical margins treated with surgical resection and radiation therapy. In patients with negative surgical margins the addition of radiation therapy did not statistically improve overall survival.
Figure 4:
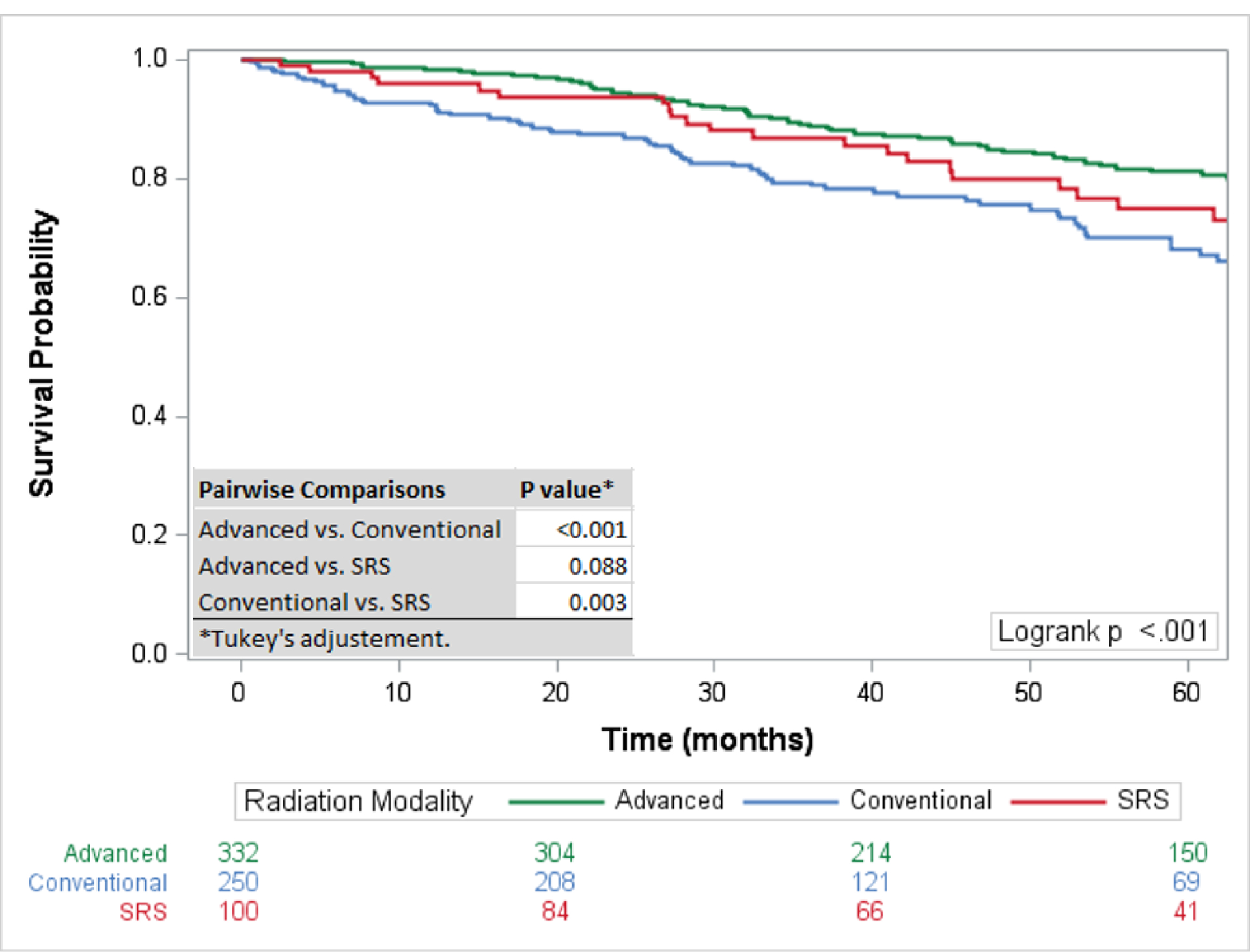
Overall Survival by Radiation Modality: Advanced radiation therapy and stereotactic radiation therapy demonstrated improved survival compared to conventional radiation therapy. No statistically significant improved survival was observed between advanced radiation therapy and stereotactic radiation therapy.
Figure 5:
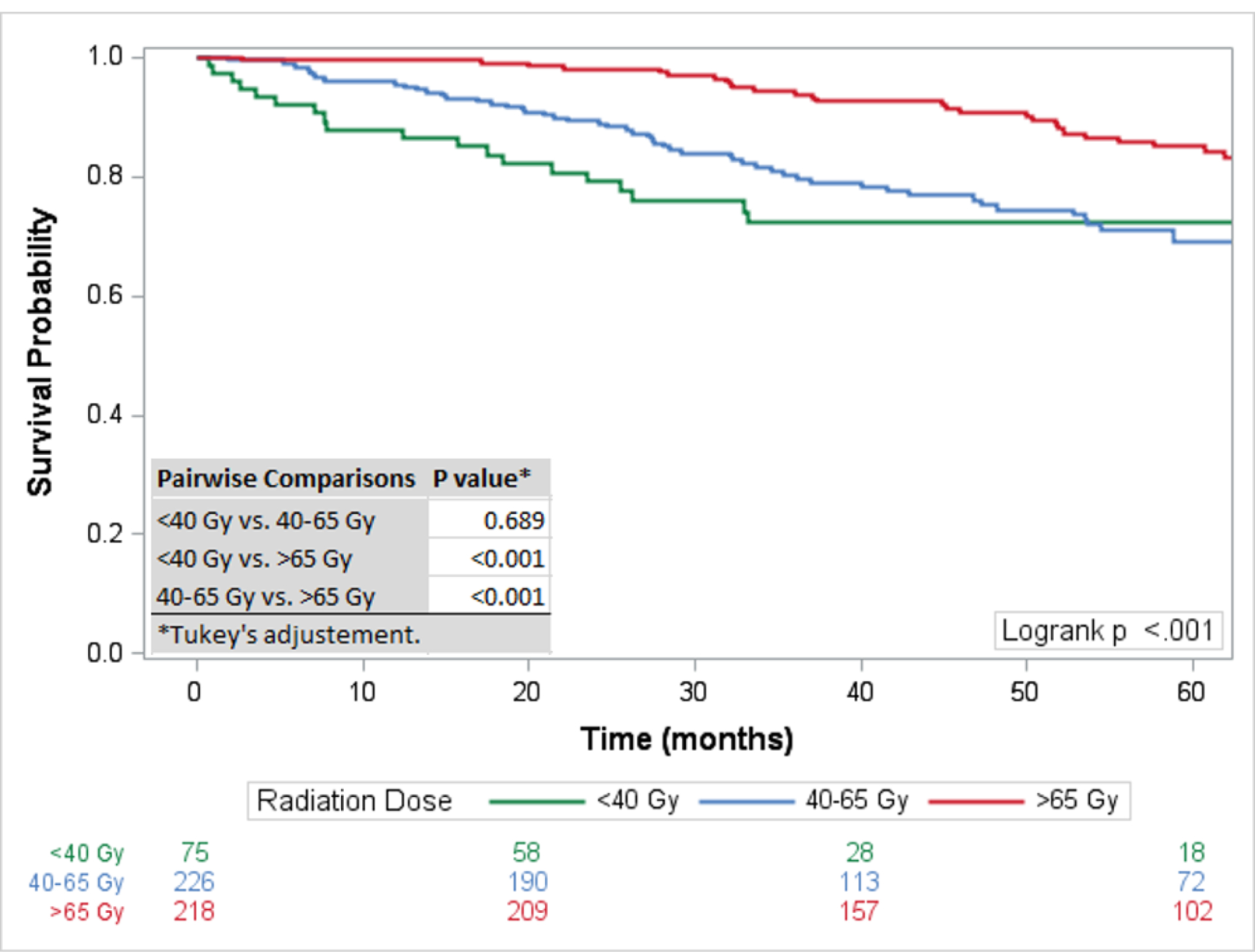
Overall Survival by Radiation Dose: Radiation dose >65Gy was associated with statistically significant improved survival compared to radiation doses of 40–65Gy and <40Gy.
Patient demographics and tumor characteristics between treatment groups were obtained and documented (Table 1). Sociodemographic characteristics were largely similar among treatment groups, with the exception of patients treated with high dose RT being younger (median: 52 years, Q1–Q3: 36–64, p<0·001), having private insurance (65·0%, p=0·001), and being treated at academic centers (82·1%, p<0·001). RT was utilized for 43·3% of skull base chordomas, 29·2% of mobile spine chordomas, and 27·5% of sacral chordomas (Table 2). Advanced RT techniques were utilized most frequently for skull base chordomas (51·9%, p<0·001), and least commonly for sacral chordomas (19·2%) (Table 2). Similarly, high dose RT was used most frequently for skull base chordomas, compared to low dose and palliative (51·8%, p<0·001), and least commonly for sacral chordomas (20·2%) (Table 1).
Table 1:
Baseline patient and tumor characteristics
| Treatment Group | ||||||
|---|---|---|---|---|---|---|
| Variable | Overall (n=1,478) | None (n=116) | Sx Only (n=680) | Sx + RT (n=571) | RT Only (n=111) | P value |
| Age, years | 54 (19) | 66 (18) | 52 (19) | 50 (19) | 70 (16) | <0.001 |
| Female sex | 604 (40.9%) | 49 (42.2%) | 273 (40.1%) | 231 (40.5%) | 51 (45.9%) | 0.691 |
| Race | 0.604 | |||||
| Caucasian | 1,252(84.7%) | 103 (88.8%) | 573 (84.3% | 485 (84.9%) | 91 (82.0%) | |
| Black | 88 (6.0%) | 4 (3.4%) | 43 (6.3%) | 34 (6.0%) | 7 (6.3%) | |
| Asian | 77 (5.2%) | 7 (6.0%) | 30 (4.4%) | 32 (5.6%) | 8 (7.2%) | |
| Other | 61 (4.1%) | 2 (1.7%) | 34 (5.0%) | 20 (3.5%) | 5 (4.5%) | |
| Comorbidity Score ≥ 2 | 50 (3.4%) | 4 (3.4%) | 23 (3.4%) | 17 (3.0%) | 6 (5.4%) | 0.590 |
| Income above median | n=1,458 | n=115 | n=671 | n=564 | n=108 | 0.163 |
| 920 (63.1%) | 67 (58.3%) | 409 (61.0%) | 374 (66.3%) | 70 (64.8%) | ||
| Insurance | n=1,418 | n=114 | n=629 | n=565 | n=110 | <0.001 |
| Private | 772 (54.4%) | 34 (29.8%) | 363 (57.7% | 346 (61.2%) | 29 (26.4%) | |
| Government | 593 (41.8%) | 78 (68.4%) | 241 (38.3% | 195 (34.5%) | 79 (71.8%) | |
| Uninsured | 53 (3.7%) | 2 (1.8%) | 25 (4.0%) | 24 (4.2%) | 2 (1.8%) | |
| Academic Facility | n=1,113 | n=102 | n=495 | n=412 | n=104 | <0.001 |
| 783 (70.4%) | 51 (50.0%) | 362 (73.1%) | 316 (76.7%) | 54 (51.9%) | ||
| Tumor Size > 5 cm | n=1,124 | n=78 | n=533 | n=427 | n=86 | <0.001 |
| 551 (49.0%) | 46 (59.0%) | 285 (53.5%) | 166 (38.9%) | 54 (62.8%) | ||
| Tumor Site | <0.001 | |||||
| Skull | 567 (38.4%) | 29 (25.0%) | 244 (35.9% | 280 (49.0%) | 14 (12.6%) | |
| Spine | 360 (24.4%) | 27 (23.3%) | 133 (19.6% | 167 (29.2%) | 33 (29.7%) | |
| Pelvis | 551 (37.3%) | 60 (51.7%) | 303 (44.6% | 124 (21.7%) | 64 (57.7%) | |
| Metastases | n=1,401 | n=111 | n=639 | n=542 | n=109 | <0.001 |
| 28 (2.0%) | 5 (4.5%) | 8 (1.3%) | 4 (0.7%) | 11 (10.1%) | ||
| Surgical Margins | n=1,049 | n=464 | n=358 | <0.001 | ||
| Positive | 292 (27.8%) | 104 (22.4%) | 188 (52.5%) | |||
| Negative | 530 (50.5%) | 360 (77.6%) | 170 (47.5%) | |||
| None | 227 (21.6%) | NA | NA | |||
| RT Modality | <0.001 | |||||
| Advanced | 332 (22.5%) | 298 (52.2%) | 34 (30.6%) | |||
| Conventional | 250 (16.9%) | 193 (33.8%) | 57 (51.4%) | |||
| SRS | 100 (6.8%) | 80 (14.0%) | 20 (18.0%) | |||
| None | 796 (53.9%) | NA | NA | |||
| 1RT Dose, | n=519 | n=432 | n=87 | <0.001 | ||
| <40 Gy | 75 (14.5%) | 53 (12.3%) | 22 (25.3%) | |||
| 40–65 Gy | 226 (43.5%) | 182 (42.1%) | 44 (50.6%) | |||
| >65 Gy | 218 (42.0%) | 197 (45.6%) | 21 (24.1%) | |||
Note:
Dose groups only given if RT modality is Advanced or Conventional. Sx=Surgery; RT=Radiation. Values reported as mean (SD) or as count (percentage).
A multivariable Cox PH model for the entire cohort revealed age > 65, comorbidity index ≥ 2, government insurance, no health insurance, tumor size > 5cm, metastatic disease, non-surgical treatment, a positive surgical margin, and treatment at a community center compared to an academic center to be associated with significantly decreased survival (Tables 3A and 3B). In comparing types of treatments patients who were treated with advanced radiotherapy techniques with a total radiation dose >65Gy had improved survival compared to patients treated with conventional EBRT with a total radiation dose <65Gy (HR 0·52: 95% CI: 0·33–0·84, p=0·007).
Table 3:
Multivariable analysis to identify risk factors associated with overall survival (a). Individual treatment comparisons were evaluated within the multivariate model to identify how treatment independently impacted survival (b).
| Parameter | HR (95% CI) | P value |
|---|---|---|
| Age (years) | ||
| <45 | Reference | |
| 45–60 | 1.17 (0.85, 1.62) | 0.337 |
| >60 | 2.25 (1.60, 3.16) | <0.001* |
| Comorbidity Score ≥ 2 | 1.64 (1.01, 2.66) | 0.044* |
| Community / Network Facility vs Academic | 1.46 (1.14, 1.85) | 0.002* |
| Income below median | 1.19 (0.95, 1.48) | 0.125 |
| Insurance | ||
| Private | Reference | |
| Government | 1.71 (1.27, 2.31) | <0.001* |
| Not Insured | 1.87 (1.02, 3.45) | 0.044* |
| Race | ||
| White | Reference | |
| Asian | 0.82 (0.48, 1.39) | 0.457 |
| Black | 0.76 (0.46, 1.26) | 0.282 |
| Other | 0.60 (0.32, 1.15) | 0.124 |
| Male Sex | 1.13 (0.91, 1.41) | 0.253 |
| Tumor Size ≥ 5 cm | 1.52 (1.16, 1.99) | 0.002* |
| Metastatic | 3.08 (1.86, 5.11) | <0.001* |
| Margins | ||
| Negative | Reference | |
| Positive | 1.54 (1.11, 2.13) | 0.011* |
| No Surgery | 2.06 (1.52, 2.78) | <0.001* |
| RT Modality: Dose (Gy) | ||
| None | Reference | |
| Advanced: <40 | 1.33 (0.56, 3.15) | 0.512 |
| Advanced: 40–65 | 1.10 (0.72, 1.68) | 0.666 |
| Advanced: >65 | 0.78 (0.52, 1.18) | 0.241 |
| Advanced: SRS | 0.91 (0.58, 1.42) | 0.666 |
| Conventional: <40 | 1.80 (1.10, 2.93) | 0.019* |
| Conventional: 40–65 | 1.25 (0.88, 1.77) | 0.221 |
| Conventional: >65 | 1.02 (0.58, 1.80) | 0.945 |
| Comparison | HR (95% CI) | P value |
|---|---|---|
| RT: Advanced >40 Gy vs Conventional | 0.71(0.49, 1.02) | 0.061 |
| RT: Advanced >65 Gy vs Conventional | 0.60 (0.38, 0.93) | 0.024* |
| RT: Advanced >65 Gy vs Conventional ≤65 Gy | 0.52 (0.33, 0.84) | 0.007* |
| RT Advanced: >65 Gy vs ≤65 Gy | 0.65 (0.35, 1.19) | 0.161 |
| RT Dose: >65 Gy vs ≤65 Gy | 0.67 (0.43, 1.03) | 0.071 |
| RT Modality: Advanced vs Conventional | 0.77 (0.53, 1.11) | 0.164 |
| RT: No vs. Yes | 0.88 (0.69, 1.12) | 0.315 |
| Surgery: No vs. Yes | 1.66 (1.28, 2.15) | <0.001 |
Statistical Significance
Note: RT=Radiation Therapy.
Statistical Significance
DISCUSSION:
It has not been established if wide surgical resection alone, surgical resection and therapeutic RT, or surgical resection and therapeutic RT only in the setting of R1/R2 surgical resections is the optimal treatment. The results from this study demonstrate that therapeutic RT increases the OS in the setting of positive surgical margins; however, we did not observe a survival benefit with RT in the setting of negative surgical margins. In addition, high dose RT and PBT were associated with improved survival rates. The multivariable analysis demonstrated advanced RT with a cumulative dose greater than 65Gy had improved survival compared to patients treated with EBRT.
The 5-year survival of the entire cohort was 76%, which was improved over previous database reports ranging from 61–68%.2, 4, 5 Recently published studies have reported 5-year survival rates of 81–92.7% with ion based radiotherapy.6–9 Our study included patients treated with all treatment methods, and not just the most advanced ion-based therapies and wide resections used in these recent studies.
En bloc resection has remained the preferred technique to manage chordomas.10 Intralesional tumor excision and positive surgical margins have been associated with poor local tumor control and decreased OS.11–13 Despite the mortality benefit of en bloc resection, it has been estimated that only 50% of patients are candidates for wide surgical excisions (R0 resection).1 In the current study surgical resection was independently associated with improved OS. The Chordoma Global Consensus Group recommends en bloc surgical resection as the preferred management (level of evidence IV, recommendation B).10 However, monotherapy with RT alone was mentioned as an alternative to surgical management (level of evidence V, recommendation C).10 Recent studies have shown 81–91% 5-year survival rates for patients managed with high dose ion-based radiotherapy alone.7–9, 14–16 Despite the potential for selected chordomas to be managed by RT alone, in the current study when surgical resection and therapeutic RT was compared to therapeutic RT alone, surgical resection had a significant survival benefit.
When investigating patients with positive surgical margins, therapeutic RT resulted in significantly improved survival rates (p=0·047) with estimated 5-year survival of 82·3% compared to 70·6% for surgery alone. The OS rate was higher in patients with negative surgical margins treated with therapeutic RT, but this did not reach statistical significance (88% vs 85%, p=0·889). Despite this study not demonstrating a survival benefit for RT with negative surgical margins certain patients with unfavorable biologic features (tumor size >5cm) or following intralesional resection may benefit from therapeutic RT. The survival of patients with positive surgical margins treated with therapeutic RT approximated the OS of patients with negative surgical margins. This result suggests a surgical resection with a planned positive margin to minimize the sequelae from surgery could be acceptable when therapeutic RT is planned. However, our results support R0 resection to obtain negative surgical margins when major surgical morbidity can be avoided. An interesting finding was skull base chordomas had significantly improved survival compared to mobile spine and sacral chordomas despite having a greater rate of positive surgical margins. Possible explanations for this finding were that skull base chordomas had a smaller tumor burden, patients were on average younger, and patients with skull base chordomas were treated more frequently with advanced and high dose RT. Chordomas have classically been considered radioresistant. With advancement in radiation technology, effective RT doses are now able to be safely delivered.6, 8, 16–19 The advent of ion-based radiotherapies has made high dose RT less morbid, and recent studies have demonstrated greater survival with radiation doses greater than 65–70Gy.17 In the current study, high dose radiation therapy demonstrated improved OS compared to low dose or palliative radiation doses. The modality of RT was also associated with survival. PBT had the greatest 5-year survival, and conventional EBRT had the lowest survival. A statistically significant difference in survival was observed between PBT and EBRT; however, a statistically significant survival difference was not observed between PBT, SRS, and IMRT.
The multivariable analysis identified independent factors associated with decreased survival including tumor size >5cm, non-private medical insurance, metastatic disease, and positive surgical margin. Patients managed at academic centers were associated with improved mortality. Boriani et al in a retrospective review of patients undergoing en bloc resection for primary spinal tumors found significantly decreased OS and increased local recurrence rates in patients managed at non-specialized centers.20 In our study, patients managed at academic centers were more likely to receive advanced and high dose RT. Our results suggest that chordoma patients should be managed at academic centers capable of providing high dose RT.
This study has a number of limitations. The NCDB does not report local tumor recurrence, and the only outcome variable we could investigate was OS. The NCDB does not differentiate between primary and locally recurrent disease. The inclusion of recurrent chordomas underestimates the survival rates for primary tumors. Another limitation of the NCDB is the inability to determine why individual patients were treated with radiation alone compared to surgical resection. One could assume these patients were not medically fit for surgery or deferred surgical intervention; however, this information is impossible to obtain when utilizing the NCDB. Finally, incomplete data reporting for each of the investigated variables could result in selection bias; however, a multiple imputation statistical model was utilized to minimize this error.
The results of this study provide the most up to date survival data for chordoma patients managed with contemporary techniques. Our results support the use of advanced and high dose RT, compared to low dose and EBRT. A clear association was observed for improved survival with therapeutic RT following R1/2 surgical resection. In the setting of R0 surgical resection this analysis did not find a survival benefit with RT. These results support the current practice of recommending adjuvant RT for chordoma patients in the setting of positive surgical margins. In summary, the results of this study support the use of RT to treat chordomas, and provide further clinical equipoise for randomized controlled trials in the future.
Key Points:
Therapeutic radiotherapy, defined as external beam radiation therapy, proton beam radiation therapy, or intensity modulated radiation therapy with a total dose >65Gy or stereotactic radiation therapy, improved survival in patients with positive surgical margins.
Radiation dose greater than 65Gy was associated with improved survival
Proton beam radiation therapy was associated with improved survival compared to external beam radiation therapy.
Acknowledgments
The manuscript submitted does not contain information about medical device(s)/drug(s).
Outstanding Investigator Award from the NCI (R35 CA197616) funds were received in support of this work.
Relevant financial activities outside the submitted work: board membership, grants.
REFERENCES:
- 1.Walcott BP, Nahed BV, Mohyeldin A, et al. Chordoma: current concepts, management, and future directions. The Lancet Oncology. 2012;13(2):e69–76. [DOI] [PubMed] [Google Scholar]
- 2.Lee IJ, Lee RJ, Fahim DK. Prognostic Factors and Survival Outcome in Patients with Chordoma in the United States: A Population-Based Analysis. World neurosurgery. 2017;104:346–55. [DOI] [PubMed] [Google Scholar]
- 3.Dea N, Fisher CG, Reynolds JJ, et al. Current treatment strategy for newly diagnosed chordoma of the mobile spine and sacrum: results of an international survey. Journal of neurosurgery Spine. 2018;30(1):119–25. [DOI] [PubMed] [Google Scholar]
- 4.Pan Y, Lu L, Chen J, et al. Analysis of prognostic factors for survival in patients with primary spinal chordoma using the SEER Registry from 1973 to 2014. J Orthop Surg Res. 2018;13(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun HH, Hong X, Liu B, et al. Survival analysis of patients with spinal chordomas. Neurosurgical review. 2018. [DOI] [PubMed] [Google Scholar]
- 6.Rotondo RL, Folkert W, Liebsch NJ, et al. High-dose proton-based radiation therapy in the management of spine chordomas: outcomes and clinicopathological prognostic factors. Journal of neurosurgery Spine. 2015;23(6):788–97. [DOI] [PubMed] [Google Scholar]
- 7.Imai R, Kamada T, Araki N. Carbon Ion Radiation Therapy for Unresectable Sacral Chordoma: An Analysis of 188 Cases. International journal of radiation oncology, biology, physics. 2016;95(1):322–7. [DOI] [PubMed] [Google Scholar]
- 8.Aibe N, Demizu Y, Sulaiman NS, et al. Outcomes of Patients With Primary Sacral Chordoma Treated With Definitive Proton Beam Therapy. International journal of radiation oncology, biology, physics. 2018;100(4):972–9. [DOI] [PubMed] [Google Scholar]
- 9.Demizu Y, Jin D, Sulaiman NS, et al. Particle Therapy Using Protons or Carbon Ions for Unresectable or Incompletely Resected Bone and Soft Tissue Sarcomas of the Pelvis. International journal of radiation oncology, biology, physics. 2017;98(2):367–74. [DOI] [PubMed] [Google Scholar]
- 10.Stacchiotti S, Sommer J. Building a global consensus approach to chordoma: a position paper from the medical and patient community. The Lancet Oncology. 2015;16(2):e71–83. [DOI] [PubMed] [Google Scholar]
- 11.Hsieh PC, Xu R, Sciubba DM, et al. Long-term clinical outcomes following en bloc resections for sacral chordomas and chondrosarcomas: a series of twenty consecutive patients. Spine. 2009;34(20):2233–9. [DOI] [PubMed] [Google Scholar]
- 12.Stacchiotti S, Casali PG, Lo Vullo S, et al. Chordoma of the mobile spine and sacrum: a retrospective analysis of a series of patients surgically treated at two referral centers. Annals of surgical oncology. 2010;17(1):211–9. [DOI] [PubMed] [Google Scholar]
- 13.Gokaslan ZL, Zadnik PL, Sciubba DM, et al. Mobile spine chordoma: results of 166 patients from the AOSpine Knowledge Forum Tumor database. Journal of neurosurgery Spine. 2016;24(4):644–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mima M, Demizu Y, Jin D, et al. Particle therapy using carbon ions or protons as a definitive therapy for patients with primary sacral chordoma. The British journal of radiology. 2014;87(1033):20130512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen YL, Liebsch N, Kobayashi W, et al. Definitive high-dose photon/proton radiotherapy for unresected mobile spine and sacral chordomas. Spine. 2013;38(15):E930–6. [DOI] [PubMed] [Google Scholar]
- 16.Kabolizadeh P, Chen YL, Liebsch N, et al. Updated Outcome and Analysis of Tumor Response in Mobile Spine and Sacral Chordoma Treated With Definitive High-Dose Photon/Proton Radiation Therapy. International journal of radiation oncology, biology, physics. 2017;97(2):254–62. [DOI] [PubMed] [Google Scholar]
- 17.Gatfield ER, Noble DJ, Barnett GC, et al. Tumour Volume and Dose Influence Outcome after Surgery and High-dose Photon Radiotherapy for Chordoma and Chondrosarcoma of the Skull Base and Spine. Clinical oncology (Royal College of Radiologists (Great Britain)). 2018;30(4):243–53. [DOI] [PubMed] [Google Scholar]
- 18.Yasuda M, Bresson D, Chibbaro S, et al. Chordomas of the skull base and cervical spine: clinical outcomes associated with a multimodal surgical resection combined with proton-beam radiation in 40 patients. Neurosurgical review. 2012;35(2):171–82; discussion 82–3. [DOI] [PubMed] [Google Scholar]
- 19.Sahgal A, Chan MW, Atenafu EG, et al. Image-guided, intensity-modulated radiation therapy (IG-IMRT) for skull base chordoma and chondrosarcoma: preliminary outcomes. Neuro-oncology. 2015;17(6):889–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lador R, Bandiera S, Gasbarrini A, et al. Treatment of Spinal Tumors in a High Volume Center has Direct Impact on Local Recurrence, Morbidity, and Mortality. Clinical spine surgery. 2017;30(8):E1074–e81. [DOI] [PubMed] [Google Scholar]


